Abstract
Purpose
To investigate changes of peripheral blood biomarkers and their impact on clinical outcome following treatment with ipilimumab in advanced melanoma patients.
Experimental Design
Changes in blood counts and the frequency of circulating immune cell populations analyzed by flow cytometry were investigated in 82 patients to compare baseline values with different time-points after starting ipilimumab. Endpoints were overall survival (OS) and best clinical response. Statistical calculations were done by Wilcoxon-matched pairs tests, Fisher exact test, Kaplan–Meier analysis, and Cox regression analysis.
Results
Increases in absolute lymphocyte counts (ALC) 2 to 8 weeks (P = 0.003) and in percentages of CD4+and CD8+T cells 8 to 14 weeks (P = 0.001 and P = 0.02) after the first dose of ipilimumab were correlated with improved survival. These associations did not meet significance criteria, when conservatively adjusted for multiple testing, but were additionally correlated with clinical responses (all P < 0.05). However, validation is required. Increases in all three factors were observed in 36% of patients, who had a favorable outcome and survival probabilities of 93.3% and 63.8% at 12 and 24 months, respectively. A partial or complete response was observed in 71% of these patients compared with only 8% in patients with decreases in ≥1 of the 3 factors, respectively. Changes of regulatory T cells or myeloid-derived suppressor cells were not associated with OS.
Conclusions
Increases of ALC observed 2 to 8 weeks after initiation of ipilimumab and delayed increases in CD4+ and CD8+ T cells reflect changes associated with positive outcome. These changes represent surrogate marker candidates and warrant further validation.
Introduction
Treatment with the inhibitory anti–cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) antibody ipilimumab represented a major breakthrough in melanoma therapy and was the first systemic treatment ever that proved to prolong survival in late-stage patients (1, 2). Despite these encouraging results, objective response rates for treatment with ipilimumab are rather low, whereas many patients are at high risk of potentially severe treatment-related side effects (3). Administration of ipilimumab blocks CTLA-4 and enhances the antitumor function of T cells (4). However, the exact mode of action of ipilimumab-mediated tumor rejection remains incompletely understood.
The number of available therapeutic approaches for metastatic melanoma has increased recently (5, 6). This emphasizes the need for robust biomarkers because predictive biomarkers may affect treatment selection or sequence, if determined before starting treatment. Moreover, biomarkers measured early during treatment or changes comparing later values to the baseline findings can potentially predict side effects or outcome before disease response is otherwise able to be assessed radiographically. These surrogate markers may help to decide whether to continue an ongoing treatment or to change to alternative options early. In addition, specific changes occurring during treatment can improve the understanding of the beneficial mode of action of the applied drug.
Changes to the absolute lymphocyte count (ALC) and frequencies of several immune cell subpopulations have been described during treatment with ipilimumab. These include changes to myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg; refs. 7–11) which may qualify as surrogate marker candidates for outcome of ipilimumab treatment. Conflicting data exist on the role of ALC in this respect. An increase in ALC has been associated with improved overall survival (OS) and clinical benefit in several studies (12–15). However, differing results have also been reported (16). An increase in absolute eosinophil counts (AEC) has been described to be associated with favorable OS (12) or clinical response (17). MDSCs are strong modifiers of T-cell responses (18), and their frequencies have been inversely associated with OS in melanoma and different other cancer entities (19–21). Lower pre-treatment levels of MDSCs have been associated with tumor responses (7, 9, 17), and a greater decrease in MDSCs after 6 weeks was related to improved progression-free survival (10). Ipilimumab therapy resulted in early decreases of MDSCs and reduced their inhibitory function (8). Tregs constitutively express high amounts of CTLA-4 (22) on their surface, making them direct potential targets of ipilimumab. However, also here, conflicting data have been reported on the dynamic changes in frequencies of circulating Tregs and their impact on outcome under treatment (10, 11).
Other biomarkers have also been implicated in monitoring ipilimumab treatment, including Ki67, a marker for dividing cells the expression of which was found to be increased on CD4+ and CD8+ T cells during and after ipilimumab therapy (23). Similar to Ki67, inducible T-cell costimulator (ICOS) expression on CD4+ T cells has also been described as a pharmacodynamic marker for ipilimumab therapy (24). Patients with an increase in the number of circulating ICOS+ T cells at week 7 were more likely to experience disease control and have improved survival (25).
In an earlier study, we had focused on the identification of baseline biomarkers, as analyzed before starting ipilimumab (26). In the present study, we investigated changes in routine blood counts and frequencies of circulating immune populations, including Tregs and MDSCs, in late-stage melanoma patients following treatment with ipilimumab. Our aim was to identify associations of specific changes with clinical response and OS to serve as surrogate biomarker candidates and to better understand the beneficial mode of action of ipilimumab.
Materials and Methods
Patients
Clinical data and peripheral blood mononuclear cells (PBMC) were provided by five clinical centers (Essen, Nantes, Naples, New York, and Tuebingen). Inclusion criteria were stage IV melanoma (no uveal or mucosal melanoma), treatment with at least one dose of ipilimumab at 3 or 10 mg/kg in the metastatic (not adjuvant) setting, and availability of cryopreserved PBMCs before and at one or more time-points after start of ipilimumab. Detailed patient characteristics are presented in Table 1. All patients gave written informed consent for biobanking, and for use of biomaterials and clinical data for scientific purposes. This study was approved by the Ethics Committee, University of Tuebingen (approval 524/2012B02).
Table 1.
