Abstract
Purpose
The purpose of this study was to develop a method for isolating, culturing, and characterizing cells from patient-derived membranes in proliferative vitreoretinopathy (PVR) to be used for drug testing.
Methods
PVR membranes were obtained from six patients with grade C PVR. Membrane fragments were analyzed by gross evaluation, fixed for immunohistologic studies to establish cell identity, or digested with collagenase II to obtain single cell suspensions for culture. PVR-derived primary cultures were used to examine the effects of methotrexate (MTX) on proliferation, migration, and cell death.
Results
Gross analysis of PVR membranes showed presence of pigmented cells, indicative of retinal pigment epithelial cells. Immunohistochemistry identified cells expressing α-smooth muscle actin, glial fibrillary acidic protein, Bestrophin-1, and F4/80, suggesting the presence of multiple cell types in PVR. Robust PVR primary cultures (C-PVR) were successfully obtained from human membranes, and these cells retained the expression of cell identity markers in culture. C-PVR cultures formed membranes and band-like structures in culture reminiscent of the human condition. MTX significantly reduced the proliferation and band formation of C-PVR, whereas it had no significant effect on cell migration. MTX also induced regulated cell death within C-PVR as assessed by increased expression of caspase-3/7.
Conclusions
PVR cells obtained from human membranes can be successfully isolated, cultured, and profiled in vitro. Using these primary cultures, we identified MTX as capable of significantly reducing growth and inducing cell death of PVR cells in vitro.
Keywords: PVR, proliferative vitreoretinopathy, retinal detachment, methotrexate, apoptosis
A complication of vitreo-retinal surgery for retinal detachment is a condition known as proliferative vitreoretinopathy (PVR), in which cells grow uncontrollably beneath or on top of the retina.1 These cells, together with the extracellular matrix that they produce, comprise membranes that adhere to the retina and contract, resulting in traction and recurrent retinal detachment. PVR occurs in 5% to 10% of all rhegmatogenous retinal detachment cases, and it is especially prevalent after retinal detachment associated with open globe injury, where it occurs in approximately 50% of cases.2,3
Migration and proliferation of retinal cells, the formation of contractile membranes, and subsequent retinal detachment are hallmarks of PVR.4 This gradual progression of PVR from the presence of vitreous cells to full-thickness retinal folds has been described by the Retina Society classification scheme.5 Currently, the treatment of advanced PVR is vitreo-retinal surgery to remove these tractional membranes.6 There are no specific therapeutic agents used for the prevention or treatment of PVR.7,8 A variety of pharmacologic agents have been tested for the treatment of PVR including anti-inflammatory drugs,9–12 antiproliferative agents,13–16 antineoplastics,17,18 and anti-growth factor agents,8 and none have demonstrated reproducible clinical success.
Studies from animal models and limited human data indicate that PVR is a complex condition resulting from aberrant migration and proliferation of specific cell types into compartments within the eye where they do not belong.19 The most accepted view of the mechanisms leading to PVR involves migration and proliferation of retinal pigment epithelial (RPE) cells, exposure to cytokines released by retinal trauma, breakdown of the blood–retinal barrier, and infiltration of inflammatory cells.20 This is followed by proliferation and invasion of the intravitreal space with the formation of contractile membranes. Current in vitro models of PVR rely primarily on RPE cell cultures to mimic early PVR, where RPE cells are hypothesized to undergo epithelial to mesenchymal transition (EMT) into fibroblast-like cells.21 In vivo models rely on the injection of a wide variety of cells and factors that are presumed to be involved in PVR membrane formation, including fibroblasts,22,23 RPE,24,25 and blood.26
It is critical to consider that other cell types including glial cells and immune system cells, mainly macrophages, have been shown to play a role in PVR. Specifically, Müller glia has been shown to proliferate inside the retina in PVR cases, and also cells within PVR membranes have been shown to be reactive to glial markers.27,28 Macrophages coming from blood have been shown to be present in PVR membranes where they are thought to play a significant role in secretion of inflammatory cytokines.29,30 Misregulation of critical signaling molecules including transforming growth factor-β (TGF-β)/connective tissue growth factor (CTGF),31 a master regulator of fibrosis, and a significant increase in the abundance of proinflammatory molecules including interleukin-1 β (IL-1β) and tumor necrosis factor-α (TNF-α) are characteristic of PVR and play critical roles in promoting cell growth and extracellular matrix deposition.32,33
Here, we developed a methodology for isolation and primary culture of cells derived from human PVR membranes, including multiple cell types, and established protocols to use these cultures to examine the cell biology of PVR membranes and to test the efficacy of potential therapies.
Materials and Methods
Study Population
This study was performed at the Schepens Eye Research Institute of Massachusetts Eye and Ear, and research protocols were approved by the Institutional Review Board at Massachusetts Eye and Ear for the collection of surgical specimens and for the retrospective analysis of the clinical data. All research protocols adhered to the tenets of the Declaration of Helsinki, and each patient signed a consent form and Health Information Portability and Accountability Act (HIPAA) authorization before participation within the study.
Six patients were recruited from Massachusetts Eye and Ear who had grade C PVR and were undergoing surgery for repair of retinal detachment and removal of PVR membranes. Patients had to be at least 18 years old and could not be pregnant.
Human Fundus Photography
Photographs of patients with PVR were obtained using an ultra-wide field Optos imaging system (Optos, Dunfermline, Scotland).
Processing of Surgically Removed Human PVR Membranes
Immediately after surgery, PVR membranes were placed in a specimen cup containing calcium-free and magnesium-free balanced salt solution for transportation. Membranes were divided into pieces under sterile biosafety level 2 conditions to be used for immunohistochemistry or for cell culture.
Light Microscopy
Unprocessed PVR membranes were transferred into a culture dish in 1× sterile tissue culture grade phosphate buffered saline (PBS). Phase contrast images were taken using an EVOS FL automated stage live cell imaging system (AMAFD1000R; Life Technologies, Woburn, MA, USA).
