Abstract
We previously found that L-tyrosine (L-TYR) but not D-TYR administered by reverse dialysis elevated catecholamine synthesis in vivo in medial prefrontal cortex (MPFC) and striatum of the rat (Brodnik et al., 2012). We now report L-TYR effects on extracellular levels of catecholamines and their metabolites. In MPFC, reverse dialysis of L-TYR elevated in vivo levels of dihydroxyphenylacetic acid (DOPAC) (L-TYR 250 – 1000 μM), homovanillic acid (HVA) (L-TYR 1000 μM) and 3-methoxy-4-hydroxyphenylglycol (MHPG) (L-TYR 500 – 1000 μM). In striatum L-TYR 250 μM elevated DOPAC. We also examined L-TYR effects on extracellular dopamine (DA) and norepinephrine (NE) levels during two 30 min pulses (P2 and P1) of K+ (37.5 mM) separated by t = 2.0 h. L-TYR significantly elevated the ratio P2/P1 for DA (L-TYR 125 μM) and NE (L-TYR 125 – 250 μM) in MPFC but lowered P2/P1 for DA (L-TYR 250 μM) in striatum. Finally, we measured DA levels in brain slices using ex-vivo voltammetry. Perfusion with L-TYR (12.5 – 50 μM) dose-dependently elevated stimulated DA levels in striatum. In all the above studies, D-TYR had no effect. We conclude that acute increases within the physiological range of L-TYR levels can increase catecholamine metabolism and efflux in MPFC and striatum. Chronically, such repeated increases in L-TYR availability could induce adaptive changes in catecholamine transmission while amplifying the metabolic cost of catecholamine synthesis and degradation. This has implications for neuropsychiatric conditions in which neurotoxicity and / or disordered L-TYR transport have been implicated.
1. Introduction
Tyrosine 3-monooxygenase (EC 1.14.16 .2) (tyrosine hydroxylase - TH) stereospecifically catalyzes hydroxylation of L-tyrosine (L-TYR) and is considered to be the rate-limiting step in the synthesis of catecholamines (Fitzpatrick, 1999;Kaufman, 1995;Nagatsu et al., 1964). Conventionally, TH is thought to be near full saturation by L-TYR; hence acute changes in the rate of TYR-hydroxylation are attributed almost exclusively to changes in TH activity (Fitzpatrick, 1999;Kaufman, 1995;Reed et al., 2010;Zigmond, 1988). This tenet must be reconciled, however, with persistent evidence that at least under certain conditions, L-TYR availability affects catecholamine synthesis and efflux.
Elevated brain L-TYR levels were first reported to increase L-dihydroxyphenylalanine (DOPA) (tissue accumulation measured ex vivo) by Wurtman and co-workers (Wurtman et al., 1974). The consistency and physiological relevance of this finding, however, were challenged. Other groups variously reported that several-fold increases in tissue L-TYR levels at best marginally elevated brain tissue DOPA (Carlsson and Lindqvist, 1978;Nissbrandt and Carlsson, 1987) or that DOPA accumulation modestly increased within a very narrow range of elevated TYR concentrations (Badawy and Williams, 1982). In all these studies however, tissue DOPA accumulation was measured after systemic administration of aromatic amino acid decarboxylase (AADC) inhibitors that concomitantly inhibit TYR-transaminase (EC 2.6.1.5) and hence raise brain L-TYR levels per se (Dyck, 1987). In the one study where AADC was inhibited by microwave irradiation rather than pharmacologically, systemic administration of L-TYR markedly elevated tissue DOPA levels in the rat brain (Westerink et al., 1982).
Administration of AADC inhibitors via reverse dialysis permits measurement of DOPA accumulation in vivo (Westerink et al., 1990) without perturbing L-TYR levels (Bongiovanni et al., 2008). We applied this approach to the medial prefrontal cortex (MPFC) and striatum (Brodnik et al., 2012) and postulated that as the concentration of L-TYR increased, DOPA levels would initially increase and then plateau due to inhibition of TH by L-TYR (Lloyd and Kaufman, 1974;Quinsey et al., 1998;Ribeiro et al., 1992), or by the end-product DA, given that inhibition of AADC by NSD-1015 remains incomplete (Neff et al., 2006). We were surprised, however, that in striatum increasing concentrations of L-TYR (62.5 – 1000 μM) incrementally elevated DOPA levels without evidence of inhibition (Brodnik et al., 2012). In MPFC, L-TYR concentrations (31.75 – 500 μM) increased L-DOPA levels with maximal elevation induced by L-TYR range (62.5 – 125 μM) (Brodnik et al., 2012). D-TYR, the enantiomer for which TH has negligible activity (Nagatsu et al., 1964), had no effect in either region (Brodnik et al., 2012). We concluded that increases in L-TYR availability within the physiological range increased brain regional DOPA accumulation and by implication DOPA synthesis in vivo, via a stereospecific TH-mediated mechanism (Brodnik et al., 2012). The functional implications of these findings depend on the fate of the additional DOPA generated.
Under usual conditions, most DOPA is rapidly decarboxylated to DA which is then metabolized to dihydroxyphenylacetic acid (DOPAC) or homovanillic acid (HVA) or is converted to norepinephrine (NE) (Eisenhofer et al., 2004;Kopin, 1985). Thus, in the absence of AADC inhibition, L-TYR-induced elevations in DOPA synthesis would be expected to elevate levels of catecholamines or their metabolites. Experimentally elevated brain L-TYR levels have indeed been reported to increase tissue levels of DOPAC, HVA, NE or the NE metabolite 3-Methoxy-4-hydroxyphenylglycol (MHPG) in the MPFC, a region characterized by high basal rate of TH activation and DA synthesis (Morrow et al., 1996;Tam et al., 1990) as well as in other brain regions after TH activation (Chance et al., 1990;Milner and Wurtman, 1986). Since both released and unreleased catecholamines enter the same degradative pathway (Eisenhofer et al., 2004;Gesi et al., 2001) however, tissue metabolite levels alone provide incomplete information about the underlying pathways.
For this reason we have now examined how L-TYR concentrations within the range that increased in vivo DOPA levels stabilized by AADC inhibition (Brodnik et al., 2012), affect in vivo catecholamine and metabolites levels when metabolic pathways are not pharmacologically perturbed. We postulated that increases in L-TYR availability would raise extracellular levels of metabolites of DA and/ or NE, confirming that L-TYR elevations affect catecholamine synthesis under baseline conditions. We further postulated that increased L-TYR availability would elevate K+ stimulated extracellular levels of DA or NE, indicating an expansion of the functional pool of releasable catecholamines. Finally, we hypothesized that inhibitory DA receptors belonging to the type 2 family (D2R) (Anzalone et al., 2012;Wolf and Roth, 1990;Wu et al., 2002;Zhang and Sulzer, 2012) would limit L-TYR effects on DA efflux.
2.0 Methods
2.1 Subjects
Male Sprague-Dawley rats (Harlan, IN) (250–350 g) were housed two to a plastic cage (30 × 30 × 36 cm) with sawdust bedding and maintained on a standard 12h on/off (7:00H/19:00H) light cycle with food and water ad libitum in an AALAC accredited facility. All procedures were conducted in accordance with current guidelines (National Research Council, 2011;Zhang and Sulzer, 2012), and were approved by the Institutional Animal Care and Use Committee at the LSC-DVAMC (in vivo studies) or at the Drexel University College of Medicine (ex vivo studies).
2.2 Chemicals
For in vivo studies L-TYR and D-TYR (Sigma-Aldrich) were dissolved in Dulbecco’s PBS (122 mM NaCl, 3.0 mM KCl, 1.2 mM MgSO4, 0.4 mM KH2PO4, 25.0 mM, NaHCO3, 1.2 mM CaCl2, and 5.0 mM glucose, pH 7.4) immediately before use. In K+ stimulation studies, constant osmolality was maintained by an equimolar lowering of the concentration of NaCl as the concentration on of KCl was raised. For ex vivo studies, L-TYR, D-TYR and/or (−) quinpirole hydrochloride (Tocris) were dissolved in artificial cerebrospinal fluid (aCSF; 126 nM NaCl, 2.5 nM KCl, 1.2 nM NaH2PO4, 2.4 nM CaCl2, 1.2 nM MgCl2, 25 nM NaHCO3, 11 nM glucose, 0.4 nM l-ascorbic acid, pH adjusted to 7.4) immediately before use.
2.3 In vivo microdialysis
2.3.1 Surgery
Anesthesia was induced in a gas chamber with 4.5% isoflurane (Baxter) flushed with 2.0 l/min O2 at 1 bar. Anesthetized rats were weighed, shaved, and placed into a stereotaxic frame (Kopf) where anesthesia was maintained with 3% isoflurane delivered through a nosecone. A guide cannula and microdialysis probe (PAN 30 kDa, MWCO, 320-μm OD, 4-mm active membrane; Bioanalytical Science) were implanted in either the MPFC (probe terminating: M/L ± 0.70 mm, A/P + 3.20 mm, V/D −7.00 mm, relative to bregma) or the striatum (probe terminating: M/L ± 3.20 mm, A/P + 1.00 mm, D/V − 7.00 mm). The probe was then connected via polyethylene tubing to a swivel and perfusion pump, and rats were placed in a 30 × 30 × 35-cm Plexiglas enclosure with sawdust bedding and food and water ad libitum.
2.3.2 Microdialysis
One day after surgery, the microdialysis probe was perfused with Dulbecco’s PBS at the rate of 10 μl/min for 15 minutes and then at 1.3 μl/min for the remainder of the study. Following a 1 hour equilibration period, samples were collected every 30 minutes. Three baseline samples were collected beginning at 10:00 am. Changes in perfusate composition started at 11:30 am (t= 0). For studies without K+ stimulation, the inflow line was switched to one containing PBS with a specified concentration of TYR (62.5 – 1000 μM) for a period of 3h. After the collection at 3.0 h, the perfusate line was switched back to one containing PBS only and samples were collected for an additional 2.0 h. In studies of stimulated efflux, the perfusate was switched for a 30 min period to one containing K+ (25–100mM) starting at t = 0.5 h and again at t = 3.0 h. There were thus two 30 min pulses of high K+ perfusion separated by an interval of 2.0 h. From t = 1.0 h onwards the perfusate also contained TYR (0 – 250 μM), In other words. TYR was administered during the second but not the first high K+ pulse (Fig. 3).
Figure 3.

K+ effects on dopamine (DA) efflux in vivo. Various K+ concentrations were administered by reverse microdialysis in two 30 min pulses (P1 and P2), 2 h apart in medial prefrontal cortex (MPFC) or striatum. Time course of DA levels in response to K+ 37.5 mM in MPFC (A) and striatum (B). Peak DA levels (P1 and P2) in MPFC (C) or striatum (D) in response to different K+ concentrations. Ratio P2/P1 in MPFC (E) or striatum (F). Mean ± SEM. n = 5/group.
2.3.3 Sample analysis
Microdialysate samples (10 μl) were analyzed by HPLC coupled to electrochemical detection. Separation was achieved with a 100 × 4.6-mm reversed-phase C18 column with 3-μm particles (Agilent Technologies Microsorb-MV). For analysis of the catecholamine metabolites DOPAC, HVA, and MHPG the mobile phase consisted of 12.5 mM citrate, 20 mM acetate, and 0.1 mM EDTA with 10% (v/v) methanol adjusted to pH 2.8, and for analysis of catecholamines DA and NE the mobile phase consisted of 12.5 mM citrate, 20 mM acetate, and 0.1 mM EDTA with 10% (v/v) acetonitrile adjusted to pH 5.0–6.0 and with 0–3.0 mM octylsulfonic acid adjusted as a modifier according to (Brodnik and Jaskiw, 2015;Eisenhofer et al., 2004). The mobile phase was pumped at 0.5 mL/min. The ECD had a glassy carbon electrode set at a potential of 0.78V for analysis of metabolites, and at 0.50 for analysis of catecholamines relative to the Ag/AgCl reference electrode. The detection limit for DA was 50 fg/10 μL at a 3:1 signal-to-noise ratio.
2.3.4 Histology
At the end of each microdialysis session, the rats were euthanized (IP Euthasol, Virbac Animal Health), decapitated, and their brains removed. Brains were frozen at −40°C before being cut on a cryostat at 50-μm intervals for probe placement verification. Data from rats in which the probe extended beyond the target region (Fig. 1) were discarded.
Figure 1.
Location of tips of microdialysis probes or carbon fiber microelectrodes.
Microdialysis probes were implanted within the (A) Medial prefrontal cortex
(MPFC) or the (B) striatum, and were entirely contained within the shaded
regions  . Microelectrodes were
placed in (B) striatum as designated by the darker shaded regions
. Microelectrodes were
placed in (B) striatum as designated by the darker shaded regions
 .
.
2.4 Ex vivo voltammetry
Slices containing the striatum were prepared from naive rats following decapitation without pretreatment. Brains were removed and transferred into ice-cold oxygenated aCSF containing 3.175 μM L-TYR to prevent the potential TYR depletion that may develop when tissue is stimulated in a TYR-free medium (Buyukuysal and Mogol, 2000). A vibrating microtome was used to produce 300 μm thick coronal sections, which were then transferred into a testing chamber and flushed with aCSF with 3.175 μM L-TYR (32°C) flowed at 1.0 ml/min. Following 30 min of equilibration, a carbon fiber microelectrode (150–200 μm length × 7.0 μm diameter) and a bipolar stimulating electrode (Plastics One, Roanoke, VA) were placed in the dorsolateral striatum (Fig. 1). DA release was elicited every 5 min using a single electrical pulse (600 μA, 4.0 ms, monophasic), and was recorded as previously described (Brodnik and Espana, 2015;Espana et al., 2010). Once the stimulated DA response was stable for three successive stimulations (less than 10% variation) experimental manipulation began. In the first experiment, L-TYR (3.125, 6.25, 12.5, 25, 50 μM) was applied cumulatively to brain slices via perfusate. In the second experiment, slices were perfused with L-TYR (3.125 or 25 μM) or D-TYR 25 μM for 1.0 h following baseline collections. In the third experiment, slices were perfused for 45 min with the relatively D2R-selective agonist quinpirole at a concentration (66 μM) that maximally stimulates release-modulating autoreceptors in brain slices (Mateo et al., 2005) and then with both quinpirole and L-TYR for an additional hour.
Stimulated DA release and reuptake were characterized according to Demon Voltammetry and Analysis Software (Yorgason et al., 2011). The concentration of stimulated DA release was assessed by comparing the peak oxidation potential on voltammograms with electrode calibrations of 3.0 μM DA.
