Abstract
Purpose
The objective was to determine velopharyngeal (VP) status of stop consonants and vowels produced by young children with repaired cleft palate (CP) and typically developing (TD) children from 12 to 18 months of age.
Method
Nasal ram pressure (NRP) was monitored in 9 children (5 boys, 4 girls) with repaired CP with or without cleft lip and 9 TD children (5 boys, 4 girls) at 12, 14, and 18 months of age. VP status was categorized as open or closed for oral stops and vowels in three contexts—consonant–vowel syllables, vowel–consonant–vowel syllables, and isolated vowels—on the basis of the presence or absence of positive nasal ram pressure.
Results
At 12 months of age, TD children produced 98% of stops and vowels in syllables with VP closure throughout the entire segment compared with 81% of stops and vowels for children with CP (p < .0001). There were no significant group differences at 14 or 18 months of age.
Conclusions
TD children exhibit consistent VP closure for stop consonants and vowels at 12 months of age. Some children with repaired CP do not achieve consistent closure until 14 months of age, approximately 3 to 4 months following palate repair.
The velopharyngeal (VP) mechanism plays an important role in early speech development by signaling phonetic contrasts between nasal and oral consonants. Despite its important role, there has been relatively little research on the time course of VP closure during infancy and early childhood. Available evidence indicates that the velopharynx is open for cries during birth (Bosma, Truby, & Lind, 1965) and early infancy (Stark & Nathanson, 1974; Wasz-Hockert, Lind, Vuorenkoski, Partanen, & Valanne, 1968). Kent and Murray (1982) suggested that VP closure might be achieved by 4 months of age. On the basis of perceptual and acoustic analyses, they characterized a low-frequency component (i.e., the nasal formant) in the spectrogram of corresponding acoustic signals, which was less prominent in the vocalizations of 6- to 9-month-old infants compared with 2- to 3-month-old infants. Compared with adults, 2- to 3-month-old infants have a broader oral cavity, a more anterior tongue position, a shorter pharynx, and a more superior larynx that leads to approximation of the epiglottis to the velum (Kent & Murray, 1982). Proximity of the epiglottis to the velum causes nasal resonant vocalizations or what Oller (1986) called “quasi-resonant nuclei” (p. 28). During the first 6 months of life, the infant's vocal tract undergoes significant anatomical restructuring toward a more adultlike configuration with lowering of the larynx and epiglottis at about 4 to 6 months of age (Sasaki, Levine, Laitman, & Crelin, 1977). At this time, vocalizations of the infant are less characterized by nasal resonance, and the infant produces vocalizations with “fully resonant nuclei” (Oller, 1986, p. 28).
In contrast to acoustic and perceptual methods to infer VP function, Thom, Hoit, Hixon, and Smith (2006) used nasal ram pressure (NRP) to determine the status of the VP port in infants. This technique detects localized nasal air velocity sensed at the anterior nares through a relatively noninvasive procedure that does not interfere with speech production. Thom et al. reported that the velopharynx was closed or partially closed during cries and screams at 2 to 6 months of age and that laughs were produced with an open velopharynx. Although Thom et al. reported that VP closure increased with age from 2 to 6 months for syllabic-like oral sounds, consistent closure was not yet achieved at 6 months of age.
Information regarding VP function of older children and adults during speech production is less equivocal. Thompson and Hixon (1979) studied 92 typically developing (TD) children aged 3 to 18 years and 20 adults using a nasal mask and pneumotachometer. All speakers produced syllables including /ti/, /di/, and /ni/. Thompson and Hixon reported that nasal airflow was present during production of nasal consonants by all speakers and absent during production of oral consonants for all but one of the speakers. Zajac (2000) used the pressure-flow technique to obtain rates of nasal airflow and estimates of VP port size during speech production of 181 TD children aged 6 to 16 years and 42 adults. Similar to Thomson and Hixon, Zajac reported essentially complete VP closure during production of the syllable /pi/ for all but three speakers.