Patient and treatment characteristics
| Factor | Category | Total (n = 82)
|
|
|---|---|---|---|
| n | (%) | ||
| Clinical site | Essen | 3 | 3.7 |
| Nantes | 5 | 6.1 | |
| Naples | 20 | 24.4 | |
| New York | 43 | 52.4 | |
| Tuebingen | 11 | 13.4 | |
| Gender | Male | 51 | 62.2 |
| Female | 31 | 37.8 | |
| Age | ≤50 years | 23 | 28.0 |
| >50 years | 20 | 24.4 | |
| >60 years | 19 | 23.2 | |
| >70 years | 20 | 24.4 | |
| Median age | 60 years | ||
| M category (AJCC) | M1a | 11 | 13.4 |
| M1b | 18 | 22.0 | |
| M1c | 53 | 64.6 | |
| Visceral involvement | Soft tissue only | 14 | 17.1 |
| Lung | 29 | 35.4 | |
| Other organs | 39 | 47.6 | |
| LDH | Elevated | 36 | 44.4 |
| Normal | 45 | 55.6 | |
| Unknown | 1 | ||
| Treatment background | CA-184-128 (3 mg/kg, local IL2) | 11 | 13.4 |
| CA-184-169 (3 or 10 mg/kg) | 2 | 2.4 | |
| Early access program (3 mg/kg) | 45 | 54.9 | |
| Regular prescription (3 mg/kg) | 24 | 29.3 | |
| Doses applied | 2 | 7 | 8.5 |
| 3 | 10 | 12.2 | |
| 4 | 65 | 79.3 | |
| Best clinical response (irRC) | Complete response | 3 | 4.1 |
| Partial response | 12 | 16.2 | |
| Stable disease | 6 | 8.1 | |
| Progressive disease | 53 | 71.6 | |
| Unknown | 8 | ||
| Salvage treatment after ipilimumab | BRAF/MEK inhibitors | 8 | 9.8 |
| PD-1 antibodies | 9 | 11.0 | |
| Chemotherapy | 12 | 14.6 | |
| Others | 3 | 3.7 | |
| No salvage treatment | 56 | 68.3 | |
Abbreviation: AJCC, American Joint Committee on Cancer.
Study design
This was an exploratory analysis of pooled data including patients who received ipilimumab at different treatment backgrounds. All peripheral blood parameters were derived from blood draws taken within 28 days before the first dose (baseline) and at different time-points after initiation of ipilimumab. To allow robust statistical analysis, samples taken after baseline were classified as “early” if taken between 2 and 8 weeks or as “delayed” if taken later than 8 up to 14 weeks after the first dose. An individual early increase or decrease of the respective parameter was defined by any change (independent from its magnitude) comparing the baseline value with that obtained at the early time-point after start of treatment, whereas an individual delayed increase or decrease was defined by comparing the baseline value with the value obtained at the later time-point. Clinical responses were categorized according to the immune-related response criteria (irRC; ref. 27) as either complete response, partial response, stable disease, or progressive disease. Best overall response (bOR) was defined as the best achieved response between starting ipilimumab therapy and progression or starting a new systemic treatment, considering all available tumor assessments within this time period. A clinical response was defined if bOR was complete response or partial response, while clinical benefit additionally included stable disease.
Flow cytometry
PBMCs were isolated using Ficoll/Hypaque density gradient centrifugation, cryopreserved according to institutional standard protocols, and subsequently stored in liquid nitrogen or at −80°C until shipment on dry ice. All samples were kept in liquid nitrogen upon arrival in Tuebingen. PBMCs were analyzed by flow cytometry as described previously (26). Briefly, PBMCs were thawed and immediately analyzed via flow cytometry. Fc receptors were blocked with human IgG (Gamunex; Talecris), and dead cells were excluded by ethidium monoazide labeling (EMA, Biotinum). Staining was performed separately for the analysis of myeloid cells/MDSCs and T cells/Tregs. Antibody panels and gating strategies are presented in Supplementary Table S1 and Supplementary Fig. S1. Data were acquired with a BD LSR-II and FACS-Diva software V6.1.3 (BD). Counts of CD4+, CD8+, and CD3− T cells were calculated based on their proportion among ALC.
Statistical analysis
Follow-up time was defined from the date of the first dose of ipilimumab to the date of last follow-up or death. Survival probabilities were calculated according to the Kaplan–Meier method. Comparisons were done using log rank tests. Cox proportional hazard regression analysis was applied to determine the relative impact of confirmed single factors, which was reported by means of HRs. Patients with missing data in variables analyzed in the given model were excluded. Values of routine blood counts and immune cell frequencies at different time-points were compared using Wilcoxon-matched pairs tests. Associations between bOR and respective biomarker categories were analyzed by χ2 and Fisher exact tests. P values <0.05 were considered statistically significant. Bonferroni correction was applied separately for the analysis of differences between biomarkers at different time-points and the associations of changes with OS. The factor chosen was 44 (22 markers, 2 time-points). Thus, raw P values < 0.0011 are considered significant when adjusting for a type 1 error of 0.05. Analyses were carried out using SPSS 22 (IBM) and Prism 5 (GraphPad).
Results
Patients’ characteristics
Samples and clinical data of 82 patients were provided by five different clinical centers (Table 1). The median age at the start of anti–CTLA-4 therapy was 60 years, and 62.2% were male. The M-category M1a was assigned to 11 (13.4%), M1b to 18 (22.0%), and M1c to 53 patients (64.6%). Lactate dehydrogenase (LDH) levels were normal at the start of ipilimumab in 45 (55.6%), elevated in 36 (44.4%), and unknown in the remaining patient. All but 2 patients received ipilimumab at 3 mg/kg every 3 weeks, either in the early access program (45; 54.9%), after marketing approval as a regular prescription (24; 29.3%), or in trial CA184-128 (11; 13.4%). Sixty-five patients (79.3%) underwent all four cycles of ipilimumab. Seventy-four patients had available data on bOR.
Of these, 20.3% experienced a clinical response and 28.4% were defined as having clinical benefit. Median OS was 8 months after starting ipilimumab for the entire cohort, 31 months for patients alive at the date of last follow-up, and 6 months for those who died. All deaths were melanoma-related. In total, 215 blood samples were provided. Baseline PBMCs were available for all 82 patients, and additional samples at early (2–8 weeks, median 42 days) or later (8–14 weeks, median 77 days) time-points after start of ipilimumab were provided for 73 or 60 patients, respectively. OS was no different for patients investigated for early or delayed changes (P = 0.338) nor in any of three comparisons between patients who subsequently received BRAF/MEK inhibitors, chemotherapy, PD-1 antibodies, or other treatments, compared with patients with other salvage treatments, respectively (all P > 0.100).
