Abstract
Much recent research on adolescent decision making has sought to characterize the neurobiological mechanisms that underlie the proclivity of adolescents to engage in risky behavior. One class of influential neurodevelopmental models focuses on the asynchronous development of neural systems, particularly those responsible for self-regulation and reward seeking. While this work has largely focused on the development of prefrontal (self-regulation) and striatal (reward processing) circuitry, the present article explores the significance of a different region, the anterior insular cortex (AIC), in adolescent decision making. Although the AIC is known for its role as a cognitive-emotional hub, and is included in some models of adult self-regulation and reward seeking, the importance of the AIC and its maturation in adolescent risk taking has not been extensively explored. In this article we discuss evidence on AIC development, and consider how age-related differences in AIC engagement may contribute to heightened risk taking during adolescence. Based on this review, we propose a model in which the engagement of adolescents in risk taking may be linked in part to the maturation of the AIC and its connectivity to the broader brain networks in which it participates.
Keywords: Adolescence, Risky decision making, Neural development, Anterior insula
Introduction
Adolescence is a period characterized by high rates of mortality and accidental injury that result from heightened risk taking. Compared to adults, adolescents evince higher rates of violent and nonviolent crime [1–3], automobile crashes and fatalities [4], unprotected sex [5], and initial experimentation with tobacco, alcohol and illicit drugs [6]. In order to create and implement educational programs that are effective at decreasing risk taking during adolescence, it is important that we understand the mechanisms that contribute to the increased tendency of adolescents to engage in these behaviors.
While there is a considerable body of prior work exploring the psychosocial factors that contribute to heightened risk taking during adolescence, researchers have recently focused greater attention on characterizing the neurobiological mechanisms that might underlie this hallmark trait of adolescence. Much of this work has focused on patterns of development in the prefrontal cortex (PFC), a locus for self-regulatory signaling, and the striatum, a focal point for reward signaling. The current article attempts to extend current neurodevelopmental models by considering how maturation taking place within another brain region, the anterior insular cortex (AIC), may also contribute to heightened risk taking during adolescence. Although the AIC is known for its role in the integration of sensory information into cognitive and affective processes, and is a key structure in some models of self-regulation and reward seeking [7–10], the development of the AIC and its role in adolescent risk taking remain largely underexplored.
Current Neurobiological Theories of Adolescent Decision Making
In order to understand how the development of the AIC and its networks might impact decision making, it is useful to first consider current neurodevelopmental theories of adolescent decision making, particularly in the context of risk taking. Around the onset of puberty, the human brain is known to undergo a major reorganization of neural structures, networks, and functioning [11, 12]. While this dramatic neural reorganization begins early in adolescence, it does not occur uniformly across the brain [13]. The distinct trajectories of maturation exhibited by different brain regions and networks have been the focus of several theories of adolescent risk taking [14–17].
One class of models in particular, known as ‘dual systems’ models, has occupied a prominent position in the field [14, 17, though see also 18, 19]. Dual systems models highlight the asynchronous development of the neural systems that underlie sensation seeking – the tendency to seek varied, novel, complex and intense sensations and experiences [20] – and self-regulation – the deliberate modulation of one’s thoughts, feelings or actions in the pursuit of planned goals [21, 22]. Within this framework, the maturation of reward centers in the brain, localized to midline dopaminergic regions, including the striatum, is thought to occur early in adolescence, around the time of puberty, and is hypothesized to evoke an increase in reward sensitivity that results in more frequent sensation seeking [for reviews, see 23, 24]. The major changes in this reward-processing system are thought to occur prior to the completion of a more prolonged period of maturation exhibited by the brain regions involved in self-regulation, notably the lateral PFC (lPFC) [for review, see 22]. The crux of the dual system hypothesis is that the maturational imbalance during adolescence between heightened sensation seeking and a still-maturing capacity for self-regulation makes adolescents particularly susceptible to engage in risky behaviors [for review, see 25].
Some theories of adolescent development have extended this basic model to also include brain regions involved in harm avoidance [for full review, see 15, 26]. One such view, referred to as the triadic model, highlights the blunted engagement of the amygdala during adolescence (recent reviews also consider the potential role of the thalamus and insula in the harm avoidance network) [26]. Thus, within this framework, the tendency of adolescents to engage in risky behavior is due not only to an imbalance between self-regulation and sensation seeking, but also to their inability to engage harm avoidance circuitry during decision making.
A shared characteristic of the dual system and triadic models is that they both treat adolescent risk taking as the result of interactions among separate brain networks, especially those involved in deliberative and affective information processing. However, the mechanisms that govern the interactions among these brain systems are poorly understood. Thinking about the mechanisms that might dictate how and when deliberative and affective brain networks are engaged is what first led us to consider the potential importance of the AIC in the normative development of decision making. This region is thought to act as a hub involved in the integration of cognitive and emotional information [10], and to have structural and functional connections to regions that feature prominently in current developmental models of adolescent decision making (e.g., the IPFC and striatum). Thus, we believe that the AIC is very likely to play a part in the development of processes that involve the interplay of cognitive and affective systems, as is thought to occur in the context of risky decision making. Our focus on the AIC was also motivated by the frequency with which activations in the AIC are found, but not discussed, in the developmental literature. While other structures serving hub-like functions (e.g., the thalamus) may also be relevant to adolescent decision making, activation of the AIC seemed to us to be especially pervasive, and thus our purpose in this paper was to consider its possible developmental role. The functions of other hub-like structures may be worthy of future consideration, but this was beyond the scope of our review.
Structure and Function of the Insula
The insula has, for the most part, eluded consideration in neurodevelopmental theories of decision making [though see 27, 28], yet it is implicated in a markedly diverse range of functions that are relevant to current theories of adolescent decision making, such as attention [7], sexual attraction/romance [29], language [30], social experiences [31] and motivation/reward [8], among others [for review, see 32]. Located at the base of the sylvian fissure, this large cortical area separates the frontoorbital, frontoparietal and temporal opercular regions [33]. The insula is known to receive direct input from the somato-sensory cortex and to then project outputs to both cortical and subcortical regions, including the PFC (lPFC and ventral medial PFC, vmPFC), striatum, anterior cingulate cortex (ACC), amygdala, and superior temporal sulcus [34–36]. Thus, the insula sends its outputs to many of the brain structures that are essential to deliberative and affective processing and that are central components of the dual systems and triadic models of adolescent decision making.
