Abstract
Mycoplasma pulmonis is a wall-less eubacterium belonging to the Mollicutes (trivial name, mycoplasmas) and responsible for murine respiratory diseases. The genome of strain UAB CTIP is composed of a single circular 963 879 bp chromosome with a G + C content of 26.6 mol%, i.e. the lowest reported among bacteria, Ureaplasma urealyticum apart. This genome contains 782 putative coding sequences (CDSs) covering 91.4% of its length and a function could be assigned to 486 CDSs whilst 92 matched the gene sequences of hypothetical proteins, leaving 204 CDSs without significant database match. The genome contains a single set of rRNA genes and only 29 tRNAs genes. The replication origin oriC was localized by sequence analysis and by using the G + C skew method. Sequence polymorphisms within stretches of repeated nucleotides generate phase-variable protein antigens whilst a recombinase gene is likely to catalyse the site-specific DNA inversions in major M.pulmonis surface antigens. Furthermore, a hemolysin, secreted nucleases and a glyco-protease are predicted virulence factors. Surprisingly, several of the genes previously reported to be essential for a self-replicating minimal cell are missing in the M.pulmonis genome although this one is larger than the other mycoplasma genomes fully sequenced until now.
INTRODUCTION
Mycoplasmas are prokaryotes that cause slowly progressive, chronic diseases in human and animals. Mycoplasmal infections in humans are associated with a variety of respiratory and urogenital diseases, and those in farm animals are responsible for important economic losses (1–3). Because current antibiotic treatments often fail to eradicate mycoplasmas from the host, better control of these infections is needed. As for other pathogens, it is expected that the availability of fully sequenced genomes will help in understanding the physiology and the pathogenesis mechanisms of these bacteria. This should subsequently allow the development of new and efficient means for the control of these pathogens.
Mycoplasmas (class Mollicutes) lack a cell wall but are related to Gram-positive eubacteria from which they evolved by a drastic reduction of genome size, resulting in the loss of many biosynthetic abilities. They are, thus, considered as the best representatives of the concept of a minimal cell (4). Three mycoplasma genomes have been sequenced to date, two from closely related species, Mycoplasma pneumoniae (Mpn) (5) and Mycoplasma genitalium (Mge) (6), and the third from Ureaplasma urealyticum (Uu) (7), which belongs to the same phylogenetic group.
Mycoplasma pulmonis, the subject of our work, is the etiologic agent of murine respiratory mycoplasmosis in rats and mice. This natural pathogen is considered as the most useful model for studying mycoplasmal respiratory infections, including those commonly caused by Mpn in humans. Mpn is a leading cause of respiratory tract infections, especially in children and young adults. Although it has been estimated that only 3–10% of Mpn-infected patients develop pneumonia, up to 30% of cases involving all age groups may be caused by this bacterium (for review see 8). In accordance with observations made during Mpn infections, innate immunity provides anti-M.pulmonis defence of the lungs and humoral immunity plays a role against the dissemination of the infection (9). A key factor in the ability of mycoplasmas to establish a chronic infection is their genome flexibility, which allows them to produce a highly variable mosaic of surface antigens (10,11). One of the better-understood systems exhibiting this antigenic variation is the one responsible for the expression of the Vsa lipoproteins in M.pulmonis (12). Here we report the complete M.pulmonis genome sequence. Its analysis revealed hypervariable domains relevant to pathogenesis and provides clues for deciphering the respective roles of putative virulence factors.
MATERIALS AND METHODS
Mycoplasma strain
The strain UAB CT of M.pulmonis was kindly provided by Maureen Davidson (University of Florida, Gainesville, FL). This strain was passaged twice in a modified PPLO broth medium in our laboratory and the obtained stock named UAB CTIP. Growth medium contained per liter: 22.5 g of PPLO broth powder (Difco), 0.2 g of salmon sperm DNA (Sigma), 200 ml of horse serum, 5 ml of Isovitalex solution (Becton Dickinson), 10 ml of 25% (w/v) glucose, 4 ml of 1% (w/v) phenol red and 120 mg of ampicillin. Genomic DNA was isolated by lysing mycoplasma cells with SDS and proteinase K followed by phenol–chloroform extractions. The same DNA preparation was used for the construction of the different libraries.
Construction of genomic libraries
A preliminary library, named A (1–2 kb inserts obtained by partial Sau3AI digestion of genomic DNA and cloned into pUC18), was constructed for a pilot study. We have subsequently generated three other libraries of different insert size to favor the scaffold formation between sequence contigs during the assembly stage. Library C was a short insert (2–3 kb) library constructed by partial Sau3AI digestion followed by gel sizing and ligation to the BamHI site of pUC18. Library R was made by partial Tsp509I digestion, sizing of fragments to 5–6 kb and insertion into the EcoRI site of the pBAM3 vector (derived from pBluescript II SK+; R.Heilig, unpublished material). Library B was a ‘miniBAC’ library, constructed by partial Tsp509I digestion of genomic DNA, sizing >15 kb and ligation to the gel-purified 8.7 kb EcoRI-linearized pBACe3.6 BAC vector.
Shotgun sequencing and sequence assembly
Cycle sequencing was performed, from both ends of the plasmid and miniBAC clones using flanking vector sequences as primers. Dye primer reactions were analyzed on a Li-Cor 4200L (4000 clones from library R) and dye terminator reactions on an ABI 377 (500, 2000 and 1500 clones, from libraries A, C and B, respectively). The whole genome sequence assembly was performed using the PHRED and PHRAP software packages (13). A total of 6018 end sequences from library R (mean length 930 nt; 5.8 genome equivalents) and 574, 3840 and 1920 reads from libraries A, C and B, respectively (mean length 600 nt; 4.0 genome equivalents), were incorporated into contigs. Moreover, the vsa-containing region, spanning 10–15 kb, revealed a high level of heterogeneity between the subclones, which hindered the correct sequence assembly and was attributed to sequence heterogeneity in the M.pulmonis culture used for the genomic DNA preparation. To circumvent this problem, we performed restriction digest analysis of all the miniBACs covering this region to identify an over-represented population of clones and selected two of them for subsequent subcloning and complete sequencing. Finally, the five individual assemblies were combined to form a consensus, which was used to generate the full genomic sequence.
