Brown adipose tissue (BAT) is an organ specialized to fuel nonshivering thermogenesis for the defense of high body temperature of many eutherian mammals in the cold. Cold-induced sympathetic stimulation of brown adipocytes activates lipolysis, glucose uptake, and mitochondrial biogenesis, with the mitochondrial biogenesis providing a powerful cellular engine for heat generation. At the mitochondrial inner membrane, the energy of nutrients such as glucose and lipids is converted into a proton gradient, but instead of storing the potential energy in the generation of ATP, uncoupling protein 1 (UCP1) catalyzes an inducible proton leak to release the energy of the proton gradient directly as heat. The central role of UCP1 for nonshivering thermogenesis in rodents has been confirmed in knockout mice (1, 2), and the majority of research efforts on BAT and UCP1 centers on the physiological consequences of thermogenesis, including the regulation of body weight for treatment of obesity and other metabolic diseases. Although it appears that UCP1’s function to release the proton motive force as the final energy conversion step may only affect thermogenesis, reduction of the proton motive force will inevitably impact other mitochondrial and cellular processes. Reducing proton motive force by mitochondrial proton leak would alter the redox state of the respiratory chain and potentially reduce the production of reactive oxygen species (ROS) (3). The absence of UCP1 in BAT could be problematic, given the high concentration of respiratory chain complexes in cold-acclimated brown fat mitochondria and the minor concentrations of ATP synthase, with the latter not being able to release the enhanced, cold-induced proton motive force. Although the physiological consequences in UCP1 knockout mice have been almost exclusively attributed to thermogenesis, there may be further consequences on mitochondrial ROS biology and downstream effects that have been widely ignored. In PNAS, Kazak et al. (4) explore cold-induced molecular differences of BAT lacking UCP1 and show that mitochondrial calcium buffering is compromised through ROS production in an UCP1-dependent manner, demonstrating that UCP1 ablation is about more than thermogenesis.
Mitochondrial ROS production has a major functional impact in all cells that has led to the emergence of new theories and biological disciplines such as the “free radical theory of aging” addressing the damage by ROS in aging (5) and the idea of ROS being an important signaling molecule. The sites of ROS production have been intensively explored in recent years, demonstrating that there are 11 molecular sites that are capable of producing ROS, at least in isolated mitochondria (6). The magnitude of ROS depends on the concentration of electron transport chain (ETC) complexes and their redox state, with the latter being affected by the concentration of electrons that are donated by substrates to the ETC and by their ability to be passed on from complex to complex so as to reduce oxygen finally to water. How effectively these electrons can be passed along the ETC also depends on the proton gradient generated by the same ETC. The higher the proton gradient, the more this gradient would stall the ETC, with the electrons possibly “overreducing” their carriers and prematurely escaping the ETC complexes toward oxygen, generating either superoxide or hydrogen peroxide (3). Thus, interspersed literature claiming that ROS production is positively associated with the rate of electron flux and oxygen consumption cannot be substantiated with the current understanding of bioenergetics.
More than 20 y ago, it was already suggested that reduction of the proton motive force by uncoupling agents impacts mitochondrial production of ROS (7). Endogenous proton leak was proposed as the mechanism to reduce ROS (8), and a similar impact on ROS by UCP1 has been verified in isolated brown fat mitochondria by two laboratories (9, 10). However, the involvement of UCP1 in ROS biology has been disputed based mainly on conflicting studies, claiming that ROS directly activates UCPs, thus providing an elegant feedback mechanism to prevent excessive mitochondrial ROS levels (reviewed in ref. 11). Other studies have reported that this feedback mechanism does not exist (12–14) or is mediated by other carriers, such as the adenine nucleotide translocase (ANT) (15). The superoxide dismutase 2 (SOD2) overexpressor mouse, exhibiting reduced superoxide levels, represented an in vivo model that did not show quantitative differences in thermogenic responses compared with wild-type mice (16). The pros and cons of such a mechanism have been critically reviewed recently (17), but new proteomic approaches have brought in novel data showing that ROS-induced modifications of UCP1 are physiologically relevant (18). Although some critiques remain (19), the issue of whether specific ROS-mediated UCP1 modifications possess physiological relevance may only be settled by expanding analysis, e.g., introducing critical ROS-modified amino acids using in vivo CRISPR/Cas approaches.