Researchers have identified two distinct subregions of the insula, the anterior and posterior portions, which differ from each other in both structure and function. The posterior insula receives an abundance of projections directly from somatosensory regions and is involved in visceral sensations such as pain [32]. The anterior portion of the insula also receives direct projections from somatosensory regions, but is involved in the conscious representation of these bodily sensations and in the regulation of arousal [32, 37]. Accordingly, one view of AIC function highlights its involvement in the subjective experience of arousal (feelings experienced as a result of an event or stimulus) [38–40]. For example, a study by Lovero et al. [41] (2009) showed that increased AIC activation (as reflected in BOLD signal) during anticipation of touch was positively associated with self-reported ratings of the intensity of the stimulus. The relationship between AIC anticipatory engagement and self-reported ratings of the intensity of the experience suggests that the AIC directly impacts the way in which an event is experienced, including the subjective arousal and emotionality evoked by the experience [42, 43].
More recently, theorists have proposed that AIC function goes beyond simply interpreting signals of arousal, and have argued that this region acts as an ‘integrative hub’ which is centrally involved in coordinating the recruitment of task- and context-relevant brain networks in response to arousal [10]. That is, at least in adulthood, this region participates in multiple brain networks and varies its functional involvement with these networks according to the nature of the stimulus environment and task demands. The hub model thus highlights the ability of the AIC to process and interpret physiological signals and then project information regarding the experience to the specific brain networks that are most suited to achieve the desired behavioral response, a role that is particularly important in situations that require an interplay of cognitive and emotional processes, often referred to as cognitive-emotional interactions [10, 44, 45]. The notion that the AIC may govern the engagement of specialized brain networks to which it is connected finds support in evidence that activation of the AIC precedes, and directly influences, activation of prefrontal regions during response inhibition [44] and activation of the striatum during reward anticipation [27]. Such findings are especially relevant to neurodevelopmental models of decision making, since they implicate the AIC in the initiation of activity within the separate deliberative and affective brain circuits that are the focus of current theories of adolescent decision making.
Indeed, activity in the AIC is a common feature in studies of cognitive control and inhibitory processing [7, 46–50] and reward sensitivity [8, 9]. Moreover, recent neuroimaging evidence shows that the role of the AIC in cognitive and affective interactions influences how individuals make decisions that involve risk [51, 52]. Consistent with this notion, the way in which recruitment of the AIC influences adult decision making and, in particular, whether it predicts risk taking or risk aversion, is dependent on past experiences, task performance, and individual differences in risk preference and strategy. For instance, compared to individuals who are more inclined toward risk taking, adults who are risk averse show increased AIC engagement when taking risks [52]. The increase in AIC recruitment during risk taking in risk averse individuals may be the result of a desire to integrate a greater amount of cognitive and affective information before making a decision. While the adaptive nature of AIC recruitment during decision making does not lead to a simple explanation of its role, it is clear that it is important for integrating emotional and cognitive information during the decision-making process and it is likely that differences in engagement of this region have significant implications for understanding individual differences in risk-taking behavior.
Defining the Boundaries of the AIC
Evidence of AIC involvement in the interplay between cognitive and affective processes makes this region a prime candidate for further investigation in studies of developmental changes in the neural underpinnings of decision making. Few studies of adolescent risk taking and decision making explicitly focus on the AIC. However, in many instances information regarding AIC engagement is contained in tables or supplementary material. These studies often implicate the AIC, but their discussion and interpretation of findings typically focus on other regions. To explore how the AIC might contribute to the development of decision making, we reviewed a corpus of prior developmental neuroimaging studies investigating structural and functional connectivity, response inhibition, anticipation of reward and risky decision making, looking specifically for information regarding age-related differences in AIC activation and connectivity.
Since the AIC has seldom been treated as a target region in the developmental studies we identified in this review, clusters of activation occurring proximal to the AIC, as well as connections that involve this region, are not always labeled accordingly. For example, BOLD activations with peak coordinates that are spatially consistent with the location of the AIC on a stereotaxic atlas are variably referred to as occurring in the inferior frontal gyrus, the pars opercularis (of the inferior frontal gyrus), the frontoinsular cortex, or just the insula (with no specificity as to the anterior-posterior dimension). These alternative designations may reflect the following: (1) the conceptual expectations of the authors of the study (e.g., a hypothesis that activation would occur in the inferior frontal gyrus rather than the AIC), (2) limitations of the probabilistic atlas labels included in many fMRI software packages, (3) an attempt to accommodate the spatial resolution limits of fMRI by using generic labels [53, 54] or (4) compensation for possible distortions due to large vasculature (middle cerebral artery) that passes through the junction between the AIC and the operculum [55–57].
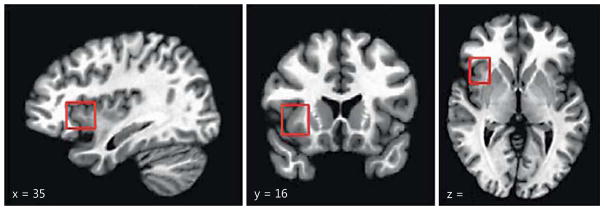
Because our goal was to investigate the possibility that the AIC is a more consistently implicated structure in adolescent decision making than previously recognized, for the purposes of the current paper we disregarded the specific labels used in prior studies and adopted an inclusive strategy that defined the AIC as a region falling within the following stereotaxic boundaries: x = ±45 to ±25, y = +27 to +5, z = +12 to −9 (Montreal Neurological Institute, MNI, atlas; see fig. 1). With the exception of the posterior edge, which marks the approximate midway point between the anterior and posterior margins of the insula, these boundaries mark the furthest extent in each dimension where the insular cortex (gray matter) is present on the MNI atlas. All activations hereafter referred to as the AIC have peak voxels within these boundaries. Because there are important structural and functional differences between the anterior portion of the insula in nonhuman species and the AIC in humans, our review only includes studies of humans [10, 58].