Finishing and validation
One cloning gap (39 bp) and several regions with uncertainties were resolved by sequencing duplicate PCR products. To fullfil the sequence quality criteria (a minimum of three reads for each position, sequencing on both strands or using two types of chemistry and a PHRAP quality value of 40 or more), a total of 1144 finishing reactions, requiring 481 primers, were performed. The assembly was validated by restriction digest analysis, both on total genomic DNA by pulsed-field gel electrophoresis using the enzymes EagI, KpnI, NruI and SacII, and on a selection of overlapping miniBACs and plasmid clones using the enzymes EcoRI, EcoRV, HindIII and BssHII.
Identification of CDSs and annotation
The annotation was mainly performed using the integrated computer environment specialized for large-scale sequence annotation, Imagene (14). The see text on p.1 were identified using GLIMMER, software for compositional analysis with an interpolated Markov model (15). Putative coding sequences (CDSs) extracted from 300 kb of the M.pulmonis genome were used to train the Markov model. Once trained, the model was applied to the genome using 100 bp as the cut-off value for the smallest CDSs. A total of 875 CDSs were detected using this method but some of them were removed because either they significantly overlapped other CDSs and/or they were of small size (100–150 bp) with a deduced polypeptide presenting no detectable similarity with sequences and patterns in databases. CDSs in intergenic spaces were also searched using BLASTX2 (16). The CDSs were numbered starting with dnaA as the first gene (MYPU_10010) and recD as the last (MYPU_7820).
Search of homology of the products deduced from these CDSs was performed using BLASTP2 (16) against SWISS-PROT and SP-TrEMBL. Additional investigations with CDSs showing no homologs using this first strategy were performed using FASTA (17) and Smith and Waterman algorithms. Motifs were also searched on translated genes using the ProfileScan server (http://www.isrec.isb-sib.ch/software/PFSCAN_form.html) against the databases PROSITE, Pfam-A and Gribskov (18–20). For putative membrane proteins and ABC transporters, transmembrane domains were searched using TMpred (21). In the comparison of mollicute genomes, two genes were regarded as homologous if the proteins they encode were similar both in sequence and in size. For this, we made pair-wise comparisons of all proteins of all proteome pairs, filtering potential homologs using a threshold in BlastP of E-value <10–5 and in maximal difference of protein lengths of 20%. Subsequently, we aligned the sequences, using a variant of the classical dynamic programming algorithm for global alignment, where one counts 0-weight for gaps at both ends of the largest sequence, using the BLOSUM62 matrix (22). Finally, we retained pairs of proteins presenting similarity >40%. The set of orthologous genes (supposed to have diverged following a speciation event) was defined by adding a further criterion of double best hit, i.e. two genes are defined as orthologous if they are homologous and if they are the best match of one another in the respective genomes. For the comparaison of genomes, we also used the information provided in the KEGG catalog of genes for Mpn, Mge and Uu (http://star.scl.genome.ad.jp/kegg/kegg2.html#genes) (23). Terminators were searched using PETRIN (24). The tRNAs were located using the software fastRNA (25) and rRNA by detecting regions of homology with mycoplasmal rRNAs. Analysis of codon usage through factorial correspondence analysis was done using software developed at the Atelier de BioInformatique (26). The search for close repeats was done through the use of the KMR algorithm in windows as explained elsewhere (27). Dot-plots were drawn by using the program dotter from the Sanger Center (28).
Database submission and web-based access to the sequences
These sequence data have been submitted to the EMBL/GenBank/DDBJ databases under accession number AL445566.
The information of individual CDSs, data retrieval and graphical views are available on the web (http://genolist.pasteur.fr/MypuList). This server, which has an organization identical to that described for Subtilist, the Bacillus subtilis-dedicated web server (29), also provides the possibility of performing similarity searches, using FASTA or BLAST, and motif searches using pattern matching.
RESULTS AND DISCUSSION
General features of the genome
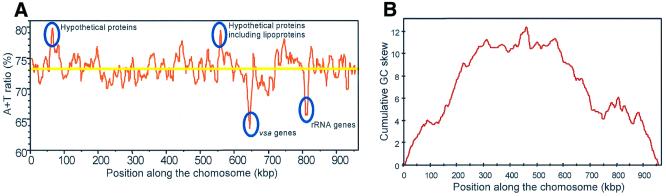
The general features of the genome are listed in Table 1. The genome size is 963 879 bp and its average G + C content is 26.6%, which is, along with that of Uu, the lowest of all bacterial genomes (7). In M.pulmonis, the base composition is rather homogeneous along the entire chromosome (Fig. 1A), with regions corresponding to rRNA and vsa genes showing the lowest A + T values. The average A + T content of the coding regions is 73% with 85.3% at the third position of the codon. An analysis of the codon usage among the 782 identified CDSs using factorial correspondence analysis (26) indicated that the code is homogeneously used among all the CDSs, the only exception being the vsa genes and a few other lipoprotein genes (data not shown). This result suggests that there was, if any, a low level of horizontal transfer during the recent evolution of this mollicute. Like all species belonging to the genus Mycoplasma, M.pulmonis reads UGA as a tryptophan codon instead of a stop codon. In fact, the UGA codon is strongly preferred to UGG (91.2 versus 8.8%). There is also a strong preference for the usage of the UAA stop codon (86.2%) as compared with 13.8% for UAG, the other stop codon. This bias confirms the association between the use of these two stop codons and the G + C% of the entire genome which was recently reported for different bacterial species (31). The use of only two stop codons is in accordance with the finding of a single peptide chain release factor-encoding gene (prfA), capable of recognizing both UAA and UAG.
Table 1. General features of the M.pulmonis genome.
| Length (bp) | 963 879 |
| G + C ratio | 26.6% |
| Putative protein coding sequences (CDSs) | 782 |
| Coding region (% of chromosome size) | 91.4 |
| Average CDS length (bp) | 1115 |
| CDSs with functional assignmenta | 486 |
| CDSs with matches to conserved hypothetical proteins | 92 |
| CDSs without significant database match | 204 |
| Ribosomal RNA operons | 1b |
| TRNAs | 29c |
aFunctional assignment was performed using the classification scheme from Riley (30).
bThe 5S rRNA gene is located 350 kb upstream of the 16S rRNA and 23S rRNA gene cluster.
cThis set of tRNAs corresponds to all amino acids.
Figure 1.