2.5 Statistics
In microdialysis studies, analyte levels were first expressed as a percentage of baseline, defined as the average of three consecutive pre-treatment levels. Unstimulated levels of catecholamines or metabolites were analyzed by ANOVA (time as repeated measure + treatment as main measure). If the main effects were significant, the following comparisons were made by Dunnett’s post-hoc t-tests: i) 3.0 h levels v. baseline, 4.5 h levels v. baseline, 4.5 h levels v. 3.0 h levels. In all except one experiment (Fig. 2B), TYR was administered by reverse dialysis for 3.0 h. Thus 4.5 h levels were collected 1.5 h after cessation of TYR administration.
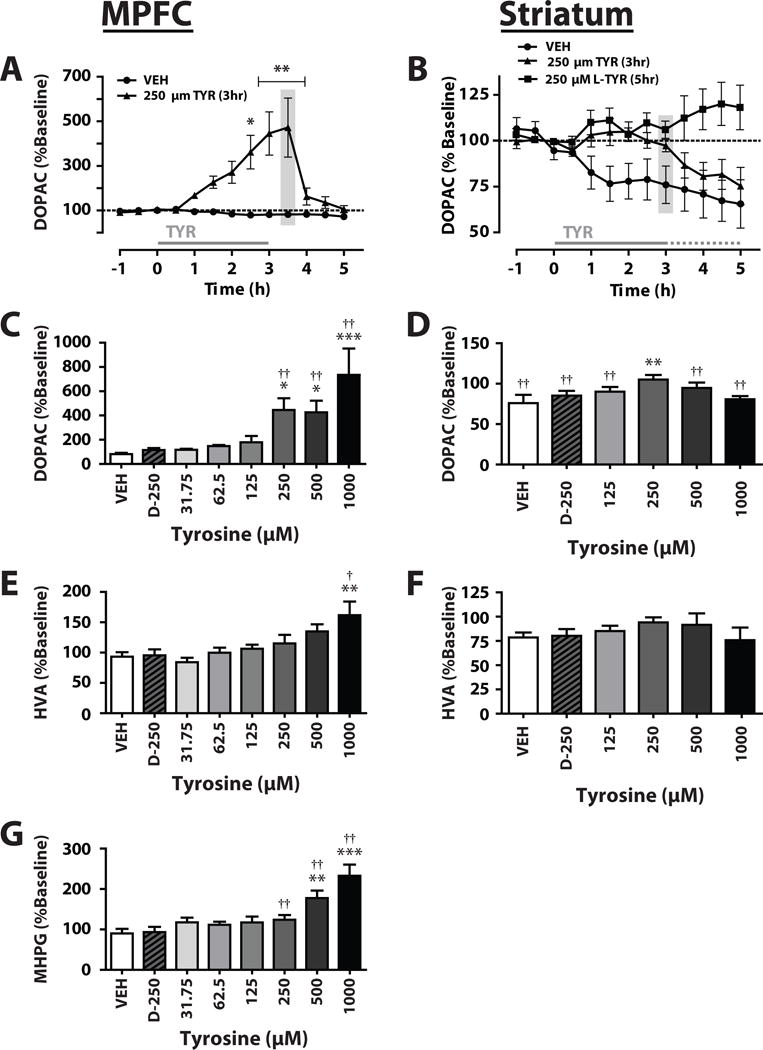
Figure 2.
Tyrosine (TYR) effects on catecholamine metabolites levels in vivo. TYR (31.75 – 1000 μM) was administered into the medial prefrontal cortex (MPFC) or striatum by reverse microdialysis. Time course of DOPAC during administration of L-TYR 250 μM within (A) MPFC or (B) striatum. DOPAC in MPFC (C) or striatum (D) after 3.0 h of vehicle (VEH) or TYR. HVA in MPFC (E) or striatum (F) after 3.0 h of VEH or TYR. MHPG in MPFC (G) after 3.0 h of VEH or TYR. Significantly different from baseline: * p < 0.05, ** p < 0.01, *** p < 0.001. Significantly different from VEH-treated group, † p < 0.01, †† p < 0.001. Mean ± SEM, n = 5/group.
For in vivo studies of stimulated efflux, the maximum DA or NE levels reached during the first (P1) and second K+ pulses (P2) were calculated as a % of baseline. Since the data for P1 and P2 were not normally distributed we compared P1 to P2 using the Wilcoxon rank sum test (Figs. 4 D, E), and then tested for TYR effects on the P2/P1 ratios using one-way ANOVA followed by Dunnett’s post-hoc tests (Figs. 4 G–I). Pearson correlations were calculated for P2 vs. P1 across different K+ as well as TYR concentrations (Table 2).
Figure 4.
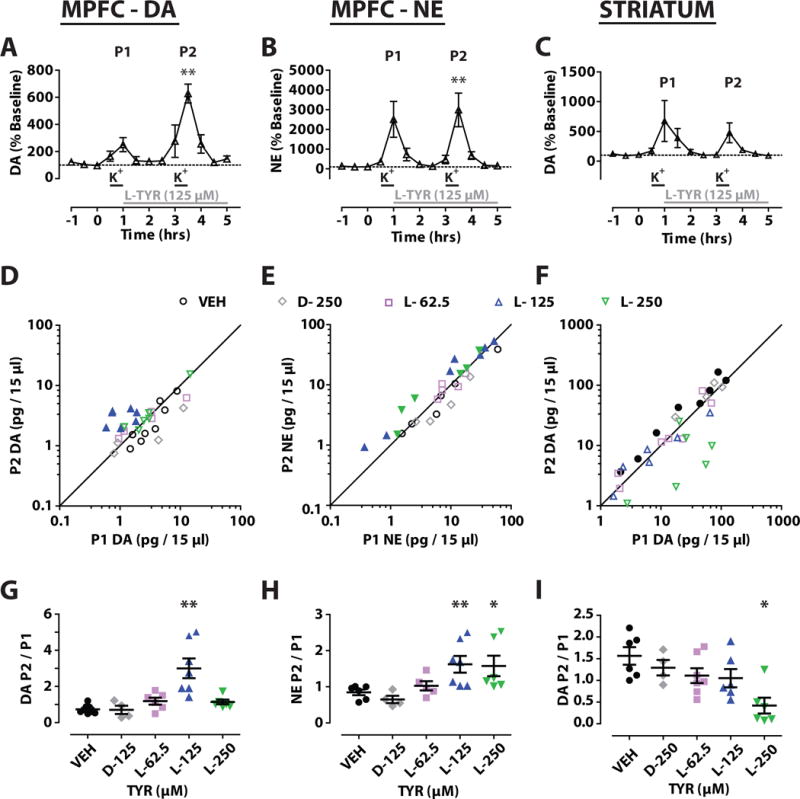
Tyrosine (TYR) effects on K+ induced efflux of catecholamines in vivo. K+ 37.5 mM was administered by reverse microdialysis in two 30 min pulses (P1, P2) 2 h apart (–) while TYR was administered continuously from the end of P1 (–) in medial prefrontal cortex (MPFC) and striatum. Time course for DA or NE and L-TYR 125 μM (A, B, C). Bivariate scatter plots (D, E, F) for extracellular levels of DA or NE for P1 and P2 with filled makers denoting TYR concentrations at which P1 and P2 were significantly different (p < 0.05) Column scatter plots of P2/P1 ratios for DA or NE (G, H, I). Significantly different from vehicle (VEH) treated animals. (* p < 0.05, ** p < 0.01). Mean ± SEM. n = 5/group.
Table 2.
Significant Pearson Correlations for P2 v P1 (calculated as % baseline)
| A | K+ (mM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | 37.5 | 50 | 100 | ALL | ||||||
| MPFC | % DA | 0.94* | 0.88* | 0.92*** | ||||||
| Striatum | % DA | 0.91* | 0.95* | 0.92*** | ||||||
| B | Tyrosine (μM) | |||||||||
| Region | 0 | L-62.5 | L-125 | L-250 | D-125 | ALL | ||||
| MPFC | % DA | 0.96*** | 0.83* | 0.99** | 0.70*** | |||||
| MPFC | % NE | 0.99*** | 0.97** | 0.96* | 0.89*** | |||||
| striatum | % DA | 0.88* | 0.98** | 0.99** | 0.64** | |||||
p < .05,
<0.001,
< 0.0001 (only significant correlations shown)
Two 30 min intervals (P1, P2) of reverse dialysis with high K+ were separated by an interval of 2.0 h. In initial studies (A) a range of K+ concentrations was examined. Then (B), K+ (37.5mM) was applied during both P1 and P2 while tyrosine (TYR) (0 – 250 μM) was applied during P2 (Fig. 3). (medial prefrontal cortex = MPFC)
For ex vivo studies, DA levels in response to changing TYR concentrations were analyzed by a repeated measures one-way ANOVA. A one -way ANOVA was also used to compare effects of L-TYR 25 μM v. D-TYR 25 μM. Finally, paired t-tests were used to compare the effects of quinpirole v. baseline and of quinpirole v. (quinpirole + L-TYR 25 μM). Statistical analysis was performed using SPSS (IBM). All data in figures and tables are expressed as mean ± SEM.
3. Results
3.1 L-TYR elevates in vivo catecholamine metabolite levels
Basal levels of DA, DOPAC and HVA were measured in both MPFC and striatum; NE and MHPG were quantified only in MPFC (Table 1). In the MPFC, there were no significant TYR effects on DA levels (SI Table 1). For all analyses in which a significant main effect was observed, we performed two separate post-hoc analyses. First, an independent one-way ANOVA with time as a repeated measure was applied to determine if metabolite levels changed significantly from baseline across the duration of treatment. Second, a Dunnett’s post-hoc test was conducted to determine if metabolite levels differed from vehicle treated groups. For DOPAC, the time × treatment effect was significant (F(108, 58) = 3.842, p < 0.001). Post-hoc tests showed that MPFC DOPAC levels after 3.0 h of reverse dialysis with L-TYR 250 μM, 500 μM or 1000 μM were significantly higher than those at baseline (Fig. 2C) or those at t = 4.5 h (i.e. 1.5 h after reverse dialysis of TYR had ceased) (Fig. 2A, SI Table 2). MPFC DOPAC levels after 3.0 h reverse dialysis with L-TYR 1000 μM were significantly higher than those of vehicle perfused rats (Fig. 2C). For HVA the effect of time × treatment was significant (F(108, 58) = 1.59, p < 0.001). HVA levels after 3.0 h reverse dialysis with L-TYR 1000 μM were significantly higher than i) those at baseline, ii) those of VEH- treated rats at t = 3.0 h (Fig. 2E), iii) those at t = 4.5 h (SI Table 3). For MHPG the effect of time × treatment was significant (F(108, 58) = 2.85, p < 0.001). Post-hoc tests showed that MHPG levels after 3.0 h reverse dialysis with L-TYR 250 μM, 500 μM or 1000 μM were higher than those at baseline (Fig. 2G) or those collected at 4.5 h (SI table 4). In addition, MHPG levels after 3.0 h of reverse dialysis with L-TYR 500 μM or 1000 μM were significantly higher than those of vehicle perfused rats (Fig. 2G). MPFC levels of DOPAC, HVA, and MHPG remained stable in VEH-treated animals. D-TYR (250 – 1000 μM) had no significant effects (Fig. 2 and SI Table 1).
Table 1.
Brain Regional Levels in vivo
| nM | DA | DOPAC | HVA | NE | MHPG |
|---|---|---|---|---|---|
| MPFC | 0.31± 0.02 | 521.5 ± 70.5 | 190.1 ± 17.9 | 0.315 ± 0.3 | 49.4 ± 4.0 |
| striatum | 3.5 ± 0.3 | 3141.2 ± 258.7 | 1498.5 ± 121.9 |
In striatum there were no significant overall L-TYR effects on DA or HVA (Fig. 2F, SI Table 3). DOPAC levels showed a significant time × treatment effect (F(72, 42) = 2.21, p < 0.05). By t = 3.0 h, DOPAC levels in VEH treated animals were significantly lower than at baseline (Fig. 2B, 2D). The same was evident after L-TYR 125 μM, 500 μM, 1000 μM and after D-TYR 250 μM (Fig. 2D). However, L-TYR 250 μM prevented the decline at t= 3.0 h and produced DOPAC levels higher than any of the other treatments (Figs. 2B, 2D). One way ANOVA with time as a repeated measure for each of the treatments confirmed changes in DOPAC levels for all but the L-TYR 250 μM group. To examine this further, we administered TYR 250 μM for 5.0 μh to an additional group of animals, (Fig. 2B). The one way ANOVA with time as a repeated measure showed that in the group receiving TYR 250 μM there was no significant change in DOPAC levels (F(12,60) = 1.20, p = 0.30).
3.2 Determining optimal K+ concentrations for stimulated efflux in vivo
We examined a range of K+ concentrations (12.5 – 100 mM) on DA efflux using a two pulse paradigm (two 30 min K+ pulses 2.0 h apart) (Ripley et al., 1997) (Figs. 3A, B). Overall, the K+ response curve fit a sigmoidal function both in the MPFC and striatum (Figs. 3C, D). The ratio of peak response amplitude for the second potassium pulse (P2) to the first (P1) did not vary significantly across K+ concentrations in the MPFC (F(4,24) = 0.83, p = 0.52) or striatum (F(4, 24) = 1.49, p = 0.24) (Figs. 3E,F). K+ 37.5 mM was the minimal concentration that showed a significant and high Pearson correlation (r > 0.9, p < .05) for P2 vs. P1 in both MPFC and striatum (Table 2), and was used for further studies.
3.3 L-TYR elevates K+ stimulated in vivo catecholamine efflux in MPFC but not in striatum
For MPFC DA, Wilcoxon comparisons showed significant differences between P1 and P2 only for L-TYR 125 μM (Z = 28, p < 0.05) (Figs. 4A, 4D, 4G). One-way ANOVA also revealed a significant effect of treatment on the P2/P1 ratio (F (26, 4) = 10.361, p < 0.001), and post-hoc tests showed that L-TYR 125 μM L-TYR significantly increased P2/P1 relative to the VEH (Figs. 4D, 4G). For MPFC NE, Wilcoxon comparisons showed significant differences between P1 and P2 for L-TYR 125 μM (Z = 28, p < 0.05) and L-TYR 250 μM (Z = 21, p < 0.05). In addition, a one-way ANOVA revealed a significant effect of treatment on P2/P1 ratios (F (23, 4) = 4.56, p < 0.01); P2/P1 was significantly higher in the L-TYR 125 μM and 250 μM groups compared to VEH (Figs. 4E, 4H). There were generally high and significant correlations between P1 and P2 for MPFC DA as well as for MPFC NE (Table 2A). Due to the technical challenges, separate animals were often used to measure DA and NE. For that reason, we are not reporting correlations between DA and NE within animals.