Dynamic changes in biomarkers after the start of ipilimumab
Twenty-two factors were investigated (15 immune populations analyzed by flow cytometry and 7 routine blood counts; Table 2). Two comparisons were performed separately for each factor to investigate dynamic changes on ipilimumab treatment. Early or delayed changes were analyzed by comparison of baseline levels and the corresponding levels at the early (2–8 weeks) or later (8–14 weeks) time-points after starting treatment. Significant changes (P < 0.05) were observed in 26 of 44 comparisons (Table 2). The following changes remained significant when Bonferroni-adjusted significance level was considered due to multiple testing (P < 0.0011): Changes upon treatment with ipilimumab were most prominent for CD4+Ki67+ and CD8+Ki67+ T cells (baseline vs. early and baseline vs. late; P < 0.001) where the majority of patients were found to have increased frequencies after ipilimumab treatment. An early or delayed decrease in CD4+Ki67+ T cells was observed in only 3 of 50 and 6 of 47 patients, respectively. An early or delayed decrease of CD8+Ki67+ T cells was also restricted to a minority (8 of 50 and 12 of 47 patients, respectively). Absolute and relative eosinophil counts as well as two phenotypes of Tregs (CD4+CD127lowCD25+FoxP3+ and CD4+CD25+FoxP3+) were increased at the early and the later time-point. Frequencies of proliferating (CD4+ CD127lowCD25+FoxP3+CD45+Ki67+) Tregs were significantly increased only at the early time-point. Nonclassical (Lin−CD14−CD16+HLA-DR+) monocytes were decreased at the later time-point only.
Table 2.
Quantitative changes of circulating immune cell populations and their associations with OS
| Change | Change BL vs. earlya
|
Change BL vs. delayedb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Pairwise comparison (P value)c | Median Survival (months) | Difference in survival (P value)d | N | Pairwise comparison (P value)c | Median survival (months) | Difference in survival (P value)d | ||
| Abs. leucocyte counts (cells/μL) | ↗ | 45 | 0.005 | 10 | 0.043 | 44 | <0.001 | 10 | 0.595 |
| ↘ | 26 | 6 | 16 | 9 | |||||
| Abs. lymphocyte counts (cells/μL) | ↗ | 48 | 0.005 | 12 | 0.003 | 38 | 0.100 | 13 | 0.193 |
| ↘ | 23 | 4 | 22 | 8 | |||||
| Rel. lymphocyte counts (%) | ↗ | 41 | 0.469 | 8 | 0.066 | 25 | 0.120 | 12 | 0.516 |
| ↘ | 30 | 6 | 35 | 10 | |||||
| Abs. monocyte counts (cells/μL) | ↗ | 44 | 0.707 | 7 | 0.429 | 40 | 0.112 | 12 | 0.489 |
| ↘ | 27 | 6 | 19 | 8 | |||||
| Rel. monocyte counts (%) | ↗ | 27 | 0.009 | 7 | 0.914 | 25 | 0.007 | 9 | 0.688 |
| ↘ | 44 | 7 | 34 | 11 | |||||
| Abs. eosinophil counts (cells/μL) | ↗ | 52 | <0.001 | 7 | 0.555 | 42 | <0.001 | 13 | 0.047 |
| ↘ | 19 | 7 | 18 | 8 | |||||
| Rel. eosinophil counts (%) | ↗ | 51 | <0.001 | 8 | 0.479 | 41 | 0.001 | 12 | 0.513 |
| ↘ | 20 | 7 | 19 | 9 | |||||
| CD4+ T cells (% of lymphocytes) | ↗ | 37 | 0.002 | 8 | 0.254 | 36 | 0.016 | 14 | 0.001 |
| ↘ | 17 | 11 | 12 | 4 | |||||
| CD8+ T cells (% of lymphocytes) | ↗ | 29 | 0.081 | 11 | 0.129 | 28 | 0.156 | 14 | 0.020 |
| ↘ | 21 | 6 | 19 | 8 | |||||
| CD8+CD103+ T cells (% of CD8+) | ↗ | 32 | 0.042 | 8 | 0.446 | 33 | 0.085 | 12 | 0.242 |
| ↘ | 22 | 7 | 15 | 5 | |||||
| CD8+Ki67+ T cells (% of CD8+) | ↗ | 42 | <0.001 | 8 | 0.861 | 35 | <0.001 | 11 | 0.335 |
| ↘ | 8 | 7 | 12 | 7 | |||||
| CD4+Ki67+ T cells (% of CD4+) | ↗ | 47 | <0.001 | 8 | 0.905 | 41 | <0.001 | 9 | 0.228 |
| ↘ | 3 | 7 | 6 | 22 | |||||
| CD4+CD25+FoxP3+ Tregs (% of CD4+) | ↗ | 39 | <0.001 | 7 | 0.377 | 34 | 0.001 | 9 | 0.963 |
| ↘ | 15 | 8 | 14 | 8 | |||||
| CD4+CD127lowCD25+FoxP3+ Tregs (% of CD4+) | ↗ | 36 | 0.002 | 9 | 0.994 | 34 | 0.001 | 11 | 0.755 |
| ↘ | 14 | 7 | 13 | 8 | |||||
| CD4+CD127lowCD25+FoxP3+CD45RA−Ki67+ proliferating Tregs (% of CD4+) | ↗ | 41 | <0.001 | 8 | 0.924 | 31 | 0.015 | 11 | 0.794 |
| ↘ | 9 | 8 | 16 | 9 | |||||
| CD4+CD127lowCD25+FoxP3+CD45RA+Ki67− nonproliferating Tregs (% of CD4+) | ↗ | 17 | 0.030 | 7 | 0.424 | 17 | 0.189 | 14 | 0.953 |
| ↘ | 33 | 9 | 30 | 9 | |||||
| Lin−CD14+HLA-DR−/low MDSCs (% of all cells) | ↗ | 27 | 0.149 | 10 | 0.744 | 26 | 0.244 | 9 | 0.395 |
| ↘ | 32 | 8 | 27 | 14 | |||||
| Lin−CD14−CD15+CD11b+ MDSCs (% of all cells) | ↗ | 28 | 0.238 | 7 | 0.129 | 27 | 0.682 | 9 | 0.254 |
| ↘ | 30 | 14 | 23 | 14 | |||||
| CD14+ monocytes (% of all cells) | ↗ | 21 | 0.008 | 8 | 0.987 | 22 | 0.642 | 9 | 0.500 |
| ↘ | 38 | 8 | 31 | 13 | |||||
| Lin−CD14+CD16−HLA-DR+ classical monocytes (% of all cells) | ↗ | 22 | 0.011 | 7 | 0.328 | 21 | 0.062 | 14 | 0.943 |
| ↘ | 37 | 13 | 32 | 10 | |||||
| Lin−CD14+CD16+HLA-DR+ monocytes (% of all cells) | ↗ | 26 | 0.179 | 8 | 0.200 | 27 | 0.388 | 10 | 0.757 |
| ↘ | 33 | 13 | 26 | 12 | |||||
| Lin−CD14−CD16+HLA-DR+ nonclassical monocytes (% of all cells) | ↗ | 29 | 0.976 | 9 | 0.705 | 19 | 0.001 | 9 | 0.654 |
| ↘ | 30 | 7 | 34 | 13 | |||||
NOTE: Bold letters indicate significant differences between groups when accounting for multiple testing (P < 0.0011).