A + T ratio and cumulated GC skew along the M.pulmonis chromosome. (A) The curve indicates the A + T ratio along the M.pulmonis chromosome. This ratio was calculated with a sliding window of 10 kb with a step of 1 kb. The yellow line indicates the average A + T ratio for the genome. The identity of regions showing unusual A + T ratio is indicated. (B) The curve indicates the cumulated GC skew along the M.pulmonis chromosome starting at position 1 as defined in the text. The transition in GC skew is easily identified around position 1, the putative origin of replication of the chomosome. In contrast, the other transition which would correspond to the termination of replication is more difficult to identify because there are distinct peaks in the region centered around 460 kb.
The M.pulmonis genome encodes a single set of rRNA genes. In contrast to Mpn, Mge and Uu, the three genes are not organized as an operon: the 16S rRNA and 23S rRNA genes are adjacent, without intervening tRNA genes, which seems to be common to all mollicutes, the phytoplasmas excepted. The 5S rRNA is located 350 kb from the two other rRNA genes, such a separation having previously been described for two other mycoplasmas, Mycoplasma hyopneumoniae and Mycoplasma flocculare (32). The set of 29 tRNA genes with specificity for all amino acids was identified in the M.pulmonis genome and currently represents the smallest set of tRNAs among the sequenced bacterial genomes. Likewise, the four sequenced mollicute genomes share a single gene for a sigma factor, the one encoding σ70.
With the software Petrin, that uses Escherichia coli default parameters, we were able to identify 281 ρ-independent terminators in the genome of M.pulmonis. Although these terminators were detected in the genome of Mpn (5), they have not been reported in the other sequenced mycoplasma genomes and they have been predicted not to exist in such low G + C genomes (33).
Origin of replication
The origin of replication of the M.pulmonis chromosome has not been experimentally identified. However, as described for other bacterial genomes, but not for the Mpn and Mge genomes, the tandem arrangement of dnaA and dnaN genes was identified. However, the two other genes, gyrA and gyrB, commonly found in the vicinity of the origin of replication, are located elsewhere on the genome. We have designated as base 1 of the M.pulmonis genome the first non-coding base upstream dnaA. We identified five putative DnaA boxes (consensus sequence 5′-TTATCCACA-3′), three being upstream dnaA and the two other downstream of this gene. This organization was not found in the Mpn, Mge and Uu genomes (5–7), but is reminiscent of that described for other mollicutes including Spiroplasma citri (34) and Mycoplasma capricolum (35). It is, therefore, possible that this divergence from the typical eubacterial oriC is restricted to the phylogenetic cluster of mycoplasma species that includes Mpn, Mge and Uu.
The analysis of the G + C skew, defined as [(G – C)/(G + C)] (36), on the M.pulmonis genome provided additional evidence that the origin of replication was properly located (Fig. 1B). There was indeed a marked inversion of G + C skew upstream of dnaA. This bias is stronger than in Mpe and Mge but weaker when compared with other bacterial genomes (37). On both sides of the putative origin of replication, there is also a strong bias in the polarity of the genes transcribed on the two DNA strands.
Comparison with other sequenced mollicute genomes
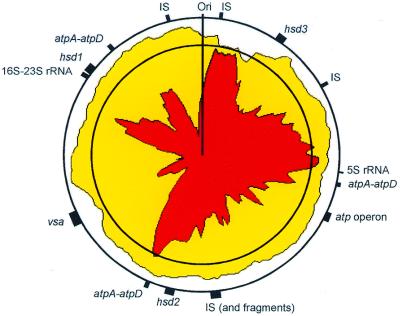
The M.pulmonis genome is currently the largest completely sequenced genome from a mollicute. Consequently, the number of CDSs identified is larger than for the other three mollicutes: 782 as compared with 483, 688 and 605 for Mpn (38), Mge and Uu, respectively (Table 2). This increased number of genes is not evenly distributed in all the functional categories. In some categories, including those governing nucleotide metabolism, intermediary metabolism and protein synthesis and fate, the number of genes is equivalent in the four mollicute genomes (Table 2). In these categories, these genomes appear to approach, to a similar level, the concept of a minimal cell. Furthermore, and quite surprisingly, in contrast to Mpn, Mge and Uu which share the same set of genes for transcription, M.pulmonis has adopted in this category a still more minimalist organization. Indeed, the gene rpoE encoding the δ subunit of RNA polymerase is lacking; this gene has been shown by mutagenesis experiments to be dispensable in B.subtilis (39). The larger number of genes in M.pulmonis as compared with the other three mollicutes can be mainly attributed to an increased number of membrane proteins, in particular those devoted to transport. It is difficult to predict from the sequence analysis the specificity of ABC (ATPase) transporters (40), 56 M.pulmonis CDSs were found to potentially encode subunits for these transporters as compared with 32 and 33 CDSs for Mpn and Uu, respectively. Differences were also found in the ‘Energy metabolism’ category (Table 2) and are due, in part, to the presence of four CDSs encoding α (atpA) and β (atpD) ATP synthase subunits. A set of atpA-atpD genes is located within the operon encoding the other six subunits of the ATP synthase and the three other atpA-atpD tandem copies are scattered on the chromosome (Fig. 2).
Table 2. Functional classification of proteins in the completely sequenced mollicute genomes.
| Functional category |
M.pulmonis (964 kb) |
M.genitalium (580 kb) |
M.pneumoniae (816 kb) |
U.urealyticum (752 kb) |
| Amino acid biosynthesis | 0 | 0 | 0 | 1 |
| Purine, pyrimidine, nucleoside and nucleotide metabolism | 23 | 19 | 19 | 20 |
| Fatty acid and phospholipid metabolism | 15 | 8 | 9 | 8 |
| Biosynthesis of co-factors, prosthetic groups and carriers | 10 | 4 | 8 | 12 |
| Central intermediary metabolism | 9 | 7 | 6 | 13 |
| Energy metabolism | 55 | 33 | 39 | 24 |
| Transport and binding proteins | 83 | 33 | 44 | 48 |
| DNA metabolism | 66 | 29 | 46 | 46 |
| Transcription | 12 | 13 | 13 | 17 |
| Protein synthesis | 97 | 90 | 91 | 91 |
| Protein fate | 22 | 21 | 17 | 12 |
| Regulatory functions | 4 | 5 | 8 | 6 |
| Cell envelope | 60 | 29 | 54 | 19 |
| Cellular processes | 11 | 6 | 11 | 13 |
| Other categories | 8 | 0 | 3 | 18 |
| Unknown | 11 | 12 | 46 | 22 |
| Hypothetical | ||||
| Database match | 92 | 168 | 188 | 106 |
| No database match | 204 | 6 | 86 | 171 |
| Total number of proteins | 782 | 483 | 688 | 613 |
Figure 2.