Cell Isolation and Culture of PVR Membranes
PVR membranes were transferred into 500 μL PBS buffer containing collagenase II (Worthington, Lakewood, NY, USA) and 0.25% bovine calf serum (Hyclone, Logan, UT, USA) and incubated at 37°C for 60 minutes to digest the membrane into a single cell suspension. The membrane was resuspended by gentle pipetting to aid the digestion at a 30-minute time point. Subsequently, the membrane was washed once in Dulbecco's modified Eagle's medium (DMEM; Lonza, Walkersville, MD, USA) containing 1% l-glutamine, 5% bovine calf serum (Hyclone), 2.5% wt/vol nystatin, 100 U/mL penicillin G, and 100 μg/mL streptomycin and spun by centrifugation for 3 minutes at 1200 rpm at room temperature. PVR membrane cells (C-PVR) were resuspended in 250 μL PVR medium containing endothelial basal medium 2 (EBM2; Lonza), EGM-2 Bullet Kit, 100 U/mL penicillin, 100 μg/mL streptomycin (Lonza), 2 mM l-glutamine (Lonza), and 12% fetal bovine serum (FBS; final concentration obtained by adding the serum aliquot included with the EGM-2 Bullet Kit and additional FBS; Atlanta Biologicals, Flowery Branch, GA, USA) and seeded onto 48-well plates.
Immunohistochemistry of PVR Membranes
PVR membranes were fixed in 10% formalin in Dulbecco's PBS (1×; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2PO4, 1.47 mM KH2PO4, pH 7.4) overnight at room temperature and stored in PBS at 4°C until paraffin embedding. Serial sections (6 μm) were cut, deparaffinized in 100% xylene, rehydrated in a series of ethanol, and washed in PBS. Sections were processed for immunohistochemistry using the following antibodies: α-smooth muscle actin (1:100; Dako, Carpinteria, CA, USA), glial fibrillary acidic protein (GFAP) (1:500; Dako), cytokeratin (1:250; Abcam, Cambridge, UK), Bestrophin-1 (BEST-1; 1:150; Abcam), and CD14 (1:50; Abcam). Slides were incubated in 3% H2O2 in methanol to block endogenous peroxidases, and then heat-induced epitope retrieval was performed according to manufacturer's instructions and as described previously.34,35 Slides were then blocked in TNB protein blocking solution (Perkin Elmer, Waltham, MA, USA) and incubated in primary antibody overnight at 4°C. The following day, slides were incubated with biotinylated secondary antibody and then incubated in alkaline phosphatase–conjugated avidin (Vectastain ABC-AP Universal Kit; Vector Laboratories, Burlingame, CA, USA). Expression of markers was visualized using the Vector Red chromogenic substrate kit (Vector Laboratories) and counterstained using Gil no. 3 hematoxylin (Sigma-Aldrich Corp., St. Louis, MO, USA).
Induction of Invasive Phenotype in Culture
C-PVR cells grew as a confluent monolayer on 48-well plates and were cultured in PVR medium. A transformation from a monolayer to invasive cell clusters was triggered by switching P1 cultures from PVR medium to a differentiation medium containing αMEM, N1 supplement, taurine (250 μg/mL), hydrocortisone (20 ng/mL), triodo-thyronin (13 pg/mL), and 5% FBS for 72 hours. Membranes grown in vitro by incubation of C-PVR cells with this conditional medium were harvested and fixed in 10% formalin in PBS overnight prior to paraffin embedding and immunohistochemistry as described above.
Immunofluorescence
Cells from PVR membranes were seeded on a four-well Chamber Slide System for 24 to 72 hours (Thermo Fisher Scientific, Waltham, MA, USA). Cells were fixed with 4% paraformaldehyde (PFA) for 10 minutes, washed with PBS, permeabilized with 0.25% Triton X-100 in PBS for 5 minutes, and blocked (10% goat serum in PBS) for 1 hour. To identify specific cell types, C-PVR was incubated with a fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-SMA primary antibody (smooth muscle actin, 1:150; Sigma-Aldrich Corp.), rabbit polyclonal anti-GFAP primary antibody (glial fibrillary acidic protein, 1:150; Dako), rabbit anti-F4/80 antibody (1:200; Abcam), and rabbit anti-cytokeratin antibody (1:200; Abcam). Cells were incubated with the primary antibody overnight at 4°C followed by incubation with goat anti-rabbit Alexa Fluor 594 secondary antibody (1:300; Life Technologies) or with goat anti-mouse Alexa Fluor 488 secondary antibody (1:300; Life Technologies) for 2 hours at room temperature. Cells were washed with PBS three times, and coverslips were mounted onto the chamber slides using a Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies). Images were obtained using a Zeiss Axioskop 2 MOT Plus microscope (Carl Zeiss, Inc., Oberkochen, Germany).
Assessing Effects of Methotrexate on C-PVR Cells
Thirty thousand C-PVR cells per well were plated in 48-well plates. The next day, C-PVR cells were washed with PBS and treated with PVR media alone (control) or in PVR media with methotrexate (MTX; 100, 200, or 400 μM; Sigma-Aldrich using triplicates for each condition). PVR media were replaced every 72 hours, and the cells were washed with PBS before media change for 6 weeks. Cultures were fixed with 4% paraformaldehyde for 10 minutes prior to staining. Cell nuclei were labeled with Hoechst 33342 diluted in PBS 1:200 (ImmunoChemistry Technologies, LLC, Bloomington, MN, USA). Nuclei were counted using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The quantification of cell density is reported as an average of cells per high-powered field of human PVR cultures, which were cultured in triplicate.
Assessment of Proliferation
C-PVR cells were cultured on cover slips at a confluency of 30,000 cells per well and treated with different concentrations of MTX (100, 200, and 400 μM) or vehicle. At the 24-, 48-, and 72-hour time points, cells were washed and fixed with 4% PFA for 10 minutes. Cells were permeabilized with 0.5% Triton X-100 in PBS for 5 minutes and blocked (10% goat serum in PBS) for 1 hour at room temperature. Proliferation was evaluated by incubating cells with anti-Ki67 antibody (1:50; Novus Biologicals, Cambridge, United Kingdom). The primary antibody was prepared in antibody dilution buffer (5% goat serum) and incubated overnight at 4°C. Cells were incubated with goat anti-rabbit Alexa Fluor 594 secondary antibody (Life Technologies) at 1:300 dilution for 2 hours at room temperature. The cover slips were washed and mounted onto slides using Prolong Gold Antifade Reagent with DAPI. Images were obtained using a Zeiss Axioskop 2 MOT Plus microscope (Carl Zeiss, Inc.). The quantification is reported as an average of Ki67-positive cells to the number of DAPI-positive cells per high-powered field of human C-PVR cultures, which were cultured in triplicate.