Although the above studies show that VP closure for oral stop consonants is achieved by at least 3 years of age for children without a cleft, it is not known if consistent VP closure occurs earlier, perhaps at the time of stop emergence. This question is of greater importance for children with repaired cleft palate (CP). In such cases, it is not unusual for the emergence of stop consonants to be delayed well into the second year of life following palate repair (Chapman, 2008; Chapman, Hardin-Jones, & Halter, 2003; Jones, Chapman, & Hardin-Jones, 2003). Assuming that palatal surgery results in a structurally competent VP mechanism, it is not known if children with repaired CP achieve VP closure soon after palatal surgery or if it develops over time. Zajac, van Aalst, Vallino, and Napoli (2011) suggested the latter. They reported that only 70% of 2-year-old children with repaired CP exhibited consistent VP closure during stop production as determined by NRP. That study, however, included some children that subsequently required secondary surgical management for VP inadequacy (Krochmal, Zajac, Alhudaib, Emodi, & van Aalst, 2013). The purpose of this preliminary study was to determine (a) VP status of stop consonants and vowels produced by TD children and children with repaired CP at 12, 14, and 18 months of age and (b) if differences exist between the children at any of the ages.
Method
Participants
As part of a longitudinal, multisite project aimed at investigating VP function of children with repaired CP, 18 children were identified for this preliminary analysis. These children were selected because they had completed study visits up to 18 months of age. Nine children (five boys and four girls) had repaired CP, and nine children were TD without CP (five boys and four girls). Parents of all children signed informed consent as part of institutional review board oversight. Eight of the children (two CP, six TD) were studied at the Craniofacial Center, University of North Carolina at Chapel Hill, six (five CP, one TD) were studied at Nationwide Children's Hospital, Columbus, OH, and four (two CP, two TD) were studied at A. I. duPont Hospital, Wilmington, DE. All were from American English–speaking families. On the basis of information obtained from medical records and parent reports, all participants were in good general health. None of the participants were born prematurely or diagnosed with any known syndromes, including Pierre Robin sequence. Three children with CP also had repaired unilateral cleft lip. Of the nine children with CP, five had clefts of the hard and soft palate, and four had clefts of the soft palate only. All children with CP had undergone a single surgery to repair the palate by at least 11 months of age (range = 7 to 11 months, M = 9.8 months). Oral examinations were conducted at each study visit (e.g., 12, 14, and 18 months of age) to rule out the presence of oronasal fistulae or surgical dehiscence of the palate. All participants also underwent sound-field audiologic assessments at 12 months of age and were found to have normal hearing in the better ear. Last, as reported in the Results section, it should be emphasized that only children determined to have adequate VP function at 18 months of age were selected for the analysis.
NRP Procedures
NRP of all participants was monitored during spontaneous and elicited speech at 12, 14, and 18 months of age using identical equipment and procedures. Data were collected in quiet, child-friendly rooms. Study visits were scheduled at the time of day when the child's behavior was most alert as reported by parents. The child sat in a booster chair at a table. A two-pronged, nasal cannula (AirLife, infant or pediatric size) was inserted into the nares and looped around the ears of the child. The prongs of the cannula did not occlude the nostrils. The end of the cannula was connected to a bidirectional, differential pressure transducer (Setra, model 239, range ±1 in. WC) referenced to atmosphere. This transducer was part of the PERCI-SARS system (Version 3.2, Microtronics, Inc., Chapel Hill, NC) and provided both filtered (80 Hz low-pass) and unfiltered outputs. Following Thom et al. (2006), the pressure transducer was not calibrated because NRP is essentially a measure of localized air velocity, not volume airflow, and the purpose of the study was simply to determine the status of the VP port as open or closed. Audio signals were obtained using an omnidirectional condenser microphone (Shure, model MX150) attached to the clothing of the infant under the chin.