Density of coding sequences along the M.pulmonis chromosome. Yellow indicates the density of coding sequences in both strands of the sequence and red the density of the coding sequences in the clockwise strand (published sequence). This density was calculated with a sliding window of 50 kb. The first circle represents 100%, the second (inner circle) is 80%. The positions of rRNA genes and of other genes discussed in the text are indicated by black boxes.
Of the 179 M.pulmonis CDSs, which do not have an ortholog in the Mpn, Mge and Uu genomes, 123 encode putative proteins showing no significant homology in the databases. Additionally, eight other M.pulmonis CDSs encode conserved hypothetical proteins with the closest homologs among the polypeptides deduced from Gram-positive bacterial genomes, as expected from the mycoplasmal phylogenetic position. One noteworthy exception is Mypu_4730, which shares homology with a putative Thermotoga maritima polypeptide. Some other M.pulmonis polypeptides with no ortholog deduced from the Mpn, Mge and Uu genomes include ribosomal protein S1 (Mypu_1300), glucokinase (GlcK; Mypu_2280), 5′-nucleosidase precursor (UshA; Mypu_0550) and signal peptidase I (SipS; Mypu_6300). It should be noted that re-annotating the Mpn genome suggested that one of the identified proteases in this organisms (MPN386) could act as a signal peptidase I (38). Mycoplasma pulmonis also seems to have the ability to metabolize sugars other than those metabolized by Mpn, Mge and Uu. Indeed, a set of genes involved in the catabolism of maltodextrins has been identified; these genes are clustered together in one region of the genome and encode a maltodextrin ABC transport system (Mypu_6390–6410), a β-d-phosphate-glucomutase (PgmB; Mypu_6350), a dextrinase with unknown specificity (Mypu_6330) and an α-amylase 3 (AmyC, Mypu_6320). This finding suggests that M.pulmonis can use glycogen as a source of energy and carbon which is in accordance with reports that all glucose-fermenting mycoplasmas ferment glycogen. However, these results were based on experiments using media containing serum or serum fractions, which contain saccharolytic enzymes (41). Therefore, the use of complex sugars by M.pulmonis will have to be experimentally verified. Interestingly, the closely located Mypu_6220 CDS potentially encodes a polypeptide that belongs to the LacI family of transcriptional regulators. This polypeptide, with no ortholog in Mpn, Mge and Uu, together with the heat-inducible transcription repressor (Mypu_1420) is the only indication for transcriptional regulation in this mycoplasma.
Among the M.pulmonis CDSs for which an ortholog was found in Uu but not in Mpn, are CTP synthase (Mypu_6660), cardiolipin synthetase (Mypu_6650) and a poorly understood enzyme (PslX; Mypu_1620) involved in the phospholipid metabolism. In addition, six IS-elements including three which are incomplete were found in the M.pulmonis genome.
Although the gene order in Mpn and Mge is highly conserved (42), this is the case neither in Uu (7) nor in M.pulmonis (data not shown). The regions of syntheny between the four genomes consist in most cases of genes encoding products such as ribosomal proteins, ABC transporters or ATP synthase subunits.
Comparison with the set of genes for a minimal cell
The Mge genome is the smallest bacterial genome and as such is considered as the best representative for the concept of a minimal cell (43). With the assumption that genes conserved across large phylogenetic distances are likely to be essential, a minimal set of genes for a living cell was defined by others (4). Among the 256 Mge genes belonging to this minimal set (http://www.ncbi.nlm.nih.gov/Complete_Genomes/Minset/), eight genes do not have an ortholog in the M.pulmonis genome [MG278, guanosine 5′-diphosphate 3′-diphosphate pyrophosphohydrolase (spoT); MG453, UTP-glucose-1-phosphate uridylyltransferase (gtaB); MG053, phosphomannomutase (cpsG); MG063, 1-phosphofructokinase (fruK); MG393, heat shock protein (groES); MG392, heat shock protein (groEL); MG382, uridine kinase (udk); MG118, UDP-glucose 4-epimerase (galE)]. Although the Mpn and Mge genomes have genes for the synthesis of the heat shock proteins-chaperonins GroEL and GroES, the absence of these genes has also been reported for Uu (7). GroEL has also been shown to be dispensable for Mpn in vitro (44). The fact that the M.pulmonis genome lacks fruK but has a probably functional fruA gene has to be regarded in the light of some recently reported data showing that in the mollicute S.citri, fructose catabolism requires fruA but not fruK (45). Another striking absence in M.pulmonis is that of SpoT, an enzyme playing a key role in the stringent response. Strong evidence supports that there is only one relA/spoT product in Gram-positive bacteria (in contrast to two in Gram-negative bacteria) that displays activity for both synthesis and degradation of (p)ppGpp (46). SpoT was shown by transposon mutagenesis to be dispensable for Mpn and Mge (44). There are only a few examples of eubacteria, which lack the stringent response, in particular Helicobacter pylori (47) and Chlamydia trachomatis (48).