For striatum DA, P2 was significantly greater than P1 in the VEH- treated group (Wilcoxon; Z = 36, p < 0.05) (Fig. 4F). However, no significant differences were observed between P1 and P2 for any concentration of L-TYR or D-TYR. A one-way ANOVA revealed a significant effect of treatment on the P2/P1 ratio (F (24, 4) = 4.90, p < 0.01). P2/P1 was significantly lower in the L-TYR 250 μM treated group than in VEH-treated animals (Fig. 4I). There were generally high and significant correlations between P1 and P2 for striatal DA (Table 2A).
3.4 L-TYR elevates DA efflux ex vivo in the striatum
Increasing concentrations of L-TYR in the medium progressively increased evoked DA (Fig. 5A) and a repeated measures one-way ANOVA confirmed an overall treatment effect on DA (F = (16,4) = 12.047, p < 0.001). Post-hoc tests demonstrated that L-TYR 12.5 μM – 50 μM increased evoked DA efflux relative to controls (3.125 μM) (Fig. 5B). In a separate experiment comparing L-TYR 3.125 μM, L-TYR 25 μM, and D-TYR 25 μM, a one-way ANOVA showed significant effects of treatment (F (12, 2) = 10.31, p < 0.01). Dunnett’s post-hoc t-tests revealed that stimulated DA efflux was significantly greater during perfusion with L-TYR 25 μM than in the other two groups; the effect of D-TYR 25 μM was not different from that of controls (Fig. 5C). In the final experiment, quinpirole 0.66 μM (calculated as free base) added to the control medium after baseline collections were completed, significantly lowered stimulated DA release by ~45% relative to baseline (t(4) = 4.144, p < 0.05) (Fig. 5D). L-TYR 25 μM introduced following 45 min of perfusion with quinpirole 0.66 μM did not alter stimulated DA release compared to quinpirole alone (t(4) = −0.357, p = 0.739).
Figure 5.
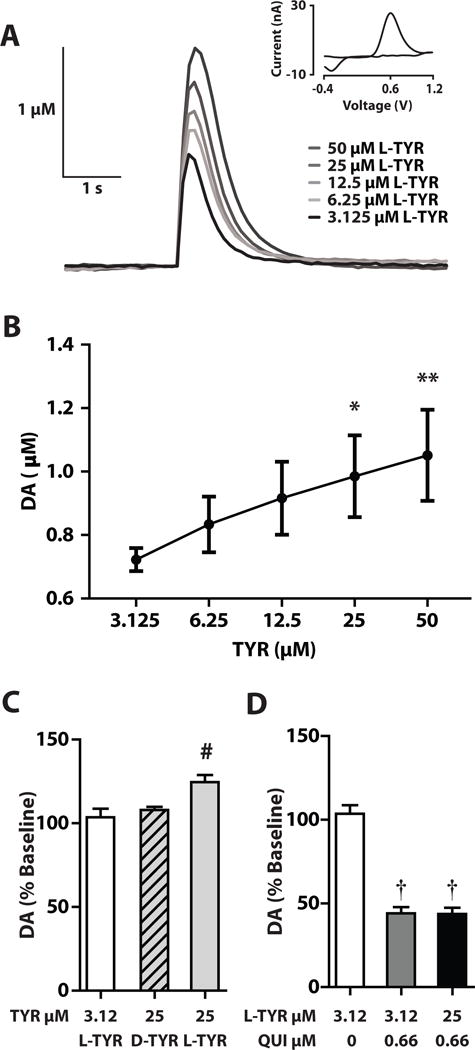
Tyrosine (TYR) effects on striatal DA levels ex vivo. (A) Traces of stimulated DA from striatal slices following bath application of increasing concentrations of L-TYR with inset cyclic voltammogram. (B) Peak stimulated DA levels after incremental increases in L-TYR. (C) Peak stimulated DA (% baseline) after L-TYR 3.125 μM, L-TYR 25 μM or D-TYR 25 μM. (D) Peak stimulated DA(% baseline) after quinpirole (QUI) 0.66 μM alone or with L-TYR 25 μM. (Mean ± SEM, n = 5 per group). Significantly different from TYR 3.125 μM (* p < 0.05, ** p < 0.01). Significantly different from other groups, a, (* p < 0.05). Significantly different from baseline, († p < 0.05). Mean ± SEM. n = 5/group.
4. Discussion
4.1 Overall
We examined concentrations of L-TYR previously reported to elevate in vivo DOPA levels stabilized by AADC inhibition (Brodnik et al., 2012). Now, elevated L-TYR availability raised in vivo DOPAC and MHPG levels in the MPFC and DOPAC levels in the striatum under baseline conditions (Fig. 2). Furthermore, L-TYR elevation augmented K+-induced DA and NE levels in MPFC but lowered in vivo DA levels in striatum (Fig. 4). Finally, administration of L-TYR elevated stimulated DA efflux from striatal slices in vitro; this was blocked by a presynaptic dose of a D2R agonist (Fig. 5). In all cases, D-TYR had no effect. Our initial hypothesis, that elevations of brain regional L-TYR availability would elevate metabolite levels as well as stimulated efflux of DA and NE (Figs. 2, 4, 5) was fully met with respect to MPFC and partially met for striatum. The findings must be interpreted in light of both theoretical and technical considerations.
4.2 L-TYR levels
L-TYR is transported across biological membranes by a family of large neutral amino acid (LNAA) carriers (del Amo et al., 2008;Uchino et al., 2002) expressed by all classes of brain cells (Heckel et al., 2003; Ishizuka et al., 2008). As extracellular concentrations of a given LNAA increase, intracellular concentrations increase as well (Mains and Patterson, 1973; Tsai and Lee, 1996). Thus, L-TYR introduced by reverse dialysis should readily raise intracellular L-TYR levels in all brain cells. Given usual probe recovery for L-TYR (15 – 30%) (Bongiovanni et al., 2003; Choi et al., 2001) and average L-TYR levels of 2 μM in microdialysate from rat brain (Bongiovanni et al., 2006; Bongiovanni et al., 2008; Bongiovanni et al., 2010; Jaskiw et al., 2005; Jaskiw et al., 2006; Jaskiw et al., 2008b; McFarlane et al., 2011) we estimate basal L-TYR levels in rat brain interstitial fluid to be ~ 7–14 μM. That falls within the range of L-TYR levels reported in rat cerebrospinal fluid (2–19 μM) (Bongiovanni et al., 2010; Kornhuber et al., 1986; Reichel et al., 1995; Therrien and Butterworth, 1991).
L-TYR levels in rodent brain tissue can be raised up to 7-fold by experimental diets (Block and Harper, 1991; Thurmond et al., 1980) though increases up to 3-fold are more commonly described (Choi et al., 2001; Fernstrom and Faller, 1978; Peters and Harper, 1987; Voog and Eriksson, 1992). Our goal was to achieve a comparable elevation in vivo. During reverse dialysis, 10% or less of an administered drug reaches the surrounding extracellular space (Westerink and De Vries, 2001). Homogeneous diffusion is not assured (Benveniste et al., 1990). However, assuming that L-TYR levels in interstitial fluid near the microdialysis probe are ~ 5–10% of those of perfusate (Westerink and De Vries, 2001), and given basal levels of L-TYR in interstitial fluid ~ 10 μM, we estimated that perfusate concentrations of L-TYR in the range 200 – 400 μM would raise local extracellular L-TYR levels approximately 3-fold. We previously showed that after systemic administration of L-TYR, increases in extracellular and tissue L-TYR were highly comparable (Bongiovanni et al., 2003;Bongiovanni et al., 2010).
4.3 D-TYR as non-enzymatic control
In mammalian tissue, L-TYR is hydroxylated enzymatically either by TH (Fitzpatrick, 1999; Kaufman, 1995;Nagatsu et al., 1964) or by monophenol monooxygenase (EC 1.14.18.1) (tyrosinase), an enzyme not expressed in adult rodent brain (Rios et al., 1999). L-TYR can also be hydroxylated non-enzymatically under neurotoxic conditions (Breier et al., 2006), presumably via the Fenton reaction (Maskos et al., 1992). TH-mediated vs. non-enzymatic mechanisms are often distinguished through administration of a TH-inhibitor, typically α-methyltyrosine or one of its analogues (Nagatsu et al., 1964). As a synthetic LNAA however, α-methyltyrosine also competes with L-TYR for binding to LAT-1, the main LNAA membrane transporter (Kopka et al., 2001). LAT-1, like other LNAA transporters from the L-family is an obligatory 1:1 heteroexchanger; for every LNAA molecule carried in one direction across a membrane, another LNAA molecule must be transported in the opposite direction (del Amo et al., 2008;Uchino et al., 2002). Administration of α-methyltyrosine by reverse dialysis would inhibit TH directly but would in addition deplete intracellular L-TYR. This depletion could per se lower indices of DA synthesis, metabolism and efflux (Biggio et al., 1976; Bongiovanni et al., 2008;Bongiovanni et al., 2012;Fernstrom and Fernstrom, 1994;Jaskiw et al., 2005;Jaskiw and Bongiovanni, 2004;McTavish et al., 1999). Thus, any in vivo α-methyltyrosine-induced attenuation of L-TYR effects could reflect the net influence of two separate mechanisms.
Another approach involves exploiting the high stereospecificity of TH for L-TYR over D-TYR (Fitzpatrick, 1999;Kaufman, 1995;Nagatsu et al., 1964). While D-TYR readily enters brain tissue (Chirigos and Greengard, 1960), it interacts relatively weakly with the LNAA membrane transporter (Kanai and Endou, 2001;Kawai et al., 1999) and induces little heteroexchange with L-TYR (Richelson, 1974). Thus administration of D-TYR should not per se significantly affect intracellular L-TYR kinetics. Since L-TYR and D-TYR are equally likely to be hydroxylated via the Fenton reaction (Wittbjer et al., 1996), an absence of D-TYR effects would implicate TH-mediated hydroxylation of L-TYR. We tested a single concentration (250 μM) of D-TYR corresponding to the L-TYR concentration (250 μM) which affected DOPAC and MHPG in the medial prefrontal cortex (Fig. 2A, 2C, 2G), DOPAC in striatum (Fig. 2B, 2D and for which we generated the most extensive time course (Fig. 2A, 2B). Possible effects of higher D-TYR concentrations cannot be precluded.
4.4 Catecholamine metabolite levels as indices of DA synthesis
Administration of L-TYR by reverse dialysis did not affect extracellular levels of DA (SI Tables 1, 2) but elevated catecholamine metabolites, albeit with region-specific differences (Fig. 2). These must be understood in relation to catecholamine kinetics. Cytosolic TH is present in both soluble and membrane bound forms associated with different regulatory properties (Cartier et al., 2010; Kuczenski and Mandell, 1972). Approximately 80% of DOPA generated from the hydroxylation of L-TYR in striatal terminals is rapidly converted to DA by AADC (Cumming et al., 1998). Some of the newly formed DA is metabolized by mitochondrial monoamine oxidase (MAO) via a short-lived aldehyde intermediate to DOPAC, which can be transported from the cytosol into the extracellular space by a carrier for acidic metabolites (Lamensdorf et al., 2000;Markov et al., 2008). The remaining DA is packaged into vesicles by the type 2 vesicular monoamine transporter (Eisenhofer et al., 2004;Westerink, 2006). Depolarization-induced calcium influx then promotes fusion of vesicles with the plasma membrane and release of DA into the synaptic cleft (Denker and Rizzoli, 2010).
Synaptic vesicles preferentially take up freshly synthesized DA (Westerink, 2006). Increased DA availability, such as follows elevated DA synthesis, raises the DA content of individual vesicles intended for the active zone and increases stimulation-induced quantal DA release (Pereira and Sulzer, 2012;Westerink, 2006). Together these processes increase coupling between DA synthesis and stimulation-induced DA release (Westerink, 2006). The extracellular DA can be taken up by one of the catecholamine transporters and either re-released or metabolized (Eisenhofer et al., 2004;Westerink, 2006). Some vesicular DA may leak into the cytosol where it can also be converted to DOPAC (Eisenhofer et al., 2004). Extraneuronal DA can be metabolized to homovanillic acid (HVA) through the action of catechol-O-methyltransferase (COMT) (Eisenhofer et al., 2004;Westerink, 2006). The relative contributions of uptake and metabolism to net extracellular DA levels vary between DA terminal fields and can be modified pharmacologically (Westerink, 2006).
Due to the overlapping and interconnected metabolic pathways, extracellular levels of DOPAC and HVA could reflect intracellular metabolism of i) newly synthesized DA before it is sequestered (Wallace and Traeger, 2012), ii) DA that has leaked from storage vesicles into the cytosol, iii) extracellular DA recaptured by reverse transport (Eisenhofer et al., 2004;Wallace and Traeger, 2012). Thus metabolite levels can be affected by DA reuptake (Zhuang et al., 2001) as well as by the activity of MAO or COMT (Huotari et al., 2002;Okada et al., 2011). L-TYR can inhibit TH activity directly (Lloyd and Kaufman, 1974;Quinsey et al., 1998;Ribeiro et al., 1991) but is not known to interact with any of the other enzymes or transporters that affect intra- or extracellular levels of DA or its metabolites. Thus, we conclude that L-TYR induced elevations of extracellular DOPAC or HVA necessarily reflect an increase in net DA synthesis.
4.5 L-TYR administration elevates brain regional catecholamine metabolites
The mesocortical DA innervation is characterized by a high fraction of activated TH (Iuvone and Dunn, 1986), a greater dependence of DA synthesis on TYR availability (Bradberry et al., 1989;Tam et al., 1990), rapid DA turnover (Bannon et al., 1981a), tight coupling between DA synthesis and efflux (Galloway et al., 1986), and a high rate of DA efflux per terminal (Cass and Gerhardt, 1995;Garris and Wightman, 1994). These characteristics make MPFC DA synthesis and efflux relatively sensitive to L-TYR availability (Tam et al., 1990). The regulation of extracellular DA levels in the MPFC is also distinct (Budygin et al., 1999;Ciliax et al., 1995;Kaenmaki et al., 2010;Masana et al., 2011). Compared to striatum, the MPFC is characterized by a lower density of DA terminals but greater density of NE terminals (Audet et al., 1988;Descarries et al., 1987;Doucet et al., 1986;Plantje et al., 1987). The NE transporter has a higher affinity for DA than for NE (Gu et al., 1994). Even though most TH (Miner et al., 2003;Schmidt and Bhatnagar, 1979) and DOPA synthesis (Wolf et al., 1986) in MPFC is associated with DA terminals, NE terminals are a major determinant of extracellular mesocortical DA (Carboni et al., 2006;Di Chiara et al., 1992;Yamamoto and Novotney, 1998) and DOPAC levels (Devoto et al., 2001;Devoto et al., 2004).
