Abstract
Cardiovascular disease (CVD) is the leading cause of death in the industrialized world. Aging is the most predictive risk factor for CVD and is associated with arterial inflammation which contributes to increased CVD risk. Although age-related arterial inflammation has been described in both humans and animals, only a limited number of inflammatory mediators, cytokines and chemokines have been identified. In this investigation we sought to determine whether lifespan extending interventions, including crowded litter early life nutrient deprivation (CL), traditional lifelong caloric restriction (CR) and lifelong Rapamycin treatment (Rap) would attenuate age-related arterial inflammation using multi analyte profiling. Aortas from Young (4–6 months), Old (22 months), Old CL, Old CR and Old Rap mice were homogenized and cytokine concentrations were assessed using Luminex Multi Analyte Profiling. Chemokines involved in immune cell recruitment, such as CCL2, CXCL9, CXCL10, GMCSF and MCSF, were increased in Old vs. Young (p < 0.05). The age-related increase of CXCL10 was prevented by CR (p < 0.05 vs. Old). MSCF concentrations were lower in aortas of Rap treated mice (p < 0.05 vs. Old). Interleukins (IL), IL-1α, IL-1β and IL-10, were also greater in Old vs. Young mice (p < 0.05). These data demonstrate selected lifespan extending interventions can prevent or limit age-related increases in selected aortic chemokines.
Keywords: cytokine, chemokine, caloric restriction, rapamycin, aorta, interleukin
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the industrialized world. Aging is the most predictive risk factor for CVD and most CVD are a result of arterial dysfunction [1]. In particular, arterial aging manifests itself though decreased endothelium dependent dilation (EDD) and increased stiffness of the large arteries [1–5]. Arterial inflammation is also observed with age in both humans and animals and is associated with functional impairment [3, 6–11]. Age-related arterial inflammation appears to involve endothelial cells [7], smooth muscle cells [8] and accumulation of immune cells in the perivascular space [10]. Treatment with anti-inflammatory interventions has been shown to improve arterial function in older adults [12, 13]. Despite these observations, there is limited information about how arterial chemokines and cytokines may be altered by age.
Crowded litter early life nutrient deprivation (CL) [14], lifelong caloric restriction (CR) [15, 16], and lifelong rapamycin treatment (Rap) [17–19] have all been shown to extend lifespan in mice. Notably, CR and Rap have also been shown to improve age-related vascular dysfunction including improvements in large artery stiffness and EDD [20–23]. In this investigation, we sought to gain a more comprehensive profile of the cytokines and chemokines produced by the aged artery through the use of Multi-Analyte Profiling (MAP). In addition, we sought to determine whether lifespan extending interventions CL, CR or Rap would prevent age-related changes in the arterial inflammatory profile.
We hypothesized that aging would result in increased arterial concentrations of pro-inflammatory cytokines/chemokines and decreased concentrations of anti-inflammatory cytokines. Further, we hypothesized that life extending interventions would prevent or retard these age-related changes in the arterial inflammatory profile.
2. METHODS
2.1 Mice
All procedures conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Committee on Use and Care of Animals at the University of Michigan, University of Utah, and Veteran’s Affairs Medical Center-Salt Lake City (VAMC-SLC). All mice were housed in standard mouse cages with ad libitum access to water and food (with exceptions noted below) on a 12:12 light:dark cycle. Young mice were housed at the VAMC-SLC animal facilities and all old mice regardless of treatment group were housed at the University of Michigan animal facilities. Five groups of mice were employed in this study; 1) Young (4–6 month) B6D2F1 mice, n = 8. 2) Old (22 month) genetically heterogeneous offspring of CB6F1 female and C3D2F1 male (UM-HET3) mice, n = 29. 3) Old Crowded litter (CL) UM-HET3 mice, n = 10. Litters for these mice were culled to eight, and an additional four mice from separate litters were added, resulting in a 50% increase in litter size as previously described [14, 24]. 4) Old Caloric Restriction (CR) mice, n = 19. Starting at 4–5 weeks of age these mice received 66–70% of the food a mouse of similar age and sex would consume ad lib as described [25]. 5) Old Rapamycin (Rap) treated mice, n = 23. Starting at 9 weeks of age mice were provided with a diet containing 14 ppm (mg of drug per kg of food). The old control group is the largest (n = 29) as we pooled control mice for each anti-aging intervention. Dates of birth for all old mice were between October 19, 2009 and March 18, 2010, old mice were sacrificed at 22 months of age. Young mice had dates of birth between January 4, 2011 and April 25, 2014 and were sacrificed at 4–6 months of age.