Correlating Proliferation of PVR Membranes and C-PVR
Cell proliferation of PVR membranes and C-PVRs from clinical cases 3 and 5 was determined by immunofluorescence staining using an antibody against Ki67, a proliferation marker used as described above. Primary antibody rabbit anti-human Ki67 antibody (1:100; Novus Biologicals) and cell nuclei were labeled with Hoechst 33342 diluted in PBS 1:200 (ImmunoChemistry Technologies).
Assessment of Caspase-Mediated Apoptosis
Cell death of C-PVR cells treated with MTX was measured using fluorochrome-labeled inhibitor of caspase-3 and caspase-7 (FAM FLICA CASPASE 3&7 Assay kit; ImmunoChemistry Technologies).36 Three different time points were tested: 2, 4, and 6 weeks. After each time point, cells were protected from light and pretreated with fluorescent-labeled inhibitor of caspases (FLICA) compound and PVR media for 1 hour at 37°C and 5% CO2. Following incubation, the cell nuclei were stained with Hoechst 33342 diluted in PVR media 1:200 (ImmunoChemistry Technologies) for 10 minutes at 37°C; cultures were washed with EBM2 media and fixed with 4% PFA for 10 minutes. Nuclei were counted using Image J software (National Institutes of Health). Positive cells for apoptosis were counted manually. The quantification of cell density is reported as an average of cells per high-powered field of human C-PVR cultures, which were cultured in triplicate. The quantification of apoptosis is reported as the percentage of positive cells for apoptosis.
Scratch Wound Assay and TNF-α Effect on C-PVR Cells
To detect and measure cell migration, 6 × 105 C-PVR were cultured per well using 12-well plates. Cultures were maintained 5 to 6 days to permit formation of a confluent monolayer. The monolayer was scratched with a sterile 200-μL pipette tip, and a single wound was generated per well. Cells were washed with PBS to remove debris and then exposed to the specific treatment: PVR media alone (control) or in PVR media with MTX (100, 200, or 400 μM). Images were taken 0, 6, 12, 24, 48, 72, and 96 hours after wounding using an EVOS FL automated stage live cell imaging system (Life Technologies). All the experiments were performed in triplicate, and migration was measured as the percentage scratch closure related to time 0 using the TScratch Matlab module (ETH Zurich, Zurich, Switzerland).37 To determine the effect of TNF-a (PeproTech, Rocky Hill, NJ, USA) on C-PVR cells, a scratch wound assay was performed as described above. For this experiment, cells were exposed to specific treatments: PVR media (control), 0.2% BSA in water (vehicle), 400 μM MTX, 1 ng/mL TNF-α, 1 ng/mL TNF-α plus 400 μM MTX, 10 ng/mL TNF-α, and 10 ng/mL TNF-α plus 400 μM MTX. Images were taken 0, 6, 12, 24, 48, 72, and 96 hours after wounding. All the experiments were performed in triplicate, and migration was measured as the percentage of scratch closure related to time 0 using the TScratch Matlab module.37
Imaging
Images for immunohistochemistry of PVR membranes, assessing effects of MTX on C-PVR cells, the scratch wound assay, and the TNF-a effect on C-PVR cells were taken using an EVOS FL automated stage live cell imaging system (Life Technologies).
Statistical Analysis
Experiments were repeated at least three times and included at least three technical replicates. Statistical analyses were performed using the GraphPad Prism 5 software (GraphPad, Inc., La Jolla, CA, USA). Statistical significance was tested by multiple ANOVA and Tukey's test for group comparison. Data are presented as the mean ± SEM. P < 0.05 was considered statistically significant.
Results
Clinical Demographics
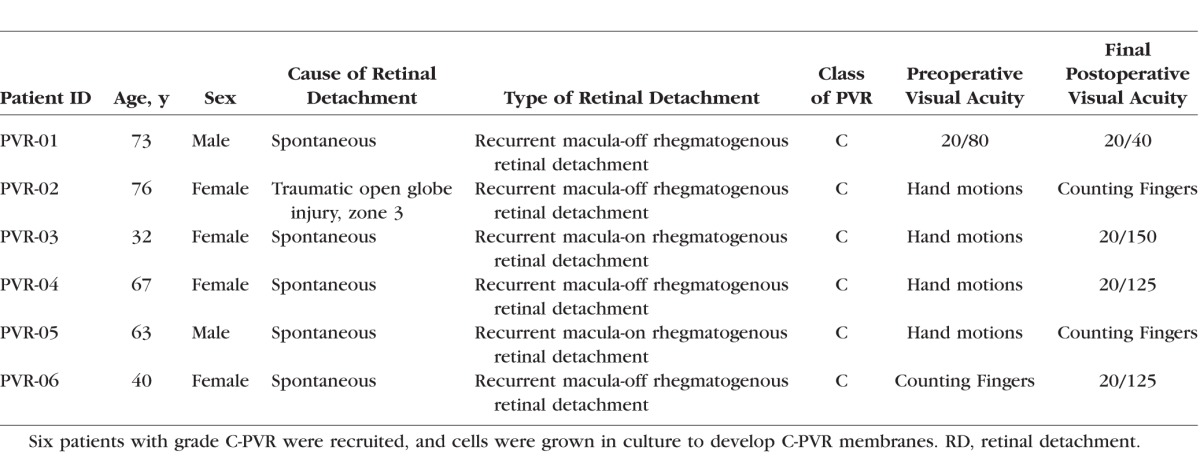
Six patients with grade C PVR that required surgical excision were enrolled in the study. The demographics of the patients are summarized in the Table. All patients had recurrent rhegmatogenous retinal detachment due to PVR. One patient had a recent history of a zone 3 open globe injury with rhegmatogenous retinal detachment and retinal incarceration in the scleral wound. This patient underwent vitrectomy, but returned with recurrent rhegmatogenous retinal detachment due to PVR.
Table.
