Abstract
Lipoate serves as a cofactor for the glycine cleavage system (GCS) and four 2-oxoacid dehydrogenases functioning in energy metabolism (α-oxoglutarate dehydrogenase [α-KGDHc] and pyruvate dehydrogenase [PDHc]), or amino acid metabolism (branched-chain oxoacid dehydrogenase, 2-oxoadipate dehydrogenase). Mitochondrial lipoate synthesis involves three enzymatic steps catalyzed sequentially by lipoyl(octanoyl) transferase 2 (LIPT2), lipoic acid synthetase (LIAS), and lipoyltransferase 1 (LIPT1). Mutations in LIAS have been associated with nonketotic hyperglycinemia-like early-onset convulsions and encephalopathy combined with a defect in mitochondrial energy metabolism. LIPT1 deficiency spares GCS deficiency and has been associated with a biochemical signature of combined 2-oxoacid dehydrogenase deficiency leading to early death or Leigh-like encephalopathy. We report on the identification of biallelic LIPT2 mutations in three affected individuals from two families with severe neonatal encephalopathy. Brain MRI showed major cortical atrophy with white matter abnormalities and cysts. Plasma glycine was mildly increased. Affected individuals’ fibroblasts showed reduced oxygen consumption rates, PDHc, α-KGDHc activities, leucine catabolic flux, and decreased protein lipoylation. A normalization of lipoylation was observed after expression of wild-type LIPT2, arguing for LIPT2 requirement in intramitochondrial lipoate synthesis. Lipoic acid supplementation did not improve clinical condition nor activities of PDHc, α-KGDHc, or leucine metabolism in fibroblasts and was ineffective in yeast deleted for the orthologous LIP2.
Keywords: LIPT2, encephalopathy, pyruvate dehydrogenase, α-oxoglutarate dehydrogenase, lipoic acid, metabolic flux, hyperglycinemia
Main Text
Lipoic acid (LA) is an essential cofactor of major mitochondrial enzyme complexes (Figure S1), including the glycine cleavage system (GCS) and four 2-oxoacid dehydrogenases, namely pyruvate dehydrogenase (PDHc; pyruvate oxidation), α-oxoglutarate dehydrogenase (α-KGDHc; Krebs cycle), branched chain α-oxoacid dehydrogenase (BCKDHc; leucine, isoleucine, and valine catabolism), and 2-oxoadipate dehydrogenase (2-OADH, lysine catabolism). This cofactor is covalently bound to a conserved lysine residue of the E2 subunits of PDHc, BCKDHc, 2-OADH, and α-KGDHc as well as the H protein of GCS. In addition, lipoylation of the E3-binding protein (E3BP) of PDHc has been observed. Based on studies in yeast, the LA biosynthesis pathway involves mitochondrial fatty acid synthesis up to eight carbon length (octanoyl moiety bound to an acyl carrier protein, ACP) and three lipoate-specific sequential enzymes, LIPT2, LIAS, and LIPT1.1, 2, 3, 4, 5 In brief, LIPT2 transfers an octanoyl moiety from octanoyl-ACP on the GCS H protein, LIAS adds two sulfur atoms to produce a lipoyl residue, and LIPT1 transfers lipoyl residues from the GCS H protein to the E2 subunits of PDHc, BCKDHc, 2-OADH, and α-KGDHc (Figure S1).
We and others have recently shown that mutations involving LIAS (MIM: 614462) and LIPT1 (MIM: 616299) lead to severe clinical conditions.6, 7, 8 Because LIAS is an iron-sulfur cluster (ISC) protein, lipoylation deficiency is a prominent feature of defective iron-sulfur [4Fe-4S] cluster cofactor biosynthesis (e.g., NFU1 [MIM: 605711], BOLA3 [MIM: 614299], IBA57 [MIM: 615330], LYRM4 [MIM: 615595]9, 10, 11, 12, 13, 14, 15). ISC cofactors also participate in electron transfer reactions and are required for respiratory chain complexes I, II, and III,16, 17 thereby accounting for combined lipoic acid and OXPHOS deficiency.11, 18 Furthermore, defects in mitochondrial transport of S-adenosylmethionine (SAM), mediated by SLC25A26, result in defective LA synthesis, since SAM is a substrate for the lipoic acid synthetase (MIM: 616794).19 Here we report on three individuals from two unrelated families (Figure S2) with a lipoate-related disease involving α-oxoacid dehydrogenase dysfunction due to mutations in LIPT2, encoding the enzyme that catalyzes the first dedicated step of the LA biosynthesis pathway. This study was approved by the local ethics committees. Informed consent was obtained from the parents.
Individuals presented with severe neonatal-onset encephalopathy and abnormal EEG summarized in the Supplemental Note (see also Figure S3 and Table S1). Both clinical and radiological findings were reminiscent of LIPT1 deficiency,7, 8 whereas first-line biochemical findings were abnormal yet little specific.