Fig. 1.
MNI boundaries of the AIC. The AIC was defined as all peak coordinates within the following boundaries: x = ±45 to ±25; y = +27 to +5; z = +12 to −9.
To determine the papers to include in our analyses we performed PubMed searches using various combinations of the following keywords: ‘adolescent, adolescence, development, reward, reward anticipation, response inhibition, inhibitory control, decision making, risk taking, risky decision making’. Additional citations mentioned in the papers produced by these searches were also considered for inclusion. Further criteria for inclusion in our analysis were the use of nonclinical adolescent and adult participants, and the explicit testing of developmental differences during the relevant phases for each task type. Candidate studies could examine developmental differences via subtractive group comparisons (e.g., adolescent vs. adult participants) or trajectory analyses (e.g., linear age-related changes). In our exploration of reward tasks we only included papers that focused on the anticipatory phase of reward processing (rather than outcome-related differences). Similarly, we focused on the decision-making phase of risk-taking tasks rather than on postdecision feedback. Using these criteria we identified 15 papers on response inhibition, 12 on reward anticipation and 6 on risky decision making for inclusion in our review. Only activations that survived statistical corrections were included. Results from any publications originally reporting Talairach coordinates were converted to MNI coordinates in order to standardize the comparison of studies.
Developmental Changes in AIC Structure and Connectivity
We began our search for developmental differences in AIC function by examining whether the AIC exhibits evidence of changes in either structure or connectivity across the period of adolescence. Similar to findings from whole brain studies, which show different timing and rates of development in various regions [59], different patterns of development have been documented within different areas of the insular cortex [60, 61]. Using cortical thickness measurements as a marker of neural maturity (thinner cortex is associated with greater neural maturity), Shaw et al. [60] (2008) found that the most anterior portion of the insula (not anatomically defined in the paper, however) exhibited a linear pattern of thinning across adolescence, suggesting that this region undergoes ongoing maturation across this developmental period, similar to that typically found in regions linked to cognitive control (e.g. the IPFC) [28, 62]. Meanwhile, slightly posterior and ventral portions of the insula evinced a curvilinear pattern of thinning, similar to pruning patterns found in reward-related regions (the striatum) [63]. Importantly, the subregions identified by Shaw et al. [60] closely resemble subregions identified in a recent meta-analysis of adult insular function during cognitive and emotional processes [64]. In particular, the dorsal portion of the AIC, which according to Chang at al. [64] is commonly recruited during cognitive processes, is similar to the region showing a linear developmental pattern in the paper by Shaw et al. [60], whereas the ventral portion of the AIC, which according to Chang et al. [64] was recruited during affective processes, corresponds to the insular region exhibiting a curvilinear developmental trajectory in the study by Shaw et al. [60]. Different developmental trajectories within the AIC, particularly in subregions implicated in cognitive and affective processes, suggest that ongoing development of the AIC during adolescence may differentially impact cognitive and affective functioning.
A recent investigation of insular development using diffusion tensor imaging [61] likewise demonstrates disparate developmental trends in the different fiber projections from the insula. In this study, only linear changes were considered across the three cross-sectional age groups examined: early adolescents (aged 12), adolescents (aged 16) and adults (aged 20–30). A general pattern of decreased fiber density across development, which might be interpreted as the downstream consequence of cortical pruning, was observed in several insular projections, including the connection from the AIC to several frontal regions, whereas no changes in fiber density were seen in connectivity to subcortical regions such as the striatum [61]. This also suggests that the development of the AIC may have different implications for cognitive and affective processes during adolescence.
The continuing maturation of the AIC during adolescence is also evident in changes in its pattern of functional connectivity with cognitive control and reward-processing regions. For example, a study examining resting state connectivity within a network including the AIC, the dorsal ACC, the anterior PFC and the thalamus found that although the network was already established by adolescence, inter-network strength, including the specific pathway between the AIC and PFC, continued to increase with age [65]. Whereas that study pointed most specifically to connections between the AIC and an anterior portion of the PFC, other studies using functional connectivity analyses show that AIC-IPFC connectivity also changes across development. One study, for example, found that the AIC and IPFC are coactivated to a greater degree in adults, compared to adolescents, during a cognitive challenge task [66].
The aforementioned studies, showing changes with development in AIC connectivity to cognitive control regions, may have important implications for the development of self-regulatory behaviors during this time. Indeed, it was recently discovered that age-related thinning of the AIC is significantly correlated with decreases in self-reported impulsivity across adolescence and into early adulthood [28]. Although these findings suggest that the maturation of impulse control may be AIC specific, it is possible that the relation between the development of the AIC and the development of impulse control reflects the strengthening of the entire cognitive control network during adolescence, including the connection between the AIC and IPFC.
Connectivity studies suggest that the AIC is not only involved in the activation of the brain’s cognitive control network but also in reward and affective processing. Specifically, the AIC has been shown to act in cooperation with several reward-processing areas (ACC, striatum and thalamus [27, 67–69]) and, importantly, its involvement with these regions appears to be dependent on age [69]. Using a resting-state connectivity approach, Stevens et al. [69] (2009) demonstrated that a network composed of the insula, striatum, ACC and temporal lobe was already operational by adolescence but, like the control network observed by Fair et al. [65] (2007), the interconnectivity of this network continued to strengthen with increases in age into young adulthood. Task-dependent connectivity findings also point to developmental changes in the relationship between the AIC and the striatum, but suggest that this connection may have reached a state of maturity by midadolescence that is adequate to support adult-like functions [27, 67]. For example, Cho et al. [27] (2013) found that, already by adolescence, activation of the AIC is able to evoke subsequent activation within the striatum during anticipation of reward. Not only is the relationship between the AIC and striatum established by adolescence, but the strength of connectivity (as indexed by co-activation) between the insula and the striatum during reward anticipation did not differ from that of adults.