In the current study, Kazak et al. (4) observe that cold-induced BAT of UCP1 knockout mice has reduced mitochondrial power, seen as reduced respiratory chain subunit concentrations and decreased expression of genes involved in oxidative phosphorylation, thus providing the molecular basis for previous functional observations demonstrating reduced maximal respiration rates in isolated UCP1 knockout mitochondria (10). Increased mitochondrial ROS levels might cause the reduction of the ETC, because mainly mitochondrial, but not nuclear-encoded, ETC subunit expression was disturbed. Importantly, Kazak et al. (4) point out that not all differences in mitochondrial respiration between wild-type and UCP1 knockout mice can be solely attributed to differences in mitochondrial proton leak. That notion is important because UCP1 knockout mice are now widely distributed to investigate BAT mechanisms for the treatment of metabolic diseases. Indeed, mitochondrial uncoupled respiration is only an indirect measure of the mitochondrial proton leak, because proton leak respiration is also a function of maximal substrate oxidation capacity. Only when maximal respiration is constant does the measurement of proton leak respiration give a semiquantitative readout for proton leak (20, 21). Thus, precise measurements of proton conductance would require the simultaneous assessment of proton motive force, which is still the gold standard to quantify the proton leak rates (22).
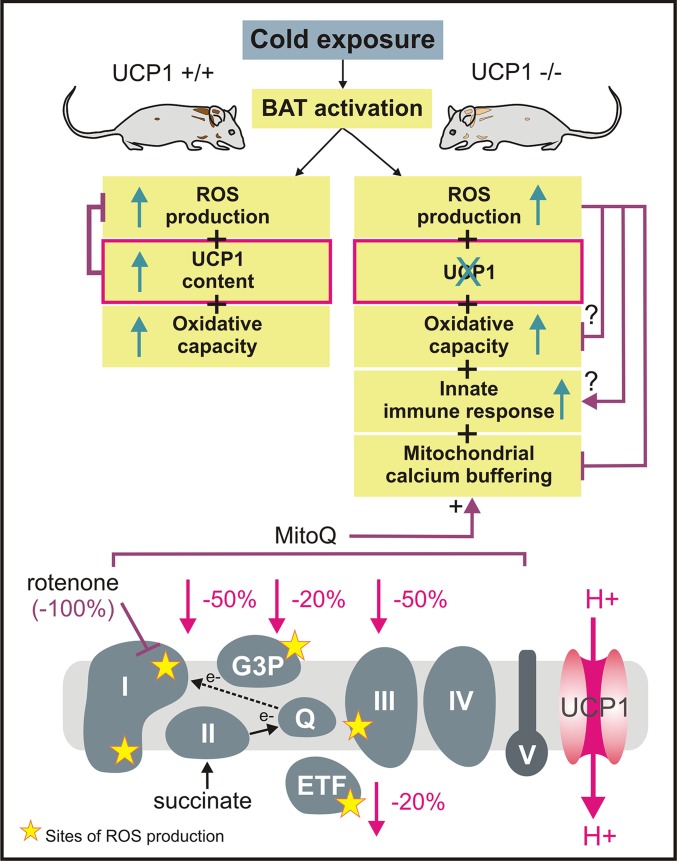
Kazak et al. (4) observe enriched expression of innate immunity genes in UCP1-ablated BAT, supporting a relationship between the immune system and adaptive thermogenesis. So far, it remains unclear whether these gene expression changes are only signs of dysfunctional cells or whether they would support nonshivering thermogenesis (23) or not (24). Kazak et al. (4) establish causality between mitochondrial calcium loading and ROS. UCP1-lacking BAT mitochondria appear more sensitive to extramitochondrial calcium levels that are not explained by differences in the permeability transition pore however (25). Strikingly, eliminating ROS production from the IQ site of respiratory complex I or using the antioxidant MitoQ restores calcium handling of BAT mitochondria. Fig. 1 depicts that ROS production from the IQ site is sensitive to mitochondrial uncoupling and the ROS production by reverse-electron transfer (RET) from this site is blocked by using rotenone in the current study.