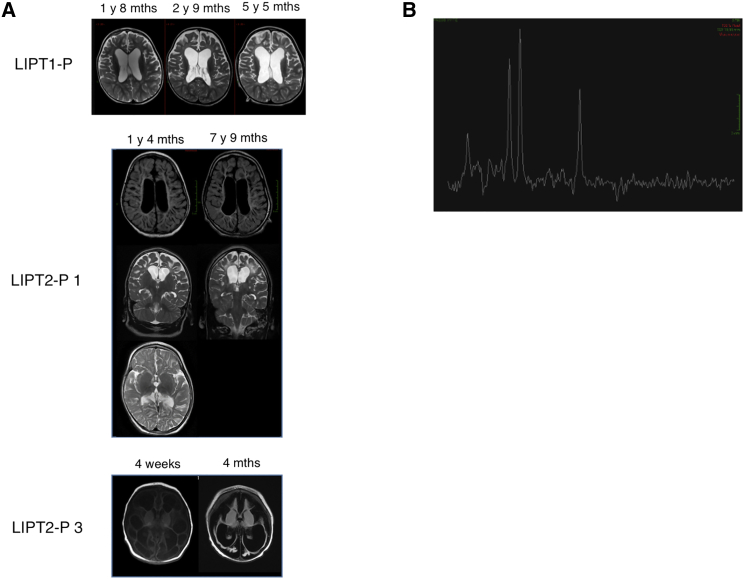
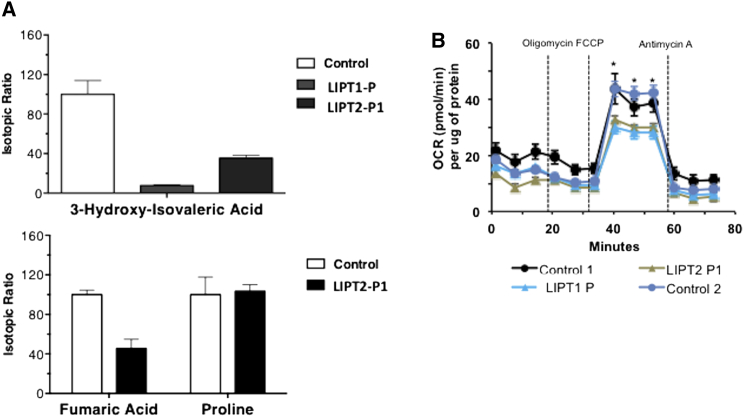
Individual P1 is alive with no episodes of metabolic decompensation. Brain MRI showed marked supra-tentorial cortical atrophy with ventricular dilatation, bi-frontal white matter abnormalities, and delayed myelination (Figure 1A, middle), similar to those observed in a previously described LIPT1-deficient individual7 (Figure 1A, top). MRS spectroscopy with a long TE (144) showed a lactate peak (Figure 1B). Laboratory testing showed hyperlactatemia with a high lactate/pyruvate ratio at 16 months of age, but CSF lactate was normal as well as bicarbonates and urinary organic acid analysis except for a slight increase in lactate (Table S2). Moderate biochemical abnormalities in plasma amino acids included moderate hyperglycinemia contrary to LIPT1 deficiencies previously described,7, 8 increased alanine and decreased branched-chain amino acids (Table S2). At that time, these results were not suggestive of combined α-oxoacid dehydrogenase deficiency or E3 deficiency (MIM: 246900) but rather suggested denutrition. During follow-up, the lactate levels normalized (age 5 years). As in LIPT1 deficiency,7, 8 no elevations were noted for α-hydroxy or α-oxoadipic acids, thus contrasting with the large increases and sometimes massive amounts observed in typical forms of NFU1 deficiency.9 Mitochondrial respiratory chain activities in skeletal muscle and liver and E3 subunit activity measured in fibroblast homogenates were normal (data not shown).20 Using blood DNA, Sanger sequencing did not identify pathogenic gene variations in PDHA1 (MIM: 312170), PDHB (MIM: 614111), PDHX (MIM: 245349), DLAT (MIM: 245348), DLD,21 LIAS, BOLA3, NFU1, and LIPT1. Subsequently, exome sequencing was performed as previously described7 and resulted in a list of 35 candidate genes, including only one encoding a mitochondrial protein, LIPT2. In P1, the two heterozygous c.89T>C transition and c.377T>G transversion were found in LIPT2 (GenBank: NM_001144869.2; see below). To ensure that LIPT2 mutations led to a decrease in lipoic-acid-dependent enzymatic activities, we measured oxygen consumption using pyruvate as a substrate and PDHc and α-KGDHc activities in fibroblasts.22 Both metabolic investigations were severely reduced (Tables S3 and S4) as in LIPT1-deficient individual.7 In P1 fibroblasts, consistent with a defect in both the Krebs’ cycle and PDHc activity, 1 mmol/L 13C5-labeled glutamine loading test revealed decreased fumarate versus normal proline labeling (Figure 2A), and 1 mmol/L 13C6-labeled leucine loading test (a modification of a previously described radioactive test24) revealed decreased BCKDHc activity, but to a lesser extent than in LIPT1-deficient individual (Figure 2A). Lipoic acid administration was orally tested over 3 months at a daily dosage of 25 mg/kg/day in two doses. No clinical modification was observed by the parents, and the neurological examination remained unchanged.
Figure 1.
Brain MRI and MRS Spectroscopy
(A) Brain MRIs of individuals with LIPT1 and LIPT2 mutations. As in LIPT1-deficient individual (LIPT1-P, top), brain MRI revealed supra-tentorial cortical atrophy, ventricular dilatation in P1 with LIPT2 deficiency (LIPT2-P1, middle), cortical anomalies with cystic white matter anomalies at age 4 weeks, and major cortical and subcortical atrophy with ventricular dilatation and formation of major cysts at age 4 months in P3 with LIPT2 deficiency (LIPT2-P3, bottom).
(B) MRS spectroscopy in P1 with LIPT2 deficiency. MRS spectroscopy with long TE showed a peak of lactate (1.3 ppm).
Figure 2.
Labeled Glutamine and Leucine Loading Tests and Oxygen Consumption Rates in Fibroblasts from Control Subjects and Individuals with LIPT1 and LIPT2 Deficiencies
(A) Labeled to natural ratios for 3-hydroxyisovaleric acid after a 13C6 leucine loading test in fibroblasts of an individual with LIPT1 deficiency and LIPT2-P1 and for fumaric acid and proline after a 13C5 glutamine loading test in LIPT2-P1 and control fibroblasts. Labeled leucine and glutamine loading tests are consistent with decreased BCKDHc activity and Krebs cycle activity defects. Labeled amino acids were acquired from Eurisotop. Organic acids derived from labeled substrates were measured by gas chromatography-mass spectrometry (GC-436 Scion-TQD, Brüker Daltonics). Results are presented as means ± SD of triplicates.