At sacrifice aortas were dissected, homogenized and lysed for protein analysis as previously described [26, 27]. Aortic protein concentrations were determined by BCA protein Assay (ThermoFisher). A total of 7μg of aortic protein was loaded onto two separate Milliplex MAP mouse cytokine/chemokine plates (Millipore), the plates was prepared according to manufacturer’s protocols and read on a Luminex MAGPIX system. From each sample, the following cytokine concentrations were assessed: CC ligand (CCL)2, CCL3, CCL4, CCL5, CCL11, CXC ligand (CXCL)2, CXCL5, CXCL9, CXCL10, Granulocyte Colony Stimulating Factor (GCSF), Granulocyte Macrophage Colony Stimulating Factor (GMCSF), Interferon (IFN)-γ, Interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-3, IL-5 IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-15, IL-17, Leukemia Inhibitory Factor (LIF), Macrophage Colony Stimulating Factor (MCSF), Tumor Necrosis Factor (TNF)-α and Vascular Endothelial Growth Factor (VEGF).
2.2 Statistical Analysis
As individual data points for a number of analytes were at or below the level of detection of the MAP assay and because the data for several analytes were not normally distributed, differences were assessed with the Kruskal-Wallis one-way ANOVA on ranks. Using this analysis allowed for appropriate statistical comparison where calculation of mean and standard error was not possible. Dunn’s pairwise comparison post hoc test was employed to determine group differences. Adjusted p values of p < 0.05 were considered statistically significant.
3. RESULTS
The following cytokines were undetectable in the majority of aortas regardless of group: CCL4, CXCL5, GCSF, IL-3, IL-4, IL-5, IL-7 IL-12p40, IL-15, IL-17, LIF and VEGF (data not shown). CCL3, CCL5, CCL11, CXCL2, IFN-γ, IL-2, IL-6, IL-8, IL-9 and IL-12p70 did not exhibit significant increases in Old compared to Young control aortas (Table 1). CCL11 was increased in Old CL vs. Young (p < 0.05, Table 1). CCL3 and IL-8 were both increased in Old CR compared to Young (p < 0.05, Table 1). CCL5 and IL-9 were both increased in Old CL compared to Old CR.
Table 1.
Aortic Chemokine/Cytokine Concentrations (ng/mg aortic protein)
| Young | Old | Old CL | Old CR | Old Rap | |
|---|---|---|---|---|---|
| n | 8 | 29 | 10 | 19 | 23 |
| CCL3 | 1.00 (1.00, 1.51) | 3.40 (0.83, 14.67) | 1.45 (0.66, 6.73) | 10.34 (6.99, 13.30)* | 3.04 (0.09, 8.73) |
| CCL5 | 0.33 (0.33, 0.34) | 0.33 (0.33, 2.59) | 0.33 (0.33, 0.62) | 1.36 (0.47, 2.49)† | 0.33 (0.33, 1.99) |
| CCL11 | 0.30, (0.30, 6.61) | 9.32 (3.73, 13.82) | 13.44 (7.17, 16.29)* | 4.41 (3.26, 5.61) | 10.91 (3.77, 13.09) |
| CXCL2 | 0.64 (0.64, 1.29) | 1.50 (1.06, 2.33) | 1.56 (1.06, 2.99) | 1.50 (1.06, 3.14) | 1.06 (1.06, 2.33) |