The low-pass filtered NRP signal, the unfiltered NRP signal, and the audio signal were digitized by an A/D converter (Data Translation, model 9804) to a computer. The NRP and audio signals were digitized at 2 kHz and 20 kHz, respectively, using TF32 Lab Automation software (Milenkovic, 2005). The filtered NRP signal was obtained to facilitate interpretation of relative NRP levels of nasal and oral speech segments. Because the filtered NRP signal introduced a slight delay relative to the audio signal, the unfiltered signal was obtained to facilitate interpretation of NRP timing events to speech segments. In addition, a video camera (Sony, HDR CX380) with 8.9-megapixel resolution and a built-in zoom microphone was mounted on a tripod and positioned in front of the participant. Participants were video recorded to confirm placement of the nasal cannula, document any head or body movements that may affect NRP, and determine the intended targets, if known, of the child's productions. At least 15 min of NRP and speech data were recorded during each visit. A standardized set of toys was used to elicit vocalizations during the recordings. The toys targeted each of the oral stop consonants /p b t d k g/ in the initial and final word positions. All targeted words are listed in the Appendix.
Data and Statistical Analyses
All data analysis occurred at the primary study site at the University of North Carolina at Chapel Hill. Stop consonants and vowels in either consonant–vowel (CV) or vowel–consonant–vowel (VCV) syllables and isolated vowels were first identified from the video recordings. Although only stop consonants are reported, nasal consonants were also identified to facilitate NRP interpretation of some stop segments as described later. In VCV syllables, both the initial and final vowels were included in the analysis. Isolated vowels were included only if durations were longer than approximately 100 ms. All speech segments were included only if the nasal cannula was in place, the child was not engaged in excessive head or body movement (i.e., head shaking or body bouncing), and there was not simultaneous talking of the parent and/or investigator that masked the child's speech. In addition, speech segments were excluded if whispered, produced as part of whining and/or crying, or adjacent to a nasal consonant. Relative to the latter, vowels in a nasal vowel syllable and stops adjacent to a nasal consonant were not analyzed.
TF32 Lab Automation software (Milenkovic, 2005) was then used to determine the status of the velopharynx during the identified speech segments on the basis of NRP. The software was configured to simultaneously display the audio signal, the filtered NRP signal, the unfiltered NRP signal, and a spectrogram of the audio signal. Similar to procedures described by Thom et al. (2006), utterances were segmented into breath groups using negative NRP peaks, indicating inspirations. At times, breath groups were difficult to identify on the basis of NRP if the participant maintained an open mouth posture. In those instances, the breath group was confirmed by listening to the audio signal for evidence of audible inspiration.
For all vowels, VP status was coded as (a) closed throughout if NRP was zero (atmospheric) during the entire vowel segment, (b) closed partially if NRP was zero during at least part of the vowel segment, and (c) open throughout if NRP was positive during the entire vowel segment. It should be noted that NRP was rarely exactly zero due to slight moment-to-moment fluctuations and voicing. When voicing occurred, the unfiltered signal tended to oscillate slightly above and below zero with the filtered signal showing slight positive fluctuations at times (see Results). Following Thom et al. (2006), these slight offsets were considered artifacts in the presence of VP closure. As further indicated by Thom et al., brief (100 ms) instances of positive NRP either at the beginning or end of an isolated vowel were considered normal artifacts if these occurred as part of the onset or offset of the breath group. Figures in Results show examples of vowels that were coded as closed throughout and closed partially, respectively.
For oral stop consonants, VP status was coded as (a) closed if NRP was zero throughout the entire segment and (b) open if NRP was positive during any part of the segment. Stops were coded in a strictly binary manner because the relatively short duration of the stop segments precluded reliable coding of partial closure. As for vowels, slight positive fluctuations were considered as artifact. VP status of some stops in CV syllables was difficult to determine if produced at the beginning of a breath group. As indicated previously, onset effects of the breath group were ignored unless NRP extended into the vowel segment. In these cases, the unfiltered NRP signal was used to determine the VP status of the stop—that is, the stop was considered closed if the unfiltered NRP signal was zero (or slight oscillations around zero) prior to the onset of the vowel. Last, in some cases, a small positive NRP peak occurred during a stop segment that could not confidently be considered as artifact. In these cases, the segment was considered open if the relative NRP value from the participant's filtered signal exceeded 15% of the median NRP value obtained for the participant during production of nasal consonants. We admit this was an arbitrary criterion and was used infrequently. Figures in Results also show examples of stops that were coded as closed and open, respectively.