While admittedly limited, the existing literature suggests that during adolescence the AIC is still undergoing maturational changes (e.g. changes in cortical thickness and connectivity) that impact its ability to process information and its involvement with cognitive control and reward-processing regions. Continuing development of the AIC may be particularly relevant to the emergence of self-regulatory behaviors, including impulse regulation, during this period of life.
Activation of the AIC in Association with Response Inhibition
In the developmental decision-making literature, the maturation of brain regions supporting response inhibition is thought to underlie an eventual decline in risk-taking behavior [for review, see 22 ]. The literature on behavioral response inhibition consistently demonstrates that the ability to successfully inhibit a response improves linearly from childhood through adulthood [70–72] and, though the evidence is a bit mixed, this behavioral trend is generally found to coincide with linear increases in the focal recruitment of the IPFC across this developmental period [73–80, though see also 81–84].
Indications of a strengthening connection between the AIC and IPFC during adolescence [66], and of a link between AIC cortical thinning and self-reported impulse control [28], suggest that AIC maturation may also be essential to the growth of the ability to inhibit unintended or undesirable responses. A specific prediction derived from this view is that, in parallel to age-dependent changes in IPFC activation, the developmental neuroimaging literature should contain evidence of weaker (or less focal) AIC recruitment among adolescents relative to adults.
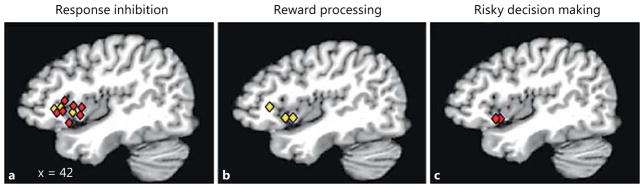
Upon inspection of the developmental response inhibition literature, we find considerable evidence of developmental differences in AIC recruitment [73–77, 79, 84, 85]. The stereotaxic peaks for AIC sites showing developmental differences are plotted in figure 2a. Of the studies reporting on age-dependent effects in AIC activation during response inhibition, seven studies report significantly weaker functional engagement of the AIC in adolescents compared to adults [73–75, 77, 79, 84, 85], while only three find stronger AIC activation among adolescents than adults [75–77]1. For example, using the Simon task (spatial or motor Stroop), Rubia et al. [77] (2006), demonstrated that during incongruent trials, which require suppression of a prepotent response, adults recruited the AIC significantly more than did adolescents, an effect that was also correlated with age when treated as a continuous variable. Across all subjects, increased AIC activation was also related to fewer incongruent task errors. The same age- and performance-related effects were observed in the IPFC. The common patterns obtained for the AIC and IPFC are in line with the structural and connectivity data discussed earlier.
Fig. 2.
Peak coordinates of developmental differences in AIC recruitment during response inhibition (a), reward processing (b) and decision making (c). Red diamonds represent age-related increases in AIC activation while yellow diamonds represent age-related decreases in AIC activation.
Age-related differences in AIC recruitment during response inhibition suggest that the functionality of the AIC, at least as it is related to response inhibition, is still maturing during adolescence 2. In fact, from the studies we found it seems that age-related increases in AIC recruitment during adolescence are just as consistent as age-related increases in IPFC recruitment (see table 1), with several studies showing concomitant developmental differences in both the AIC and IPFC [73–77, 79, 84]. The pervasiveness of AIC differences across developmental studies of response inhibition suggests that maturation of the IPFC is not uniquely explanatory of age-related improvements in response inhibition. Instead, the development of this capacity may be the result of continuing maturation of the cognitive control network more broadly, and especially the interrelationship between the AIC and the IPFC.
Table 1.
Developmental neuroimaging studies
| Author | Year | Task | Sample | Developmental differences | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| lPFC (Brodmann area 8, 9, 44, 45, 46) | Striatum | AIC | AIC coordinates (MNI) | ||||||
|
| |||||||||
| x | y | z | |||||||
| Response inhibition | |||||||||
| Adleman [73] | 2002 | Stroop | 7–22 | Increase with age | Increase with age | Increase with age1 | |||
| Andrews-Hanna [74] | 2011 | Stroop | 14–25 | Adults > adolescents | n.s. | Adults > adolescents | −42 | 22 | 2 |
| Booth [81] | 2003 | Go/no-go | 9–11 | Children > adults | Children > adults | n.s. | |||
| Bunge [75] | 2002 | Flanker | 8–12 | Adults > early adolescents | Adults > early adolescents | Adults > early adolescents (R) | 34 | 17 | −9 |
| Early adolescents > adults (L) | −42 | 27 | 4 | ||||||
| Go/no-go | Adults > early adolescents | n.s. | n.s. | ||||||
| Casey [82] | 1997 | Go/no-go | 7–12 | Early adolescents > adults | n.s. | n.s. | |||
|
| |||||||||
| Casey [117] | 2002 | Stimulus-response compatibility | 7–11 | n.s. | Adolescents > adults | n.s. | |||
| Cohen [113] | 2010 | Stop signal task | 9–19 | n.s. | n.s. | n.s. | |||
| Luna [83] | 2001 | Antisaccade | 8–30 | Adolescents > adults | n.s. | n.s. | |||
| Marsh [76] | 2006 | Stroop | 7–57 | Increase with age | Increase with age | Decrease with age | −36 | 13 | 1 |
| Rubia [77] | 2000 | Stop task | 12–19 | Adults > adolescents | Adolescents > adults | Adults > adolescents (L) | −40 | 6 | 5 |
| Adolescents > adults (R) | 38 | 22 | 6 | ||||||
| Rubia [79] | 2006 | Simon task | 10–17 | Adults > adolescents | Adults > adolescents | Adults > adolescents | 32 | 19 | 11 |
| Switch task | Adults > adolescents | Adults > adolescents | Adults > adolescents | −32 | 7 | −2 | |||
| Go/no-go | Adults > adolescents | Adults > adolescents | n.s. | ||||||
|
| |||||||||
| Rubia [85] | 2007 | Stop task | 10–17 | n.s. | Increase with age | Increase with age | 43 | 27 | 2 |