Abbreviations: Abs., absolute; BL, baseline; MST, median survival time; Rel., relative.
Early defines changes between BL and a second time-point, ranging from 2 to 8 weeks after start of ipilimumab. ↗: increase between BL and follow-up, ↘: decrease between BL and follow-up.
Delayed defines changes between BL and a third time-point, ranging from 8 to 14 weeks after start of ipilimumab.
Wilcoxon-matched pairs test.
Kaplan–Meier analysis.
Patients with unchanged values were included in the category “increase.”
Associations of marker changes with OS
Next, we analyzed whether dynamic changes in biomarkers in the individual patient are associated with clinical outcome. For this, we compared OS of patients with an increase (or unchanged values) with those with a decrease of the respective factor. This analysis was separately performed for early (values from blood draw weeks 2–8 compared with baseline values) and delayed (values from blood draw weeks 8–14 compared with baseline values) changes and for all 25 factors (Table 2). Intriguingly, neither changes in MDSCs nor Tregs were significantly associated with OS (Table 2). Of note, by analyzing the biomarker values at a definite time-point (not the relative changes over time), patients with lower than median frequencies of MDSCs or higher than median frequencies of Tregs at baseline, 2 to 8 weeks, and 8 to 14 weeks had higher OS compared with patients with high frequencies of MDSCs or low frequencies of Tregs (Supplementary Table S2).
Changes of only five factors significantly correlated with prognosis (P < 0.05; Tables 2 and 3). An early increase of absolute leucocyte counts (Fig. 1A) or ALC (Fig. 1B) and delayed increases in AECs (Fig. 1C) and frequencies of CD4+ T cells (Fig. 1D) or CD8+ T cells (Fig. 1E) were significantly associated with improved OS. Differences in OS were most significant for changes in ALC and CD4+ T cells. Patients with an early increase in ALC had a median survival of 12 months as compared with 4 months for the others (P = 0.003). The 1-year survival rate was 51.9% for patients with an increase in ALC, but only 13.0% for those where ALC decreased. Similar results were obtained for patients who experienced a delayed increase in frequencies of CD4+ T cells, with a median survival of 14 versus 4 months for the others (P = 0.001). A delayed increase in frequencies of CD8+ T cells indicated the best chance for long-term survival with a 2-year survival rate of 41.4%. In contrast, the 2-year survival rate was only 5.3% for patients with a delayed decrease in CD8+ T cells (Table 3). When correcting for multiple testing, the associations of the five changes with OS were above the adjusted level of significance (P < 0.0011).
Table 3.
Changes significantly associated with OS and their correlation with clinical responses
|
|
OS according to Kaplan–Meier
|
Response rate
|
Benefit rate
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Total n | Change | N (%) | % Dead | 1-year survival rate (95% CI) | 2-year survival rate (95% CI) | P value | % (n) | P value | % (n) | P value |
| Abs. leucocyte counts (cells/mL) Earlya | 71 | Increase | 45 (63.4) | 71.1 | 46.7 (32.1–61.2) | 32.9 (19.0–46.8) | 0.043 | 25.6 (11/43) | 0.356 | 30.2 (13/43) | 0.769 |
| Decrease | 26 (36.6) | 92.3 | 26.9 (9.9–44.0) | 4.5 (0.0–13.0) | 14.3 (3/21) | 23.8 (5/21) | |||||
| Abs. lymphocyte counts (cells/μL) Earlya | 71 | Increase | 48 (67.6) | 72.9 | 51.9 (37.7–66.1) | 29.3 (16.0–42.6) | 0.003 | 29.5 (13/44) | 0.047 | 36.4 (16/44) | 0.037 |
| Decrease | 23 (32.4) | 91.3 | 13.0 (00–26.8) | 8.7 (0.0–20.2) | 5.0 (1/20) | 10.0 (2/20) | |||||
| Abs. eosinophil counts (cells/μL) Delayedb | 60 | Increase | 42 (70.0) | 66.7 | 54.7 (39.6–69.7) | 34.1 (19.3–48.8) | 0.047 | 25.6 (10/39) | 0.480 | 35.9 (14/39) | 0.182 |
| Decrease | 18 (30.0) | 94.4 | 33.3 (11.6–55.1) | 11.1 (0.0–25.6) | 14.3 (2/14) | 14.3 (2/14) | |||||
| CD4+ T cells (% of lymphocytes) Delayedb | 48 | Increase | 36 (75.0) | 66.7 | 58.3 (42.2–74.7) | 31.1 (15.4–46.8) | 0.001 | 36.4 (12/33) | 0.040 | 45.5 (15/33) | 0.008 |
| Decrease | 12 (25.0) | 100 | 8.3 (0.0–24.0) | 8.3 (0.0–24.0) | 0.0 (0/10) | 0.0 (0/10) | |||||
| CD8+ T cells (% of lymphocytes) Delayedb | 47 | Increase | 28 (59.6) | 60.7 | 56.9 (38.5–75.3) | 41.4 (22.7–60.1) | 0.020 | 45.8 (11/24) | 0.005 | 50.0 (12/24) | 0.049 |
| Decrease | 19 (40.4) | 94.7 | 31.6 (10.7–52.5) | 5.3 (0.0–15.3) | 5.6 (1/18) | 16.7 (3/18) | |||||
Abbreviations: Abs., absolute; CI, confidence interval.
Early defines changes between baseline and a second time-point, ranging from 2 to 8 weeks after start of ipilimumab.
Delayed defines changes between baseline and a third time-point, ranging from 8 to 14 weeks after start of ipilimumab.
Best overall tumor response was investigated as either clinical response (complete response or partial response) or benefit (complete response, partial response, or stable disease) defined via irRC.