Highly recombinogenic loci in the M.pulmonis genome
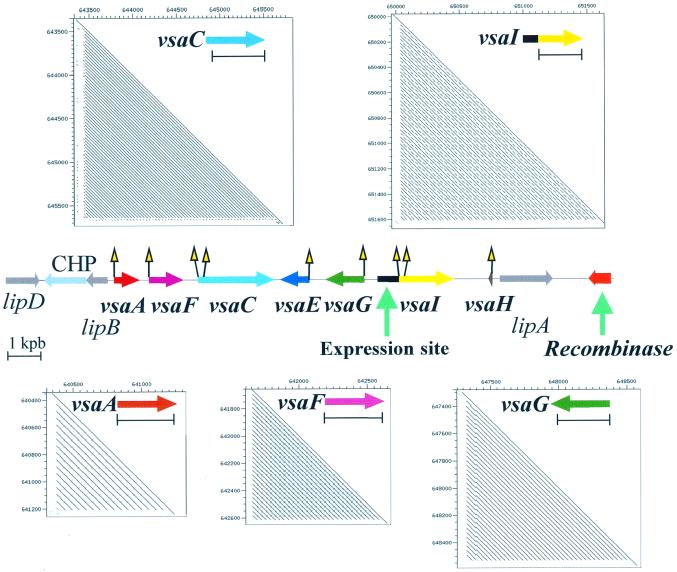
The vsa locus encodes highly variable surface lipoprotein antigens. It has been shown in strain KD735-15 that high-frequency, site-specific DNA inversions serve to regulate the phase-variable expression of individual vsa genes (12). Only one vsa gene, located in the expression site, is transcribed at a given time, the other vsa genes being silent. A model involving DNA strand exchange at conserved vsa recombination sites (vrs boxes) has been proposed to mediate these inversions (12). The completed genomic sequence allows for the first time analysis of the complete vsa locus of the UAB CTIP strain and reveals seven vsa genes (Fig. 3), with four of them (vsaA, vsaC, vsaE and vsaF) being previously described in the strain KD735-15 containing 11 vsa genes (12). As expected from such a highly recombinogenic locus, neither the gene order nor the number of repeated units (see below) in individual genes are conserved between strains. The expressed gene in the sequenced locus is the newly described vsaI gene. At the 5′-end of each vsa gene a vrs box was identified, an additional vrs box laying within the genes vsaC and vsaI (Fig. 3). The dot-plot analysis of this locus highlights repeated elements within each individual vsa gene; for example, a 57 bp unit (19 amino acids) is repeated 27 times in the vsaI gene. There are also common repeats between different vsa genes such as between vsaF and vsaG (data not shown). Within the vsa locus, three lipoprotein-encoding CDSs (lipA, lipB and lipD; Fig. 3) were also identified. Although the deduced sequences share significant similarity with Vsas, they do not exhibit a repeated C-terminus nor vrs boxes within their genes. Furthermore, it was predicted that the putative site-specific recombinase that promotes DNA inversion within this locus would be a member of the λ integrase family. Indeed a gene, the product of which potentially encodes this type of recombinase, was located immediately downstream of the vsa locus (Fig. 3). Experiments are underway to confirm that this gene is indeed responsible for the variability of cell surface antigens and also to determine if antigenic variation confers some protection from the host’s immune system.
Figure 3.
General organization of the vsa locus and visualization of repeats within individual vsa genes Within this locus encoding seven vsa genes, the transcribed gene is vsaI as indicated by the location of the expression site which is fused to this gene. The vrs boxes which are the sites for DNA site-specific inversions are indicated by yellow triangles. We suggest that the recombinase responsible for the rearrangements within this locus is encoded by the gene located at the 3′-end. In addition to the vsa genes, three genes putatively encoding lipoproteins (LipA, LipB and LipD) and a gene encoding a conserved hypothetical protein (CHP) were identified. The repeats within each vsa gene were individually analyzed by dot-plot. Within each of the five boxes, the bracket below the colored gene indicates the region of the gene with repeated units.
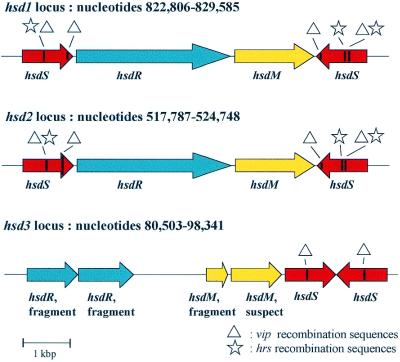
Previous studies have shown that M.pulmonis strain KD735-15 contains at least two highly recombinogenic hsd loci that code for type I restriction and modification systems (49). The UAB CTIP genome has two hsd loci similar to that of KD735-15 and an additional third hsd locus that is not completely functional (Fig. 4). At the hsd3 locus, the two hsdS are most likely functional but the hsdR and hsdM have been disrupted by frameshift mutations and are predicted to be non-functional. The three loci are not co-located on the chromosome (Fig. 2) and, in contrast to the vsa locus, no CDS encoding a putative recombinase involved in the site-specific DNA inversions was found in their vicinity or elsewhere on the chromosome. Although detected in the Mpn and Mge genomes, the hsd systems do not seem to be active in these species. In addition to these hsd genes, we identified a complex locus of a type III RM system and CpG methylase genes that were not identified previously within the genus Mycoplasma.
Figure 4.
Comparative organization of the three hsd loci encoding type I restriction modification systems in M.pulmonis. The organization of the three hsd is depicted on this figure. Within the hsdS genes the two types of recombination sequences are indicated by stars (hrs recombination sequences) and by triangles (vip recombination sequences). The position of the hsd loci on the genome is indicated and can also be found on a circular representation of the genome in Figure 2.
Lipoproteins, polymorphisms and repeats
In addition to the expressed vsa product (VsaI), 55 other lipoprotein CDSs (7% of the total number of CDSs), were identified as compared with 46, 21 and 42 in Mpn, Mge and Uu, respectively. Very few of these lipoproteins had orthologs in the databases and a putative function could be attributed to only three of them: MYPU_1390 and MYPU_6930 as membrane nucleases, and MYPU_6320 as an amylase. Three other lipoproteins with unknown function (MYPU_0070, MYPU_3430 and MYPU_5260) have orthologs in other mycoplasmas. However, several of the lipoproteins could be classified into highly homologous families. Two of the enzymes involved in the maturation of bacterial lipoproteins could be deduced from the genome, the prolipoprotein diacylglycerol transferase (lgt) and the prolipoprotein signal peptidase (lsp). A third enzyme, the apolipoprotein:phospholipid N-acyl transferase (lnt), was not found, which substantiates the finding that lipoprotein acylation may not be complete in some mycoplasma species (50,51). However, one cannot rule out that other types of acylation occur, similar to that recently described for Borrelia burgdorferi (52).