L-TYR 250–1000 μM produced a greater than five-fold elevation of MPFC DOPAC (Fig. 2C) and a more modest elevation of MHPG (Fig. 2G). One possibility is that both elevations reflect catecholamine synthesis exclusively within NE terminals. On the other hand, since extracellular DA is a major precursor for extracellular MPFC NE (Carboni et al., 2006;Di Chiara et al., 1992;Yamamoto and Novotney, 1998) and extracellular DA levels were not concomitantly elevated (SI Table 1) it is more likely that the administered L-TYR elevated DA synthesis within DA terminals as well as NE synthesis within NE terminals. In brain regions where the DA innervation predominates, changes in extracellular DA and DOPAC can be dissociated, suggesting that extracellular DOPAC reflects mainly cytosolic metabolism of newly synthesized DA rather than of DA recaptured from extracellular fluid (Jones et al., 1998;Yadid et al., 2000). By extension, this would suggest that the L-TYR induced elevation of catecholamine metabolites in MPFC under baseline conditions (Fig. 2C) reflects increased turnover rather than release of DA and NE.
In striatum, DOPAC levels in our control (VEH-treated) rats declined significantly from morning through the afternoon (Figs. 2B, C), consistent with circadian rhythms. Striatal extracellular DOPAC levels in rats typically peak during the dark phase and decline to a nadir during the light phase (Marquez de et al., 2000;Paulson and Robinson, 1994). Our critical finding is that L-TYR 250 μM abolished this decline (Figs. 2B, 2D). Furthermore, extracellular DOPAC levels that remained stable during the 3.0 h period of L-TYR 250 μM administration began to decline immediately after L-TYR was discontinued (Fig. 2B). When the duration of L-TYR 250 μM administration was extended, DOPAC levels at t = 5.0 h were about twice as high as those in the VEH group (Fig 2B). These striatal changes were more modest than those in MPFC, where L-TYR concentrations over a wider range (250–1000 μM) elevated DOPAC 4 – 7-fold (Figs. 2A, C). Since the striatum has a very sparse NE innervation (Doucet et al., 1986;Plantje et al., 1987), the changes in striatal DOPAC must be attributed to DA terminals.
4.6 Inhibition of TH activity
We posited that at higher L-TYR concentrations, increases in MPFC DA synthesis would be limited by overlapping mechanisms that inhibit TH activity. Cytosolic DA competes with reduced tetrahydrobiopterin for binding of Fe+2, and in addition forms a nearly irreversible, inactive TH-DA-Fe+3 complex (Dunkley et al., 2004;Kumer and Vrana, 1996). Furthermore, mesocortical DA terminals possess release-modulating autoreceptors of the D2R type; their stimulation enlarges the cytosolic DA pool inhibitory to TH (Galloway et al., 1986;Wolf et al., 1986). We found, however, that both extracellular MPFC DOPAC and MHPG increased along with increasing concentrations of L-TYR 250–1000 μM (Fig. 2). Furthermore, DOPAC levels began to rise shortly after introduction of perfusate containing L-TYR 250–1000 μM and continued to do so throughout the perfusion period (e.g. Fig. 2A) without any evidence of inhibition (Figs. 2A, C, E). One possibility is that intra-terminal MAO rapidly metabolized the newly-synthesized DA, effectively preventing significant expansion of the inhibiting DA pool. Another explanation may derive from the relatively high fraction of phosphorylated TH in MPFC (Iuvone and Dunn, 1986). Whereas unphosphorylated TH possessed both high affinity (Kd < 10 nM) and low affinity (Kd ~ 90–100nM) DA binding sites, phosphorylation abolishes the high affinity site, thus promoting dissociation of the inhibitory TH-DA-Fe+3 complex (Gordon et al., 2008).
L-TYR itself can stereospecifically inhibit TH activity. This has been demonstrated in vitro for the free enzyme at L-TYR concentrations ≥ 30–100 μM (Lloyd and Kaufman, 1974;Quinsey et al., 1998;Ribeiro et al., 1991) as well as in cultured PC12 cells at intracellular L-TYR concentrations exceeding 400–500 μM (DePietro and Fernstrom, 1998). Cytosolic L-TYR levels in brain cells can be estimated at ~ 170 μM (Table 3), assuming homogeneous distribution within and across different brain cells. We posit that reverse dialysis of L-TYR 250 μM elevated intracellular L-TYR ~ 3-fold, into the range ~ 500 μM. Therefore, reverse dialysis of L-TYR (500–1000 μM) should have raised intracellular L-TYR levels beyond the 400–500 μM range at which TH inhibition has been demonstrated in other catecholaminergic cell types (DePietro and Fernstrom, 1998).
Table 3.
Affinity (Km) of TH v. Levels of aromatic amino acids in rat brain
| Kma (μM) | Tissueb (ng/mg protein) | Intracellularc (μM) | |
|---|---|---|---|
| L-tyrosine | 8.6 – 10.8 | 1.1 | 172 |
| L-phenylalanine | 49.3 – 160 | 0.85 | 130 |
| L-tryptophan | 210 – 540 | 0.20 | 31 |
cloned rat tyrosine hydroxylase (TH) expressed heterologously and assayed in presence of tetrahydrobiopterin (Daubner et al., 1992;Fitzpatrick, 1991),
average tissue levels in rat medial prefrontal cortex and striatum (Bongiovanni et al., 2010),
calculated for 122 mg/g protein (Banay-Schwartz et al., 1992), 78% water content (Schwab et al., 1997) in rat brain tissue and assuming homogeneous distribution of amino acids in intracellular water.
Inhibitory effects of L-TYR, however, vary depending on the source of TH. Rat brain TH is less sensitive to L-TYR inhibition than TH purified from bovine adrenal medulla (Kaufman, 1974). Furthermore, no one, to our knowledge, has examined whether phosphorylation state affects the sensitivity of TH to L-TYR-mediated inhibition. There is also the possibility of metabolic channeling. Limited data suggest that newly acquired L-TYR can be compartmentalized into a functionally distinguishable metabolic pool rather than being homogenously distributed in cytosol (Menniti and Diliberto, Jr., 1989;Schallreuter et al., 2004;Shiman and Gray, 1998). Could the additional L-TYR administered by reverse dialysis been sequestered away from TH? That is unlikely. The L-TYR-induced elevations of DOPAC indicate that TH was indeed exposed to the enlarged pool of L-TYR. However, we cannot preclude the possibility that high TH activity in MPFC prevented local L-TYR concentrations from reaching inhibitory levels. Several groups, including our own have demonstrated that TH activity can affect local L-TYR levels (Bongiovanni et al., 2006;Jaskiw et al., 2008a; Westerink and Wirix, 1983).
In striatum, only L-TYR 250M had an effect on DOPAC (Fig. 2D) and that was modest compared to the MPFC (Fig. 2B). The striatum is characterized by a higher fraction of unphosphorylated TH (Iuvone and Dunn, 1986) which should be more sensitive to inhibition by cytosolic DA (Dickson and Briggs, 2013). Furthermore, inhibitory D2R autoreceptors on nigrostriatal DA terminals regulate DA synthesis, DA efflux and DA uptake (Bannon et al., 1981b;Chiodo et al., 1984;Wolf and Roth, 1990;Wu et al., 2002). D2R that tonically inhibit local DA efflux have also been identified on non-DA striatal neurons as well (Anzalone et al., 2012). In combination, these mechanisms likely prevented higher concentrations of L-TYR from elevating extracellular DOPAC. It is noteworthy, however, that even the highest L-TYR concentrations tested did not lower DOPAC below control levels (Fig. 2), as might have been expected had L-TYR-mediated inhibition been involved (Katz et al., 1976;Kaufman, 1974;Reed et al., 2010).
Other investigators reported that L-TYR doses (25 – 200 mg/kg IP) which elevated L-TYR levels 1.5 - 6 fold had no effect on MPFC tissue DOPAC or HVA (Tam et al., 1990). Similarly, striatal tissue DOPAC levels did not change in response to an L-TYR dose (250 mg/ kg IP) that elevated tissue L-TYR levels ~ 5-fold (Westerink et al., 1982;Westerink and Wirix, 1983). We note, however, tissue that tissue and extracellular striatal DOPAC levels can be dissociated (Phebus et al., 1995). Extracellular DOPAC may be a more sensitive index of perturbations in DA systems (Gagnaire et al., 2006).
In early microdialysis studies, systemically administered L-TYR was reported to modestly elevate extracellular striatal DA but not DOPAC (Acworth et al., 1988;During et al., 1988;During et al., 1989). Those data, however, were collected under anesthesia, within a few hours of probe implantation. Such potential confounds (Westerink et al., 1988) (Adachi et al., 2000;Hamilton et al., 1992;Westerink and De Vries, 1988) are avoided in most modern studies. There is a single report that reverse dialysis of L-TYR 100 μM elevated extracellular DA levels in striatum of awake animals by ~ 30% (Fusa et al., 2002). We have not been able to detect significant changes of such modest size by in vivo microdialysis. In our laboratory, a wide range of L-TYR doses administered systemically or by reverse dialysis did not affect extracellular DA levels in MPFC or striatum (Bongiovanni et al., 2003;Jaskiw et al., 2001;Jaskiw et al., 2004;Jaskiw et al., 2005) (SI Table 1, 2). However, we did not previously examine extracellular catecholamine metabolites in vivo.
The current investigation was intended to complement our earlier study on DOPA levels (Brodnik et al., 2012). The L-TYR dose-response curves for DA metabolites (Fig. 2) and for NSD 1015-stabilized extracellular DOPA levels (Brodnik et al., 2012) turned out to be different. In the MPFC, DOPA levels were elevated by L-TYR 31.75 – 500 μM, with highest elevations induced by L-TYR 62.5 – 125 μM (Brodnik et al., 2012). In the current study only concentrations L-TYR > 250 μM elevated MPFC DOPAC and MHPG (Fig. 2A, 2C). In contrast, while the L-TYR-induced elevation in striatal DOPA levels in vivo was dose-dependent for the range 62.5 – 1000 μM (Brodnik et al., 2012), only L-TYR 250 μM raised DOPAC levels in striatum (Figs. 2B, 2D). Overall, NSD 1015 stabilized DOPA levels in MPFC and striatum were elevated by a wider range of L-TYR concentrations (Brodnik et al., 2012) than those affecting DA metabolite levels (Fig. 2). Differences in end-product inhibition of TH could be implicated. By inhibiting AADC, NSD 1015 elevates tissue (Carlsson et al., 1972) and extracellular DOPA (Westerink et al., 1990) levels. DOPA, however, is at best a weak inhibitor of TH (Almas et al., 1992;Karobath, 1971) with a binding affinity only about 1/1000th of that of DA (Ramsey and Fitzpatrick, 2000). TH derived from native tissue has been found complexed with DA, NE and epinephrine but never with L-DOPA (Andersson et al., 1992;Haavik et al., 1988). Thus, the wide range of L-TYR concentrations that elevates NSD-1015 stabilized DOPA levels in vivo could reflect a secondary pharmacological attenuation of DA-mediated inhibition. While typical systemic doses of NSD 1015 lower striatal but not MPFC tissue DA levels (Berry et al., 1994;Galloway et al., 1986;Nakamura et al., 1992;Tam et al., 1990), their effects on the DA pool inhibitory to TH have not been examined.
4.7 K+ stimulation
High K+-induced depolarization of DA terminals, promotes calcium influx, thereby increasing DA turnover and exocytotic efflux (Bustos et al., 1976;Imperato and Di Chiara, 1984;Kapatos and Zigmond, 1979;Ripley et al., 1997;Westerink et al., 1989) of both newly synthesized as well as recycled DA (Bustos et al., 1972). We addressed the considerable inter-subject variability of such effects by comparing sequential stimulations within the same animal (Jaskiw et al., 2008b;Ripley et al., 1997). Of course, unlike sequential trains of physiologic depolarizations that promote vesicle fusion at the active zone of terminals, reverse dialysis of high K+ imposes a non-physiological clamped depolarization that induces prolonged ectopic exocytosis (Tibbs et al., 1989;Valtorta et al., 1990) of multiple neurotransmitters (Ghijsen et al., 2003;Hertz and Peng, 1992;Verhage et al., 1991) across a relatively large volume of brain tissue. It affects protein synthesis (Wedege et al., 1977), ECF LNAA levels (Jaskiw et al., 2008b) and influences glial cells as well as neurons (Hertz and Peng, 1992). Since most high K+ effects are dose-dependent, our goal was to use the minimum K+ concentration that would yield consistent catecholamine efflux across two successive pulses. As expected (Stanford et al., 2000), peak stimulated DA efflux in striatum increased with increasing K+ concentration (Fig. 3D). We observed similar effects on MPFC DA efflux (Fig. 3C), confirming previous observations from brain slices (Ohmori et al., 1991). As K+ concentrations were elevated 37.5mM - 50 mM, DA efflux increased markedly (Figs. 3C, 3D). Across this range, the experimental variance but not the ratio P2/P1 also increased, prompting us to select K+ 37.5 mM for additional studies.
In MPFC, L-TYR 125 μM increased P2/P1 for DA and for NE by 3-fold and 1.5 fold respectively (Fig. 4). The P2/P1 for NE was also increased 1.5 fold by L-TYR 250 μM (Fig. 4). In contrast, D-TYR 125 μM had no effect (Fig. 4). The data suggest that an estimated 2–3 fold elevation of ECF L-TYR levels potently enhances stimulation-induced DA and NE efflux. This is consistent with earlier studies in which pharmacologically stimulated MPFC extracellular DA levels were also elevated when L-TYR levels were elevated 2–3 fold (Bongiovanni et al., 2003;Jaskiw et al., 2001;Jaskiw et al., 2004). The dissociation between DA and NE effects in response to L-TYR 250 μM (Figs. 4G, H) suggests a contribution from NE terminals independent of that from DA. We note that the L-TYR concentration (125 μM) that elevated K+-stimulated DA efflux (Fig. 4G) was below the threshold (L-TYR 250 μM) for elevating unstimulated DOPAC and MHPG levels (Fig. 3C). This is not surprising. K+-induced stimulation changes the kinetic properties of TH (Harada et al., 1996;Haycock et al., 1998) and hence of DA-mediated feedback mechanisms on TH catalytic activity.