Figure 1.
OS according to changes in ALC, leucocyte counts, eosinophil counts, and CD4+ and CD8+ frequencies during ipilimumab therapy. Kaplan–Meier analysis of OS according to early changes (increase vs. decrease) of absolute leucocyte counts (A), ALC (B), and delayed changes in AECs (C) and changes in percentage of CD4+ (D) and CD8+ (E) T cells observed during ipilimumab treatment. Censoring is indicated by vertical lines; P values were calculated by log-rank statistics.
Associations of changes with clinical response
Next, we analyzed the correlation of changes with clinical responses considering those factors which were significantly associated with OS. Note that 29.5% and 36.4% of patients with early increase in ALC experienced a clinical response and clinical benefit, respectively. In contrast, clinical responses and benefit were observed in only 5.0% or 10.0% of patients with an early decrease in ALC. The response and benefit rates were 36.4% (12/33) and 45.5% (15/33), respectively, for patients with delayed increasing frequencies of CD4+ T cells. None of the patients who showed a decrease in CD4+ T cells had a clinical response or benefit. Similar results were also found for patients with delayed increasing frequencies of CD8+ T cells. Their response rate was 45.8% (11/24), and benefit rate was 50.0% (12/24). No such association with tumor response was found for changes in absolute leukocyte counts or AEC (Table 3).
Further characterization of early changes in ALC
An early increase of ALC was identified here as the only early factor correlating with clinical response and also being associated with OS (Fig. 1B and Table 3). We analyzed the evolution of ALC over time in different outcome groups regarding OS (OS ≤6 months vs. 7–18 months vs. >18 months) and tumor response (Supplementary Fig. S2A–S2C). Here, ALC analyzed between 2 to 8 weeks did not change compared with baseline levels in patients who died within 6 or 18 months, but were significantly increased in patients surviving >18 months (Supplementary Fig. S2A). ALC were only significantly increased in patients with clinical benefit or response (Supplementary Fig. S2B and S2C), but remained unaltered in the respective complementary subgroups.
Next, we used flow cytometry to further characterize the lymphocyte subpopulations responsible for the increase in ALC and thereby for the favorable outcome. Thus, we calculated counts of CD4+ or CD8+ T cells and CD3− lymphocytes [the majority of which are likely to be B cells or natural killer (NK) cells]. Within the group of patients with an early increase of ALC, 91.2% of patients had an increase in absolute numbers of CD4+ T cells. An increase of absolute counts of CD8+ T cells or CD3− cells was observed in 91.2% or 67.7% of patients (Supplementary Fig. S3A). A total of 85.3% had increases in both CD4+ and CD8+ T cells, and 52.9% had increase in all three analyzed subpopulations. Patients with increases in CD4+, CD8+, or CD3− counts had a longer survival compared with those without the respective increase, but these values were not statistically significant if each subset was analyzed separately (Supplementary Fig. S3B–S3D). Thus, all three investigated cellular subpopulations seem to contribute to the early increase in ALC and to the association of the early ALC increase with OS.
Further characterization of delayed changes in CD4+ and CD8+ T cells
Frequencies of CD4+ T cells and CD8+ T cells were the only delayed changes associated with OS and additionally correlated with clinical response/benefit. According to Cox regression analysis, both changes had an independent impact on OS if considered in combination (HR 2.9; P = 0.004 for CD4+ T cells; HR 2.0; P = 0.044 for CD8+ T cells). Analyzing different outcome groups, frequencies of CD4+ T cells examined in weeks 8 to 14 did not change compared with baseline levels in patients who died within 6 or 18 months, but were found to be significantly increased in patients surviving >18 months (Supplementary Fig. S2D). A similar trend was seen for delayed changes in frequencies of CD8+ T cells (P = 0.065; Supplementary Fig. S2G). Significantly increasing frequencies of CD4+ and CD8+ T cells 8 to 14 weeks after starting treatment were only observed in the subgroup of patients experiencing clinical benefit (Supplementary Fig. S2E and S2H) or clinical responses (Supplementary Fig. S2F and S2I), but not in the complementary groups, respectively.
In line with the findings based on the analysis of frequencies, absolute numbers of CD4+ T cells were also found to be increased in a majority of the patients at the later time-point (32 of 48 patients, 66.7%; P = 0.018). In addition, there was a strong trend for an association of increases in absolute counts with improved OS (P = 0.061; Supplementary Fig. S3E). Patients with a delayed increase in CD8+ counts had a better OS, but despite apparent separation of survival curves, this difference was not statistically significant (P = 0.148; Supplementary Fig. S3F). No association with prognosis was observed for delayed changes in absolute numbers of CD3− lymphocytes (P = 0.810; Supplementary Fig. S3G).
Early increases in ALC in combination with delayed increases of CD4+ and CD8+ T-cell frequencies characterize patients with an excellent outcome
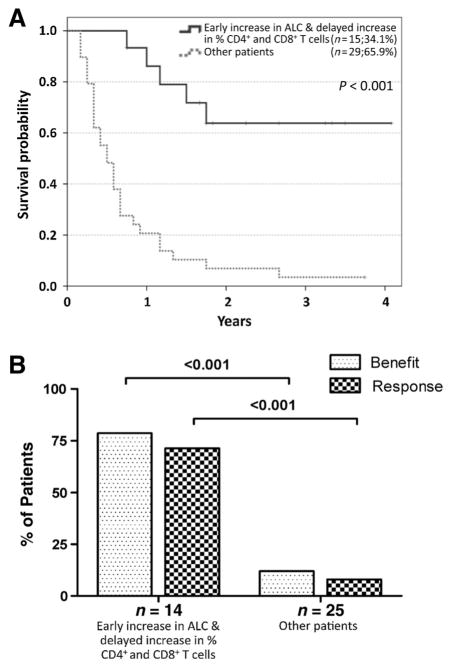
Patients with an early decrease in ALC and/or a delayed decrease of CD4+ T cells (47.7%) had a 1-year survival rate of 9.5%, and the objective response rate was 6%. In contrast, 23 of 44 (52.3%) patients with an early increase in ALC and a delayed increase in CD4+ T cells had a 1-year survival rate of 78.3% and a response rate of 50%. Interestingly, no further deaths were observed among 8 patients of this group after surviving the first 2 years following the first dose (Fig. 2). In 23 patients with an increase in ALC and CD4+ T cells, OS was markedly different between patients further stratified according to changes in CD8+ T cells (P < 0.001; Supplementary Fig. S4). Patients with delayed increases of CD8+ T cells in addition to increases in ALC and CD4+ T cells had a 1-year OS probability of 93.3% and response rates of 71.4% in (Fig. 3A). In line with those findings, a clinical response was observed in 10 of 14 (71.4%) patients with an increase in all three factors, whereas this was seen in only 2 of 25 (8.0%) patients who showed a decrease in at least one of these three factors, respectively (Fig. 3B).