Clinical Demographics
Characterization by Immunohistochemistry of PVR Membranes and C-PVR
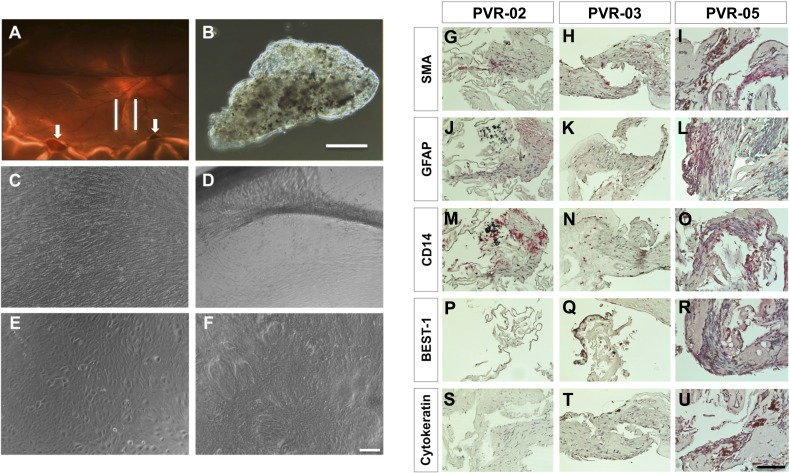
A total of six grade C PVR membranes were surgically excised from patients with retinal detachment (Fig. 1A) and processed in the laboratory. Under light microscopy, PVR membranes grossly appeared to consist of pigmented and nonpigmented cells embedded in a fibrous matrix (Fig. 1B). We examined the cellular constituents of PVR membranes using immunohistochemistry in specimens PVR-02, PVR-03, and PVR-05. Staining of these samples revealed positive localization of SMA a marker of myofibroblasts.38 CD14, a marker expressed by most tissue macrophages,39 displayed more robust expression in PVR-02 compared with PVR-03. BEST-1, a marker for RPE cells,40 did not show expression in PVR-02 yet was localized to the pigmented cells of PVR-03, suggesting these cells were derived from the RPE. Both cytokeratin, found in epithelial cells,41 and GFAP, found in astrocytes,42 were not expressed in PVR-02, whereas PVR-03 showed low expression for both markers. Interestingly, PVR-05 showed positive signal for all these markers (Figs. 1G–1U).
Figure 1.
Culture of human PVR membranes and histopathology of PVR membranes. (A) Fundus photograph of the left eye of a patient, case 6 (PVR-06) with recurrent retinal detachment; note presence of a gas bubble within the eye from previous retinal surgery. There is a band of pigmented PVR along the inferior arcade (outlined by white lines). Also note inferior retinal holes in area of detached retina (white arrows). (B) Phase contrast view of the PVR membrane after surgical excision from PVR-06. Scale bar denotes 500 μm. (C) C-PVR cells from case 3 (PVR-03) after 1 week in culture. (D) After 4 weeks in culture, note the formation of bands between the membrane and the rim of the culture dish. (E) C-PVR cells from case 5 (PVR-05) were similarly confluent after 1 week. (F) After 4 weeks in culture, cells grew on top of each other with loss of cell contact inhibition. Scale bar denotes 100 μm. (G–U) Light micrographs of PVR membranes from three different cases (PVR-02, PVR-03, and PVR-05) using primary antibodies (all in red) against SMA (G–I), GFAP (J–L), CD14 (M–O), BEST-1 (P–R), cytokeratin (S–U), and counterstained with hematoxylin (blue). Scale bar denotes 100 μm.
Establishment of C-PVR Primary Cultures
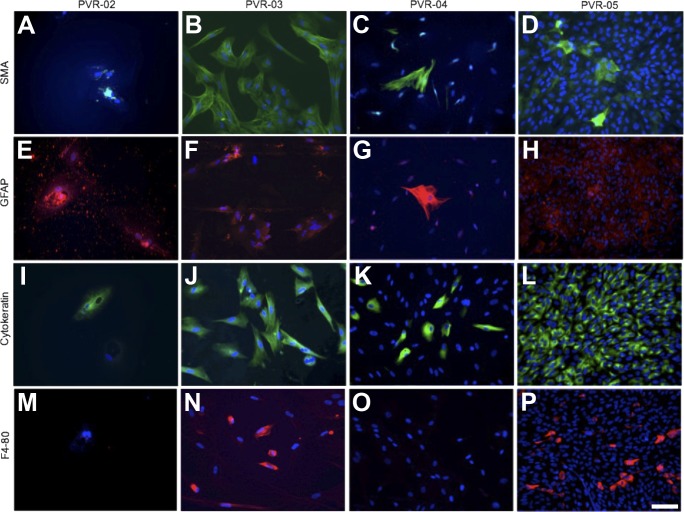
Cells from these PVR membranes, which we called C-PVR, were successfully isolated in each case and cultured as described in the Methods section (Figs. 1C–1F; Supplementary Fig. S2). Distinct populations of C-PVR cells contained pigmented granules characteristic of RPE cells (Fig. 1B). SMA-positive cells were abundant in C-PVR derived from PVR-03, PVR-04, and PVR-05 but not from PVR-02 (Figs. 2A–2D), suggesting the presence of myofibroblasts in culture. Other cell populations, including GFAP-positive cells, were also identified in the cell culture for PVR-02, PVR-03, and PVR-04 (Figs. 2E–2G), with nonspecific staining of PVR-05 (Fig. 2H), suggesting the presence of stellate shaped glial cells. Cytokeratin was positive for all cells in culture for PVR-03 and PVR-05 but was rare in PVR-02 and PVR-04 (Figs. 2I–2L), whereas F4-80, a marker for macrophages and microglial cells, was positive in PVR-03 and PVR-05 but negative in PVR-02 and PVR-04 cultures (Figs. 2M–2P). These findings suggest that C-PVR cells retain the expression of some markers expressed in the patient-derived tissue.
Figure 2.
Characterization of cultured cells by immunofluorescence. Immunofluorescence of C-PVR cells from four different cases using primary antibodies against (A–D) SMA all in green, (E–H) GFAP all in red, (I–L) cytokeratin (intermediate filaments in epithelial cells) all in green, and (M–P) F4/80 (immune cells) all in red and counterstained with DAPI (blue). Scale bar denotes 100 μm.