In striatum, in contrast to MPFC, no elevation of P2/P1 was evident. Indeed, reverse dialysis of L-TYR 250 μM significantly lowered P2/P1 (Figs. 4F, 4I). This was unexpected. Although even basal in vivo DA levels are sufficiently high enough to exert some tonic autoinhibition via D2R (Moquin and Michael, 2009), we previously found that a systemic dose of L-TYR that elevated brain L-TYR levels 2–3 fold, augmented haloperidol-induced striatal DA levels (Bongiovanni et al., 2003;Jaskiw et al., 2001). Similarly to high K+, haloperidol administration induces TH phosphorylation (Haycock, 1987;Salvatore et al., 2001), elevates DA synthesis (Zivkovic et al., 1975) and increases DA efflux (Jaskiw et al., 2001;Moghaddam and Bunney, 1990;Pehek and Yamamoto, 1994). Phosphorylation does not, however, affect either uptake of L-TYR into synaptosomes (Goldstein et al., 1976; Harris et al., 1975) or the affinity of TH for L-TYR (Daubner and Fitzpatrick, 1993). One possibility is that during reverse dialysis of L-TYR 250 μM, the rate of K+-induced DA synthesis exceeds that of internal metabolism and release, thereby expanding the intraterminal DA pool inhibitory to TH. As a group, our data are consistent with greater inhibitory regulation of DA efflux in striatum relative to MPFC (Budygin et al., 1999;Ciliax et al., 1995;Kaenmaki et al., 2010;Masana et al., 2011).
We also note that P2/P1 for striatal DA was ~ 1.5 in the vehicle-treated group (K+ 37.5 mM, VEH) and that P2 was significantly larger than P1 by paired, non-parametric tests uncorrected for multiple comparisons (Fig. 4I). Even though the P2/P1 ratio did not differ between VEH, L-TYR 62.5–125 μM and D-TYR 250 μM, this still raises the possibility of some carryover effects from P1 to P2. Pharmacologically induced acute activation of striatal TH typically ceases within 60 min of drug administration and does not affect the amount of TH protein for at least 6 h (Neff et al., 2006). In most studies of DA efflux, DA terminals appear to be fully recovered 60 min after cessation of an initial stimulus (Michael et al., 1987;Ripley et al., 1997). However, both the activity and protein expression of AADC can be increased within 60 min of a pharmacological stimulus and persist for 3.0 h (Neff et al., 2006). In our current study, the second K+ exposure began 2.0 h after the end of the first K+ exposure, that is, within the period of time during which AADC activity could still be expected to be elevated above baseline levels.
4.8 Ex vivo voltammetry
Given technical challenges in distinguishing relative contributions of DA and NE to the catechol signal, we did not conduct ex vivo studies in MPFC. In brain slices from striatum, increasing L-TYR concentrations (12.5 – 50 μM) produced a dose-dependent increase in evoked synaptic overflow with no evidence of inhibition (Figs. 5A, 5B). Prior to drawing any mechanistic implications, critical differences between the in vivo and ex vivo models must be considered. In the intact brain, LNAAs can be readily replenished by transport across the BBB. In contrast, intracellular stores of free L-TYR in tissue slices are dependent on net protein catabolism and uptake from the incubating medium (Jones and McIlwain, 1971). L-TYR levels in tissue slices fall by ~ 33% within 15 min of incubation in a TYR-free medium (Buyukuysal and Mogol, 2000). To prevent this, our control medium contained TYR 3.125 μM. We posit, given the 5–10% estimated delivery of L-TYR in vivo (Westerink and De Vries, 2001), that intracellular L-TYR concentrations in brain slices during incubation with L-TYR 6.25 – 50 μM overlap with those reached during reverse dialysis concentrations of L-TYR 12.5 – 1000 μM.
The ex vivo synaptic overflow represents the net result of efflux and uptake in response to a relatively brief 4 ms stimulation intended to model physiological depolarization (Kennedy et al., 1992). This contrasts with the clamped 30 min K+ depolarization used to generate in vivo data. Functionally distinct pools of vesicles have been identified by their response to different experimental conditions (Fowler and Staras, 2015). In general, as the duration of a stimulus increases, both the size of individual neurotransmitter pools and the number of different neurotransmitter pools mobilized for fusion increase as well (Fowler and Staras, 2015;Ghijsen et al., 2003). Within 5 min, high K+ -induces widespread ectopic exocytosis; the fusion of vesicles, which is normally limited to the active zone, becomes extended over a relatively large area of the synaptic membrane (Valtorta et al., 1990). The ex vivo DA levels reflect less extensive mobilization of synaptic pools and hence are likely to be less tightly coupled to ongoing DA synthesis and hence to L-TYR availability.
In slices of striatum, stimulated DA synthesis and/ or synaptic overflow in striatum increased concomitantly with increasing L-TYR availability (Figs. 5A, B). In other words, L-TYR augmented striatal DA efflux could not be inhibited despite two groups of potent regulatory mechanisms, namely a) inhibition of TH by L-TYR and/or the intraterminal DA pool and b) D2R-mediated inhibition of TH / DA efflux / DAT. While endogenous DA acting on D2R-autoreceptors can inhibit synaptic DA overflow within 100 ms (Kennedy et al., 1992;Palij et al., 1990) the detection of such effects depends on the experimental design (Kennedy et al., 1992;Phillips et al., 2002).
cAMP-dependent kinase promotes phosphorylation and hence activation of TH (Briggs et al., 2014). We examined a concentration of the D2R agonist quinpirole (0.66 μM) that exceeds both the threshold for inhibiting striatal DA efflux in vivo (12.5 nM) in the rat (Brodnik et al., 2013) as well as the threshold (100 nM) for inhibiting cAMP-induced phosphorylation in slices of mouse striatum (Lindgren et al., 2003). Quinpirole 0.66 μM inhibited synaptic overflow of DA to ~ 45% baseline (Fig. 5D). That effect is within the range of published dose-response curves (Cragg et al., 1997;Mateo et al., 2005;Palij et al., 1990). In addition, quinpirole blocked the ability of L-TYR 25 μM to elevate synaptic DA overflow (Fig. 5D). Since phosphorylation of TH does not per se affect TH affinity for L-TYR (Daubner and Fitzpatrick, 1993), the data suggest that quinpirole effects are mediated by inhibition of DA efflux and / or inhibition of phosphorylation / activation of TH (Lindgren et al., 2003). This also confirms that the presence of functional inhibitory D2R does not prevent the L-TYR augmented evoked DA overflow under baseline conditions (Fig. 5B).
4.9 Enzyme affinity v. amino acid substrate levels
TH has a high and stereoselective affinity for L-TYR but can also hydroxylate the other aromatic amino acids, L-phenylalanine and L-tryptophan (Teigen et al., 2007) (Table 2). Experimental determination of amino acid substrate affinity (Km) is highly dependent on assay conditions, particularly on the nature and concentration of the tetrahydropterin cofactor (Daubner et al., 1993;Kaufman, 1995;Minami et al., 1992;Teigen et al., 2007). We identified two studies in which the affinity of rat TH for each of its three amino acid substrates was determined in vitro under the same conditions and in the presence of the natural cofactor tetrahydrobiopterin (Daubner et al., 1992;Fitzpatrick, 1991) (Table 2). Using brain tissue levels from our laboratory (Bongiovanni et al., 2010), reference values for protein and water content in rat brain (Banay-Schwartz et al., 1992;Schwab et al., 1997) and assuming homogeneous distribution in intracellular water, we estimated free intracellular amino acid levels (Table 3). The relative substrate affinities confirm that under usual conditions, brain TH would almost exclusively hydroxylate L-TYR rather than L-phenylalanine or L-tryptophan. However, the in vitro data also indicate that TH operates under a marked excess of L-TYR (Table 3) and hence that elevation of basal L-TYR availability should not affect DA synthesis (Kaufman and Kaufman, 1985). While a full treatment of the apparent discrepancy between the in vitro and in vivo data are beyond the scope of this paper, we note that i) the assumption of homogeneous amino acid distribution can be challenged, ii) high TH activity may affect L-TYR levels (Bongiovanni et al., 2006) and that iii) individual in vitro enzyme characterization studies typically examine only a few of the many factors that may be involved in TH regulation in vivo (Tekin et al., 2014).
5. Conclusion
Our three sets of experiments offer complementary information about the effect of increasing L-TYR availability on forebrain catecholamine systems. Across all experiments, effects were seen for L-TYR but not D-TYR, confirming a stereospecific enzyme-mediated mechanism. The simplest study, and the most physiologically relevant, was the first in which elevated levels of L-TYR potently elevated extracellular DOPAC and NE levels in MPFC and more modestly elevated DOPAC levels in striatum. The effects were evident when estimated brain L-TYR level were ~ 2–3 fold above basal, a range readily attained through dietary means (Choi et al., 2001;Fernstrom and Faller, 1978;Peters and Harper, 1987;Voog and Eriksson, 1992). Since L-TYR is not known to interact with vesicular transporters, catecholamine receptors, uptake mechanisms or any of the enzymes that catabolize DA or NE, we conclude that at least some of the additional L-TYR was converted enzymatically to DA. The corollary, of course, is that contrary to the prevailing understanding derived largely from in vitro studies (Fitzpatrick, 1999;Kaufman, 1995;Reed et al., 2010;Zigmond, 1988), in vivo data do not confirm that TH is fully saturated by its L-TYR substrate under baseline conditions. This is consistent with our previous report on L-TYR augmented in vivo DOPA accumulation (Brodnik et al., 2012).
Also consistent with our earlier reports (Bongiovanni et al., 2003;Jaskiw et al., 2001;Jaskiw et al., 2004;Jaskiw et al., 2005), elevation of L-TYR did not affect unstimulated in vivo DA levels in either MPFC or striatum. We also conclude that under unstimulated conditions, most additional DA or NE synthesized from administered L-TYR is promptly metabolized, either before it is first packaged in vesicles or shortly afterwards. Thus, the increased catecholamine turnover suggested by earlier brain tissue studies synthesis (Chance et al., 1990;Milner and Wurtman, 1986;Morrow et al., 1996;Tam et al., 1990) is now confirmed in vivo.
Reverse dialysis with high K+ perfusate induces a clamped depolarization that mobilizes and releases multiple vesicular stores. When such conditions are applied to the MPFC, at least some of the administered L-TYR is converted to DA that is then released into the extracellular space; a fraction of the latter is thought to be taken up by NE terminals and converted to NE. It is noteworthy, that K+-evoked DA or NE levels in MPFC were not inhibited at any L-TYR concentration. In striatum in contrast, a concentration of L-TYR that elevated striatal DA synthesis and metabolism in the unstimulated state, depressed K+ stimulated efflux, suggesting an L-TYR-driven expansion of the DA pool inhibitory to TH.
The ex-vivo data demonstrate that in the absence of control provided by afferents, L-TYR readily increases synaptic DA overflow in the striatum. We posit that the brief electrical pulses used to promote DA efflux ex vivo (Fig. 5) are less potent in activating TH activity and DA synthesis than the prolonged K+ depolarization in vivo (Figs. 3, 4) and hence do not expand the inhibitory intracellular DA pool. This raises the possibility that transient DA efflux elicited by physiological stimuli in vivo could be amplified by elevations of L-TYR. Such elevations are within the physiological range. Cerebrospinal fluid L-TYR levels in non-human primates vary up to 5-fold in response to dietary changes (Grimes et al., 2009). Given the high concordance between brain intracellular, brain extracellular and cerebrospinal LNAA levels (Bongiovanni et al., 2010), similar fluctuations can be expected in human brain parenchyma.
Our conclusions must be understood within the technical limitations of our experimental design and techniques. We could not measure intracellular L-TYR directly. The in vivo data represent average analyte efflux measured over 30 min collections. Possible effects of L-TYR availability on phasic catecholamine efflux over much shorter time periods (Marinelli and McCutcheon, 2014) remain to be explored. A K+- induced clamped 30 min depolarization differs strikingly from a physiological action potential that lasts a few milliseconds. While ex vivo voltammetry allowed application of more physiological 4 ms stimulus durations, brain slices are both deafferented and disconnected from the circulatory system. We did not measure cofactors (e.g. tetrahydrobiopterin) known to affect TH kinetics or other neurotransmitters (e.g. serotonin, glutamate) that affect DA efflux. We examined only single concentrations D-TYR and of quinpirole.
These shortcomings notwithstanding, our data unequivocally demonstrate that even under unstimulated conditions, physiologically relevant elevations of L-TYR can increase catecholamine synthesis and metabolism in MPFC and striatum. This imposes a metabolic cost. At neutral pH, DA is unstable and prone to autoxidation (Bisaglia et al., 2014). The regular metabolic pathway for DA involves generation of the highly toxic intermediate 3,4-dihydroxyphenylacetaldehyde (Eisenhofer et al., 2004). Both processes generate quinones and highly reactive radical oxygen species that can attack thiol groups and damage cellular components (Bisaglia et al., 2014). High concentrations of DOPAC alone inhibit mitochondrial respiration (Gautam and Zeevalk, 2011). Thus, elevated L-TYR availability could promote oxidative stress and neurodegeneration, particularly if protective mechanisms were compromised. Oxidative stress in subcortical DA terminal fields has been implicated in neurodegenerative disorders such as Parkinson’s disease (Bisaglia et al., 2014). More recently, indices of increased oxidative stress have also been detected in the prefrontal cortex of patients with schizophrenia, bipolar disorder and autism (Kim et al., 2014;Sajdel-Sulkowska et al., 2011), conditions in which dysregulated L-TYR transport (Bongiovanni et al., 2013;Fernell et al., 2007;Flyckt et al., 2001;Hagenfeldt et al., 1987;Olsson et al., 2006;Persson et al., 2009;Wiesel et al., 1991;Wiesel et al., 1999) would expose the brain to abnormally elevated levels of L-TYR (Wiesel et al., 1999). These disorders are also associated with working memory deficits (McGrath et al., 2016) and dendritic abnormalities in pyramidal cells of the prefrontal cortex (Konopaske et al., 2014;Phillips and Pozzo-Miller, 2015).