Figure 2.
OS and best tumor response based on combined changes in ALC and frequencies of CD4+ T cells. Kaplan–Meier analysis of OS for patients based on combined early changes in ALCs and delayed changes of frequencies of CD4+ T cells. Patients were grouped based on whether both factors showed an increase versus patients where one or no factor increased (A). Best overall tumor response was investigated as either clinical benefit (complete response, partial response, or stable disease) or response (complete response or partial response) defined via irRC. The best overall tumor response was not available for 5 of 44 patients with complete biomarker data (changes in ALC, CD4+ T cells, and CD8+ T cells; B).
Figure 3.
OS and best tumor response based on combined changes in ALC and frequencies of CD4+ and CD8+ T cells. Kaplan–Meier analysis of OS for patients based on combined early changes in ALCs and delayed changes of frequencies of CD4+ and CD8+ T cells. Changes were defined merely as increase versus decrease. Patients were grouped based on whether all three factors showed an increase versus patients with an increase in 0–2 factors (A). Best overall tumor response was investigated as either clinical benefit (complete response, partial response, or stable disease) or response (complete response or partial response) defined via irRC. The best overall tumor response was not available for 5 of 44 patients with complete biomarker data (changes in ALC, CD4+ T cells, and CD8+ T cells; B).
Similar associations of ALC, CD4+, and CD8+ T cells with outcome were observed when separately analyzing 69 patients receiving ipilimumab monotherapy at 3 mg/kg bodyweight, or for the 51 patients with available biomarker data at all three time-points (Supplementary Table S3).
Patients with clinical benefit according to the week-12 tumor assessment usually do not receive further active treatment until disease progression. Only 1 of 11 patients with clinical benefit at week 12 and increases in ALC, CD4+T cells, and CD8+T cells died during follow-up. However, 2 of 3 patients with decreases in at least one cell subset died despite showing clinical benefit at the first tumor assessment (Supplementary Fig. S5).
Discussion
In this study, we focused on the analysis of changes in circulating immune populations after starting ipilimumab treatment for stage IV melanoma. After accounting for multiple testing, significant dynamic changes were observed for relative eosinophil counts, CD4+Ki67+ and CD8+Ki67+ T cells, CD4+ (CD127low)CD25+FoxP3+ Tregs, and Lin−CD14−CD16+HLA-DR+ nonclassical monocytes. As no association with OS was found, none of these changes qualifies as a potential biomarker candidate. Most likely the observed changes are at least partially a consequence of ipilimumab treatment, but due to the uncontrolled design of our study, no definitive explanation can be provided here. In line with our analysis, Wang and colleagues had previously reported increasing frequencies of Ki67+CD4+ and Ki67+CD8+ T cells in patients treated with ipilimumab, but these were not correlated with clinical outcome (23). However, decreasing frequencies of Ki67+CD4+ T cells expressing the transcription factor EOMES at 6 months were significantly associated with relapse in the same study. In our study, we did not analyze EOMES expression.
The lack of correlations between these relative biomarker changes following therapy and outcome does not rule out a potential association of the absolute numbers or frequencies of these cell populations at a certain time-point with prognosis. This is especially true for MDSCs that were proposed as baseline biomarkers for ipilimumab treatment outcome earlier (9, 26). MDSCs have been proposed for their potential predictive (9, 26) but also surrogate (7, 10) function in metastatic melanoma patients receiving ipilimumab. Recently, increasing monocytic MDSCs were found during ipilimumab in nonresponders, whereas responding patients showed a decrease (17). In the current study, we did not find associations between changes of Lin−CD14+HLA-DR−/low MDSCs and outcome. However, low MDSCs were associated with improved OS at all analyzed time-points, which is in line with earlier findings (9, 26). We did not observe dynamic decreases of granulocytic (Lin−CD14−CD15+CD11b+) MDSCs during ipilimumab as recently reported by Pico de Coaña and colleagues (28). A difference that might explain the divergent findings is that the authors analyzed freshly isolated (not cryopreserved) cells.
Previous studies have reported conflicting results on circulating Tregs in ipilimumab-treated patients. No dynamic changes were observed by Weber and colleagues (29), but decreasing frequencies have been reported in other studies (28, 30). In contrast with these earlier findings, we found a significant increase in all analyzed Treg phenotypes (except the nonproliferating Ki67− subtype). However, these changes were small and not associated with OS, whereas an association between progression-free survival and an early temporary increase in circulating Tregs after 6 weeks was found by Tarhini and colleagues in the neoadjuvant setting (10). Based on these differing results, the prognostic role of Tregs for patients treated with ipilimumab remains controversial.
An early increase in ALC occurring within 2 to 8 weeks after the first cycle of ipilimumab was associated with best overall response and with improved survival. Even if the association with OS (P = 0.003) failed to reach the significance level when adjusted for multiple testing (P = 0.0011), we do not expect this observation to be a false positive as similar findings have already been reported by other groups (12, 14, 15). An absolute increase in ALC of more than 200 cells/μL between the first and second doses was associated with improved survival in a study of Delyon (12). Similar to our analysis, an early increase of ALC in general (14) or by more than 1.35-fold (15) at week 6 compared with baseline was associated with longer OS. However, an increase in ALC was associated with outcome but not specifically predictive for ipilimumab’s treatment benefit in improving OS in a retrospective analysis of randomized controlled trial data (31). Thus, the role of ALC as a surrogate marker candidate remains unclear and needs to be further analyzed prospectively in the future.