| IFN-γ | 0.49 (0.49, 0.49) | 0.49 (0.34, 0.49) | 0.35 (0.34, 0.49) | 0.49 (0.49, 0.49) | 0.49 (0.34, 0.49) |
| IL-2 | 0.27 (0.27, 0.27) | 0.30 (0.21, 0.69 | 0.27 (0.07, 0.62) | 0.36 (0.27, 0.63) | 0.44 (0.27, 0.61) |
| IL-6 | 0.39 (0.39, 0.69) | 0.48 (0.33, 1.03) | 0.33 (0.33, 0.50) | 0.66 (0.39, 0.76) | 0.39 (0.33, 0.54) |
| IL-8 | 0.86 (0.41, 1.13) | 2.00 (0.63, 3.27) | 0.89 (0.61, 1.63) | 2.19 (1.60, 2.96)* | 1.20 (0.70, 2.10) |
| IL-9 | 46.92 (31.64, 64.79) | 30.0 (17.64, 121.49) | 20.57 (10.76, 65.81) | 97.00 (56.11, 131.83)† | 29.29 (17.00, 94.41) |
| IL-12p70 | 0.24 (0.21, 0.36) | 0.74 (0.23, 1.07) | 0.51 (0.31, 0.87) | 0.74 (0.40, 0.94)* | 0.50 (0.22, 0.74) |
CL: crowded litter, CR: caloric restriction, Rap: Rapamycin. Differences were assessed with Kruskal-Wallis one-way ANOVA with Dunn’s pairwise comparisons.
Different from Young,
different from Old CL, p < 0.05 Data are medians (25th percentile, 75th percentile).
Chemokines principally involved in immune cell recruitment and activation CCL2, CXCL9, CXCL10, GMCSF and MCSF were increased in Old aortas vs. Young (Figure 1). CCL2 and GMCSF were not altered by any intervention (Figure 1A, D). The age-related increase in CXCL10 was abolished by CR (p < 0.05 vs. Old, Figure 1C). MCSF was not altered by CL or CR but was similar to Young in the Old Rap group (p < 0.05 vs. Old, Figure 1E). Aortic concentrations of IL-1α, IL-1β and IL-10 were increased with age (Young vs. Old p < 0.05, Figure 2), these cytokines were not altered by any of the life extending interventions. To assess whether these observations were confounded by differences in the two multiplex plate runs, each plate was analyzed separately. We found that directional changes were preserved regardless of which plate was excluded, to reach statistical significance some differences required inclusion of both plates.
Figure 1. Age-related increases in aortic concentrations of cytokines involved in immune cell recruitment.
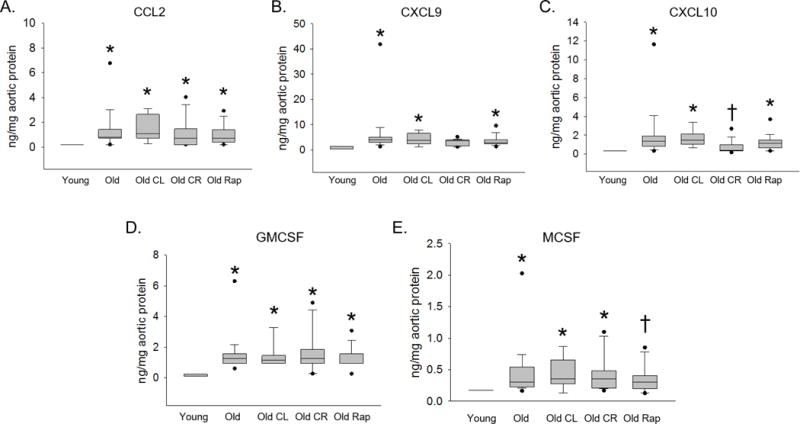
Aortas from Young (4–6 months), Old (22 months), Old crowded litter (CL), Old caloric restriction (CR) and Old Rapamycin treated (Rap) mice were homogenized and chemokine and cytokine concentrations were assessed using Luminex Multi Analyte Profiling data for chemokines CCL2 (A), CXCL9 (B), CXCL10 (C), GMCSF (D) and MCSF (E) are shown. n = 8–29/group. Differences were assessed with Kruskal-Wallis one-way ANOVA on ranks and Dunn’s pairwise comparison post hoc test. An asterisk (*) indicates different from Young, † different from Old, p ≤ 0.05. Data are expressed as box and whisker plots where the horizontal line within the box indicates the median, boundaries of the box indicate the 25th- and 75th -percentile, the whiskers indicate the 5th- and 95th-percentile, dots represent outlying values.