This is particularly interesting in light of our findings that increased L-TYR availability markedly elevated stimulated DA and NE levels in MPFC (Figs. 2, 3). Stress can also elicit catecholamine efflux in the prefrontal cortex of rodents and primates (Pani et al., 2000;Vaessen et al., 2015). Chronic stress-elicited catecholaminergic efflux is known to induce working memory deficits and loss of dendritic complexity in the MPFC (Hains et al., 2009). Our data suggest that this pathophysiological process could be amplified by elevated L-TYR availability and conversely, attenuated by appropriate interventions.
Supplementary Material
Exogenously introduced L-tyrosine is converted to catecholamines in vivo
Tyrosine hydroxylase does not behave as though it were fully saturated in vivo
L-tyrosine availability affects catecholamine neurotransmission and metabolism
Implications for neurotransmission and neurotoxicity in health and disease
Acknowledgments
The in vivo experiments were supported by MERIT grant 1 I01 BX000381-01 (to GEJ) by the Office of Research and Development, Medical Research Service of the Department of Veterans Affairs (DVAMC) and conducted at the LSCDVAMC. The ex vivo experiments were supported by DA031900 grant (to RAE) by the National Institute on Drug Abuse and by the Dean’s Fellowship for Excellence in Collaborative or Themed Research (to ZDB) from the Drexel University College of Medicine Graduate school of Biomedical Sciences and Professional Studies and were conducted at Drexel University. No funding source played any role in the collection, analysis, interpretation or publication of the data.
Appendix A. Supplementary data
Supplementary data related to this article is attached.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Acworth IN, During MJ, Wurtman RJ. Processes that couple amino acid availability to neurotransmitter synthesis and release In: Amino Acid Availability and Brain Function in Health and Disease, Vol H20, G Huether ed. Springer-Verlag; Heidelberg: 1988. pp. 117–136. [Google Scholar]
- Adachi Y, Uchihashi Y, Watanabe K, Satoh T. Halothane anesthesia decreases the extracellular level of dopamine in rat striatum: a microdialysis study in vivo. J Anesth. 2000;14:82–90. doi: 10.1007/s005400050072. [DOI] [PubMed] [Google Scholar]
- Almas B, Le Bourdelles B, Flatmark T, Mallet J, Haavik J. Regulation of recombinant human tyrosine hydroxylase isozymes by catecholamine binding and phosphorylation. Structure/activity studies and mechanistic implications. Eur J Biochem. 1992;209:249–255. doi: 10.1111/j.1432-1033.1992.tb17283.x. [DOI] [PubMed] [Google Scholar]
- Andersson KK, Vassort C, Brennan BA, Que L, Jr, Haavik J, Flatmark T, Gros F, Thibault J. Purification and characterization of the blue-green rat phaeochromocytoma (PC12) tyrosine hydroxylase with a dopamine-Fe(III) complex. Reversal of the endogenous feedback inhibition by phosphorylation of serine-40. Biochem J. 1992;284(Pt 3):687–695. doi: 10.1042/bj2840687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De MC, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S, Descarries L. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J Comp Neurol. 1988;274:307–318. doi: 10.1002/cne.902740302. [DOI] [PubMed] [Google Scholar]
- Badawy AA, Williams DL. Enhancement of rat brain catecholamine synthesis by administration of small doses of tyrosine and evidence for substrate inhibition of tyrosine hydroxylase activity by large doses of the amino acid. Biochem J. 1982;206:165–168. doi: 10.1042/bj2060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banay-Schwartz M, Kennesey A, DeGuzman T, Lajtha A, Palkovits M. Protein content of various regions of rat brain and adult and aging human brain. Age. 1992;15:51–54. [Google Scholar]
- Bannon MJ, Bunney EB, Roth RH. Mesocortical dopamine neurons: rapid transmitter turnover compared to other brain catecholamine systems. Brain Res. 1981a;218:376–382. [PubMed] [Google Scholar]
- Bannon MJ, Michaud RL, Roth RH. Mesocortical dopamine neurons: Lack of autoreceptors modulating dopamine synthesis. Mol Pharmacol. 1981b;19:270–275. [PubMed] [Google Scholar]
- Benveniste M, Clements J, Vyklicky L, Jr, Mayer ML. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MD, Scarr E, Zhu MY, Paterson IA, Juorio AV. The effects of administration of monoamine oxidase-B inhibitors on rat striatal neurone responses to dopamine. Br J Pharmacol. 1994;113:1159–1166. doi: 10.1111/j.1476-5381.1994.tb17119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G, Porceddu ML, Gessa GL. Decrease of homovanillic, dihydroxyphenylacetic acid and cyclic-adenosine-3′,5′-monophosphate content in the rat caudate nucleus induced by the acute administration of an aminoacid mixture lacking tyrosine and phenylalanine. J Neurochem. 1976;26:1253–1255. doi: 10.1111/j.1471-4159.1976.tb07014.x. [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Filograna R, Beltramini M, Bubacco L. Are dopamine derivatives implicated in the pathogenesis of Parkinson’s disease? Ageing Res Rev. 2014;13:107–114. doi: 10.1016/j.arr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Block KP, Harper AE. High levels of dietary amino and branched-chain alpha-keto acids alter plasma and brain amino acid concentrations in rats. J Nutr. 1991;121:663–671. doi: 10.1093/jn/121.5.663. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Kirkbride B, Newbould E, Durkalski V, Jaskiw GE. Relationships between large neutral amino acid levels in plasma, cerebrospinal fluid, brain microdialysate and brain tissue in the rat. Brain Res. 2010;1334:45–57. doi: 10.1016/j.brainres.2010.03.111. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Kyser AN, Jaskiw GE. Tyrosine depletion lowers in vivo DOPA synthesis in ventral hippocampus. Eur J Pharmacol. 2012;696:70–76. doi: 10.1016/j.ejphar.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Leonard S, Jaskiw GE. A simplified method to quantify dysregulated tyrosine transport in schizophrenia. Schiz Res. 2013;150:386–391. doi: 10.1016/j.schres.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Newbould E, Jaskiw GE. Tyrosine depletion lowers dopamine synthesis and desipramine-induced prefrontal cortex catecholamine levels. Brain Res. 2008;1190:39–48. doi: 10.1016/j.brainres.2007.10.079. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE. Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and in vivo microdialysate levels in the rat. J Neurochem. 2003;87:310–317. doi: 10.1046/j.1471-4159.2003.02007.x. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Young D, Newbould E, Jaskiw GE. Increased striatal dopamine synthesis is associated with decreased tissue levels of tyrosine. Brain Res. 2006;1115:26–36. doi: 10.1016/j.brainres.2006.07.074. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Karasic DH, Deutch AY, Roth RH. Regionally-specific alterations in mesotelencephalic dopamine synthesis in diabetic rats: association with precursor tyrosine. J Neural Transm. 1989;78:221–229. doi: 10.1007/BF01249231. [DOI] [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs GD, Bulley J, Dickson PW. Catalytic domain surface residues mediating catecholamine inhibition in tyrosine hydroxylase. J Biochem. 2014;155:183–193. doi: 10.1093/jb/mvt110. [DOI] [PubMed] [Google Scholar]
- Brodnik Z, Bongiovanni R, Double M, Jaskiw GE. Increased tyrosine availability increases brain regional DOPA levels in vivo. Neurochem Int. 2012;61:1001–1006. doi: 10.1016/j.neuint.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Brodnik Z, Double M, Jaskiw GE. Presynaptic autoreceptor regulation of extracellular dopamine levels in medial prefrontal cortex and striatum during tyrosine depletion. Psychopharmacology. 2013;227:363–371. doi: 10.1007/s00213-013-2977-0. [DOI] [PubMed] [Google Scholar]
- Brodnik ZD, Espana RA. Dopamine uptake dynamics are preserved under isoflurane anesthesia. Neurosci Lett. 2015;606:129–134. doi: 10.1016/j.neulet.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Jaskiw GE. Effect of mobile phase pH on the function of other optimization parameters in an HPLC–ECD assay of biogenic amines and their metabolites. J Liq Chromatogr Relat Technol. 2015;38:467–471. [Google Scholar]
- Budygin EA, Gainetdinov RR, Kilpatrick MR, Rayevsky KS, Mannisto PT, Wightman RM. Effect of tolcapone, a catechol-O-methyltransferase inhibitor, on striatal dopaminergic transmission during blockade of dopamine uptake. Eur J Pharmacol. 1999;370:125–131. doi: 10.1016/s0014-2999(99)00084-9. [DOI] [PubMed] [Google Scholar]
- Bustos G, Kuhar MJ, Roth RH. Effect of gamma-hydroxybutyrate and gamma-butyrolactone on dopamine synthesis and uptake by rat striatum. Biochem Pharmacol. 1972;21:2649–2652. doi: 10.1016/0006-2952(72)90233-x. [DOI] [PubMed] [Google Scholar]
- Bustos G, Roth RH, Morgenroth VH., III Activation of tyrosine hydroxylase in rat striatal slices by K+-depolarization–effect of ethanol. Biochem Pharmacol. 1976;25:2493–2497. doi: 10.1016/0006-2952(76)90455-x. [DOI] [PubMed] [Google Scholar]
- Buyukuysal RL, Mogol E. Synthesis and release of dopamine in rat striatal slices: requirement for exogenous tyrosine in the medium. Neurochem Res. 2000;25:533–540. doi: 10.1023/a:1007572328295. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Vacca C, Di Chiara G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem. 2006;96:473–481. doi: 10.1111/j.1471-4159.2005.03556.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Davis JN, Kehr W, Lindqvist M, Atack CV. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedebergs Arch Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Dependence of 5-HT and catecholamine synthesis on precursor amino-acid levels in rat brain. Naunyn-Schmiedberg’s Arch Pharmacol. 1978;303:157–164. doi: 10.1007/BF00508062. [DOI] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, Egana L, Torres GE. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995;65:201–207. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- Chance WT, Foley-Nelson T, Nelson JL, Fischer JE. Tyrosine loading increases dopamine metabolite concentrations in the brain. Pharmacol Biochem Behav. 1990;35:195–199. doi: 10.1016/0091-3057(90)90226-8. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Chirigos MA, Greengard P. Uptake of tyrosine by rat brain in vivo. J Biol Chem. 1960;235:2075–2079. [PubMed] [Google Scholar]
- Choi YH, Fletcher PJ, Anderson GH. Extracellular amino acid profiles in the paraventricular nucleus of the rat hypothalamus are influenced by diet composition. Brain Res. 2001;892:320–328. doi: 10.1016/s0006-8993(00)03267-4. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S, Rice ME, Greenfield SA. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:863–873. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- Cumming P, Ase A, Kuwabara H, Gjedde A. [3H]DOPA formed from [3H]tyrosine in living rat brain is not committed to dopamine synthesis. J Cereb Blood Flow Metab. 1998;18:491–499. doi: 10.1097/00004647-199805000-00004. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Fitzpatrick PF. Alleviation of catecholamine inhibition of tyrosine hydroxylase by phosphorylation at serine40. Adv Exp Med Biol. 1993;338:87–92. 87–92. doi: 10.1007/978-1-4615-2960-6_17. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- Daubner SC, Lohse DL, Fitzpatrick PF. Expression and characterization of catalytic and regulatory domains of rat tyrosine hydroxylase. Protein Sci. 1993;2:1452–1460. doi: 10.1002/pro.5560020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35:161–174. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Frontiers in Synaptic Neuroscience. 2010;2:1–12. doi: 10.3389/fnsyn.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePietro FR, Fernstrom JD. The effect of phenylalanine on DOPA synthesis in PC12 cells. Neurochem Res. 1998;23:1011–1020. doi: 10.1023/a:1021044708116. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pira L, Longu G, Gessa GL. Mirtazapine-induced corelease of dopamine and noradrenaline from noradrenergic neurons in the medial prefrontal and occipital cortex. Eur J Pharmacol. 2004;487:105–111. doi: 10.1016/j.ejphar.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda GL, Frau R, Carboni E. Heterologous monoamine reuptake: lack of transmitter specificity of neuron-specific carriers. Neurochem Int. 1992;20(Suppl):231S–235S. 231S–235S. [PubMed] [Google Scholar]
- Dickson PW, Briggs GD. Tyrosine hydroxylase: regulation by feedback inhibition and phosphorylation. Adv Pharmacol. 2013;68:13–21. doi: 10.1016/B978-0-12-411512-5.00002-6. [DOI] [PubMed] [Google Scholar]
- Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- During MJ, Acworth IN, Wurtman RJ. Effects of systemic L-tyrosine on dopamine release from rat corpus striatum and nucleus accumbens. Brain Res. 1988;452:378–380. doi: 10.1016/0006-8993(88)90043-1. [DOI] [PubMed] [Google Scholar]
- During MJ, Acworth IN, Wurtman RJ. Dopamine release in rat striatum: physiological coupling to tyrosine supply. J Neurochem. 1989;52:1449–1454. doi: 10.1111/j.1471-4159.1989.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Dyck LE. Effect of decarboxylase inhibitors on brain p-tyrosine levels. Biochem Pharmacol. 1987;36:1373–1376. doi: 10.1016/0006-2952(87)90097-9. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernell E, Karagiannakis A, Edman G, Bjerkenstedt L, Wiesel FA, Venizelos N. Aberrant amino acid transport in fibroblasts from children with autism. Neurosci Lett. 2007;418:82–86. doi: 10.1016/j.neulet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Faller DV. Neutral amino acids in the brain: Changes in response to food ingestion. J Neurochem. 1978;30:1531–1538. doi: 10.1111/j.1471-4159.1978.tb10489.x. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Fernstrom MH. Dietary effects on tyrosine availability and catecholamine synthesis in the central nervous system: possible relevance to the control of protein intake. Proc Nutr Soc. 1994;53:419–429. doi: 10.1079/pns19940047. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. Studies of the rate-limiting step in the tyrosine hydroxylase reaction: alternate substrates, solvent isotope effects, and transition-state analogues. Biochemistry. 1991;30:6386–6391. doi: 10.1021/bi00240a006. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–81. 355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- Flyckt L, Venizelos N, Edman G, Bjerkenstedt L, Hagenfeldt L, Wiesel FA. Aberrant tyrosine transport across the cell membrane in patients with schizophrenia. Arch Gen Psychiatry. 2001;58:953–958. doi: 10.1001/archpsyc.58.10.953. [DOI] [PubMed] [Google Scholar]