The expression of several mycoplasmal lipoprotein genes has been shown to be phase-variable (for review see 11) and one of the underlying mechanisms is due to variation in the length of homopolymeric nucleotide tracts located either in the promoter or in the coding sequence itself. A stretch of repeated T (T31) and A (A39) nucleotides is located upstream of two M.pulmonis genes encoding the putative lipoproteins MYPU_0190 and MYPU_4780, respectively. These homopolymeric tracts are highly suggestive of phase-variation in the expression of these genes because they are hotspots for frameshift mutations generated by replication slippage. Additional evidence for phase-variation was provided by polymorphisms in the sequence of these repeats. The assembly revealed variations in these tracts among sequences from otherwise identical clones. We verified that this was not due to sequencing difficulties. For MYPU_0190, T31 was found six times and T29 two times (6×T31/2×T29). The situation was more complicated for MYPU_4780, with the following polymorphism: 1×A36/2×A39/2×A40/2×A41/2×A44. The detection of variation in these homopolymeric tracts was favored because the DNA for generating the sequencing libraries (see Materials and Methods) was obtained from a M.pulmonis isolate rather than from a single clone. Should this phase-variation experimentally be confirmed, this would be the first indication that two different mechanisms (length variation in a homopolymeric tract and site-specific inversions in the vsa genes) driving phase-variation of surface antigens operate in a single mycoplasma cell.
Virulence factors
Like other mollicutes, this organism contains few recognizable genes likely to be involved in virulence. Exceptions are a hemolysin (MYPU_1710), a set of membrane-associated or secreted nucleases (MYPU_6930–6940 and MYPU_1390) and a potentially secreted protease (MYPU_1180). The hemolytic activity of M.pulmonis was previously described (53). The MYPU_1710 product has no homolog in Mpn or Mge, but in Uu, another mollicute displaying a hemolytic activity (7). This hemolysin belongs to the hlyA family, members of which are recognized virulence factors in Serpulina hyodysenteriae and in mycobacteria (54,55). Mollicutes have limited biosynthetic capacity, and membrane-associated or secreted proteases and nucleases are thought to help these microorganisms to acquire metabolic precursors from the host. The study of the nuclease produced by Mycoplasma penetrans clearly indicated that mycoplasmal nucleases could act as virulence factors (56,57). A thiol peroxidase (MYPU_7080) is also of interest because the production of hydrogen peroxide has been suggested to be a virulence factor during M.pulmonis infections (58). This enzyme was not deduced from the genomes of other mollicutes.
CONCLUSIONS
The complete sequence of the M.pulmonis genome opens a wide range of perspectives for the study of the mollicutes, and, more generally, of bacterial evolution related to pathogenicity. The analysis of this genome will be more easily exploited than those of the preceding mollicutes because of the availability of tools, transposons and oriC-based replicative plasmids, to genetically manipulate M.pulmonis (59,60) (A.Blanchard, unpublished material). Furthermore, the mouse, one of the natural hosts of M.pulmonis, appears to be a particularly useful model system to dissect mycoplasma–host interactions notably owing to the existence of mice strains differing in their susceptibility to mycoplasmal disease. Mycoplasma pulmonis belongs to a phylogenetic branch distinct from that of the other sequenced mycoplasmas. We are aware of at least six other ongoing mollicute genome projects (M.mycoides subsp. mycoides SC, M.hyopneumoniae, M.gallisepticum, M.penetrans, S.citri and S.kunkelii). From an evolutionary perspective, the availability of the genome sequence of these mycoplasmal species will make this group of organisms an ideal target for genome comparisons and studies of microbial evolution.
Still, the most striking feature of the genome of M.pulmonis is that it reveals an astonishing diversity of strategies related to the evolution of pathogenicity. It contains genes that can participate in intra-chromosomal homologous recombination, like in M.pneumoniae and M.genitalium, antigenic variation through slipped-strand mispairing of repetitive motifs in control regions, as found in H.pylori (61) and Haemophilus influenzae (62), highly variable repetitive proteins, like in Mycobacterium tuberculosis (63), and variable restriction and modification systems, as proposed for Neisseria gonorrhoea (64,65). Hence, in spite of its minimalist character in terms of biochemical potential, M.pulmonis is an extremely suited model organism to study pathogenesis and co-evolution of bacteria with the host.
This work paves the way for a wide variety of experimental studies. One obvious experiment is to inactivate the gene(s) potentially controlling the inversions within the vsa locus to confirm the contribution of this enzyme in the variability of cell surface antigens and also to determine whether antigenic variation confers protection against the host’s immune system. The fact that a single recombinase gene was found in the genome suggests that this enzyme catalyzes DNA inversions in both the vsa and hsd loci.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank J.Weissenbach and L.Montagnier for continuous support during this program, Paul Scott for carefully reading the manuscript and Sophie Oztas for technical help during the sequencing at Genoscope. This work was funded by the Institut Pasteur, Genoscope, INRA, Région Aquitaine and the Université Victor Segalen Bordeaux 2. It was also supported in part by the US Public Health Service grant AI41113 to K.D. from the National Institutes of Health.