| Rubia [78] | 2013 | Stop task | 13–19 | Increase with age | Increase with age | n.s. | |||
| Decrease with age | |||||||||
| Tamm [84] | 2002 | Go/no-go | 8–20 | Decrease with age | n.s. | Increase with age | −34 | 12 | 6 |
| Velanova [80] | 2009 | Antisaccade | 8–27 | Increase with age | n.s. | n.s. | |||
|
| |||||||||
| Reward processing | |||||||||
| Bjork [92] | 2004 | Monetary incentive delay | 12–17 | n.s. | Adults > adolescents | n.s. | |||
| Bjork [93] | 2010 | Monetary incentive delay | 12–17 | n.s. | Adults > adolescents | n.s. | |||
| Christakou [118] | 2013 | Gambling task | 12–18 | Increase with age | n.s. | n.s. | |||
| Galvan [90] | 2006 | Pirate task | 13–17 | n.a. | Adolescents > adults | n.a. | |||
| Galvan [98] | 2013 | Primary reward | 13–17 | Adolescents > adults | Adolescents > adults | Adolescents > adults | 34 | 26 | 8 |
| Geier [94] | 2010 | Reward antisaccade | 13–17 | n.s. | Adolescents > adults | n.s. | |||
|
| |||||||||
| Hoogendam [114] | 2013 | Monetary incentive delay | 10–25 | n.s. | Decrease with age | n.s. | |||
| Jarcho [99] | 2012 | Guessing task | 14 | n.s. | Adolescents > adults | Adolescents > adults | 34 | 8 | −3 |
| Krain [95] | 2006 | Card guessing task | 13–17 | n.s. | n.s. | n.s. | |||
| Padmanabhan [96] | 2011 | Reward antisaccade | 14–17 | n.a. | Adolescents > adults | n.a. | |||
| Teslovich [115] | 2013 | Rewarded random dot | 13–21 | Adolescents > adults | n.s. | n.s. | |||
| Van Leijenhorst [97] | 2010 | Slot machine | 14–15 | n.s. | Adolescents > adults | Adolescents > adults | 42 | 12 | −3 |
|
| |||||||||
| Risky decision making | |||||||||
| Bjork [102] | 2007 | Chicken game | 12–17 | n.s. | n.s. | n.s. | |||
| Chein [103] | 2011 | Stoplight | 14–18 | Adults > adolescents | Adolescents > adults2 | n.s. | |||
| Paulsen [105] | 2012 | Domino game | 14–16 | Increase with age | Increase with age | Increase with age | 36 | 16 | −6 |
| Eshel [104] | 2007 | Wheel of fortune | 9–17 | n.s. | n.s. | Adults > adolescents | −44 | 14 | −6 |
| Van Leijenhorst [116] | 2006 | Cake task | 9–12 | n.s. | n.s. | n.s. | |||
| Barkley-Levenson [101] | 2013 | Gambling task | 13–17 | n.s. | n.s. | n.s. | |||
n.s.= A whole-brain analysis was reported but no peak activations for the ROI were reported. n.a.= no whole-brain analyses were reported and therefore we were unable to assess activation in this region.
The specific peak coordinates of this activation are unavailable due to the large cluster size included in this activation map (6,109 voxels).
Ventral striatum activation in Chein et al. [103] (2011) was only significant when social context was manipulated.
Activation of the AIC in Association with Reward Processing
One of the most prominent and well-documented neurobiological characteristics of adolescence is that it coincides with a peak in reward sensitivity [for review, see 23, 24]. Increased reward sensitivity during adolescence is believed to underlie increases in sensation seeking [86], attention to social information [87], the inability to delay gratification [86] and risky decision making [88, 89]. Changes in reward sensitivity during adolescence are believed to be a result of early maturation of the brain’s reward-processing system, including the striatum, which makes adolescents hypersensitive to opportunities for, and receipt of, rewards [90, 91].
The striatum and in particular the ventral areas of the striatum, including the nucleus accumbens, have been the focal point of much neurodevelopmental inquiry related to reward anticipation and sensitivity. In fact, several of the studies that we identified on the developmental and neural underpinnings of reward processing selectively examined striatal functioning by using an ROI-based analysis [90, 94, 96]. While the use of ROI-based analyses may be important for theory-driven, a priori, hypothesis testing, failure to report whole-brain analyses precludes the possibility of identifying other regions that may also account for developmental differences in reward sensitivity, such as the AIC.
Despite this caveat, we were able to find some limited evidence consistent with the idea that the AIC is an important area in the development of reward processing, particularly during anticipation of reward (see table 1; fig. 2b) [91, 98, 99]. One study showing that the development of the AIC, alongside that of the striatum, is relevant to even basic reward processing was conducted by Van Leijenhorst et al. [91]. In the study, adolescents (aged 10–15 years) and adults (aged 18–23 years) completed a slot machine task in which they passively viewed slots that signaled possible rewards or nonrewards. When presented with the possibility of obtaining a reward, the adolescents, but not the adults, demonstrated increased recruitment of both the AIC and the striatum. The authors interpreted heightened AIC activation in adolescents as a reflection of increased physiological arousal associated with either the excitement of possibly receiving a reward or the anxiety produced by the uncertainty of receiving a reward. They go on to suggest that the coactivation of the AIC and striatum may increase the proclivity of adolescents to engage in risk-taking behaviors.
In another recent study implicating AIC maturation in the development of reward processing, Galvan and Mc-Glennen [98] (2013) used a juice administration paradigm to look at the receipt of appetitive (sucrose-water) versus aversive (salt-water) liquids in adolescents and adults. They found that receipt of an appetitive liquid increased AIC and striatal recruitment in adolescents, but not in adults. In adolescents, both AIC and striatal activation during receipt of sucrose-water was correlated with self-reported pleasure ratings. Conversely, adults showed more AIC activation during receipt of an aversive liquid (salt-water), which was unrelated to the self-reported rating of the experience. These findings suggest that the AIC may be more involved in appetitive processes during adolescence, but may then shift to involvement in harm avoidance processes in adulthood.