We observed that a culture medium containing 12% FBS, heparin, insulin-like growth factor (IGF), fibroblast growth factor (FGF), ascorbic acid, hydrocortisone, epithelial growth factor (EGF) and vascular endothelial growth factor (VEGF) (PVR medium) induced growth and survival of C-PVR cultures (Supplementary Fig. S2J). Unknown serum factors were required because C-PVR growth was reduced in PVR medium lacking serum (Supplementary Fig. S2B). After 4 weeks in culture, human cells from clinical cases 3 and 5 (PVR-03 and PVR-05) grew rapidly and displayed distinct morphologic characteristics and growth behavior in culture (Figs. 1C and 1E, respectively). After 1 week of culture, PVR-03 formed a confluent monolayer. After 4 weeks of culture in PVR medium, these cells formed bands that appeared analogous to contractile bands found in patients with PVR (Fig. 1D). Similarly, C-PVR from clinical case 5 (PVR-05) grew over 1 week to form a confluent monolayer. However, when grown over 4 weeks, these cells appeared to grow on top of one another, forming a distinct membranous structure but no bands (Fig. 1F).
Remarkably, PVR membranes and their corresponding C-PVRs showed congruent levels of proliferation measured with the Ki67 marker (Supplementary Fig. S3).43
In Vitro Membrane Formation by C-PVR
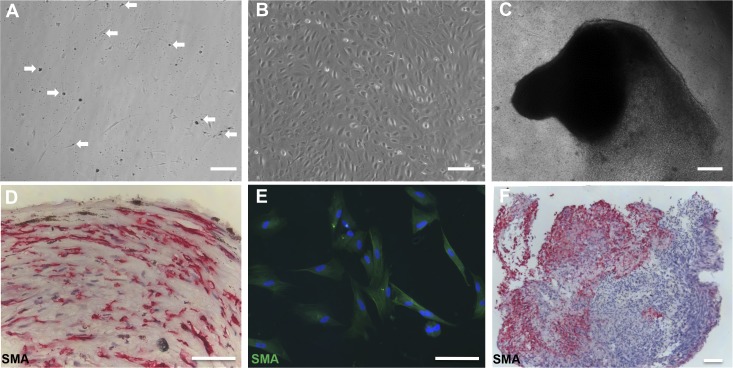
C-PVR recapitulated key features of human PVR under specific culture conditions. Initially, pigmented granules characteristically found in RPE cells were released from C-PVR cells after 48–72 hours in culture (Fig. 3A, 3B). In addition, PVR cells can form invasive membranes that break into the vitreous. Accordingly, C-PVR cells grew as a confluent monolayer on transwells in EBM-2 culture medium supplemented with SingleQuots Kit (Lonza) and 12% FBS (Figs. 1C–1F, 3B; Supplementary Fig. S1). A transformation from a monolayer to invasive cell clusters that grew out of the culture dish toward the surface was triggered by switching to a culture medium containing αMEM, N1 supplement, taurine (250 μg/mL), hydrocortisone (20 ng/mL), triodo-thyronin (13 pg/mL), and 5% FBS, a medium commonly used to induce differentiation of RPE. The invasive phenotype was characterized by the formation of cell clusters capable of invading the culture medium and the walls of the transwell resembling some aspects of the disease (Fig. 3C). The phenotypic change was reversible by switching back to the growth medium. We harvested these PVR membrane–like cell clusters for immunohistologic analysis, which revealed the presence of extracellular matrix, SMA-positive cells suggestive of the presence of myofibroblasts, and other cell types (Figs. 3D–3F).
Figure 3.
Expression of cell identity molecular markers in PVR membranes is retained in C-PVR primary cultures. (A) Specific populations of C-PVR cells contained pigmented granules (white arrows), characteristic of RPE cells. These granules were released from the cells after 48 to 72 hours. Scale bar denotes 500 μm. (B) C-PVR cells grew as a confluent monolayer in growth medium containing EBM-2 supplemented with SingleQuots Kit (Lonza) and 12% FBS. Scale bar denotes 100 μm. (C) A transformation from a monolayer to invasive cell clusters was triggered by switching to a differentiation culture medium. The phenotypic change was reversible by switching back to the growth medium. Scale bar denotes 500 μm. (D) PVR membrane from clinical case 3 was processed for immunohistochemistry using the antibody against SMA in red and counterstained with hematoxylin (blue). Scale bar denotes 100 μm. (E) Immunofluorescence of the C-PVR primary cultures showing positive cells for SMA in green, suggesting that this molecular marker is retained in C-PVR primary cultures. Nuclei are counterstained with DAPI. Scale bar denotes 100 μm. (F) Cell cluster shown in (C) was harvested and processed for immunocytochemistry using antibody against SMA. Scale bar denotes 100 μm.