Bar graphs showing the percentages of stops produced with the velopharynx closed were constructed for individual participants and for both groups of children across the three ages. Stacked bar graphs showing the percentages of vowels produced with the velopharynx closed throughout the entire segment and during part of the segment were also constructed. Binomial logistic regression with group as an independent variable (one for TD children, zero for children with CP) was applied using “events/trials” model syntax with which trials were the total number of vowels (or stops) and events were the number of vowels (or stops) that were produced with VP closure. Separate models were fitted for each combination of age (12, 14, or 18 months) and outcome (isolated vowels, vowels in CV and VCV syllables, and stops in CV and VCV syllables) to determine if TD children had greater odds to have VP closure than children with CP. Considering the high proportions of VP closure as well as the small number of participants in the study, estimation was by exact logistic regression (Mehta & Patel, 1995; Preisser & Koch, 1997), implemented using the “exact” statement in PROC Logistic, SAS v. 9.4. Significance levels were set at p = .05.
Reliability of NRP Coding
The first author (M.E.) coded all stop segments and vowels from all participants and visits. Intrarater reliability was assessed by randomly selecting six study visits (approximately 10%) and repeating the coding after a period of approximately 3 months. Four of the study visits were from children with CP, and two were from TD children. Inter-rater reliability was assessed by training an undergraduate student to repeat the coding from the six selected study visits. Reliability was assessed by calculating exact agreements between raters.
Relative to intrarater reliability, the first author achieved 98% agreement coding the two categories of closure for stops and 99% agreement coding the three categories of closure for vowels. Relative to inter-rater reliability, the first author and student achieved 88% agreement coding the two categories of closure for stops and 99% agreement coding the three categories of closure for vowels.
Results
A total number of 1,021 vowel and stop segments (186 isolated vowels, 434 vowels in CV and VCV syllables, and 401 stops in CV and VCV syllables) were coded for children with CP. A total number of 1,349 vowel and stop segments (150 isolated vowels, 637 vowels in CV and VCV syllables, and 562 stops in CV and VCV syllables) were coded for TD children. For children with CP, approximately 95% of stops were voiced with /b/, /d/, and /g/ accounting for approximately 37%, 43%, and 15% of stops, respectively. For TD children, approximately 88% of stops were voiced with /b/, /d/, and /g/ accounting for approximately 45%, 39%, and 4% of stops, respectively. Both groups of children produced vowels that mostly consisted of the central vowels (stressed /ʌ/ and unstressed /ə/), low back vowels /ɑ/, and midfront vowels /e/. These vowel types are consistent with previous findings reported for young children (see Kent & Miolo, 1995, for a review). Table 1 lists the total numbers of vowels and stops, respectively, for TD children and children with CP across the three ages and the number with VP closure.
Table 1.
Number of vowels and stop consonants produced with velopharyngeal (VP) closure (first cell entry) by typically developing (TD) children and children with cleft palate (CP) at 12, 14, and 18 months of age.