(B) Oxygen consumption rates measured in fibroblasts from healthy subjects and individuals with LIPT1 and LIPT2 deficiencies. Basal OCR levels did not differ significantly in fibroblasts from healthy control subjects and individuals with LIPT17 and LIPT2 deficiencies. By contrast, when challenged with a mitochondrial uncoupler (FCCP), fibroblasts from healthy individuals responded with the expected increase in oxygen consumption, whereas the response in cells from individuals with LIPT1 and LIPT2 deficiency was significantly lower. OCR was measured using the XF Cell Mito Stress Test Kit and XFe96 analyzer (Seahorse Bioscience, Agilent Technologies) following the manufacturer’s protocols.23 Cells were seeded at the density 30,000 cells/well in an XFe96cell culture microplate and allowed to attach for 3 hr before the measurement. Basal OCR was measured, followed by sequential treatment with Oligomycin A (1 μM), FCCP (1 μM), and Antimycin A (1 μM). Each treatment was measured every 3 min (3 min measurement) three times and a minimum of six replicates were utilized per condition. All compounds and materials were obtained from Seahorse Bioscience. Protein concentrations in each well were determined with the BCA method (Pierce) in cell lysates after the measurement. Data are presented as mean ± SEM normalized to protein content in each well. Statistical test was performed using ANOVA test; ∗p ≤ 0.05 control versus affected individuals’ cells.
Individuals P2 and P3, two siblings whose healthy parents are of German origin, died in the first year of life without any psychomotor acquisition (see Supplemental Note). Lactate concentration of individual P2 was 3.7 mmol/L at birth and increased to values between 8 and 10, in a single measurement up to 111 mmol/L (N < 1.8 mmol/L). Brain MRI showed periventricular cystic changes. A ketogenic diet was started, which resulted in a decrease of lactate concentrations but also led to a weight loss and worsening of his condition. Organic acids in urine were elevated (Table S2). Biochemical investigations in fibroblasts found decreased PDHc activity (Table S3) while respiratory chain enzyme activities were normal (data not shown). Individual P3 presented with severe lactic acidosis up to 17 mmol/L at birth. Brain MRI showed enlarged lateral ventricles and formation of cysts in the cortex and white matter of the whole cerebral structures. Furthermore, gyration of the cerebral hemispheres was decreased (Figure 1A, bottom). Metabolite investigations showed elevated lactate concentrations in blood and cerebrospinal fluid and elevated pyruvate in blood, with a lactate/pyruvate ratio of 30 (N < 20) (Table S2). Amino acid analysis in plasma showed elevation of alanine and proline, moderate increase of glycine, and decrease of branched-chain amino acids (Table S2). Investigation of organic acids in urine revealed elevation of lactate, pyruvate, and 2-oxoglutarate. A muscle biopsy performed at the age of 2 weeks revealed some variability in fiber diameters (7–19 μm) but no ragged-red or cytochrome c oxidase negative fibers. Electron microscopy showed some subsarcolemnal glycogen accumulation but normal structure of mitochondria. The activity of respiratory chain enzymes complexes I, II, III, and IV was normal (data not shown). Investigation of pyruvate dehydrogenase in fibroblasts was decreased (Table S3).
In P2, panel diagnostics for gene mutations associated with mitochondrial diseases was performed but did not reveal any abnormal result. Exome sequencing, performed as previously described,25, 26, 27 revealed compound heterozygous mutations in LIPT2 c.314T>G (p.Leu105Arg; not reported in gnomAD) and c.377T>G (p.Leu126Arg; frequency reported in gnomAD: 0.0001878), the latter being shared with P1. Sanger sequencing confirmed mutations in P1, P2, and P3 and showed that the parents were heterozygous for one of these mutations. The mutations c.314T>G and c.377T>G were predicted to be “probably damaging” by PolyPhen-2 (Table S5) and were located in a conserved domain of the protein (Figure S2). The c.89T>C variation is a SNP (rs539962457, minor allele frequency [MAF] < 0.0002/1; frequency reported in gnomAD: 0.00003238). It changes a leucine into a proline (p.Leu30Pro) at the second last position of a conserved alpha-helix, which is the predicted mitochondrial targeting sequence of LIPT2 protein and was considered as “possibly damaging” by PolyPhen. These variations were not found in more than 1,000 exome-sequencing projects performed in the Imagine Institute.