Assessing Effects of MTX on C-PVR Cells
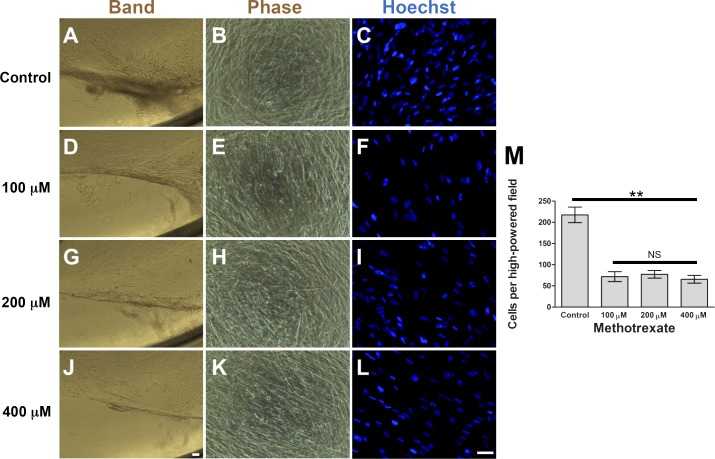
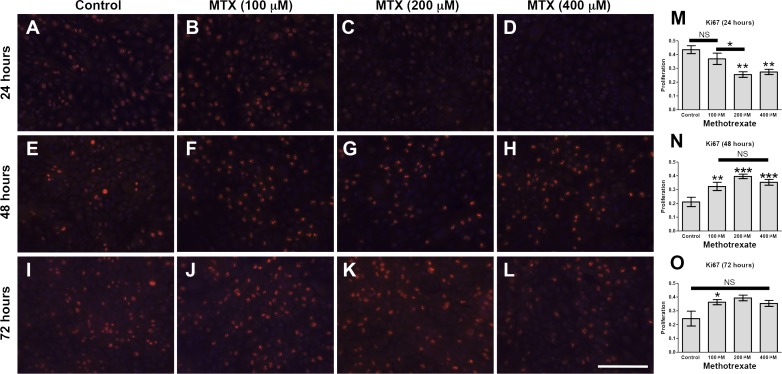
To assess the potential of C-PVR cultures in drug screening, we examined the response of these cells to MTX, a robust inhibitor of cell proliferation and inflammation.44 We found that MTX significantly reduced C-PVR band formation, cell number (Fig. 4) and proliferation (Fig. 5 and Supplementary Fig. S4). Interestingly, when we treated C-PVR cells with different concentrations of dexamethasone and daunorubicin, two drugs previously used as potential treatments for PVR,45–47 we did not see a significant reduction in proliferation (Supplementary Fig. S5). To determine whether inhibition of band formation was due to MTX effects on cell migration, we performed scratch wound healing assays. However, we found that MTX treatment at different concentrations had no effect on migration induced by our standard culture conditions (Supplementary Fig. S6) or migration induced by TNF-α treatment (1 and 10 ng/mL), a factor that has been identified in high concentrations in the vitreous during the active phase of PVR (Supplementary Fig. S7).48
Figure 4.
Effect of MTX on cell density and band formation of C-PVR. Phase contrast and Hoechst-stained immunofluorescence images of C-PVR cells from PVR-03 after 6 weeks of culture without MTX (A–C) and with 100 (D–F), 200 (G–I), and 400 μM (J–L) MTX. (M) Graph shows quantification of cell density after treatment with different concentrations of MTX or control. Error bars represent SEM. Scale bar denotes 100 μm.
Figure 5.
Effect of MTX on proliferation of C-PVR cells. Immunofluorescence images of C-PVR cells from after 24, 48, and 72 hours without (A, E, I) and with 100 (B, F, J), 200 (C, G, K), and 400 mM (D, H, L) MTX. Quantification of proliferation by Ki67 immunofluorescence (red) at 24, 48, and 72 hours (M–O, respectively). Error bars represent SEM. Scale bar denotes 100 μm.
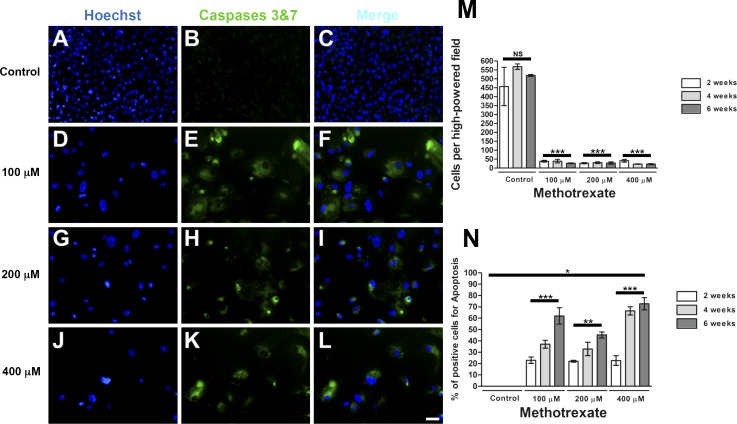
Last, we examined whether cell death contributed to the effects of MTX on C-PVR. Increasing concentrations of MTX (100, 200, and 400 μM) triggered significant cell death over time; little effect was observed after 2 weeks of exposure (Supplementary Fig. S8), whereas significant cell death was observed after 4 (Supplementary Fig. S5) and 6 weeks of treatment (Fig. 6) compared with controls.
Figure 6.
Effect of MTX on C-PVR survival. Hoechst-stained immunofluorescence images of C-PVR cells from PVR-05 after 6 weeks of culture without MTX (A–C) and with 100 (D–F), 200 (G–I), and 400 μM (J–L) MTX. (M) Quantification of cell density showing a significant reduction in cell density after 2, 4, and 6 weeks under the treatment with MTX. (N) Quantification of apoptosis showing a significant increase in the presence of Caspases 3 and 7 after different time points (2, 4, and 6 weeks) with MTX. Error bars represent SEM. Scale bar denotes 100 μm.
Discussion
Here we report the isolation, characterization, and culture of cells from patient-derived PVR membranes, a common complication of retinal detachment. PVR-derived primary cultures, which we call C-PVR, grow robustly and reproduce key features of human PVR including band formation and invasiveness under specific culture conditions. As a demonstration of experimental feasibility, we show that MTX, a commonly used US Food and Drug Administration–approved drug, can significantly inhibit proliferation and induce death of C-PVR, whereas migration was not impacted by MTX treatment. In contrast, both dexamethasone and daunorubincin (two drugs that have previously been proposed for the treatment of PVR) did not affect C-PVR proliferation. Thus, C-PVR can potentially be used for testing in vitro the efficacy of therapies and to screen for new compounds capable of halting PVR.
PVR remains one of the most common postoperative complications after retinal detachment repair. Although many investigators have proposed pathobiological mechanisms leading to the disease, little progress has been made toward developing an effective treatment. One of the major limitations has been the lack of a definite model system of the human disease for in vitro and in vivo analyses. The most widely used in vitro models involved monocultures of RPE that aim to reproduce specific aspects of PVR including EMT, proliferation, and migration.49,50 An immortalized RPE cell line derived from a human PVR was also previously reported.49 Our methodology complements these previous studies by providing protocols for the generation of robust primary cultures from PVR membranes (C-PVR cells) obtained from patients immediately after surgery. This heterogeneous mixture of cells, including RPE-derived cells, glial cells, and macrophages, likely represents the late manifestations of the disease as they were obtained from patients with grade C PVR membranes. This mixed population of cells may recapitulate the PVR “ecosystem” where RPE-derived cells, glia, and macrophages cohabitate and interact with each other.