DDBJ/EMBL/GenBank accession no. AL445566
References
- 1.Jacobs E. (1997) Mycoplasma infections of the human respiratory tract. Wien. Klin. Wochensch., 109, 574–577. [PubMed] [Google Scholar]
- 2.Taylor-Robinson D. and Furr,P.M. (1998) Update on sexually transmitted mycoplasmas. Lancet, 351, 12–15. [DOI] [PubMed] [Google Scholar]
- 3.Frey J. and Nicolet,J. (1997) Molecular identification and epidemiology of animal mycoplasmas. Wien. Klin. Wochensch., 109, 600–603. [PubMed] [Google Scholar]
- 4.Mushegian A.R. and Koonin,E.V. (1996) A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc. Natl Acad. Sci. USA, 93, 10268–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himmelreich R., Hilbert,H., Plagens,H., Pirkl,E., Li,B.C. and Herrmann,R. (1996) Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res., 24, 4420–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser C.M., Gocayne,J.D., White,O., Adams,M.D., Clayton,R.A., Fleischmann,R. D., Bult,C.J., Kerlavage,A.R., Sutton,G., Kelley,J.M. et al. (1995) The minimal gene complement of Mycoplasma genitalium. Science, 270, 397–403. [DOI] [PubMed] [Google Scholar]
- 7.Glass J.I., Lefkowitz,E.J., Glass,J.S., Heiner,C.R., Chen,E.Y. and Cassell,G.H. (2000) The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature, 407, 757–762. [DOI] [PubMed] [Google Scholar]
- 8.Mansel J.K., Rosenow,E.C.D., Smith,T.F. and Martin,J.W.,Jr (1989) Mycoplasma pneumoniae pneumonia. Chest, 95, 639–646. [DOI] [PubMed] [Google Scholar]
- 9.Cartner S.C., Lindsey,J.R., Gibbs-Erwin,J., Cassell,G.H. and Simecka,J.W. (1998) Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect. Immun., 66, 3485–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Citti C. and Rosengarten,R. (1997) Mycoplasma genetic variation and its implication for pathogenesis. Wien. Klin. Wochensch., 109, 562–568. [PubMed] [Google Scholar]
- 11.Chambaud I., Wroblewski,H. and Blanchard,A. (1999) Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol ., 7, 493–499. [DOI] [PubMed] [Google Scholar]
- 12.Shen X., Gumulak,J., Yu,H., French,C.T., Zou,N. and Dybvig,K.(2000) Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J. Bacteriol ., 182, 2900–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewing B., Hillier,L., Wendl,M.C. and Green,P. (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res., 8, 175–185. [DOI] [PubMed] [Google Scholar]
- 14.Medigue C., Rechenmann,F., Danchin,A. and Viari,A. (1999) Imagene: an integrated computer environment for sequence annotation and analysis. Bioinformatics, 15, 2–15. [DOI] [PubMed] [Google Scholar]
- 15.Salzberg S.L., Delcher,A.L., Kasif,S. and White,O. (1998) Microbial gene identification using interpolated Markov models. Nucleic Acids Res., 26, 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul S.F., Madden,T.L., Schaffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson W.R. (1990) Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol., 183, 63–98. [DOI] [PubMed] [Google Scholar]
- 18.Bateman A., Birney,E., Durbin,R., Eddy,S.R., Howe,K.L. and Sonnhammer,E.L. (2000) The Pfam protein families database. Nucleic Acids Res., 28, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bateman A., Birney,E., Durbin,R., Eddy,S.R., Finn,R.D. and Sonnhammer,E.L. (1999) Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res., 27, 260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnhammer E.L., Eddy,S.R., Birney,E., Bateman,A. and Durbin,R. (1998) Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res., 26, 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann K. and Stoffel,W. (1993) TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler, 347, 166. [Google Scholar]
- 22.Henikoff S. and Henikoff,J.G. (1993) Performance evaluation of amino acid substitution matrices. Proteins, 17, 49–61. [DOI] [PubMed] [Google Scholar]
- 23.Kanehisa M. and Goto,S. (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res., 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.d’Aubenton Carafa Y., Brody,E. and Thermes,C. (1990) Prediction of ρ-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem–loop structures. J. Mol. Biol., 216, 835–858. [DOI] [PubMed] [Google Scholar]
- 25.el-Mabrouk N. and Lisacek,F. (1996) Very fast identification of RNA motifs in genomic DNA. Application to tRNA search in the yeast genome. J. Mol. Biol., 264, 46–55. [DOI] [PubMed] [Google Scholar]
- 26.Moszer I., Rocha,E.P. and Danchin,A. (1999) Codon usage and lateral gene transfer in Bacillus subtilis. Curr. Opin. Microbiol., 2, 524–528. [DOI] [PubMed] [Google Scholar]
- 27.Rocha E.P., Danchin,A. and Viari,A. (1999) Analysis of long repeats in bacterial genomes reveals alternative evolutionary mechanisms in Bacillus subtilis and other competent prokaryotes. Mol. Biol. Evol., 16, 1219–1230. [DOI] [PubMed] [Google Scholar]
- 28.Sonnhammer E.L. and Durbin,R. (1995) A dot-matrix program with dynamic threshold control suited for genomic DNA and protein sequence analysis. Gene, 167, GC1–10. [DOI] [PubMed] [Google Scholar]
- 29.Moszer I. (1998) The complete genome of Bacillus subtilis: from sequence annotation to data management and analysis. FEBS Lett., 430, 28–36. [DOI] [PubMed] [Google Scholar]
- 30.Riley M. (1993) Functions of the gene products of Escherichia coli. Microbiol. Rev., 57, 862–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha E.P., Danchin,A. and Viari,A. (1999) Translation in Bacillus subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res., 27, 3567–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stemke G.W., Huang,Y., Laigret,F. and Bove,J.M. (1994) Cloning the ribosomal RNA operons of Mycoplasma flocculare and comparison with those of Mycoplasma hyopneumoniae. Microbiology, 140, 857–860. [DOI] [PubMed] [Google Scholar]
- 33.Ermolaeva M.D., Khalak,H.G., White,O., Smith,H.O. and Salzberg,S.L. (2000) Prediction of transcription terminators in bacterial genomes. J. Mol. Biol., 301, 27–33. [DOI] [PubMed] [Google Scholar]
- 34.Ye F., Renaudin,J., Bove,J.M. and Laigret,F. (1994) Cloning and sequencing of the replication origin (oriC) of the Spiroplasma citri chromosome and construction of autonomously replicating artificial plasmids. Curr. Microbiol ., 29, 23–29. [DOI] [PubMed] [Google Scholar]
- 35.Fujita M.Q., Yoshikawa,H. and Ogasawara,N. (1992) Structure of the dnaA and DnaA-box region in the Mycoplasma capricolum chromosome: conservation and variations in the course of evolution. Gene, 110, 17–23. [DOI] [PubMed] [Google Scholar]