The increase in ALC was due to increases in all three major lymphocyte subpopulations, namely CD4+ T cells, CD8+ T cells, and CD3− lymphocytes, and increases in all respective subpopulations showed trends for an association with OS if analyzed separately. Our data suggest that not only the T-cell responses, but also CD3− lymphocytes, are required for a beneficial mode of action of ipilimumab in the early phase of the treatment. Possible candidates for these CD3− cells would be B cells and NK cells. Other studies provided evidence that B cells or NK cells are at least partially involved in the beneficial mode of action of ipilimumab. The presence of antibodies targeting NY-ESO-1, a cancer/testis antigen, which is frequently expressed by melanoma cells, has been described in patients with clinical benefit after ipilimumab administration (32, 33). The prognostic impact of NK cells is well known in different types of cancer (34–36). NK cells were associated with tumor regression following the treatment with ipilimumab in pancreatic cancer (37). They were identified as a large population of tumor-infiltrating lymphocytes (TIL) isolated from a regressed lesion (37). Coculture of sorted NK cells isolated from TILs with an autologous tumor line resulted in IFNγ and GM-CSF production (37). In mice, efficacy of ipilimumab and IL2 in combination was dependent on both CD8+ T and NK cells. Codepletion of these subsets (but not either one alone) abrogated the therapeutic effect (38). Ipilimumab has been shown to trigger antibody dependent cellular cytotoxicity (ADCC) of CTLA-4–expressing melanoma cell lines through TNFα release by CD16+ NK cells (39) and can mediate lysis of Tregs via CD16+ nonclassical monocytes (40). B-cell depletion was found to enhance B16 melanoma outgrowth and impaired induction of IFNγ- or TNFα-secreting T cells (41). Moreover, low levels of baseline and posttreatment tumor-infiltrating CD20+ B cells tended to be associated with worse outcome of melanoma patients receiving ipilimumab (10). Further studies that specifically investigate B cells and NK cells are needed to characterize their potential role in the mode of action of ipilimumab, and as biomarkers.
Increasing frequencies of CD4+ and CD8+ T cells at the later time-point were associated with improved OS (P =0.0014 and P = 0.02). However, both stayed above the adjusted level of significance when multiple testing was accounted for (P < 0.0011). Nevertheless, we consider both as promising biomarker candidates and not as a false-positive associations for the following reasons: First, changes in CD4+ and CD8+ T cells additionally correlated with the rate of clinical response. Second, a marked difference in OS was observed, related to changes in CD8+T cells in 23 patients with increases in ALC and CD4+ T cells (Supplementary Fig. S4). However, the association of delayed increases in CD4+ and CD8+ T cells with improved OS warrants further validation.
To the best of our knowledge, this is the first report describing a direct impact of changes in frequencies of CD4+ and CD8+ T cells on clinical outcome following ipilimumab. Our findings underline its assumed beneficial mode of action, which is the drug-induced boosting of the specific T-cell response (23) either by amplification of preexisting cancer-specific T cells (10, 42) or by induction of broader T-cell responses targeting additional antigens (43).
We did not investigate which antigens are targeted by T cells, but increasing evidence indicates that the T-cell response targeting nonsynonymous individual tumor mutations is decisive for clinical responses on ipilimumab treatment (44, 45). Nevertheless, there are patients who do not experience clinical benefit upon immune-checkpoint blockade despite high individual mutational load. Our results suggest that in addition to the right target, an increase in the quantity of T cells is an important prerequisite for good outcome. The lack of an increase in (melanoma-specific) T cells might explain unresponsiveness upon treatment despite the presence of immunological targets in these patients. Adoptive transfer of autologous T cells after in-vitro expansion or concomitant treatment with nonspecific T-cell growth factors, e.g., IL2, may represent promising therapeutic approaches to increase T-cell quantity in these patients potentially resulting in increased efficacy compared with ipilimumab alone.
Finally, the combination of early increases in ALC followed by delayed increases in CD4+ and CD8+ T cells in the same patient characterizes a favorable rearrangement of the immune system and may reflect a clinically relevant shift from nonspecific to specific immunity. These patients had a clear survival benefit and significantly higher response rates compared with patients showing a decrease in at least one of these three factors. Future studies need to address the important question of whether increases in CD4+ and CD8+ T cells observed after 8 to 14 weeks persist, particularly in long-term responders without active further treatments, and whether these or similar changes in immune-cell subpopulations are likewise clinically relevant for patients treated with PD-1 antibodies alone (46) or in combination with ipilimumab (47).
Our study included 82 patients from 5 clinical sites and 3 different countries, with the intention of minimizing the risk of confounding effects and bias. Nevertheless, further validation in independent patient cohorts is warranted due to the following inherent limitations of our exploratory study: Because we analyzed changes in a broad spectrum of factors to identify potential biomarker candidates, there is an increased risk of observing false-positive associations due to multiple testing. Moreover, patients were heterogeneous regarding their treatment background. Site-specific treatment procedures, patient selection guidelines, or the inclusion/exclusion criteria in the clinical trials may lead to a selection bias. The clinical response assessment was in part performed retrospectively by the respective sites, and scans were not independently reviewed. The biomarker results, particularly regarding MDSCs, might be confounded by the use of cryopreserved PBMCs instead of freshly isolated cells (48). Thus, confirmatory studies avoiding conventional techniques of cryopreservation are desirable. Finally, it is not clear whether the observed changes are a direct consequence of ipilimumab treatment or rather a generally prognostic phenomenon.
Validation and further investigation is especially warranted because our observations may have implications for patient care in those with tumor response or stable disease in the first tumor assessment approximately 12 weeks after first dose. If all three increases are detectable, a “wait and see” strategy may be justified for patients without clinically significant overt disease progression on scans. In contrast, 3 patients with early decrease in ALC and/or delayed decrease of CD4+ or CD8+ frequencies had a poor OS despite clinical benefit at the time of the tumor assessment at week 12. Further analysis of this aspect is required to investigate if the latter patients benefit from early further treatments after completion of four doses of ipilimumab.
In conclusion, increases in ALC within 2 to 8 weeks and increases in frequencies of CD4+ and CD8+ T cells after 8 to 14 weeks are key changes associated with a favorable outcome following ipilimumab administration. These surrogate marker candidates warrant further validation and characterization. Increases in all three immune cell populations characterize patients with a favorable outcome. Although NK cells and B cells may be involved in the positive mode of action of ipilimumab in the first weeks, increasing frequencies of CD4+ and CD8+ T cells seem to be crucial for clinical benefit in the later phase of treatment. This is in agreement with the assumption that a boosted T-cell response is the basis for substantial long-term control of melanoma after ipilimumab.