| Group | Participant | Isolated vowels |
Vowels in CV and VCV syllables |
Stops in CV and VCV syllables |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) |
Age (months) |
Age (months) |
||||||||
| 12 | 14 | 18 | 12 | 14 | 18 | 12 | 14 | 18 | ||
| TD | 1 | 0/0 | 1/1 | 1/1 | 0/0 | 28/30 | 17/19 | 0/0 | 26/29 | 18/19 |
| 2 | 3/3 | 39/39 | 0/0 | 2/2 | 7/7 | 0/0 | 2/2 | 4/4 | 0/0 | |
| 3 | 1/1 | 0/1 | 0/0 | 3/3 | 9/9 | 28/28 | 2/2 | 8/8 | 20/20 | |
| 4 | 1/1 | 4/4 | 0/0 | 53/53 | 10/10 | 0/0 | 47/47 | 9/9 | 0/0 | |
| 5 | 0/0 | 1/1 | 7/7 | 7/8 | 8/8 | 16/17 | 5/5 | 8/8 | 16/16 | |
| 6 | 0/0 | 0/0 | 2/2 | 20/20 | 14/14 | 121/121 | 19/19 | 11/11 | 120/120 | |
| 7 | 6/6 | 4/4 | 8/8 | 38/39 | 37/37 | 14/14 | 35/37 | 32/32 | 14/14 | |
| 8 | 3/4 | 7/7 | 19/21 | 7/7 | 14/16 | 106/115 | 6/6 | 13/15 | 80/82 | |
| 9 | 0/0 | 19/21 | 14/18 | 0/0 | 49/49 | 11/11 | 0/0 | 46/46 | 11/11 | |
| All (%) | 14/15 (93) | 75/78 (96) | 51/57 (89) | 130/132 (98) | 176/180 (98) | 313/325 (96) | 116/118 (98) | 157/162 (97) | 279/282 (99) | |
| CP | 10 | 33/39 | 10/10 | 7/14 | 0/0 | 17/17 | 14/14 | 0/0 | 12/12 | 12/14 |
| 11 | 0/0 | 10/10 | 23/24 | 0/0 | 17/17 | 65/65 | 0/0 | 15/15 | 65/65 | |
| 12 | 2/4 | 0/1 | 4/5 | 15/21 | 3/3 | 68/76 | 14/18 | 3/3 | 62/67 | |
| 13 | 3/5 | 1/3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 14 | 0/0 | 1/1 | 8/8 | 1/3 | 6/6 | 13/13 | 0/2 | 6/6 | 10/10 | |
| 15 | 3/3 | 0/0 | 0/0 | 8/8 | 22/22 | 0/0 | 8/8 | 20/21 | 0/0 | |
| 16 | 13/21 | 11/12 | 0/0 | 14/20 | 2/2 | 10/10 | 12/18 | 2/2 | 10/10 | |
| 17 | 10/10 | 1/1 | 6/6 | 27/28 | 4/4 | 97/97 | 21/22 | 4/4 | 97/97 | |
| 18 | 0/0 | 6/6 | 3/3 | 0/0 | 7/7 | 1/1 | 0/0 | 6/6 | 1/1 | |
| All (%) | 64/82 (78) | 40/44 (91) | 51/60 (85) | 65/80 (81) | 78/78 (100) | 268/276 (97) | 55/68 (81) | 68/69 (99) | 257/264 (97) | |
Note. The second cell entry indicates total productions. Cells with zero indicate that participant did not tolerate the nasal cannula or did not produce any vowels or stops. Bold numbers indicate VP closure less than 100%. CV = consonant–vowel; VCV = vowel–consonant–vowel.
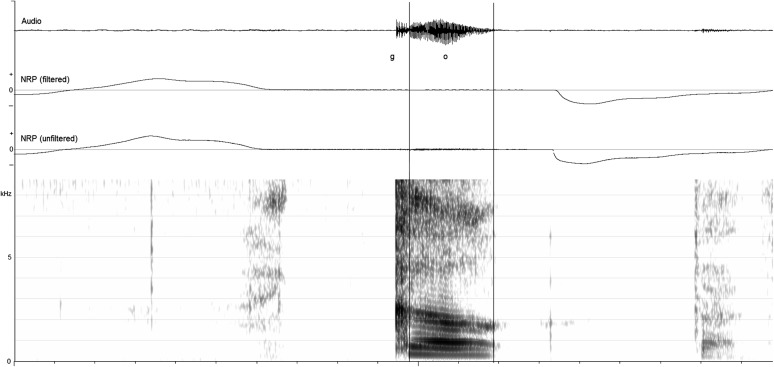
Figure 1 shows an example of a TD participant (#3) saying /go/ while playing with a car at 18 months of age that was coded as VP closed for both the stop and vowel. The figure shows the audio, filtered NRP, unfiltered NRP, and spectrogram of the audio signal. As seen in the unfiltered NRP signal, there was voicing artifact accompanying the vowel. As seen in the filtered NRP signal, there were slight positive fluctuations that were ignored.
Figure 1.