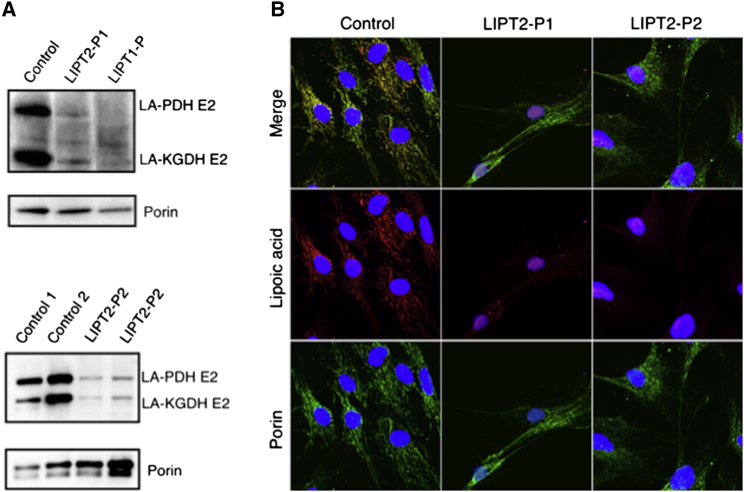
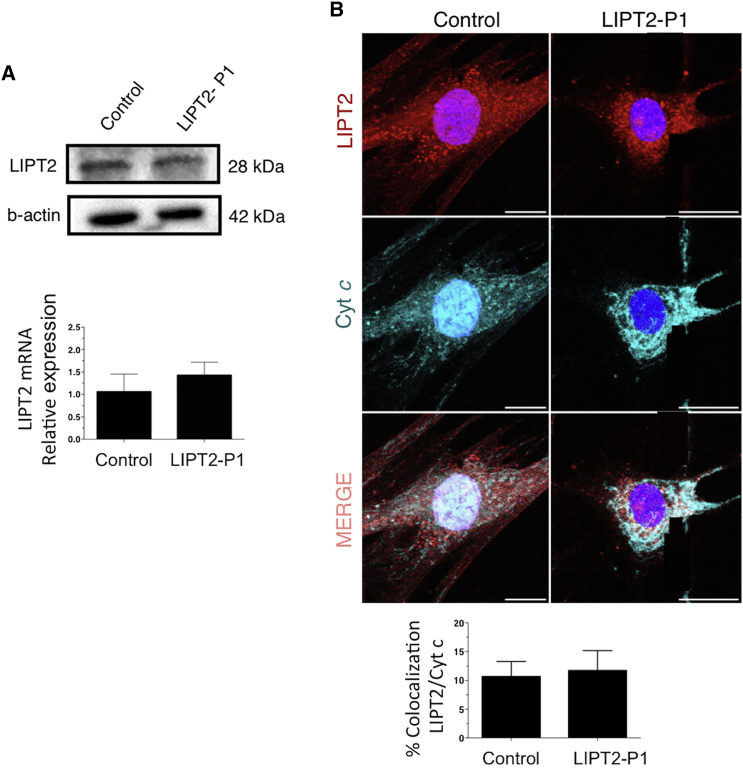
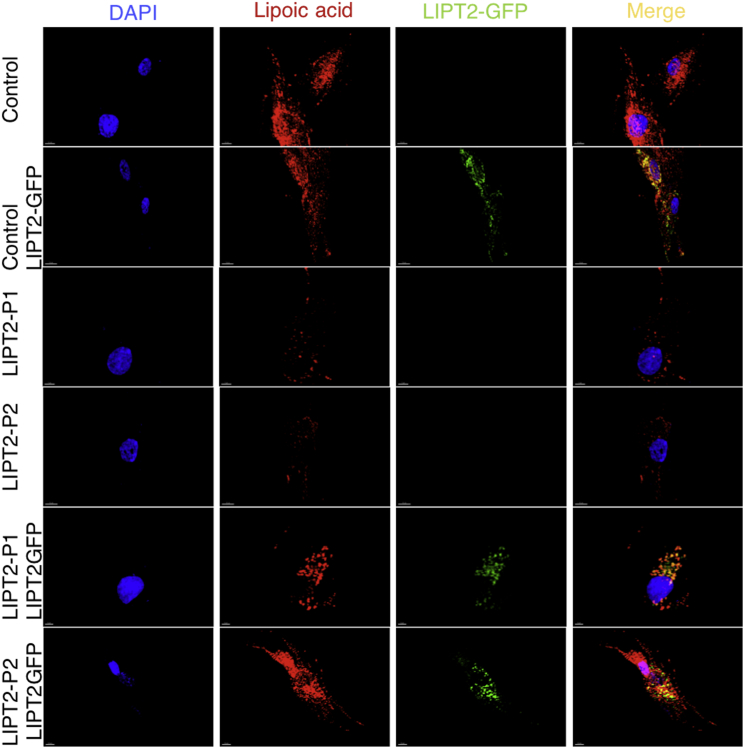
To determine whether lipoic acid metabolism was impaired in individuals with LIPT2 mutations, we used an antibody that specifically recognizes lipoic acid bound to proteins as previously described7 and anti-porin antibody. Anti-lipoate antibody detected strongly decreased levels of the expected lipoylated E2 subunits of α-oxoacid dehydrogenases in fibroblasts of individuals P1 and P2, whereas normal bands were seen in the control (Figure 3A). In fibroblasts of an individual with LIPT1 deficiency,7 these bands were undetectable (Figure 3A). Moreover, immunofluorescence staining examined by a confocal microscopy showed absence of protein lipoylation in P1 and P2 fibroblasts (Figure 3B). In P1 fibroblasts, LIPT2 mRNA expression analyzed by quantitative PCR in triplicate and normalized to the β-actin mRNA level and protein levels analyzed by western blot in triplicate were normal, suggesting that the two mutations might not affect mRNA or protein stability (Figure 4A). In P2 fibroblasts, LIPT2 mRNA was not decreased compared to control, but the variant c.314T>G was less abundant in Sanger sequencing results (Figure S4). Moreover, we examined LIPT2 localization using confocal microscopy in P1 skin fibroblasts (Figure 4B). Colocalization studies between the mitochondrial cytochrome c and LIPT2 showed a positive Pearson coefficient at 0.267 ± 0.014 for the affected individual and 0.241 ± 0.032 for the control, which demonstrated a colocalization between LIPT2 and cytochrome c. Cell surface colocalization was 11.7% ± 3.12% for P1 and 10.7% ± 2.37% for the control. Altogether, these results confirm the location of LIPT2 in mitochondria. To assess consequences of LIPT2 deficiency on mitochondria function, we measured oxygen consumption rate (OCR) in fibroblasts from healthy individuals and individuals with LIPT17 or LIPT2 deficiency using the XF Cell Mito Stress Test Kit and XFe96 analyzer (Seahorse Bioscience, Agilent Technologies) following the manufacturer’s recommendations.23 As shown in Figure 2, basal oxygen consumption rates did not differ significantly between different control and affected individuals’ fibroblasts. However, when cells were challenged with a mitochondrial uncoupler (FCCP), significant differences in OCR in control and affected individuals’ cells were revealed. As demonstrated by analyses presented on Figure 2, fibroblasts from healthy individuals had significantly higher maximal respiratory capacity than fibroblasts from individuals with either LIPT1 or LIPT2 deficiency. These observations in primary cells from affected individuals strongly suggest respiratory capacity failure downstream of mutated LIPT1 or LIPT2. Finally, to demonstrate that LIPT2 mutations are disease causing, P1 and P2 fibroblasts were transfected with a plasmid coding wild-type LIPT2-GFP. Immunostaining of protein-bound lipoic acid showed that expression of wild-type LIPT2 increased protein lipoylation in fibroblasts of P1 and P2 (Figure 5), suggesting that LIPT2 mutations are responsible for defect of protein lipoylation in fibroblasts of affected individuals.