Figure 2. Age-related increases in aortic concentrations of selected interleukins family.
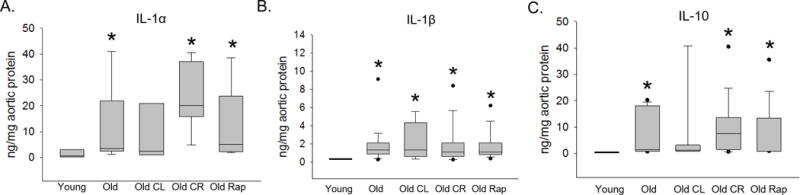
Aortas from Young (4–6 months), Old (22 months), Old crowded litter (CL), Old caloric restriction (CR) and Old Rapamycin treated (Rap) mice were homogenized and chemokine and cytokine concentrations were assessed using Luminex Multi Analyte Profiling data for Interleukin (IL)-1α (A), IL-1β (B) and IL-10 (C) are shown. n = 8–29/group. Differences were assessed with Kruskal-Wallis one-way ANOVA on ranks and Dunn’s pairwise comparison post hoc test. An asterisk (*) indicates different from Young, † different from Old, p ≤ 0.05. Data are expressed as box and whisker plots where the horizontal line within the box indicates the median, boundaries of the box indicate the 25th- and 75th -percentile, the whiskers indicate the 5th- and 95th-percentile, dots represent outlying values.
4. DISCUSSION
The major findings of this study are: 1) CCL2, CXCL9, CXCL10, GMSCF and MCSF, chemokines principally involved in immune cell recruitment and activation are increased with age in the mouse aorta. 2) Age-related increases in CXCL10 and MCSF were abolished by CR and Rap, respectively. 3) Interleukins IL-1α, IL-1β and IL-10 were increased with age, but were not impacted by lifespan extending interventions. These results demonstrate that aging is associated with increased aortic concentrations of multiple chemokines involved in immune cell recruitment and that in some cases, this increase is prevented by selected life extending interventions. Further, aging is associated with increases in both pro-(IL-1α and β) and anti-inflammatory (IL-10) Interleukins.
In the present investigation we found that aging is associated with increased aortic concentrations of CCL2, CXCL9, CXCL10, GMCSF and MSCF. CCL2, also known as Monocyte Chemoattractant Protein (MCP)-1 serves to recruit monocytes and T cells to sites of inflammation [28]. CXCL9 and CXCL10 also known as Monokine Induced by γ-Interferon (MIG) and Interferon-γ-induced Protein 10 (IP-10), respectively are closely related T cell chemoattractants [29]. GMCSF and MCSF are monocyte growth factors that induce differentiation, proliferation and recruitment of both granulocytes and macrophages and macrophages only, respectively [30].
Our observation that CCL2 is increased in the aged mouse aorta is consistent with previous observations showing increased CCL2 in aged human endothelial cells [7] and whole arteries [9] as well as rat aorta [11] and primary vascular smooth muscle cells [8]. Further, higher plasma levels of CCL2 along with CXCL9 and CXCL10 have been observed in older adults compared to young controls [31, 32]. Plasma GMSCF is not altered in older adults compared to young controls [33]; however, endothelial cells produce greater levels of GMSCF under conditions of replicative senescence [34]. Relatively little is known about MCSF and aging. This study is, to our knowledge, the first to show increased production of CXCL9, CXCL10, GMCSF and MCSF in the aged artery. The production of the above cytokines by the artery may contribute to increased arterial immune cell infiltration that has been observed with age [10,35]. Whether these immune cells directly mediate age-related arterial dysfunction is unknown, but both T cells and macrophages are implicated in mediating arterial dysfunction in experimental hypertension [36, 37]. Together these observations suggest that increased production of chemokines that recruit immune cells is an important component of arterial inflammation with age.