Supplementary Material
Translational Relevance.
An early change in absolute lymphocyte count (ALC) can be easily assessed and represents a surrogate marker candidate for outcome of ipilimumab-treated melanoma patients. After further investigation of a potential predictive impact, its analysis may help to decide whether to continue ipilimumab after two doses or to change early to alternative treatments.
Patients with early increase in ALC followed by delayed increases in CD4+ and CD8+ T cells after ipilimumab had an excellent outcome. After further validation, the assessment of these three changes in combination may facilitate more sophisticated patient counseling at the time of the tumor assessment at week 12. Patients with early decrease in ALC and/or delayed decrease of CD4+ or CD8+ frequencies had a poor prognosis despite evidence of clinical benefit at the time of the tumor assessment at week 12. Prospective studies are needed to determine whether the latter patients benefit from early alternative treatments after completion of ipilimumab.
Acknowledgments
Grant Support
This study was funded in part by Bristol-Myers-Squibb and the EU Seventh Framework Program “PRIAT” (Profiling Responders In Antibody Therapies), grant agreement no 305309.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
J. Yuan is an employee of Merck. M.A. Postow reports receiving honoraria from Bristol-Myers Squibb and Merck, and is a consultant/advisory board member for and reports receiving commercial research grants from Bristol-Myers Squibb. P. Wong is a consultant/advisory board member for Merck. B. Schilling reports receiving speakers bureau honoraria from, is a consultant/advisory board member for, and reports receiving commercial research grants from Bristol-Myers Squibb. P.A. Ascierto is a consultant/advisory board member for Amgen, Array, Bristol-Myers Squibb, MSD, Novartis, Roche-Genentech, and Ventana, and reports receiving commercial research grants from Bristol-Myers Squibb, Roche-Genentech, and Ventana. J.D. Wolchok is a consultant/advisory board member for and reports receiving commercial research grants from Bristol-Myers Squibb and Medimmune. G. Pawelec reports receiving speakers bureau honoraria from Astellas, Cellgene, Clasado, and Pfizer. C. Garbe is a consultant/advisory board member for Amgen, Bristol-Myers Squibb, Leo Pharma, Merck/MSD, Novartis, Roche, and reports receiving commercial research grants from Bristol-Myers Squibb, Novartis, and Roche, and speakers bureau honoraria and travel reimbursement from Amgen, Bristol-Myers Squibb, Leo Pharma, Merck/MSD, Novartis, and Roche. B. Weide reports receiving commercial research grants, honoraria, and travel support from Bristol-Myers Squibb. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: A. Martens, J.D. Wolchok, G. Pawelec, B. Weide
Development of methodology: A. Martens, K. Wistuba-Hamprecht, B. Weide
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Martens, K. Wistuba-Hamprecht, J. Yuan, M.A. Postow, P. Wong, M. Capone, G. Madonna, A. Khammari, B. Schilling, A. Sucker, D. Schadendorf, B. Dreno, P.A. Ascierto, J.D. Wolchok, C. Garbe, B. Weide
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Martens, K. Wistuba-Hamprecht, D. Schadendorf, P. Martus, P.A. Ascierto, J.D. Wolchok, B. Weide
Writing, review, and/or revision of the manuscript: A. Martens, K. Wistuba-Hamprecht, J. Yuan, M.A. Postow, P. Wong, A. Khammari, B. Schilling, D. Schadendorf, P. Martus, B. Dreno, P.A. Ascierto, J.D. Wolchok, G. Pawelec, C. Garbe, B. Weide
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Martens, J. Yuan, M. Capone, G. Madonna
Study supervision: A. Martens
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 7.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–57. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pico de Coana Y, Poschke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res. 2013;1:158–62. doi: 10.1158/2326-6066.CIR-13-0016. [DOI] [PubMed] [Google Scholar]
- 9.Kitano S, Postow MA, Ziegler CG, Kuk D, Panageas KS, Cortez C, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. 2014;2:812–21. doi: 10.1158/2326-6066.CIR-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE. 2014;9:e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–83. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 13.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilgenhof S, Du Four S, Vandenbroucke F, Everaert H, Salmon I, Lienard D, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013;36:215–22. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- 15.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63:449–58. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21:5453–9. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: Comparison with regulatory T cells and NY-ESO-1-or melan-A-specific T cells. Clin Cancer Res. 2014;20:1601–9. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 20.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 21.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, et al. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med. 2012;10:146. doi: 10.1186/1479-5876-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, et al. Increased frequency of ICOS+CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1:229–34. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62:1021–8. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens A, Wistuba-Hamprecht K, Foppen MG, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016 Jan 19; doi: 10.1158/1078-0432.CCR-15-2412. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 28.Pico de Coaña Y, Wolodarski M, Gentilcore G, Yoshimoto Y, Poschke I, Hansson J, et al. Ipilimumab treatment decreases circulating Tregs and GrMDSC while enhancing CD4+ T cell activation. J Transl Med. 2015;13(Suppl 1):07. [Google Scholar]
- 29.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35:89–97. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 30.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow MA, Chasalow SD, Yuan J, Kuk D, Panageas KS, Cheng M, et al. Pharmacodynamic effect of ipilimumab on absolute lymphocyte count (ALC) and association with overall survival in patients with advanced melanoma. ASCO Meeting Abstracts. 2013;31:9052. [Google Scholar]
- 32.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–83. [PubMed] [Google Scholar]
- 35.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–8. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 37.Frankel TL, Burns W, Riley J, Morgan RA, Davis JL, Hanada K, et al. Identification and characterization of a tumor infiltrating CD56(+)/CD16 (−) NK cell subset with specificity for pancreatic and prostate cancer cell lines. Cancer Immunol Immunother. 2010;59:1757–69. doi: 10.1007/s00262-010-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohlhapp FJ, Broucek JR, Hughes T, Huelsmann EJ, Lusciks J, Zayas JP, et al. NK cells and CD8+ T cells cooperate to improve therapeutic responses in melanoma treated with interleukin-2 (IL-2) and CTLA-4 blockade. J Immunother Cancer. 2015;3:18. doi: 10.1186/s40425-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J Transl Med. 2013;11:108. doi: 10.1186/1479-5876-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–5. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–16. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 44.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 47.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, White-side TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.