These two studies, which both show age-dependent differences in AIC (and striatal) activation during reward processing, illustrate that even if connectivity between the AIC and the striatum reaches an adult-like state by adolescence [27, 67], reward opportunities may still evoke stronger recruitment of both regions during adolescence.
We must acknowledge, however, that there are also several studies that do not find age-related differences in this region [90, 92, 95, 96, 100]. It is possible that both the structural and functional connectivity between the AIC and reward processing regions is adequately mature during adolescence [27] and that the AIC therefore exhibits adult-like engagement during anticipation of reward. Given the role of the AIC in cognitive-emotional interactions, another possibility is that the simplistic nature of reward-processing tasks may not require the integration of cognitive and affective information that may be a critical contributor to age differences in activation in this region. That is, age-related differences in AIC engagement may be more easily identified via tasks or paradigms that emphasize the interplay of cognitive and emotional processes, as is the case with risk taking.
Developmental Changes in AIC Involvement during Risky Decision Making
Current developmental theories posit that increases in risky behaviors during adolescence are a result of the maturational imbalance between neural systems underlying sensation-seeking and self-regulatory behaviors, which leads adolescents to disproportionately activate appetitive, reward-seeking circuitry rather than deliberative, cognitive control circuitry during risky decision making, particularly in the face of arousing, contextual influences [for review, see 25]. Only a handful of studies (see table 1) have explored age differences in brain activity during the performance of a risk-taking task [97, 101–105], and evidence for mutual involvement of both the IPFC and striatum during risky decision making is scarce. Indeed, only one study has reported age-related differences as hypothesized by current neurodevelopmental models (increased engagement of reward processing regions and less engagement of cognitive control regions in adolescents compared to adults) in both regions [103] and, at least for the striatum, the age difference was only observed when social context was manipulated. Only one other study involving a risk-taking task demonstrates age differences in both the PFC and striatum during risky decision making however, this study showed striatal engagement increasing with age [105].
When we survey the decision-making literature for evidence of age-related differences in AIC engagement, we find two studies showing increases in AIC engagement with age (see fig. 2c) [104, 105]. In the first study by Eshel et al. [104] (2007), neural responses between adolescents and adults were compared during decision making on a commonly used risk-taking task – the wheel of fortune task. In this task, participants were presented with a wheel that was divided into two colored sections varying in probability and magnitude of reward. Participants were then asked to decide which portion of the wheel they would like to bet on – the risky or safe section. Overall, there were no group differences in risk-taking behavior or in IPFC or striatal engagement during risky decision making (i.e. decision making where the risky portion of the wheel was subsequently chosen). However, adults demonstrated increased AIC activation compared to adolescents during risk taking. The authors suggested that blunted AIC engagement in adolescents may reflect difficulty integrating affective and nonaffective information into the decision-making process, particularly information related to the possible consequences of a decision.
These findings support the notion that there are differences in AIC recruitment during risky decision making between adolescence and adulthood. However, they also suggest that protracted engagement of the AIC in adolescents might be a reflection of continuing maturation of deliberative processes, including harm avoidance, rather than hypersensitivity to rewards. Finally, as seen in the response inhibition literature, maturational differences in AIC engagement during risky decision making are as prevalent as developmental differences in the recruitment of the IPFC and striatum, a pattern which we think justifies further discussion and exploration of the AIC in models of adolescent decision making and risk taking.
Integrating the AIC into a Neurodevelopmental Theory of Adolescent Decision Making
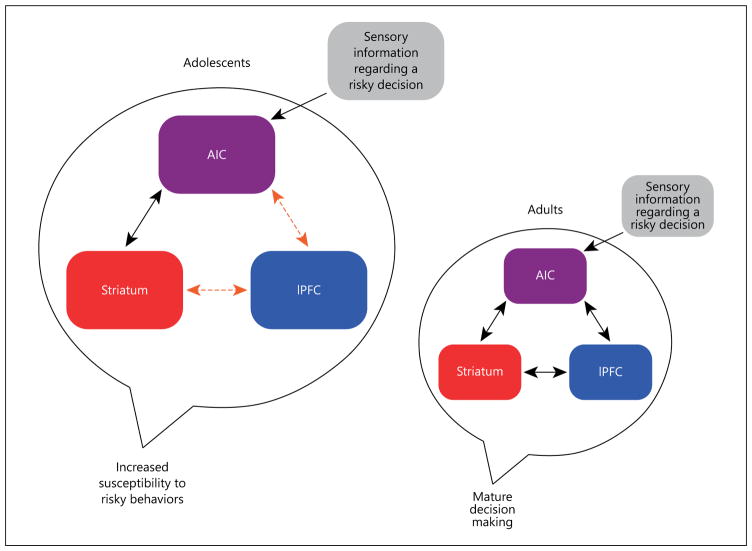
Based on the developmental neuroimaging literature reviewed here, it is clear that the structure and function of the AIC continues to mature across adolescence. Extending the current developmental perspective on adolescent decision making, we suggest that the relative immaturity of this cognitive-emotional hub may bias adolescents towards affectively driven decisions. In particular, we posit that the immaturity of the AIC-IPFC relationship, as demonstrated in developmental studies of response inhibition as well as the connectivity findings, contributes to the inability of adolescents to adequately engage the cognitive control system and the associated self-regulatory processes during risky decision making. On the other hand, the developmental literature suggests that the relationship between the AIC and striatum may already be adequately developed by mid-adolescence, as demonstrated by similar AIC responses in adolescents and adults during reward anticipation. We therefore propose that the combination of adult-like connectivity between the AIC and the striatum, and the still-developing connectivity between the AIC and pre-frontal regions, may promote risk taking in adolescence by biasing the AIC to respond more readily to affective or rewarding stimuli. In other words, we suggest that increases in affective arousal during risky decision making are more readily communicated during adolescence to reward-processing regions rather than cognitive control regions, thus increasing the likelihood that adolescents will engage in risky behaviors. Despite the adult-like state of AIC-striatal connectivity, the bias towards reward-processing regions during adolescence may result in heightened coactivation of the AIC and striatum and/or functional connectivity between these regions during decision making. Further adding to this affective bias during adolescence is the relatively weak connectivity that exists between the reward and cognitive control systems. The function of the AIC in coordinating the valuation and response to potential rewards is somewhat akin to the role played by the vmPFC in the somatic marker hypothesis [106]. Indeed, Craig [58] has suggested that the AIC may be an important missing component of that model. We note, however, that the participation of AIC in cognitive control, even in circumstances where there is no obvious demand for emotional processing (e.g. during simple response inhibition) distinguishes this area from the vmPFC in the somatic marker view of Bechara and Damasio [106].