Figure 3.
Immunoblot and Immunofluorescence Staining of Protein-Bound Lipoic Acid
(A) Deficient lipoylation of PDHc and KGDHc E2 subunits in individuals with LIPT2 and LIPT1 mutations. Protein lipoylation was studied by western blot in triplicate with an anti-lipoic acid (Abcam cat# ab58724, RRID: AB_880635, 1:1,600) and anti-porin antibody (Abcam cat# ab14734, RRID: AB_443084, 1:1,000). In fibroblasts from individuals with LIPT2 mutations, the levels of the expected lipoyl-E2 subunits of PDHc and α-KGDHc were strongly decreased, whereas they were undetectable in an individual with LIPT1 deficiency.
(B) Deficient protein lipoylation in fibroblasts of affected individuals. Immunofluorescence staining analysis by a confocal Leica TCS SP8 (Leica) under a 40× NA 1.3 oil immersion objective and acquired using LAS X software revealed decreased protein lipoylation in LIPT2-P1 and LIPT2-P2 fibroblasts (rabbit anti-lipoic acid: Abcam cat# ab58724, RRID: AB_880635, 1:1,000; mouse anti-porin: Abcam cat# ab14734, RRID: AB_443084, 1:400).
Figure 4.
Immunoblot Analysis, mRNA Expression, and Immunofluorescence of LIPT2 in Fibroblasts
(A) Fibroblasts of LIPT2-P1 and control contain similar amount of LIPT2 protein and mRNA. LIPT2 protein level was studied three times (anti-LIPT2 antibody: ab173981 Abcam, 1:500; anti-β-actin: Sigma-Aldrich cat# A5441, RRID: AB_476744, 1:10,000). LIPT2 mRNA expression was analyzed by quantitative PCR and normalized to the β-actin mRNA level (ABI PRISM 7300 Sequence Detection System instrument; TaqMan Universal PCR Master Mix from Applied Biosystem). Data are presented as mean ± SD of triplicates. For studies in LIPT2-P1 fibroblasts, primer sequences are: forward primer LIPT2: 5′-CGT GGT TTG AGC ACA TCG-3′; reverse primer LIPT2: 5′-AAG GCC ACA AGG AAA GGT G-3′.
(B) Fibroblasts from LIPT2-P1 and control exhibited colocalization of cytochrome c and LIPT2. Rabbit anti-LIPT2 (Novus Biological, Immunifluorescence [IF] 1:100) and mouse anti-cytochrome c (BD PharMingen; IF: 1:100) were used to study LIPT2 localization. The amount of co-localization between two channels was quantified using thresholded Pearson’s correlation coefficients with JACoP28 plugin from NIH FIJI software (v.1.51a). To quantify mitochondrial areas and the amount of co-localization at peripheral sites, threshold Pearson’s coefficients were calculated in three randomly chosen 100 pixel × 100 pixel squares in the cell periphery. For averaged line scans, line profiles were calculated as the mean fluorescence intensity averaged over 100 pixels. Maximum intensity projections were calculated from z stacks with 500 nm spacing between slices covering the whole cell. For analysis of immunocytochemistry experiments, a minimum of three independent experiments was performed and statistically significant estimates for each sample were obtained by choosing an appropriate sample size, correlating to 15–40 images per condition per experiment for microscopy-based quantifications. Cells were chosen arbitrarily according to the fluorescent signal in a separate channel, which was not used for quantification. Data are presented as mean ± standard deviation. Statistical tests were performed using a two-tailed, unpaired t test, without excluding samples from statistical analysis. Images were computed using the plugin FigureJ (Jerome Muitterer and Edda Zinck; v.1.10b) on FIJI software.
Figure 5.
Rescue of Protein Lipoylation in Affected Individuals with LIPT2 Deficiency
Fibroblasts from affected individuals were transfected with wild-type LIPT2. Immunostaining analysis of protein-bound lipoic acid showed increase of protein lipoylation after transfection. The following antibodies and reagents were used: rabbit anti-LIPT2 (Novus Biological, ImmunoFluorescence [IF]: 1:100), rabbit anti-lipoic acid (Abcam cat# ab58724, RRID: AB_880635; 1:1,000), and DAPI 300 nM in PBS (D1306, Molecular Probes).
Wild-type and Δlip2 strains, deleted for LIP229 (the gene orthologous to human LIPT24), were grown on glucose (YPD) and glycerol (YPG) (non-fermentable carbon source) medium for 4 days at 28°C. A clear growth defect was observed for Δlip2 cells on the respiratory glycerol medium (Figure S5A). Lipoic acid (2 ng/mL, 5 ng/mL, and 10 ng/mL) was added to the non-fermentable growth medium to test its potential therapeutic effects, but it did not improve Δlip2 growth (Figure S5A). Accordingly, fibroblasts cultures of P1 and a control were supplemented with lipoic acid (Sigma) to a final concentration of 100 μM for 3 weeks. Biochemical investigations were performed before and after 3 weeks of supplementation. As in LIPT1 individual’s fibroblasts,7 this treatment only moderately increased PDHc (276–279 pmol/min/mg of protein) and αKGDHc (1.4 ± 0.3 to 3.5 ± 0.7 nmol/min/mg of protein) activities and did not modify metabolic flux nor PDHc and αKGDHc E2 subunit lipoylation (Figures S5B and S5C).