We have previously found that lifelong CR prevents age-related arterial dysfunction [20, 22] and accumulation of T cells in both aorta and the mesenteric vasculature [35]. In the present investigation, we found that age-related increases CXCL10, which recruits T cells, was prevented by CR. We also found that treatment with Rap lowered MCSF, which recruits and serves as a growth factor for macrophages. Consistent with this observation, aortic perivascular macrophages increase with age [10, 35], and Rap treatment improves arterial function in old mice [21]. The finding that some of the lifespan extending interventions can prevent age-related increases in aortic chemokines that mediate differentiation and recruitment of inflammatory cells represents a potential mechanism by which these interventions might preserve arterial health.
We also found that concentrations of aortic IL-1α, IL-1β and IL-10 were increased with age. IL-1α and IL-1β are potent proinflammatory cytokines and modulators of innate immunity. These cytokines are produced by numerous cell types and increased circulation of IL-1α and IL-1β lead to fever, leukocyte migration and activation and, increased expression of endothelial adhesion molecules [37]. IL-1α has been shown to induce production of senescence associated secretory phenotype (SASP) cytokines (including CCL2) in cultured vascular smooth muscle cells in autocrine manner [38] and thus, may contribute to vascular inflammation with age. The increase in IL-1β is in accord with a previous observation by our group showing increased aortic IL-1β concentrations in with age in mice [10]. IL-10 is an anti-inflammatory cytokine and mice deficient in IL-10 develop arterial dysfunction at a younger age than wild type controls [39]. This suggests that the age-related increase in IL-10 may be a compensatory response in attempt to preserve arterial function with age.
Interestingly, changes in IFN-γ, IL-6, IL-8 and TNF-α described in previous investigations [6, 8–10] were absent in the present study. This may be partly explained by differences in methodology (i.e. measurement of gene expression rather than cytokine concentration, using stimulation in vitro to evoke cytokine release etc.). Further, in the present study old mice were sacrificed at 22 months, 4–5 months before median survival age [19] in the UM-HET3 mice. In a report from our laboratory, increases in IFN-γ, IL-6 and TNF-α were observed at 31 months in B6D2F1 mice which corresponds to median survival age [10]. Thus, it is conceivable that the changes observed in the present study occur with age but precede increases in proinflammatory cytokines and chemokines observed in very old mice. A limitation of the present study is that the young mice were B6D2F1 mice housed at the VAMC-SLC whereas the old mice were the UM-HET3 (offspring of CB6F1 female and C3D2F1 male) housed at the University of Michigan. Thus, it is possible that the aging differences are confounded by differences in strain and housing. In accord with the present study, we have previously found that arterial IL-1β is increased with age in B6D2F1 mice [10]. The literature also indicates that arterial CCL2 is increased with age in both rodents and humans [7–9, 11]. It should be noted that CR decreases CXCL10 and Rap treatment decreases GMCSF compared to strain matched old controls.
In the present investigation, we found that aging results in increased aortic concentrations of CCL2, CXCL9, CXCL10, GMSCF and MCSF, all cytokines involved in recruitment of immune cells. We found that CR and Rap modulated age-related changes in aortic CXCL10 and MCSF, respectively. Lastly, we found that both pro-(IL-1α and IL-1β) and anti-inflammatory (IL-10) interleukins were increased with age and not affected by life extending interventions. These results suggest that upregulation of cytokines that recruit immune cells is an important aspect of age-related arterial inflammation. Whether these cytokines directly contribute to increased arterial immune cell infiltration and how this might affect arterial function with age are unknown and important topics for future study. The interplay and precise roles of these cytokines are also important topics for further study.