We posit that the maturational imbalance between adult-like AIC-striatal connectivity and the still-developing AIC-IPFC connectivity increases the likelihood that, when faced with a risky choice, adolescents will engage in impulsive behavior in the pursuit of immediate rewards rather than more deliberative, goal-directed behaviors. See figure 3 for a graphical depiction of the proposed model.
Fig. 3.
Proposed model of AIC function during adolescent decision making. Black arrows represent structural connections that have reached an adult-like state. Orange arrows represent connection paths that are undergoing developmental changes.
Although this hypothesis requires some speculation informed by an admittedly sparse literature, the available body of research supports this proposed developmental model [28, 68, 98, 107–109]. These studies highlight the role of the AIC during the interplay of cognitive and affective processes. For example, in a recent study, Somerville et al. [68] (2011) used an affective version of a popular response inhibition task, go/no-go, to identify the influence of affective stimuli on cognitive control. In the task, participants were required to press a button every time a neutral face was presented but to withhold their response when presented with a happy face. During affectively cued inhibition trials, adolescents made significantly more inhibition errors than adults. In addition, the presentation of the affective cues elicited heightened striatal and AIC activation in adolescents compared to adults. Follow-up functional connectivity analyses demonstrated significant functional connectivity between the AIC and striatum in adolescents, but not in children or adults, during affective cues. Finally, across age groups, increased recruitment of the AIC during inhibition predicted the number of errors (i.e. stronger recruitment of the AIC during affectively cued inhibition trials resulted in worse performance). Together, the exaggerated AIC and striatal response, higher functional connectivity between these regions, and the relationship between AIC activation and poor behavioral performance in the study by Somerville et al. [68] support the notion that the increased engagement of the AIC and striatum during adolescence, which may be a downstream effect of the inability to engage the cognitive control system, may make adolescents particularly susceptible to affective interference during the interplay of cognitive and emotional processes.
Another recent study conducted by Christakou et al. [107] (2011) used a delayed discounting task to look at age-related changes in the recruitment and connectivity of the neural systems underlying reward sensitivity and decision making, including the relationships between the AIC, the striatum and the PFC. In the task, participants were asked to make a decision between two amounts of money, a smaller amount to be received immediately (between £1 and 100) and a larger amount (£100) to be received after a delay (a week, a month or a year). Behaviorally, the tendency to opt for large, delayed choices rather than small, immediate choices increased with age, a pattern seen in other studies [see 110]. Age-related decreases in impulsive choices were associated with increases in mPFC recruitment and with decreases in ventral striatum and AIC recruitment. The shift from immediate to delayed choice was also associated with increased connectivity between the AIC and the mPFC in addition to the strengthening of vmPFC and striatal connectivity. Together, these results provide support for a possible developmental shift from engagement of the AIC and striatum during impulsive choices to increased connectivity between the AIC and the PFC during more considered ones.
Conclusions and Future Directions
In conclusion, our exploration of the developmental neuroimaging literature indicates the importance of including the AIC in developmental models of risk taking and decision making, particularly in theories that highlight the interplay of cognitive and affective processes. We propose an extension of the current developmental models that focus on the maturational imbalance between the striatum and the IPFC to incorporate the AIC into this general framework. More specifically, we suggest that the relative immaturity of the AIC and its relationship with cognitive control regions leaves adolescents vulnerable to affectively driven behaviors such as reckless risk taking. This theory also may serve as a framework for exploring individual differences in risk taking during adolescence. For instance, it is reasonable to posit that individual differences in risk taking among adolescents of the same age may be correlated with differences in the degree of connectivity between the AIC and the IPFC. Other brain regions are also closely tied to cognitive-emotional interactions, such as the ACC and amygdala, and future research should explore the development of the AIC-amygdala and AIC-ACC relationships for possible influences on adolescent decision making. Nevertheless, we believe that it is critical to study the AIC in research on adolescent decision making because of its established role as a hub [10] and its demonstrated involvement in the reward processing and cognitive control systems known to mature during the transition between childhood and adulthood [27, 44, 45].
Footnotes
Bunge et al. [75] and Rubia et al. [77] show that the directionality of developmental differences in AIC engagement varies based on AIC laterality; therefore these studies have been included in both age-related increases and decreases in AIC activations
Although inconsistencies in the direction of AIC developmental differences (i.e. age-related increased vs. decreases) may complicate interpretation, this variability supports the conclusion that age-related differences reflect maturational changes in the recruitment of the AIC rather than maturational differences in neurovascular coupling [for review, see 111, 112].