Consistent with previous reports on LIAS and LIPT1 mutations,7, 30 we provide evidence that impaired attachment of lipoate on mitochondrial proteins in LIPT2-deficient individuals accounts for impaired activities of PDHc and α-KGDHc along with altered branched-chain amino acid catabolism. While lactate levels at the beginning of the investigations and brain MRI were indicative of perturbed energy metabolism, hyperlactatemia was inconstant during follow-up and pyruvate levels were not increased either in plasma or CSF in P1, thus not suggestive of PDHc deficiency prior to in vitro studies. This finding adds to the evidence that standard biochemical tests may not be fully informative to detect related energetic disorders that involve PDHc deficiency. We propose that systematic screening of PDHc or pyruvate oxidation by polarography should be performed in unresolved cases of suspected energetic diseases, or alternatively and more efficiently, that all the genes of this new pathway should be included in an NGS-based screening approach; then, enzymatic analysis can confirm the supposed deficit. Other key enzymatic complexes with similar structure and LA dependence, such as α-KGDHc, BCKDHc, and probably GCS, can be affected. Surprisingly, branched-chain amino acids concentrations were low in plasma of P1 at presentation and during follow-up despite the decrease of leucine catabolism revealed by 13C6 leucine loading test. Other essential amino acids were in the lower range of normal value. Low branched-chain amino acid concentrations were also observed in P2 and in previously reported lipoic acid synthesis defects with either LIPT17 or LIAS6 deficiency. On the other hand, it is important to note that a large proportion of individuals with related disorders such as E3 subunit31 and NFU1 deficiency13 have shown only transient or intermittent biochemical abnormalities. Therefore, there is a large heterogeneity of clinical and biochemical spectrum in these diseases. Compared to other essential amino acids, plasma levels of branched-chain amino acids are known to be most sensitive to nutritional deprivation, thus raising the possibility that feeding difficulties may mask the expected increase of branched-chain amino acids. In this context, 13C6 leucine loading test is particularly interesting to reveal BCKDHc defects when they are not obvious on plasma amino acid profiling. During follow-up, glutamate and glutamine concentrations were increased, ammonemia being normal. This is probably the consequence of increased catabolism due to denutrition and reflects α-KGDHc deficiency too.
In P1 and P2, blood glycine concentrations were increased. In P1, this increase was mild at presentation and more marked during follow-up. Blood glycine concentrations were similar or higher than those observed in some cases of NFU1 deficiency with small amounts of normal NFU1 transcript and strongly decreased but detectable E2 subunit lipoylation.14 Conversely, because LIPT1 acts downstream of GCS H lipoylation, glycine was normal in individuals with LIPT1 deficiency.7, 8 Indeed, the current model of the de novo pathway states that LIPT2 and LIAS are involved in the lipoylation of GCS H protein, whereas LIPT1 is responsible for the transfer of lipoyl moiety from lipoyl GCS H protein to the BCKDHc, PDHc, and α-KGDHc E2 subunits. Moreover, Schonauer et al. showed that, in a Δlip2 yeast strain, Gcv3 (the GCS H ortholog) was not lipoylated.4
Defects in the ISC assembly involve LA de novo biosynthesis because LIAS is an ISC-dependent enzyme, but they include multiple additional dysfunctions that involve succinate dehydrogenase and aconitase 2, two proteins from the tricarboxylic acid cycle, as well as the respiratory chain complexes I–III,16, 32 thus contrasting with normal activities in our individuals with LIPT2 mutations or those with LIAS and LIPT1 deficiency. This may explain why in individuals with [Fe-S] cluster pathway deficiencies, both neurological and non-neurological multisystem symptoms were reported.9, 10, 18 In contrast, the clinical picture of LIPT1, LIAS, and LIPT2-related individuals was mostly limited to the brain, thus similar to primary PDHc deficiencies. The much greater severity of the neurological features in the LA disorders relative to primary PDHc deficiencies might partly result from the additional α-KGDHc enzyme defect. LIPT2-P1 had less pronounced biochemical markers of energetic deficiency than individuals harboring mutations in LIPT1 and other genes, yet brain abnormalities were comparable. The white matter is frequently impaired in LA and ISC defects, including leukoencephalopathy with cysts in individuals harboring mutations in LIPT2, and white matter abnormalities and delayed myelination in LIPT1-related individuals.7, 8 We propose that the diagnostic work-up of an encephalopathy associated with white matter abnormalities and hyperglycinaemia (the latter missing only in individuals with LIPT1 mutations) should include LA mitochondrial synthesis defects, even in the absence of hyperlactatemia in the basal state.
Finally, a major challenge for this new and severe enzyme cofactor disease is therapy. Since PDHc was found decreased in P3, a ketogenic diet was tried in P2, the younger brother of P3. Lactic acidosis improved under this treatment while his clinical condition worsened and he lost weight. This is in accordance with our negative experience with ketogenic diet in an individual with NFU1 deficiency (unpublished results). Indeed, although ketogenic diet bypasses PDHc, it cannot circumvent α-KGDHc acting downstream. While lipoic acid supplementation partly rescued the growth of yeasts deficient in the LIPT1 ortholog,7 it did not restore Δlip2 yeast strain growth in agreement with previous studies.29 In P1-derived fibroblasts, functional studies did not show improvement in the presence of LA, and the clinical condition of the affected individual P1 remained unchanged under LA supplementation, as already shown in other defects of the lipoylation pathway in yeast, mouse, and human cells.4, 6, 7, 9, 16, 18 Oral LA supplementation was also ineffective in two cases of NFU1 deficiency,13, 15 supporting the proposal that in contrast to bacteria, eukaryotic cells cannot use exogenously supplied LA (salvage pathway) and they depend exclusively on de novo intramitochondrial synthesis.33, 34 Other mechanisms, such as partial redundancy between LIPT1 and LIPT2 operating either upstream of LIAS and/or in the usage of free AMP-LA as a substrate, can also be excluded.