Example of audio, filtered nasal ram pressure (NRP), unfiltered NRP, and spectrogram of /go/ produced by a typically developing child without cleft palate at 18 months of age. Slight fluctuations on the filtered NRP signal were considered as zero, and both the stop and vowel were coded as velopharynx closed.
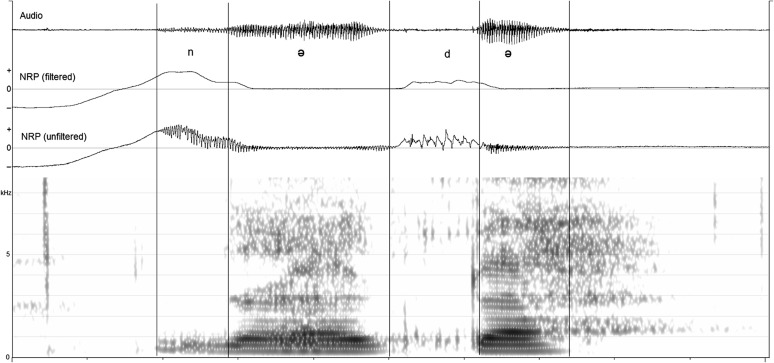
In contrast, Figure 2 shows an example of a participant with CP (#17) saying /nədə/ in response to seeing a picture of her dad at 12 months of age. The /d/ segment was coded as VP open, and the following vowel was coded as partially closed. Several observations regarding the figure should be noted. First, the vowel following the /n/ segment was not coded as it was adjacent to a nasal consonant and shows evidence of carryover nasal articulation (i.e., the NRP signal extends into the vowel). Second, there is spectral evidence of likely VP tissue flutter occurring in the stop gap of the /d/ segment. This is evidenced by the quasiperiodic striations that appear intermittently in the spectrogram, especially below 1 kHz and above 6 kHz. Zajac and Preisser (2016) reported that this type of spectral pattern often occurred during stop gaps of older children with repaired CP who exhibited obligatory (or passive) nasal turbulence. They attributed the quasiperiodic pattern to tissue vibration in the presence of incomplete VP closure, perhaps in conjunction with mucous displacement. The unfiltered NRP signal also shows a quasiperiodic pattern that roughly aligns with the spectral pattern. Related to perception, the last author (D.J.Z.) noted mild audible nasal turbulence during audio playback of this segment. Third, the second vowel segment, similar to the first, shows evidence of carryover nasal coarticulation. Of interest, although the first vowel is substantially longer in duration, the temporal extent of the carryover NRP is quite similar for both vowels, suggesting a fixed mechano-inertial effect of the velum (Ali, Hoffman, Kramer, & Daniloff, 1983).
Figure 2.
Example of audio, filtered nasal ram pressure (NRP), unfiltered NRP, and spectrogram of /nədə/ produced by a child with repaired cleft palate at 12 months of age. The oral stop was coded as velopharynx open, and the vowel was coded as velopharynx partially closed.
Stops
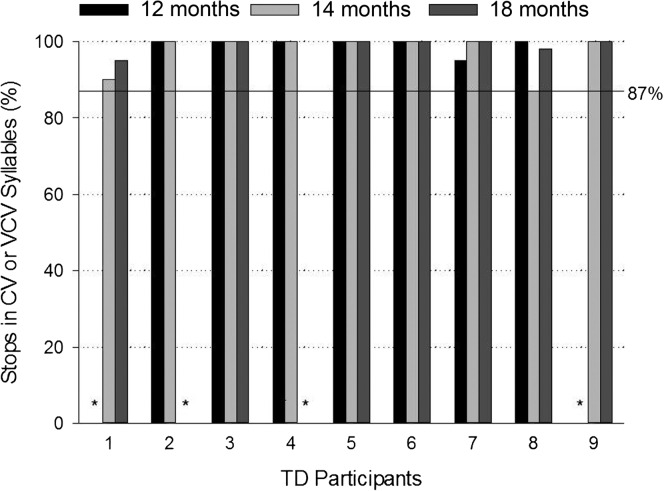
Figure 3 shows the percentages of oral stops in CV and VCV syllables produced with the velopharynx closed for individual TD children across the three ages. As seen in the figure, seven TD children (Participants 2 through 8) tolerated the nasal cannula and produced stops at 12 months of age. Of the seven children, six (Participants 2, 3, 4, 5, 6, and 8) achieved closure on all stops (100%) and one (Participant 7) achieved closure on 95% of stops. The lowest percentage of stops produced with VP closure at any age was 87% by Participant 8 at 14 months of age. The solid horizontal reference line in Figure 3 indicates this level. Missing data in Figure 3 (indicated by asterisks) occurred due to a child either not tolerating the cannula or not producing any oral stops.