References
- 1.Church JA, Petersen SE, Schlaggar BL. The ‘Task B problem’ and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piquero AR. Taking stock of developmental trajectories of criminal activity over the life course. In: Liberman A, editor. The Long View of Crime: A Synthesis of Longitudinal Research. New York: Springer; 2008. pp. 23–78. [Google Scholar]
- 3.Harris JJ, Reynell C, Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev Cogn Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twisk DAM, Stacey C. Trends in young driver risk and countermeasures in European countries. J Safety Res. 2007;38:245–257. doi: 10.1016/j.jsr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Your Risk Surveillance Survey – United States, 2011. 61. Washington: CDC; 2012. [Google Scholar]
- 6.SAMHSA. Results from the 2010 National Survey on Drug Use and Health: Mental Health Findings. 42. Rockville: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- 7.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 8.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 12.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 14.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyna VF, Farley F. Risk and rationality in adolescent decision making. Psychol Sci Public Interest. 2006;7:1–46. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- 21.Smith AR, Chein J, Steinberg L. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm Behav. 2013;64:323–332. doi: 10.1016/j.yhbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciana M, Wahlstrom D, Porter JN, Collins PF. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev Psychol. 2012;48:844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 26.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosurgery. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, et al. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2013;66:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churchwell JC, Yurgelun-Todd DA. Age-related changes in insula cortical thickness and impulsivity: significance for emotional development and decision-making. Dev Cogn Neurosci. 2013;6:80–86. doi: 10.1016/j.dcn.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- 31.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig A. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 33.Ture U, Yasargil D, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- 34.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 35.Mesulam MM, Mufson EJ. Insula of the old world monkey. III. Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 36.Mufson EJ, Mesulam MM. Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 37.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 38.Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of Galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 40.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 41.Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8:775–780. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- 44.Ham TE, de Boissezon X, Leff A, Beckmann C, Hughes E, Kinnunen KM, et al. Distinct frontal networks are involved in adapting to internally and externally signaled errors. Cereb Cortex. 2013;23:703–713. doi: 10.1093/cercor/bhs056. [DOI] [PubMed] [Google Scholar]
- 45.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- 47.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Chambers CD, Bellgrove MA, Stokes M, Henderson TR, Garavan H, Robertson IH, et al. Executive ‘brake failure’ following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 49.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 50.Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62:593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad ZS, Ropella KM, DeYoe EA, Bandettini PA. The spatial extent of the BOLD response. Neuroimage. 2003;19:132–144. doi: 10.1016/s1053-8119(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 54.Lai S, Hopkins AL, Haacke EM, Li D, Wasserman BA, Buckley P, et al. Identification of vascular structures as a major source of signal contrast in high resolution 2D and 3D functional activation imaging of the motor cortex at l. 5 T preliminary results. Magn Reson Med. 2005;30:387–392. doi: 10.1002/mrm.1910300318. [DOI] [PubMed] [Google Scholar]
- 55.Rostrup E, Law I, Blinkenberg M, Larsson HBW, Born AP, Holm S, et al. Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. Neuroimage. 2000;11:87–97. doi: 10.1006/nimg.1999.0526. [DOI] [PubMed] [Google Scholar]
- 56.Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Chang C, Thomason ME, Glover GH. Mapping and correction of vascular hemodynamic latency in the BOLD signal. Neuroimage. 2008;43:90–102. doi: 10.1016/j.neuroimage.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craig A. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 60.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, et al. Development of insula connectivity between ages 12 and 30 revealed by high angular resolution diffusion imaging. Hum Brain Mapp. 2014;35:1790–1800. doi: 10.1002/hbm.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamnes C, Østby Y, Fjell A, Westlye L, Due-Tonnessen P, Walhovd K. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 63.Teicher MH, Andersen SL, Hostetter J. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 64.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Nat Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strang N, Pruessner J, Pollack S. Developmental changes in adolescents’ neural response to challenge. Dev Cogn Neurosci. 2011;1:560–569. doi: 10.1016/j.dcn.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somerville LH, Hare T, Casey B. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- 72.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 73.Adleman N. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 74.Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS ONE. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bunge S, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marsh R, Zhu H, Schultz R, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event related tasks of cognitive control. Hum Brain Map. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, et al. Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 79.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 82.Casey B, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go/no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 83.Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 84.Tamm L, Menon V, Reiss A. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- 87.Blakemore S-J. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 88.Casey B, Jones RM, Hare T. The adolescent brain. Ann NY Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 90.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Leijenhorst L, Zanolie K, Van Meel C, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- 92.Bjork JM, Knuston B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krain AL, Hefton S, Pine DS, Ernst M, Xavier Castellanos F, Klein RG, et al. An fMRI examination of developmental differences in the neural correlates of uncertainty and decision-making. J Child Psychol Psychiatry. 2006;47:1023–1030. doi: 10.1111/j.1469-7610.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- 96.Padmanabhan A, Geier C, Ordaz S, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 98.Galvan A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. J Cogn Neurosci. 2013;25:284–296. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- 99.Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, et al. Developmental effects of decision-making on sensitivity to reward: An fMRI study. Dev Cogn Neurosci. 2012;2:437–447. doi: 10.1016/j.dcn.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 101.Barkley-Levenson EE, Van Leijenhorst L, Galvan A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev Cogn Neurosci. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paulsen DJ, Carter R, Platt M, Huettal SA, Brannon E. Neurocognitive development of risk aversion from early childhood to adulthood. Front Hum Neurosci. 2012;5:178. doi: 10.3389/fnhum.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- 107.Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54:1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 108.Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56:1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 109.Goldenberg D, Telzer EH, Lieberman MD, Fuligni A, Galvan A. Neural mechanisms of impulse control in sexually risky adolescents. Dev Cogn Neurosci. 2013;6:23–29. doi: 10.1016/j.dcn.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 111.Church JA, Petersen SE, Schlaggar BL. The ‘Task B problem’ and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harris JJ, Reynell C, Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev Cogn Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Front Hum Neurosci. 2010:4. doi: 10.3389/fnhum.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoogendam JM, Kahn RS, Hillegers MH, van Buuren M, Vink M. Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci. 2013;6:113–124. doi: 10.1016/j.dcn.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teslovich T, Mulder M, Franklin NT, Ruberry EJ, Millner A, Somerville LH, Simen P, Dustron S, Casey BJ. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Dev Sci. 2014;17:59–70. doi: 10.1111/desc.12092. [DOI] [PubMed] [Google Scholar]
- 116.van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Christakou A, Gershman SJ, Niv Y, Simmons A, Brammer M, Rubia K. Neural and psychological maturation of decision-making in adolescence and young adulthood. J Cogn Neurosci. 2013;25:1807–1823. doi: 10.1162/jocn_a_00447. [DOI] [PubMed] [Google Scholar]