In conclusion, we reported here pathogenic LIPT2 mutations, and we showed that these mutations altered lipoate binding in the E2 subunit of α-oxoacid dehydrogenases. The clinical phenotype of LIPT2 deficiency is similar to other lipoic acid synthesis defects, especially LIAS and LIPT1 deficiencies, characterized by an impressive degree of early-onset encephalopathy.
Acknowledgments
We thank Mohammed Zarhrate for his technical support. This work was supported by Fondation Lejeune (grant 2014), Fondation Bettencourt 2012, AFM 2012-2014 (number 15947), ANR 2013-2016 (ANR-13-BSV1-0020), Association contre les Maladies Mitochondriales (AMMI), Association Nos Anges, and Association Hyperinsulinisme. We are indebted to the Necker Cell Imaging Platform for helpful discussions about cellular analyses. T.B.H. work was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (grant #FKZ 01ZX1405C). Supported by the E-Rare project GENOMIT (01GM1207 and 01GM1603 to H.P., O1GML 1207 to A.R.) and Austrian Science Fonds (FWF) (I 2741-B26 to J.A.M.).
Published: July 27, 2017
Footnotes
Supplemental Data include one Supplemental Note (Case Reports), five tables, and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.07.001.
Contributor Information
Johannes A. Mayr, Email: h.mayr@salk.at.
Pascale de Lonlay, Email: pascale.delonlay@aphp.fr.
Web Resources
Clustal Omega, http://www.ebi.ac.uk/Tools/msa/clustalo/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
gnomAD Browser, http://gnomad.broadinstitute.org/
HUGO Gene Nomenclature Committee, http://www.genenames.org/
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Fujiwara K., Okamura-Ikeda K., Motokawa Y. Purification and characterization of lipoyl-AMP:N epsilon-lysine lipoyltransferase from bovine liver mitochondria. J. Biol. Chem. 1994;269:16605–16609. [PubMed] [Google Scholar]
- 2.Fujiwara K., Suzuki M., Okumachi Y., Okamura-Ikeda K., Fujiwara T., Takahashi E., Motokawa Y. Molecular cloning, structural characterization and chromosomal localization of human lipoyltransferase gene. Eur. J. Biochem. 1999;260:761–767. doi: 10.1046/j.1432-1327.1999.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Booker S.J. Unraveling the pathway of lipoic acid biosynthesis. Chem. Biol. 2004;11:10–12. doi: 10.1016/j.chembiol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Schonauer M.S., Kastaniotis A.J., Kursu V.A., Hiltunen J.K., Dieckmann C.L. Lipoic acid synthesis and attachment in yeast mitochondria. J. Biol. Chem. 2009;284:23234–23242. doi: 10.1074/jbc.M109.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiltunen J.K., Autio K.J., Schonauer M.S., Kursu V.A., Dieckmann C.L., Kastaniotis A.J. Mitochondrial fatty acid synthesis and respiration. Biochim. Biophys. Acta. 2010;1797:1195–1202. doi: 10.1016/j.bbabio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Mayr J.A., Zimmermann F.A., Fauth C., Bergheim C., Meierhofer D., Radmayr D., Zschocke J., Koch J., Sperl W. Lipoic acid synthetase deficiency causes neonatal-onset epilepsy, defective mitochondrial energy metabolism, and glycine elevation. Am. J. Hum. Genet. 2011;89:792–797. doi: 10.1016/j.ajhg.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soreze Y., Boutron A., Habarou F., Barnerias C., Nonnenmacher L., Delpech H., Mamoune A., Chrétien D., Hubert L., Bole-Feysot C. Mutations in human lipoyltransferase gene LIPT1 cause a Leigh disease with secondary deficiency for pyruvate and alpha-ketoglutarate dehydrogenase. Orphanet J. Rare Dis. 2013;8:192. doi: 10.1186/1750-1172-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tort F., Ferrer-Cortès X., Thió M., Navarro-Sastre A., Matalonga L., Quintana E., Bujan N., Arias A., García-Villoria J., Acquaviva C. Mutations in the lipoyltransferase LIPT1 gene cause a fatal disease associated with a specific lipoylation defect of the 2-ketoacid dehydrogenase complexes. Hum. Mol. Genet. 2014;23:1907–1915. doi: 10.1093/hmg/ddt585. [DOI] [PubMed] [Google Scholar]
- 9.Navarro-Sastre A., Tort F., Stehling O., Uzarska M.A., Arranz J.A., Del Toro M., Labayru M.T., Landa J., Font A., Garcia-Villoria J. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 2011;89:656–667. doi: 10.1016/j.ajhg.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haack T.B., Rolinski B., Haberberger B., Zimmermann F., Schum J., Strecker V., Graf E., Athing U., Hoppen T., Wittig I. Homozygous missense mutation in BOLA3 causes multiple mitochondrial dysfunctions syndrome in two siblings. J. Inherit. Metab. Dis. 2013;36:55–62. doi: 10.1007/s10545-012-9489-7. [DOI] [PubMed] [Google Scholar]
- 11.Ajit Bolar N., Vanlander A.V., Wilbrecht C., Van der Aa N., Smet J., De Paepe B., Vandeweyer G., Kooy F., Eyskens F., De Latter E. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum. Mol. Genet. 2013;22:2590–2602. doi: 10.1093/hmg/ddt107. [DOI] [PubMed] [Google Scholar]