- Fowler MW, Staras K. Synaptic vesicle pools: Principles, properties and limitations. Exp Cell Res. 2015;335:150–156. doi: 10.1016/j.yexcr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Fusa K, Saigusa T, Koshikawa N, Cools AR. Tyrosine-induced release of dopamine is under inhibitory control of presynaptic dopamine D2 and, probably, D3 receptors in the dorsal striatum, but not in the nucleus accumbens. Eur J Pharmacol. 2002;448:143–150. doi: 10.1016/s0014-2999(02)01911-8. [DOI] [PubMed] [Google Scholar]
- Gagnaire F, Chalansonnet M, Carabin N, Micillino JC. Effects of subchronic exposure to styrene on the extracellular and tissue levels of dopamine, serotonin and their metabolites in rat brain. Arch Toxicol. 2006;80:703–712. doi: 10.1007/s00204-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Galloway MP, Wolf ME, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther. 1986;236:689–698. [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam AH, Zeevalk GD. Characterization of reduced and oxidized dopamine and 3,4-dihydrophenylacetic acid, on brain mitochondrial electron transport chain activities. Biochim Biophys Acta. 2011;1807:819–828. doi: 10.1016/j.bbabio.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M, Santinami A, Ruffoli R, Conti G, Fornai F. Novel aspects of dopamine oxidative metabolism (confounding outcomes take place of certainties) Pharmacol Toxicol. 2001;89:217–224. doi: 10.1034/j.1600-0773.2001.d01-151.x. [DOI] [PubMed] [Google Scholar]
- Ghijsen WE, Leenders AG, Lopes da Silva FH. Regulation of vesicle traffic and neurotransmitter release in isolated nerve terminals. Neurochem Res. 2003;28:1443–1452. doi: 10.1023/a:1025606021867. [DOI] [PubMed] [Google Scholar]
- Gordon SL, Quinsey NS, Dunkley PR, Dickson PW. Tyrosine hydroxylase activity is regulated by two distinct dopamine-binding sites. J Neurochem. 2008;106:1614–1623. doi: 10.1111/j.1471-4159.2008.05509.x. [DOI] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- Haavik J, Andersson KK, Petersson L, Flatmark T. Soluble tyrosine hydroxylase (tyrosine 3-monooxygenase) from bovine adrenal medulla: large-scale purification and physicochemical properties. Biochim Biophys Acta. 1988;953:142–156. doi: 10.1016/0167-4838(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Venizelos N, Bjerkenstedt L, Wiesel FA. Decreased tyrosine transport in fibroblasts from schizophrenic patients. Life Sci. 1987;41:2749–2757. doi: 10.1016/0024-3205(87)90468-1. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ME, Mele A, Pert A. Striatal extracellular dopamine in conscious vs anesthetized rats: effects of chloral hydrate anesthetic on responses to drugs of different classes. Brain Res. 1992;597:1–7. doi: 10.1016/0006-8993(92)91498-4. [DOI] [PubMed] [Google Scholar]
- Harada K, Wu J, Haycock JW, Goldstein M. Regulation of L-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J Neurochem. 1996;67:629–635. doi: 10.1046/j.1471-4159.1996.67020629.x. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Stimulation-dependent phosphorylation of tyrosine hydroxylase in rat corpus striatum. Brain Res Bull. 1987;19:619–622. doi: 10.1016/0361-9230(87)90046-3. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M. Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site- and phosphospecific antibodies and site-directed mutagenesis. J Neurochem. 1998;71:1670–1675. doi: 10.1046/j.1471-4159.1998.71041670.x. [DOI] [PubMed] [Google Scholar]
- Heckel T, Broer A, Wiesinger H, Lang F, Broer S. Asymmetry of glutamine transporters in cultured neural cells. Neurochem Int. 2003;43:289–298. doi: 10.1016/s0197-0186(03)00014-7. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L. Effects of monoamine transmitters on neurons and astrocytes: correlation between energy metabolism and intracellular messengers. Prog Brain Res. 1992;94:283–301. 283–301. doi: 10.1016/s0079-6123(08)61758-6. [DOI] [PubMed] [Google Scholar]
- Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto PT. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Trns-striatal dialysis coupled to reverse phase high performance liquid chromatography with electrochemical detection: a new method for the study of the in vivo release of endogenous dopamine and metabolites. J Neurosci. 1984;4:966–977. doi: 10.1523/JNEUROSCI.04-04-00966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka Y, Kakiya N, Nawa H, Takei N. Leucine induces phosphorylation and activation of p70S6K in cortical neurons via the system L amino acid transporter. J Neurochem. 2008;106:934–942. doi: 10.1111/j.1471-4159.2008.05438.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Dunn AJ. Tyrosine hydroxylase activation in mesocortical 3,4-dihydroxyphenylethylamine neurons following footshock. J Neurochem. 1986;47:837–844. doi: 10.1111/j.1471-4159.1986.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Bongiovanni R. Brain tyrosine depletion attenuates haloperidol-induced striatal dopamine release in vivo and augments haloperidol-induced catalepsy in the rat. Psychopharmacology (Berl) 2004;172:100–107. doi: 10.1007/s00213-003-1619-3. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Collins KA, Pehek EA, Yamamoto BK. Tyrosine augments acute clozapine- but not haloperidol-induced dopamine release in the medial prefrontal cortex of the rat: an in vivo microdialysis study. Neuropsychopharmacology. 2001;25:149–156. doi: 10.1016/S0893-133X(01)00220-2. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Kirkbride B, Bongiovanni R. In rats chronically treated with clozapine, tyrosine depletion attenuates the clozapine-induced in vivo increase in prefrontal cortex dopamine and norepinephrine levels. Psychopharmacology (Berl) 2006;185:416–422. doi: 10.1007/s00213-005-0283-1. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Kirkbride B, Newbould E, Young D, Durkalski V, Bongiovanni R. Clozapine-induced dopamine release in the medial prefrontal cortex is augmented by a moderate concentration of locally administered tyrosine but attenuated by high tyrosine concentrations or by tyrosine depletion. Psychopharmacology (Berl) 2005;179:713–724. doi: 10.1007/s00213-004-2091-4. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Newbould E, Bongiovanni R. Gamma-butyrolactone-induced dopamine accumulation in prefrontal cortex is affected by tyrosine availability. Eur J Pharmacol. 2008a;589:106–109. doi: 10.1016/j.ejphar.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Newbould E, Bongiovanni R. Tyrosine availability modulates potassium-induced striatal catecholamine efflux in vivo. Brain Res. 2008b;1209:74–84. doi: 10.1016/j.brainres.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Simpson C, Bongiovanni R, Yamamoto BK. Tyrosine augments clozapine-induced dopamine release in the medial prefrontal cortex of the rat in vivo: effects of access to food. Neurosci Lett. 2004;357:5–8. doi: 10.1016/j.neulet.2003.11.060. [DOI] [PubMed] [Google Scholar]
- Jones DA, McIlwain H. Amino acid production and translocation in incubated and superfused tissues from the brain. J Neurobiol. 1971;2:311–326. doi: 10.1002/neu.480020404. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Mannisto PT. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114:1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Endou H. Heterodimeric amino acid transporters: molecular biology and pathological and pharmacological relevance. Curr Drug Metab. 2001;2:339–354. doi: 10.2174/1389200013338324. [DOI] [PubMed] [Google Scholar]
- Kapatos G, Zigmond MJ. Regulation of dopamine synthesis in striatal synaptosomes during depolarization. Brain Res. 1979;170:299–312. doi: 10.1016/0006-8993(79)90108-2. [DOI] [PubMed] [Google Scholar]
- Karobath M. Catecholamines and the hydroxylation of tyrosine in synaptosomes isolated from rat brain. Proc Natl Acad Sci U S A. 1971;68:2370–2373. doi: 10.1073/pnas.68.10.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz I, Lloyd T, Kaufman S. Studies on phenylalanine and tyrosine hydroxylation by rat brain tyrosine hydroxylase. Biochim Biophys Acta. 1976;445:567–578. doi: 10.1016/0005-2744(76)90111-x. [DOI] [PubMed] [Google Scholar]
- Kaufman S. Properties of the pterin-dependent aromatic amino acid hydroxylases. In: Wolstenholme GEW, Fitzsimons DW, editors. Aromatic Amino Acids in the Brain. Elsevier; Amsterdam: 1974. pp. 85–115. [Google Scholar]
- Kaufman S. Tyrosine Hydroxylase. In: Meister A, editor. Advances in Enzymology and Related Areas of Molecular Biology. Vol. 70. John Wiley & Sons Inc; New York: 1995. pp. 103–220. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Kaufman EE. Tyrosine Hydroxylase. In: Blakley RL, Benkovic SJ, editors. Chemistry and Biochemistry of the Pterins. Vol. 2. John Wiley and Sons; New York: 1985. pp. 251–352. [Google Scholar]
- Kawai K, Flores LG, Nakagawa M, Shikano N, Jinnouchi S, Tamura S, Kubodera A. Brain uptake of iodinated L-meta-tyrosine, a metabolically stable amino acid derivative. Nucl Med Commun. 1999;20:153–157. doi: 10.1097/00006231-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kim HK, Andreazza AC, Yeung PY, Isaacs-Trepanier C, Young LT. Oxidation and nitration in dopaminergic areas of the prefrontal cortex from patients with bipolar disorder and schizophrenia. J Psychiatry Neurosci. 2014;39:276–285. doi: 10.1503/jpn.130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- Kopka K, Riemann B, Friedrich M, Winters S, Halfter H, Weckesser M, Stogbauer F, Ringelstein EB, Schober O. Characterization of 3-[(123)I]iodo-L-alpha-methyl tyrosine transport in astrocytes of neonatal rats. J Neurochem. 2001;76:97–104. doi: 10.1046/j.1471-4159.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- Kornhuber ME, Kornhuber J, Kornhuber AW, Hartmann GM. Positive correlation between contamination by blood and amino acid levels in cerebrospinal fluid of the rat. Neurosci Lett. 1986;69:212–215. doi: 10.1016/0304-3940(86)90606-3. [DOI] [PubMed] [Google Scholar]
- Kuczenski RT, Mandell AJ. Regulatory properties of soluble and particulate rat brain tyrosine hydroxylase. J Biol Chem. 1972;247:3114–3122. [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intrinsic regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Hrycyna C, He LP, Nechushtan A, Tjurmina O, Harvey-White J, Eisenhofer G, Rojas E, Kopin IJ. Acidic dopamine metabolites are actively extruded from PC12 cells by a novel sulfonylurea-sensitive transporter. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:654–664. doi: 10.1007/s002100000246. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci U S A. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T, Kaufman S. The stimulation of partially purified bovine caudate tyrosine hydroxylase by phosphatidyl-L-serine. Biochem Biophys Res Commun. 1974;59:1262–1270. doi: 10.1016/0006-291x(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Mains RE, Patterson PH. Primary cultures of dissociated sympathetic neurons. II Initial studies on catecholamine metabolism. J Cell Biol. 1973;59:346–360. doi: 10.1083/jcb.59.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014;282:176–197. doi: 10.1016/j.neuroscience.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov D, Mosharov EV, Setlik W, Gershon MD, Sulzer D. Secretory vesicle rebound hyperacidification and increased quantal size resulting from prolonged methamphetamine exposure. J Neurochem. 2008;107:1709–1721. doi: 10.1111/j.1471-4159.2008.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de PB, Castaneda TR, Galindo A, Del AA, Segovia G, Reiter RJ, Mora F. Melatonin disrupts circadian rhythms of glutamate and GABA in the neostriatum of the aware rat: a microdialysis study. J Pineal Res. 2000;29:209–216. doi: 10.1034/j.1600-0633.2002.290403.x. [DOI] [PubMed] [Google Scholar]
- Masana M, Bortolozzi A, Artigas F. Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia. Int J Neuropsychopharmacol. 2011;14:53–68. doi: 10.1017/S1461145710000908. [DOI] [PubMed] [Google Scholar]
- Maskos Z, Rush JD, Koppenol WH. The hydroxylation of phenylalanine and tyrosine: a comparison with salicylate and tryptophan. Arch Biochem Biophys. 1992;296:521–529. doi: 10.1016/0003-9861(92)90606-w. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Steele J, Vinion K, Bongiovanni R, Double M, Jaskiw GE. Acute lithium administration selectively lowers tyrosine levels in serum and brain. Brain Res. 2011;1420:29–36. doi: 10.1016/j.brainres.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Braaten EB, Doty ND, Willoughby BL, Wilson HK, O’Donnell EH, Colvin MK, Ditmars HL, Blais JE, Hill EN, Metzger A, Perlis RH, Willcutt EG, Smoller JW, Waldman ID, Faraone SV, Seidman LJ, Doyle AE. Extending the ‘cross-disorder’ relevance of executive functions to dimensional neuropsychiatric traits in youth. J Child Psychol Psychiatry. 2016;57:462–471. doi: 10.1111/jcpp.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish SF, Cowen PJ, Sharp T. Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology (Berl) 1999;141:182–188. doi: 10.1007/s002130050823. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Diliberto EJ., Jr Newly synthesized dopamine as the precursor for norepinephrine synthesis in bovine adrenomedullary chromaffin cells. J Neurochem. 1989;53:890–897. doi: 10.1111/j.1471-4159.1989.tb11788.x. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Res. 1987;421:325–335. doi: 10.1016/0006-8993(87)91302-3. [DOI] [PubMed] [Google Scholar]
- Milner JD, Wurtman RJ. Catecholamine synthesis: Physiological coupling to precursor supply. Biochem Pharmacol. 1986;35:875–881. doi: 10.1016/0006-2952(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Minami M, Takahashi T, Maruyama W, Takahashi A, Nagatsu T, Naoi M. Allosteric effect of tetrahydrobiopterin cofactors on tyrosine hydroxylase activity. Life Sci. 1992;50:15–20. doi: 10.1016/0024-3205(92)90192-r. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J Neurochem. 2009;110:1491–1501. doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Tyrosine enhances behavioral and mesocorticolimbic dopaminergic responses to aversive conditioning. Synapse. 1996;22:100–105. doi: 10.1002/(SICI)1098-2396(199602)22:2<100::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial step in catecholamine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Nakamura S, Goshima Y, Yue JL, Misu Y. Transmitter-like basal and K(+)-evoked release of 3,4-dihydroxyphenylalanine from the striatum in conscious rats studied by microdialysis. J Neurochem. 1992;58:270–275. doi: 10.1111/j.1471-4159.1992.tb09306.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academis Press; Washington, D.C: 2011. [Google Scholar]