Fig. 1.
Regulation of mitochondrial ROS production in BAT. Increases in ROS levels upon adrenergic stimulation in brown adipocytes have been reported by Chouchani et al. (18). In the wild-type condition, increased ROS levels are mitigated by higher UCP1 activity (9, 10). Although causality is not fully established, reducing ROS generation may enable an increase in ETC complexes, which is supported by UCP1-dependent regulation of mitochondrial-encoded ETC subunits (4). Whether the responses of the innate immune system can be attributed to ROS remains to be elucidated. In this study, Kazak et al. (4) establish causality between reduced mitochondrial calcium buffering and increased ROS in UCP1-ablated BAT mitochondria. Mitochondrial ROS production is substantially reduced by UCP1 activity at the brown fat mitochondrial inner membrane [values are calculated using the method of Oelkrug et al. (10)]. Kazak et al. (4) use the complex I inhibitor rotenone to eliminate proton motive force dependent ROS at the IQ site and reduce overall ROS production by mitochondrially targeted antioxidant MitoQ, which are both treatments fully rescuing compromised mitochondrial calcium loading in UCP1−/− mitochondria. ETF, electron transferring flavoprotein; G3P, glycerol-3-phosphate; Q, coenzyme Q.
Clearly, the lack of UCP1 has more than just thermogenic consequences. It would be an important goal to assess all nonthermogenic effects of UCP1 for both fundamental research and translational purposes. How the presence of UCP1 affects cellular processes in human adipose tissue, or in the newly identified UCP1-positive beige adipocyte type, still remains unknown. Given some doubts about the thermogenic role of beige adipose tissue, could its recruitment of UCP1 by a variety of substances and stress factors just be an antioxidant response?
Kazak et al. (4) highlight that there is more than thermogenesis to the elimination of UCP1 in mice that needs to be considered when performing experiments using these mice. One could also consider these aspects when asking “why” and “how” UCP1-dependent proton cycling has been implemented during evolution to serve thermogenesis in eutherian mammals. Heat production in mammals is an energetically expensive characteristic, and its evolutionary development requires outweighing the energetic costs by direct benefits for species selection, such as offspring incubation (26, 27). Concerning molecular evolution, the positive effects of UCP1 on cellular ROS levels may have supported the integration of UCP1 in heat production, allowing high rates of energy turnover not bound to ATP synthesis. When did this integration for thermogenesis possibly occur? The answer is not clear so far. An UCP1 ortholog, distinct from its paralogs UCP2 and UCP3, must have existed before the divergence of lobe- and ray-finned vertebrates more than 420 million years ago because UCP1 is found in fish (28). However, the molecular function and physiological role are unknown to date. Even in marsupials, members of endothermic mammals that split about 130–150 million years ago from eutherians, the role of UCP1 in adipose tissue is unclear. The molecular phylogeny groups marsupial UCP1 close to ectothermic UCP1 orthologs (29), and classical adaptive thermogenic responses are missing in marsupials despite cold induction of UCP1 (30). From the perspective of UCP1’s evolutionary history starting in ectotherms, there should be more than thermogenesis, particularly in archetypal UCP1, and there is accumulating evidence that the UCP1-dependent effects on ROS regulation may have contributed to shape the integration of UCP1 into the high thermogenic capacity of eutherian BAT.
Acknowledgments
I thank Dr. Fabiana Perocchi for helpful edits and Dr. Rebecca Oelkrug for editing the figure for this commentary. M.J. is supported by the German Center for Diabetes Research.
Footnotes
The author declares no conflict of interest.
See companion article on page 7981.