In our model system, MTX was an effective inhibitor of C-PVR growth. MTX also halted band formation and invasiveness, features that make the disease clinically devastating. MTX is an antimetabolite and antifolate drug that has been used for cancer treatment since the 1950s.51 It is also indicated for treatment of autoimmune diseases such as rheumatoid arthritis.52 Other mechanisms of action involve direct effects on inflammatory pathways, in particular, neutralization of the effects of TNF-α and IL-1β, although the molecular mechanisms underlying these effects remain largely uncharacterized.37,53–56
The work reported here describes a model system for the study of human PVR based on the generation of primary cell cultures from human PVR composed of mixed cell populations. When used in drug screens, this added level of complexity may be beneficial at identifying potential therapies likely to work in preclinical and clinical trials for PVR.
Supplementary Material
Acknowledgments
Massachusetts Eye and Ear has filed a patent application for the use of methotrexate to prevent PVR. Dean Eliott and Tomasz Stryjewski are shareholders in Helio Vision, Inc., which has licensed the patent.
Presented in part at the Military Health System Research Symposium, Boston, MA, USA, August 18, 2015.
The authors thank Patricia D'Amore, Eric Ng, and Magali Saint-Geniez for valuable discussions and advice.
Supported by National Institutes of Health Grants R00EY021624 (JFA-V), K12EY16335 (LAK), and R21EY027061 (LAK). Foundation support from the E. Matilda Ziegler Foundation for the Blind (LAK); a Karl Kirchgessner Foundation research grant (LAK); Department of Ophthalmology, Harvard Medical School (JFA-V and LAK); National Institutes of Health P30 Center Core Grant P30EY003790. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Vision Research Program Technology/Therapeutic Development Award under Award No. W81XWH-17-2-0006. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Disclosure: D. Amarnani, None; A.I. Machuca-Parra, None; L.L. Wong, None; C.K. Marko, None; J.A. Stefater, None; T.P. Stryjewski, P; D. Eliott, P; J.F. Arboleda-Velasquez, None; L.A. Kim, None
References
- 1. Weichel ED, Colyer MH. . Combat ocular trauma and systemic injury. Curr Opin Ophthalmol. 2008; 19: 519– 525. [DOI] [PubMed] [Google Scholar]
- 2. Colyer MH, Chun DW, Bower KS, Dick JS, Weichel ED. . Perforating globe injuries during operation Iraqi Freedom. Ophthalmology. 2008; 115: 2087– 2093. [DOI] [PubMed] [Google Scholar]
- 3. Eliott D, Stryjewski TP, Andreoli MT, Andreoli CM. . Smoking is a risk factor for proliferative vitreoretinopathy after traumatic retinal detachment. Retina. 2017; 37: 1229– 1235. [DOI] [PubMed] [Google Scholar]
- 4. Martini B. . Proliferative vitreo-retinal disorders: experimental models in vivo and in vitro. Acta Ophthalmol Suppl. 1992; 201: 1– 63. [PubMed] [Google Scholar]
- 5. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983; 90: 121– 125. [DOI] [PubMed] [Google Scholar]
- 6. Ryan SJ. . Traction retinal detachment. XLIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1993; 115: 1– 20. [DOI] [PubMed] [Google Scholar]
- 7. Khan MA, Brady CJ, Kaiser RS. . Clinical management of proliferative vitreoretinopathy: an update. Retina. 2015; 35: 165– 175. [DOI] [PubMed] [Google Scholar]
- 8. Hsu J, Khan MA, Shieh WS,et al. Effect of serial intrasilicone oil bevacizumab injections in eyes with recurrent proliferative vitreoretinopathy retinal detachment. Am J Ophthalmol. 2016; 161: 65– 70. [DOI] [PubMed] [Google Scholar]
- 9. Cheema RA, Peyman GA, Fang T, Jones A, Lukaris AD, Lim K. . Triamcinolone acetonide as an adjuvant in the surgical treatment of retinal detachment with proliferative vitreoretinopathy. Ophthalmic Surg Lasers Imaging. 2007; 38: 365– 370. [DOI] [PubMed] [Google Scholar]
- 10. Ahmadieh H, Feghhi M, Tabatabaei H, Shoeibi N, Ramezani A, Mohebbi MR. . Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology. 2008; 115: 1938– 1943. [DOI] [PubMed] [Google Scholar]
- 11. Munir WM, Pulido JS, Sharma MC, Buerk BM. . Intravitreal triamcinolone for treatment of complicated proliferative diabetic retinopathy and proliferative vitreoretinopathy. Can J Ophthalmol. 2005; 40: 598– 604. [DOI] [PubMed] [Google Scholar]
- 12. Reibaldi M, Russo A, Longo A,et al. Rhegmatogenous retinal detachment with a high risk of proliferative vitreoretinopathy treated with episcleral surgery and an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol. 2013; 4: 79– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blumenkranz M, Hernandez E, Ophir A, Norton EW. . 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology. 1984; 91: 122– 130. [DOI] [PubMed] [Google Scholar]
- 14. Asaria RH, Kon CH, Bunce C,et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: results from a randomized, double-blind, controlled clinical trial. Ophthalmology. 2001; 108: 1179– 1183. [DOI] [PubMed] [Google Scholar]
- 15. Charteris DG, Aylward GW, Wong D,et al. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology. 2004; 111: 2240– 2245. [DOI] [PubMed] [Google Scholar]
- 16. Chang YC, Hu DN, Wu WC. . Effect of oral 13-cis-retinoic acid treatment on postoperative clinical outcome of eyes with proliferative vitreoretinopathy. Am J Ophthalmol. 2008; 146: 440– 446. [DOI] [PubMed] [Google Scholar]
- 17. Hardwig PW, Pulido JS, Erie JC, Baratz KH, Buettner H. . Intraocular methotrexate in ocular diseases other than primary central nervous system lymphoma. Am J Ophthalmol. 2006; 142: 883– 885. [DOI] [PubMed] [Google Scholar]
- 18. Hou H, Huffman K, Rios S, Freeman WR, Sailor MJ, Cheng L. . A novel approach of daunorubicin application on formation of proliferative retinopathy using a porous silicon controlled delivery system: pharmacodynamics. Invest Ophthalmol Vis Sci. 2015; 56: 2755– 2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charteris DG. . Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol. 1995; 79: 953– 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C, Semeraro F. . Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators Inflamm. 2013; 2013: 269787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiba C. . The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res. 2014; 123: 107– 114. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa M, Refojo MF, Marin JF, Doi M, Tolentino FI. . Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1995; 36: 2388– 2395. [PubMed] [Google Scholar]