- 12.Lim S.C., Friemel M., Marum J.E., Tucker E.J., Bruno D.L., Riley L.G., Christodoulou J., Kirk E.P., Boneh A., DeGennaro C.M. Mutations in LYRM4, encoding iron-sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum. Mol. Genet. 2013;22:4460–4473. doi: 10.1093/hmg/ddt295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nizon M., Boutron A., Boddaert N., Slama A., Delpech H., Sardet C., Brassier A., Habarou F., Delahodde A., Correia I. Leukoencephalopathy with cysts and hyperglycinemia may result from NFU1 deficiency. Mitochondrion. 2014;15:59–64. doi: 10.1016/j.mito.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer-Cortès X., Narbona J., Bujan N., Matalonga L., Del Toro M., Arranz J.A., Riudor E., Garcia-Cazorla A., Jou C., O’Callaghan M. A leaky splicing mutation in NFU1 is associated with a particular biochemical phenotype. Consequences for the diagnosis. Mitochondrion. 2016;26:72–80. doi: 10.1016/j.mito.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Tonduti D., Dorboz I., Imbard A., Slama A., Boutron A., Pichard S., Elmaleh M., Vallée L., Benoist J.F., Ogier H., Boespflug-Tanguy O. New spastic paraplegia phenotype associated to mutation of NFU1. Orphanet J. Rare Dis. 2015;10:13. doi: 10.1186/s13023-015-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouault T.A., Tong W.H. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booker S.J., Cicchillo R.M., Grove T.L. Self-sacrifice in radical S-adenosylmethionine proteins. Curr. Opin. Chem. Biol. 2007;11:543–552. doi: 10.1016/j.cbpa.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron J.M., Janer A., Levandovskiy V., Mackay N., Rouault T.A., Tong W.H., Ogilvie I., Shoubridge E.A., Robinson B.H. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am. J. Hum. Genet. 2011;89:486–495. doi: 10.1016/j.ajhg.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishita Y., Pajak A., Bolar N.A., Marobbio C.M., Maffezzini C., Miniero D.V., Monné M., Kohda M., Stranneheim H., Murayama K. Intra-mitochondrial methylation deficiency due to mutations in SLC25A26. Am. J. Hum. Genet. 2015;97:761–768. doi: 10.1016/j.ajhg.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustin P., Chrétien D., Bourgeron T., Gérard B., Rötig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 21.Imbard A., Boutron A., Vequaud C., Zater M., de Lonlay P., de Baulny H.O., Barnerias C., Miné M., Marsac C., Saudubray J.M., Brivet M. Molecular characterization of 82 patients with pyruvate dehydrogenase complex deficiency. Structural implications of novel amino acid substitutions in E1 protein. Mol. Genet. Metab. 2011;104:507–516. doi: 10.1016/j.ymgme.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Brivet M., Garcia-Cazorla A., Lyonnet S., Dumez Y., Nassogne M.C., Slama A., Boutron A., Touati G., Legrand A., Saudubray J.M. Impaired mitochondrial pyruvate importation in a patient and a fetus at risk. Mol. Genet. Metab. 2003;78:186–192. doi: 10.1016/s1096-7192(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 23.Vergnes L., Chin R., Young S.G., Reue K. Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J. Biol. Chem. 2011;286:380–390. doi: 10.1074/jbc.M110.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida I., Sweetman L., Nyhan W.L. Metabolism of branched-chain amino acids in fibroblasts from patients with maple syrup urine disease and other abnormalities of branched-chain ketoacid dehydrogenase activity. Pediatr. Res. 1986;20:169–174. doi: 10.1203/00006450-198602000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kremer L.S., Danhauser K., Herebian D., Petkovic Ramadža D., Piekutowska-Abramczuk D., Seibt A., Müller-Felber W., Haack T.B., Płoski R., Lohmeier K. NAXE mutations disrupt the cellular NAD(P)HX repair system and cause a lethal neurometabolic disorder of early childhood. Am. J. Hum. Genet. 2016;99:894–902. doi: 10.1016/j.ajhg.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolte S., Cordelières F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 29.Marvin M.E., Williams P.H., Cashmore A.M. The isolation and characterisation of a Saccharomyces cerevisiae gene (LIP2) involved in the attachment of lipoic acid groups to mitochondrial enzymes. FEMS Microbiol. Lett. 2001;199:131–136. doi: 10.1111/j.1574-6968.2001.tb10663.x. [DOI] [PubMed] [Google Scholar]
- 30.Mayr J.A., Feichtinger R.G., Tort F., Ribes A., Sperl W. Lipoic acid biosynthesis defects. J. Inherit. Metab. Dis. 2014;37:553–563. doi: 10.1007/s10545-014-9705-8. [DOI] [PubMed] [Google Scholar]
- 31.Brassier A., Ottolenghi C., Boutron A., Bertrand A.M., Valmary-Degano S., Cervoni J.P., Chrétien D., Arnoux J.B., Hubert L., Rabier D. Dihydrolipoamide dehydrogenase deficiency: a still overlooked cause of recurrent acute liver failure and Reye-like syndrome. Mol. Genet. Metab. 2013;109:28–32. doi: 10.1016/j.ymgme.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Rouault T.A. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis. Model. Mech. 2012;5:155–164. doi: 10.1242/dmm.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulo P., Martin N.C. Isolation and characterization of LIP5. A lipoate biosynthetic locus of Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:17634–17639. [PubMed] [Google Scholar]
- 34.Morris T.W., Reed K.E., Cronan J.E., Jr. Lipoic acid metabolism in Escherichia coli: the lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J. Bacteriol. 1995;177:1–10. doi: 10.1128/jb.177.1.1-10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.