References
- 1.Enerbäck S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 2.Golozoubova V, et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 3.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazak L, et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc Natl Acad Sci USA. 2017;114:7981–7986. doi: 10.1073/pnas.1705406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 8.Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 9.Dlasková A, Clarke KJ, Porter RK. The role of UCP 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta. 2010;1797:1470–1476. doi: 10.1016/j.bbabio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Oelkrug R, Kutschke M, Meyer CW, Heldmaier G, Jastroch M. Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. J Biol Chem. 2010;285:21961–21968. doi: 10.1074/jbc.M110.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echtay KS, Brand MD. 4-hydroxy-2-nonenal and uncoupling proteins: An approach for regulation of mitochondrial ROS production. Redox Rep. 2007;12:26–29. doi: 10.1179/135100007X162158. [DOI] [PubMed] [Google Scholar]
- 12.Couplan E, et al. No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J Biol Chem. 2002;277:26268–26275. doi: 10.1074/jbc.M202535200. [DOI] [PubMed] [Google Scholar]
- 13.Shabalina IG, et al. Cold tolerance of UCP1-ablated mice: A skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects. Biochim Biophys Acta. 2010;1797:968–980. doi: 10.1016/j.bbabio.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Nabben M, et al. Uncoupled respiration, ROS production, acute lipotoxicity and oxidative damage in isolated skeletal muscle mitochondria from UCP3-ablated mice. Biochim Biophys Acta. 2011;1807:1095–1105. doi: 10.1016/j.bbabio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Parker N, Affourtit C, Vidal-Puig A, Brand MD. Energization-dependent endogenous activation of proton conductance in skeletal muscle mitochondria. Biochem J. 2008;412:131–139. doi: 10.1042/BJ20080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva JP, et al. SOD2 overexpression: Enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J. 2005;24:4061–4070. doi: 10.1038/sj.emboj.7600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crichton PG, Lee Y, Kunji ER. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie. 2017;134:35–50. doi: 10.1016/j.biochi.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouchani ET, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls DG, Rial E. A novel regulatory mechanism for the brown-fat uncoupling protein? Nat Struct Mol Biol. 2016;23:364–365. doi: 10.1038/nsmb.3221. [DOI] [PubMed] [Google Scholar]
- 20.Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 2011;26:192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 21.Keipert S, Jastroch M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta. 2014;1837:1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Affourtit C, Quinlan CL, Brand MD. Measurement of proton leak and electron leak in isolated mitochondria. Methods Mol Biol. 2012;810:165–182. doi: 10.1007/978-1-61779-382-0_11. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer K, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crichton PG, Parker N, Vidal-Puig AJ, Brand MD. Not all mitochondrial carrier proteins support permeability transition pore formation: No involvement of uncoupling protein 1. Biosci Rep. 2009;30:187–192. doi: 10.1042/BSR20090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oelkrug R, et al. Brown fat in a protoendothermic mammal fuels eutherian evolution. Nat Commun. 2013;4:2140. doi: 10.1038/ncomms3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oelkrug R, Polymeropoulos ET, Jastroch M. Brown adipose tissue: Physiological function and evolutionary significance. J Comp Physiol B. 2015;185:587–606. doi: 10.1007/s00360-015-0907-7. [DOI] [PubMed] [Google Scholar]
- 28.Jastroch M, Wuertz S, Kloas W, Klingenspor M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol Genomics. 2005;22:150–156. doi: 10.1152/physiolgenomics.00070.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hughes DA, Jastroch M, Stoneking M, Klingenspor M. Molecular evolution of UCP1 and the evolutionary history of mammalian non-shivering thermogenesis. BMC Evol Biol. 2009;9:4. doi: 10.1186/1471-2148-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polymeropoulos ET, Jastroch M, Frappell PB. Absence of adaptive nonshivering thermogenesis in a marsupial, the fat-tailed dunnart (Sminthopsis crassicaudata) J Comp Physiol B. 2012;182:393–401. doi: 10.1007/s00360-011-0623-x. [DOI] [PubMed] [Google Scholar]