Figure 3.
Percentage of oral stops produced in consonant–vowel (CV) or vowel–consonant–vowel (VCV) syllables with the velopharynx closed by individual typically developing (TD) children at 12, 14, and 18 months of age. Asterisks indicate no oral stops were produced at the visit. Horizontal solid line references 87% closure.
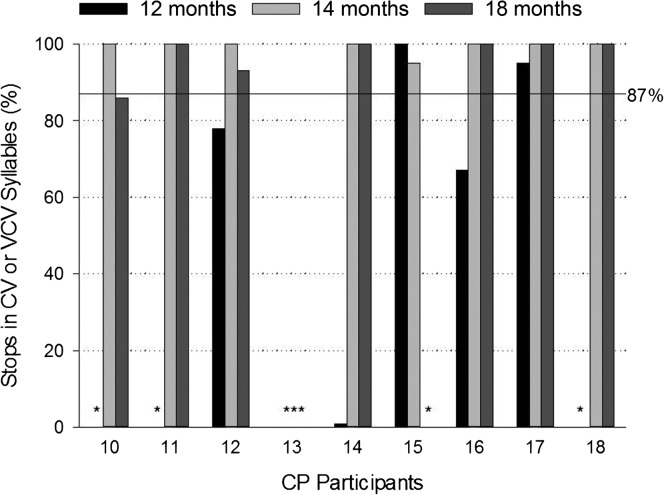
Figure 4 shows the percentages of oral stops in CV and VCV syllables produced with the velopharynx closed for individual children with repaired CP across the three ages. As seen in the figure, five children (Participants 12, 14, 15, 16, and 17) tolerated the cannula and produced stops at 12 months of age. Only two of these children (Participant 15 at 100% and Participant 17 at 95%) achieved closure levels of 87% or greater that were typical of TD children. The solid horizontal reference line in Figure 4 indicates this level. Three of the children (Participants 12, 14, and 16), however, achieved closure levels greater than 87% at 14 and 18 months of age.
Figure 4.
Percentage of oral stops produced in consonant–vowel (CV) or vowel–consonant–vowel (VCV) syllables with the velopharynx closed by individual children with cleft palate (CP) at 12, 14, and 18 months of age. Asterisks indicate no oral stops were produced at the visit. Horizontal solid line references 87% closure.
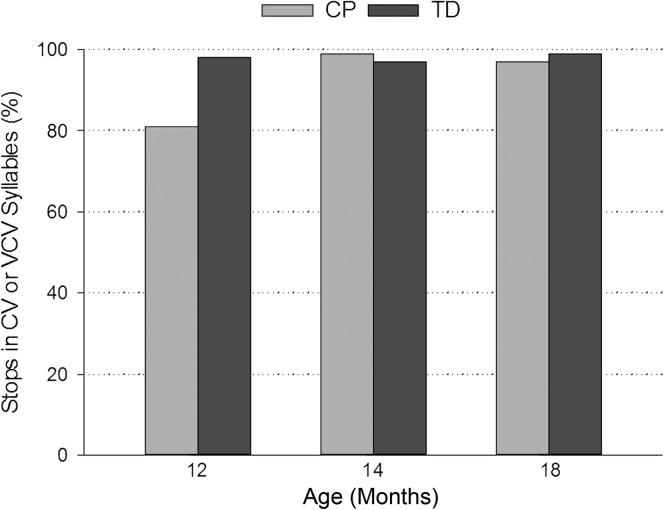
Figure 5 summarizes the stop data for the children who tolerated the nasal cannula and produced oral stops. Overall, of the seven TD children who produced stops at 12 months of age, 98% of stops were produced with VP closure. In contrast, of the five children with CP who produced stops at 12 months of age, only 81% of stops were produced with VP closure. TD children had approximately 13.5 times (95% CI [2.91, 128.00]) greater odds to have VP closure for stops in CV and VCV syllables at 12 months of age than children with CP (see Table 2, p < .0001). Both groups produced greater than 95% of stops with VP closure at 14 and 18 months of age.