- 36.Lobry I.R. (1996) Asymmetric distribution patterns in the two DNA strands of bacteria. Mol. Biol. Evol., 13, 660–665. [DOI] [PubMed] [Google Scholar]
- 37.Rocha E.P., Danchin,A. and Viari,A. (1999) Universal replication biases in bacteria. Mol. Microbiol., 32, 11–16. [DOI] [PubMed] [Google Scholar]
- 38.Dandekar T., Huynen,M., Regula,J.T., Ueberle,B., Zimmermann,C.U., Andrade,M.A., Doerks,T., Sanchez-Pulido,L., Snel,B., Suyama,M. et al. (2000) Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames. Nucleic Acids Res., 28, 3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez de Saro F.J., Yoshikawa,N. and Helmann,J.D. (1999) Expression, abundance, and RNA polymerase binding properties of the δ factor of Bacillus subtilis. J. Biol. Chem., 274, 15953–15958. [DOI] [PubMed] [Google Scholar]
- 40.Quentin Y., Fichant,G. and Denizot,F. (1999) Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol., 287, 467–784. [DOI] [PubMed] [Google Scholar]
- 41.Miles R.J. (1992) Catabolism in mollicutes. J. Gen. Microbiol., 138, 1773–1783. [DOI] [PubMed] [Google Scholar]
- 42.Himmelreich R., Plagens,H., Hilbert,H., Reiner,B. and Herrmann,R. (1997) Comparative analysis of the genomes of the bacteria Mycoplasma pneumoniae and Mycoplasma genitalium. Nucleic Acids Res., 24, 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson S.N. and Fraser,C.M. (2001) The complexity of simplicity. Genome Biol., 2, 2002.1–2002.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchison C.A., Peterson,S.N., Gill,S.R., Cline,R.T., White,O., Fraser,C.M., Smith,H.O. and Venter,J.C. (1999) Global transposon mutagenesis and a minimal Mycoplasma genome. Science, 286, 2165–2169. [DOI] [PubMed] [Google Scholar]
- 45.Gaurivaud P., Laigret,F., Garnier,M. and Bové,J.M. (2000) Fructose utilization and pathogenicity of Spiroplasma citri: characterization of the fructose operon. Gene, 252, 61–69. [DOI] [PubMed] [Google Scholar]
- 46.Wendrich T.M. and Marahiel,M.A. (1997) Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol., 26, 65–79. [DOI] [PubMed] [Google Scholar]
- 47.Scoarughi G.L., Cimmino,C. and Donini,P. (1999) Helicobacter pylori: a eubacterium lacking the stringent response. J. Bacteriol ., 181, 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephens R.S., Kalman,S., Lammel,C., Fan,J., Marathe,R., Aravind,L., Mitchell,W., Olinger,L., Tatusov,R.L., Zhao,Q. et al. (1998) Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science, 282, 754–759. [DOI] [PubMed] [Google Scholar]
- 49.Dybvig K., Sitaraman,R. and French,C.T. (1998) A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc. Natl Acad. Sci. USA, 95, 13923–13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlradt P.F., Kiess,M., Meyer,H., Sussmuth,R. and Jung,G. (1997) Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med., 185, 1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piec G., Mirkovitch,J., Palacio,S., Muhlradt,P.F. and Felix,R. (1999) Effect of MALP-2, a lipopeptide from Mycoplasma fermentans, on bone resorption in vitro. Infect. Immun., 67, 6281–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beermann C., Lochnit,G., Geyer,R., Groscurth,P. and Filgueira,L. (2000) The lipid component of lipoproteins from Borrelia burgdorferi: structural analysis, antigenicity, and presentation via human dendritic cells. Biochem. Biophys. Res. Com., 267, 897–905. [DOI] [PubMed] [Google Scholar]
- 53.Jarvill-Taylor K.J. and Minion,F.C. (1995) The effect of thiol-active compounds and sterols on the membrane-associated hemolysin of Mycoplasma pulmonis. FEMS Microbiol. Lett., 128, 213–218. [DOI] [PubMed] [Google Scholar]
- 54.Hyatt D.R., ter Huurne,A.A., van der Zeijst,B.A. and Joens,L.A. (1994) Reduced virulence of Serpulina hyodysenteriae hemolysin-negative mutants in pigs and their potential to protect pigs against challenge with a virulent strain. Infect. Immun., 62, 2244–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wren B.W., Stabler,R.A., Das,S.S., Butcher,P.D., Mangan,J.A., Clarke,J.D., Casali,N., Parish,T. and Stoker,N.G. (1998) Characterization of a haemolysin from Mycobacterium tuberculosis with homology to a virulence factor of Serpulina hyodysenteriae. Microbiology, 144, 1205–1211. [DOI] [PubMed] [Google Scholar]
- 56.Bendjennat M., Blanchard,A., Loutfi,M., Montagnier,L. and Bahraoui,E. (1997) Purification and characterization of Mycoplasma penetrans Ca2+/Mg2+-dependent endonuclease. J. Bacteriol ., 179, 2210–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bendjennat M., Blanchard,A., Loutfi,M., Montagnier,L. and Bahraoui,E. (1999) Role of Mycoplasma penetrans endonuclease P40 as a potential pathogenic determinant. Infect. Immun., 67, 4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brennan P.C. and Feinstein,R.N. (1969) Relationship of hydrogen peroxide production by Mycoplasma pulmonis to virulence for catalase-deficient mice. J. Bacteriol ., 98, 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dybvig K. and Cassell,G.H. (1987) Transposition of Gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science, 235, 1392–1394. [DOI] [PubMed] [Google Scholar]
- 60.Dybvig K., French,C.T. and Voelker,L.L. (2000) Construction and use of derivatives of transposon Tn4001 that function in Mycoplasma pulmonis and Mycoplasma arthritidis. J. Bacteriol ., 182, 4343–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G., Humayun,M.Z. and Taylor,D.E. (1999) Mutation as an origin of genetic variability in Helicobacter pylori. Trends Microbiol., 7, 488–493. [DOI] [PubMed] [Google Scholar]
- 62.Hood D.W., Deadman,M.E., Jennings,M.P., Bisercic,M., Fleischmann,R.D., Venter,J.C. and Moxon,R. (1996) DNA repeats identify novel virulence genes in Haemophilus influenzae. Proc. Natl Acad. Sci. USA, 93, 11121–11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole S., Brosch,R., Parkhill,J., Garnier,T., Churcher,C., Harris,D., Gordon,S., Eiglmeier,K., Gas,S., Barry,C.R. et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature, 393, 537–544. [DOI] [PubMed] [Google Scholar]
- 64.Parkhill J., Achtman,M., James,K.D., Bentley,S.D., Churcher,C., Klee,S.R., Morelli,G., Basham,D., Brown,D., Chillingworth,T. et al. (2000) Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature, 404, 502–506. [DOI] [PubMed] [Google Scholar]
- 65.Saunders N.J., Jeffries,A.C., Peden,J.F., Hood,D.W., Tettelin,H., Rappuolli,R. and Moxon,E.R. (2000) Repeat-associated phase-variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol., 37, 207–215. [DOI] [PubMed] [Google Scholar]