Acknowledgments
The authors would like to thank Richard A. Miller (University of Michigan) for providing the tissues from aged mice and mice exposed to interventions that extend lifespan. This work was supported by National Institute of Aging award numbers K02AG045339, R01AG050238, R01AG040297, R01AG048366, R01-022303, the Glenn Foundation for Medical Research, and by Merit Review Award 1I01BX002151 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Glossary
- CVD
Cardiovascular disease
- EDD
endothelium dependent dilation
- CL
crowded litter
- CR
caloric restriction
- Rap
Rapamycin
- IL
interleukin
- VAMC-SLC
Veteran’s Affairs Medical Center-Salt Lake City
- CCL
CC ligand
- CXC
CXC-ligand
- GCSF
Granulocyte Colony Stimulating Factor
- GMCSF
Granulocyte Macrophage Colony Stimulating Factor
- LIF
Leukemia Inhibitory Factor
- MCSF
Macrophage Colony Stimulating Factor
- TNF
Tumor Necrosis Factor
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A “Set up” for Vascular Disease. Circulation. 2003;107(1):139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of Aging-Induced Impairment of Endothelium-Dependent Relaxation: Role of Tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287(6):H2448–53. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 3.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct Evidence of Endothelial Oxidative Stress with Aging in Humans: Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-Kappab. Circ Res. 2007;100(11):1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 4.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging Progressively Impairs Endothelium-Dependent Vasodilation in Forearm Resistance Vessels of Humans. Hypertension. 1996;27(4):849–53. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part Iii: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation. 2003;107(3):490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 6.Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased Production of Tumor Necrosis Factor and Interleukin-6 by Arterial Wall of Aged Rats. Am J Physiol. 1995;268(6 Pt 2):H2288–93. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 7.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging Is Associated with Greater Nuclear Nf Kappa B, Reduced I Kappa B Alpha, and Increased Expression of Proinflammatory Cytokines in Vascular Endothelial Cells of Healthy Humans. Aging Cell. 2008;7(6):805–12. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging Enhances the Basal Production of Il-6 and Ccl2 in Vascular Smooth Muscle Cells. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(1):103–9. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-Related Telomere Uncapping Is Associated with Cellular Senescence and Inflammation Independent of Telomere Shortening in Human Arteries. Am J Physiol Heart Circ Physiol. 2013;305(2):H251–8. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic Exercise Reverses Arterial Inflammation with Aging in Mice. Am J Physiol Heart Circ Physiol. 2011;301(3):H1025–32. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R, Lakatta EG. A Local Proinflammatory Signalling Loop Facilitates Adverse Age-Associated Arterial Remodeling. PLoS One. 2011;6(2):e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear Factor-{Kappa}B Activation Contributes to Vascular Endothelial Dysfunction Via Oxidative Stress in Overweight/Obese Middle-Aged and Older Humans. Circulation. 2009;119(9):1284–92. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate Improves Vascular Endothelial Function by Reducing Oxidative Stress While Increasing Endothelial Nitric Oxide Synthase in Healthy Normolipidemic Older Adults. Hypertension. 2012;60(6):1517–23. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-Span Extension in Mice by Preweaning Food Restriction and by Methionine Restriction in Middle Age. J Gerontol A Biol Sci Med Sci. 2009;64(7):711–22. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana L, Partridge L, Longo VD. Extending Healthy Life Span–from Yeast to Humans. Science (New York, N.Y) 2010;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoro EJ. Overview of Caloric Restriction and Ageing. Mechanisms of ageing and development. 2005;126(9):913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin Fed Late in Life Extends Lifespan in Genetically Heterogeneous Mice. Nature. 2009;460(7253):392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin Slows Aging in Mice. Aging Cell. 2012;11(4):675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-Mediated Lifespan Increase in Mice Is Dose and Sex Dependent and Metabolically Distinct from Dietary Restriction. Aging Cell. 2014;13(3):468–77. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-Long Caloric Restriction Reduces Oxidative Stress and Preserves Nitric Oxide Bioavailability and Function in Arteries of Old Mice. Aging Cell. 2013;12(5):772–83. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary Rapamycin Supplementation Reverses Age-Related Vascular Dysfunction and Oxidative Stress, While Modulating Nutrient-Sensing, Cell Cycle, and Senescence Pathways. Aging Cell. 2016 doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial Effects of Lifelong Caloric Restriction on Endothelial Function Are Greater in Conduit Arteries Compared to Cerebral Resistance Arteries. Age. 2014;36(2):559–69. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-Oxidative and Anti-Inflammatory Vasoprotective Effects of Caloric Restriction in Aging: Role of Circulating Factors and SIRT1. Mechanisms of ageing and development. 2009;130(8):518–27. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of Genes Involved in Xenobiotic Metabolism Is a Shared Signature of Mouse Models with Extended Lifespan. American journal of physiology Endocrinology and metabolism. 2012;303(4):E488–95. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flurkey K, Astle CM, Harrison DE. Life Extension by Diet Restriction and N-Acetyl-L-Cysteine in Genetically Heterogeneous Mice. J Gerontol A Biol Sci Med Sci. 2010;65(12):1275–84. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor Kappab and Forkhead Box O Phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66(4):409–18. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. Sirt-1 and Vascular Endothelial Dysfunction with Ageing in Mice and Humans. J Physiol. 2011;589(Pt 18):4545–54. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte Chemoattractant Protein-1 Acts as a T-Lymphocyte Chemoattractant. P Natl Acad Sci USA. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-Gamma-Inducible Protein 10 (IP-10; CXCL10)-Deficient Mice Reveal a Role for IP-10 in Effector T Cell Generation and Trafficking. J Immunol. 2002;168(7):3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 30.Wicks IP, Roberts AW. Targeting GM-CSF in Inflammatory Diseases. Nature reviews Rheumatology. 2016;12(1):37–48. doi: 10.1038/nrrheum.2015.161. [DOI] [PubMed] [Google Scholar]
- 31.Bonfante HL, Almeida CS, Abramo C, Grunewald ST, Levy RA, Teixeira HC. CCL2, CXCL8, CXCL9 and CXCL10 Serum Levels Increase with Age but Are Not Altered by Treatment with Hydroxychloroquine in Patients with Osteoarthritis of the Knees. International journal of rheumatic diseases. 2015 doi: 10.1111/1756-185X.12589. [DOI] [PubMed] [Google Scholar]
- 32.Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic Alteration of Soluble Serum Biomarkers in Healthy Aging. Cytokine. 2007;39(2):123–9. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Ramanathan R, Kohli A, Ingaramo MC, Jain A, Leng SX, Punjabi NM, Walston JD, Fedarko NS. Serum Chitotriosidase, a Putative Marker of Chronically Activated Macrophages, Increases with Normal Aging. J Gerontol A Biol Sci Med Sci. 2013;68(10):1303–9. doi: 10.1093/gerona/glt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hohensinner PJ, Kaun C, Buchberger E, Ebenbauer B, Demyanets S, Huk I, Eppel W, Maurer G, Huber K, Wojta J. Age Intrinsic Loss of Telomere Protection Via Trf1 Reduction in Endothelial Cells. Biochimica et Biophysica Acta. 2016;1863(2):360–7. doi: 10.1016/j.bbamcr.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Trott DW, Henson GD, Ho MH, Allison SA, Lesniewski LA, Donato AJ. Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp Gerontol. 2016;(16):30348–5. doi: 10.1016/j.exger.2016.12.016. pii:S0531-5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-Positive Monocytes Mediate Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. Circulation. 2011;124(12):1370–81. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 37.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T Cell in the Genesis of Angiotensin II Induced Hypertension and Vascular Dysfunction. J Exp Med. 2007;204(10):2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garlanda C, Dinarello CA, Mantovani A. The Interleukin-1 Family: Back to the Future. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner SE, Humphry M, Bennett MR, Clarke MC. Senescent Vascular Smooth Muscle Cells Drive Inflammation through an Interleukin-1alpha-Dependent Senescence-Associated Secretory Phenotype, Arteriosclerosis. Thrombosis, and Arterioscler Thromb Vasc Biol Vascular Biology. 2015;35(9):1963–74. doi: 10.1161/ATVBAHA.115.305896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinzenbaw DA, Chu Y, Pena Silva RA, Didion SP, Faraci FM. Interleukin-10 Protects against Aging-Induced Endothelial Dysfunction. Physiol Rep. 2013;1(6):e00149. doi: 10.1002/phy2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