- 23. Andrews A, Balciunaite E, Leong FL,et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999; 40: 2683– 2689. [PubMed] [Google Scholar]
- 24. Radtke ND, Tano Y, Chandler D, Machemer R. . Simulation of massive periretinal proliferation by autotransplantation of retinal pigment epithelial cells in rabbits. Am J Ophthalmol. 1981; 91: 76– 87. [DOI] [PubMed] [Google Scholar]
- 25. Zhao HM, Sheng MJ, Yu J. . Expression of IGFBP-6 in a proliferative vitreoretinopathy rat model and its effects on retinal pigment epithelial cell proliferation and migration. Int J Ophthalmol. 2014; 7: 27– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cleary PE, Ryan SJ. . Experimental posterior penetrating eye injury in the rabbit. I. Method of production and natural history. Br J Ophthalmol. 1979; 63: 306– 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fisher SK, Lewis GP. . Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003; 43: 887– 897. [DOI] [PubMed] [Google Scholar]
- 28. Iandiev I, Uckermann O, Pannicke T,et al. Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci. 2006; 47: 2161– 2171. [DOI] [PubMed] [Google Scholar]
- 29. Kirchhof B, Sorgente N. . Pathogenesis of proliferative vitreoretinopathy. Modulation of retinal pigment epithelial cell functions by vitreous and macrophages. Dev Ophthalmol. 1989; 16: 1– 53. [PubMed] [Google Scholar]
- 30. Nagasaki H, Shinagawa K, Mochizuki M. . Risk factors for proliferative vitreoretinopathy. Prog Retin Eye Res. 1998; 17: 77– 98. [DOI] [PubMed] [Google Scholar]
- 31. Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. . TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci. 2000; 41: 2336– 2342. [PubMed] [Google Scholar]
- 32. Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. . Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014; 40: 16– 34. [DOI] [PubMed] [Google Scholar]
- 33. Rouberol F, Chiquet C. . Proliferative vitreoretinopathy: pathophysiology and clinical diagnosis [in French]. J Fr Ophtalmol. 2014; 37: 557– 565. [DOI] [PubMed] [Google Scholar]
- 34. Kim LA, Wong LL, Amarnani DS,et al. Characterization of cells from patient-derived fibrovascular membranes in proliferative diabetic retinopathy. Mol Vis. 2015; 21: 673– 687. [PMC free article] [PubMed] [Google Scholar]
- 35. Wong LL, Lee NG, Amarnani D,et al. Orbital angiogenesis and lymphangiogenesis in thyroid eye disease: an analysis of vascular growth factors with clinical correlation. Ophthalmology. 2016; 123: 2028– 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim LA, Amarnani D, Gnanaguru G, Tseng WA, Vavvas DG, D'Amore PA. . Tamoxifen toxicity in cultured retinal pigment epithelial cells is mediated by concurrent regulated cell death mechanisms. Invest Ophthalmol Vis Sci. 2014; 55: 4747– 4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. . The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005; 114: 154– 163. [DOI] [PubMed] [Google Scholar]
- 38. Skalli O, Schurch W, Seemayer T,et al. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989; 60: 275– 285. [PubMed] [Google Scholar]
- 39. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112: 1796– 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. . Bestrophin, the product of the best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000; 97: 12758– 12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chu PG, Weiss LM. . Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol. 2002; 15: 6– 10. [DOI] [PubMed] [Google Scholar]
- 42. Anlauf E, Derouiche A. . Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol (Lausanne). 2013; 4: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scholzen T, Gerdes J. . The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182: 311– 322. [DOI] [PubMed] [Google Scholar]
- 44. Chabner BA, Allegra CJ, Curt GA,et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985; 76: 907– 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang YS, Hui YN, Wiedemann P. . Role of apoptosis in the cytotoxic effect mediated by daunorubicin in cultured human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2002; 18: 377– 387. [DOI] [PubMed] [Google Scholar]
- 46. Khawly JA, Saloupis P, Hatchell DL, Machemer R. . Daunorubicin treatment in a refined experimental model of proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1991; 229: 464– 467. [DOI] [PubMed] [Google Scholar]
- 47. Koutsandrea CN, Miceli MV, Peyman GA, Farahat HG, Niesman MR. . Ciprofloxacin and dexamethasone inhibit the proliferation of human retinal pigment epithelial cells in culture. Curr Eye Res. 1991; 10: 249– 258. [DOI] [PubMed] [Google Scholar]
- 48. Limb GA, Little BC, Meager A,et al. Cytokines in proliferative vitreoretinopathy. Eye (Lond). 1991; 5 Pt 6: 686– 693. [DOI] [PubMed] [Google Scholar]
- 49. Chen XL, Bai YJ, Hu QR, Li SS, Huang LZ, Li XX. . Small interfering RNA targeted to ASPP2 promotes progression of experimental proliferative vitreoretinopathy. Mediators Inflamm. 2016; 2016: 7920631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dvashi Z, Goldberg M, Adir O, Shapira M, Pollack A. . TGF-beta1 induced transdifferentiation of rpe cells is mediated by TAK1. PLoS One. 2015; 10: e0122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR. . In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007; 2: 67– 77. [DOI] [PubMed] [Google Scholar]
- 52. Wong CA, Potter MJ, Cui JZ,et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can J Ophthalmol. 2002; 37: 211– 220. [DOI] [PubMed] [Google Scholar]
- 53. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. . Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998; 102: 322– 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. . Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxf). 2003; 42: 1189– 1196. [DOI] [PubMed] [Google Scholar]
- 55. Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. . Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000; 43: 656– 663. [DOI] [PubMed] [Google Scholar]
- 56. Seitz M, Zwicker M, Wider B. . Enhanced in vitro induced production of interleukin 10 by peripheral blood mononuclear cells in rheumatoid arthritis is associated with clinical response to methotrexate treatment. J Rheumatol. 2001; 28: 496– 501. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.