- Neff NH, Wemlinger TA, Duchemin AM, Hadjiconstantinou M. Clozapine modulates aromatic L-amino acid decarboxylase activity in mouse striatum. J Pharmacol Exp Ther. 2006;317:480–487. doi: 10.1124/jpet.105.097972. [DOI] [PubMed] [Google Scholar]
- Nissbrandt H, Carlsson A. Turnover of dopamine and dopamine metabolites in rat brain: comparison between striatum and substantia nigra. J Neurochem. 1987;49:959–967. doi: 10.1111/j.1471-4159.1987.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Koyama T, Yamashita I. Measurement of endogenous dopamine and norepinephrine release from superfused slices of rat prefrontal cortex in vitro: modulation by D2 and alpha-2 presynaptic receptors. Life Sci. 1991;48:283–289. doi: 10.1016/0024-3205(91)90356-g. [DOI] [PubMed] [Google Scholar]
- Okada M, Nakao R, Hosoi R, Zhang MR, Fukumura T, Suzuki K, Inoue O. Microdialysis with radiometric monitoring of L-[beta-11C]DOPA to assess dopaminergic metabolism: effect of inhibitors of L-amino acid decarboxylase, monoamine oxidase, and catechol-O-methyltransferase on rat striatal dialysate. J Cereb Blood Flow Metab. 2011;31:124–131. doi: 10.1038/jcbfm.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson E, Wiesel FA, Bjerkenstedt L, Venizelos N. Tyrosine transport in fibroblasts from healthy volunteers and patients with schizophrenia. Neurosci Lett. 2006;393:211–215. doi: 10.1016/j.neulet.2005.09.070. [DOI] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humphrey PP. Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res. 1990;509:172–174. doi: 10.1016/0006-8993(90)90329-a. [DOI] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108:624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Yamamoto BK. Differential effects of locally administered clozapine and haloperidol on dopamine efflux in the rat prefrontal cortex and caudate-putamen. J Neurochem. 1994;63:2118–2124. doi: 10.1046/j.1471-4159.1994.63062118.x. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Sulzer D. Mechanisms of dopamine quantal size regulation. Front Biosci. 2012;17:2740–2767. doi: 10.2741/4083. [DOI] [PubMed] [Google Scholar]
- Persson ML, Johansson J, Vumma R, Raita J, Bjerkenstedt L, Wiesel FA, Venizelos N. Aberrant amino acid transport in fibroblasts from patients with bipolar disorder. Neurosci Lett. 2009;457:49–52. doi: 10.1016/j.neulet.2009.03.095. [DOI] [PubMed] [Google Scholar]
- Peters JC, Harper AE. Acute effects of dietary protein on food intake, tissue amino acids, and brain serotonin. Am J Physiol. 1987;252:R902–R914. doi: 10.1152/ajpregu.1987.252.5.R902. [DOI] [PubMed] [Google Scholar]
- Phebus LA, Mincy RE, Clemens JA. Ischemia increases tissue and decreases extracellular levels of acid dopamine metabolites in the rat striatum: further evidence for active transport of metabolites. Life Sci. 1995;56:1135–1141. doi: 10.1016/0024-3205(95)00051-7. [DOI] [PubMed] [Google Scholar]
- Phillips M, Pozzo-Miller L. Dendritic spine dysgenesis in autism related disorders. Neurosci Lett. 2015;601:30–40. doi: 10.1016/j.neulet.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Plantje JF, Steinbusch HW, Schipper J, Dijcks FA, Verheijden PF, Stoof JC. D-2 dopamine-receptors regulate the release of [3H]dopamine in rat cortical regions showing dopamine immunoreactive fibers. Neuroscience. 1987;20:157–168. doi: 10.1016/0306-4522(87)90009-1. [DOI] [PubMed] [Google Scholar]
- Quinsey NS, Luong AQ, Dickson PW. Mutational analysis of substrate inhibition in tyrosine hydroxylase. J Neurochem. 1998;71:2132–2138. doi: 10.1046/j.1471-4159.1998.71052132.x. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Fitzpatrick PF. Effects of phosphorylation on binding of catecholamines to tyrosine hydroxylase: specificity and thermodynamics. Biochemistry. 2000;39:773–778. doi: 10.1021/bi991901r. [DOI] [PubMed] [Google Scholar]
- Reed MC, Lieb A, Nijhout HF. The biological significance of substrate inhibition: a mechanism with diverse functions. Bioessays. 2010;32:422–429. doi: 10.1002/bies.200900167. [DOI] [PubMed] [Google Scholar]
- Reichel A, Begley DJ, Ermisch A. Changes in amino acid levels in rat plasma, cisternal cerebrospinal fluid, and brain tissue induced by intravenously infused arginine-vasopressin. Peptides. 1995;16:965–971. doi: 10.1016/0196-9781(95)00065-r. [DOI] [PubMed] [Google Scholar]
- Ribeiro P, Pigeon D, Kaufman S. The hydroxylation of phenylalanine and tyrosine by tyrosine hydroxylase from cultured pheochromocytoma cells. J Biol Chem. 1991;266:16207–16211. [PubMed] [Google Scholar]
- Ribeiro P, Wang Y, Citron BA, Kaufman S. Regulation of recombinant rat tyrosine hydroxylase by dopamine. Proc Natl Acad Sci U S A. 1992;89:9593–9597. doi: 10.1073/pnas.89.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E. Studies on the transport of L-tyrosine into an adrenergic clone of mouse neuroblastoma. J Biol Chem. 1974;249:6218–6224. [PubMed] [Google Scholar]
- Rios M, Habecker B, Sasaoka T, Eisenhofer G, Tian H, Landis S, Chikaraishi D, Roffler-Tarlov S. Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J Neurosci. 1999;19:3519–3526. doi: 10.1523/JNEUROSCI.19-09-03519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Jaworski J, Randall PK, Gonzales RA. Repeated perfusion with elevated potassium in in vivo microdialysis–A method for detecting small changes in extracellular dopamine. J Neurosci Methods. 1997;78:7–14. doi: 10.1016/s0165-0270(97)00129-5. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Wazir U, Kothari S, Gibbons NC, Moore J, Wood JM. Human phenylalanine hydroxylase is activated by H2O2: a novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem Biophys Res Commun. 2004;322:88–92. doi: 10.1016/j.bbrc.2004.07.082. [DOI] [PubMed] [Google Scholar]
- Schmidt RH, Bhatnagar RK. Assessment of the effects of neonatal subcutaneous 6-hydroxydopamine on noradrenergic and dopaminergic innervation of the cerebral cortex. Brain Res. 1979;166:309–319. doi: 10.1016/0006-8993(79)90216-6. [DOI] [PubMed] [Google Scholar]
- Schwab M, Bauer R, Zwiener U. The distribution of normal brain water content in Wistar rats and its increase due to ischemia. Brain Res. 1997;749:82–87. doi: 10.1016/s0006-8993(96)01165-1. [DOI] [PubMed] [Google Scholar]
- Shiman R, Gray DW. Formation and fate of tyrosine. Intracellular partitioning of newly synthesized tyrosine in mammalian liver. J Biol Chem. 1998;273:34760–34769. doi: 10.1074/jbc.273.52.34760. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Giardina K, Gerhardt GA. In vivo microdialysis studies of age-related alterations in potassium-evoked overflow of dopamine in the dorsal striatum of Fischer 344 rats. Int J Dev Neurosci. 2000;18:411–416. doi: 10.1016/s0736-5748(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Tam SY, Elsworth JD, Bradberry CW, Roth RH. Mesocortical dopamine neurons: High basal firing rate frequency predicts tyrosine dependence of dopamine synthesis. J Neural Transm. 1990;81:97–110. doi: 10.1007/BF01245830. [DOI] [PubMed] [Google Scholar]
- Teigen K, McKinney JA, Haavik J, Martinez A. Selectivity and affinity determinants for ligand binding to the aromatic amino acid hydroxylases. Curr Med Chem. 2007;14:455–467. doi: 10.2174/092986707779941023. [DOI] [PubMed] [Google Scholar]
- Tekin I, Roskoski R, Jr, Carkaci-Salli N, Vrana KE. Complex molecular regulation of tyrosine hydroxylase. J Neural Transm. 2014;121:1451–1481. doi: 10.1007/s00702-014-1238-7. [DOI] [PubMed] [Google Scholar]
- Therrien G, Butterworth RF. Cerebrospinal fluid amino acids in relation to neurological status in experimental portal-systemic encephalopathy. Metab Brain Dis. 1991;6:65–74. doi: 10.1007/BF00999904. [DOI] [PubMed] [Google Scholar]
- Thurmond JB, Kramarcy NR, Lasley SM, Brown JW. Dietary amino acid precursors: Effects on central monoamines, aggression, and locomotor activity in the mouse. Pharmacol Biochem Behav. 1980;12:525–532. doi: 10.1016/0091-3057(80)90184-7. [DOI] [PubMed] [Google Scholar]
- Tibbs GR, Barrie AP, Van Mieghem FJ, McMahon HT, Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, Lee EH. Characterization of L-DOPA transport in cultured rat and mouse astrocytes. J Neurosci Res. 1996;43:490–495. doi: 10.1002/(SICI)1097-4547(19960215)43:4<490::AID-JNR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Uchino H, Kanai Y, Kim dK, Wempe MF, Chairoungdua A, Morimoto E, Anders MW, Endou H. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002;61:729–737. doi: 10.1124/mol.61.4.729. [DOI] [PubMed] [Google Scholar]
- Vaessen T, Hernaus D, Myin-Germeys I, van AT. The dopaminergic response to acute stress in health and psychopathology: A systematic review. Neurosci Biobehav Rev. 2015;56:241–251. doi: 10.1016/j.neubiorev.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Fesce R, Grohovaz F, Haimann C, Hurlbut WP, Iezzi N, Torri TF, Villa A, Ceccarelli B. Neurotransmitter release and synaptic vesicle recycling. Neuroscience. 1990;35:477–489. doi: 10.1016/0306-4522(90)90323-v. [DOI] [PubMed] [Google Scholar]
- Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- Voog L, Eriksson T. Relationship between plasma and brain large neutral amino acids in rats fed diets with different compositions at different times of the day. J Neurochem. 1992;59:1868–1874. doi: 10.1111/j.1471-4159.1992.tb11022.x. [DOI] [PubMed] [Google Scholar]
- Wallace LJ, Traeger JS. Dopac distribution and regulation in striatal dopaminergic varicosities and extracellular space. Synapse. 2012;66:160–173. doi: 10.1002/syn.20996. [DOI] [PubMed] [Google Scholar]
- Wedege E, Luqmani Y, Bradford HF. Stimulated incorporation of amino acids into proteins of synaptosomal fractions induced by depolarizing treatments. J Neurochem. 1977;29:527–537. doi: 10.1111/j.1471-4159.1977.tb10702.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, De Vries JB. Characterization of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantations: methodological aspects. J Neurochem. 1988;51:683–687. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, De Vries JB. A method to evaluate the diffusion rate of drugs from a microdialysis probe through brain tissue. J Neurosci Methods. 2001;109:53–58. doi: 10.1016/s0165-0270(01)00401-0. [DOI] [PubMed] [Google Scholar]
- Westerink BH, De Vries JB, Duran R. Use of microdialysis for monitoring tyrosine hydroxylase activity in the brain of conscious rats. J Neurochem. 1990;54:381–387. doi: 10.1111/j.1471-4159.1990.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Hofsteede HM, Damsma G, De Vries JB. The significance of extracellular calcium for the release of dopamine, acetylcholine and amino acids in conscious rats, evaluated by brain microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:373–378. doi: 10.1007/BF00169526. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Hofsteede RM, Tuntler J, De Vries JB. Use of calcium antagonism for the characterization of drug-evoked dopamine release from the brain of conscious rats determined by microdialysis. J Neurochem. 1989;52:722–729. doi: 10.1111/j.1471-4159.1989.tb02514.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, van Es TP, Spaan SJ. Effects of drugs interfering with dopamine and noradrenaline biosynthesis on the endogenous 3,4-dihydroxyphenylalanine levels in rat brain. J Neurochem. 1982;39:44–51. doi: 10.1111/j.1471-4159.1982.tb04699.x. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Wirix E. On the significance of tyrosine for the synthesis and catabolism of dopamine in rat brain: Evaluation by HPLC with electrochemical detection. J Neurochem. 1983;40:758–764. doi: 10.1111/j.1471-4159.1983.tb08043.x. [DOI] [PubMed] [Google Scholar]
- Westerink RH. Targeting exocytosis: ins and outs of the modulation of quantal dopamine release. CNS Neurol Disord Drug Targets. 2006;5:57–77. doi: 10.2174/187152706784111597. [DOI] [PubMed] [Google Scholar]
- Wiesel FA, Blomqvist G, Halldin C, Sjogren I, Bjerkenstedt L, Venizelos N, Hagenfeldt L. The transport of tyrosine into the human brain as determined with L-[1-11C]tyrosine and PET. J Nucl Med. 1991;32:2043–2049. [PubMed] [Google Scholar]
- Wiesel FA, Andersson JL, Westerberg G, Wieselgren IM, Bjerkenstedt L, Hagenfeldt L, Langstrom B. Tyrosine transport is regulated differently in patients with schizophrenia. Schizophr Res. 1999;40:37–42. doi: 10.1016/s0920-9964(99)00029-8. [DOI] [PubMed] [Google Scholar]
- Wittbjer A, Odh G, Rosengren E, Rorsman H. Enzymatic and non-enzymatic oxygenation of tyrosine. Pigment Cell Res. 1996;9:92–95. doi: 10.1111/j.1600-0749.1996.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Galloway MP, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex: studies in brain slices. J Pharmacol Exp Ther. 1986;236:699–707. [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–43. 323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Larin F, Mostafapour S, Fernstrom JD. Brain catechol synthesis: control by brain tyrosine concentration. Science. 1974;185:183–184. doi: 10.1126/science.185.4146.183. [DOI] [PubMed] [Google Scholar]
- Yadid G, Harvey-White JD, Kopin IJ, Goldstein DS. Estimation of striatal dopamine spillover and metabolism in vivo. Neuroreport. 2000;20(11):3367–3373. doi: 10.1097/00001756-200010200-00021. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71:274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2012;2:5–13. doi: 10.1016/j.baga.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE. A comparison of the long-term and short-term regulations of tyrosine hydroxylase activity. J Physiol (Paris) 1988;83:267–271. [PubMed] [Google Scholar]
- Zivkovic B, Guidotti A, Revuelta A, Costa E. Effects of thioridazine, clozapine and other antipsychotics on the kinetic state of tyrosine hydroxylase and on the turnover rate of dopamine in striatum and nucleus accumbens. J Pharmacol Exp Ther. 1975;194:37–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