Figure 5.
Percentage of oral stops produced in consonant–vowel (CV) or vowel–consonant–vowel (VCV) syllables with the velopharynx closed by children with cleft palate (CP) and typically developing (TD) children at 12, 14, and 18 months of age.
Table 2.
Results from exact logistic regression analysis comparing typically developing children to children with repaired cleft palate with respect to the odds that a vowel or stop has velopharyngeal closure.
| Analysis | 12 months | 14 months | 18 months |
|---|---|---|---|
| Isolated vowels | |||
| p value a | .227 | .343 | .499 |
| odds ratio | 3.90 | 2.48 | 1.50 |
| 95% CI | [0.52, 176.00] | [0.40, 17.80] | [0.44, 5.50] |
| Vowels in CV and VCV syllables | |||
| p value a | < .0001 | .201 | .574 |
| odds ratio | 14.82 | 0.43 b | 0.78 |
| 95% CI | [3.29, 137.00] | [0.00, 2.57] | [0.27, 2.11] |
| Stops in CV and VCV syllables | |||
| p value a | < .0001 | .519 | .159 |
| odds ratio | 13.52 | 0.46 | 2.53 |
| 95% CI | [2.91, 128.00] | [0.01, 4.25] | [0.57, 15.30] |
Note. CV = consonant–vowel; VCV = vowel–consonant–vowel.
On the basis of exact score test (with mid-p adjustment).
Median unbiased estimate.
Vowels
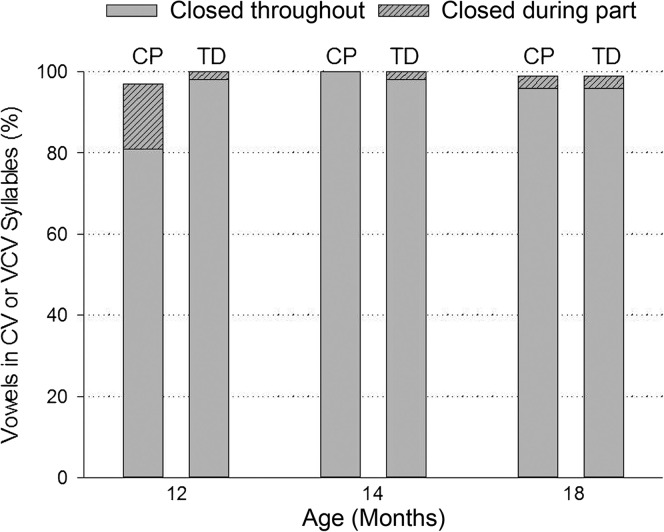
Figure 6 shows the mean percentage of vowels in CV or VCV syllables produced with the velopharynx closed throughout or during part of the vowel by both groups of children across the three ages. Similar to oral stop consonants, Figure 6 shows that seven TD children (same children as in Figure 5) produced 98% of vowels with closure throughout the entire vowel at 12 months of age compared with five children with CP (same children as in Figure 5) who produced only 81% of vowels with closure throughout the entire vowel. TD children had approximately 14.8 times (95% CI [3.29, 137.00]) greater odds to have VP closure for vowels in CV and VCV syllables at 12 months of age than children with CP (see Table 2, p < .0001). Both groups of children produced greater than 96% of vowels with closure throughout the entire vowel at 14 and 18 months of age. As further seen in Figure 6, children with CP exhibited high rates of VP closure at 12 months of age (> 95% of vowels) when only part of the vowel was considered.
Figure 6.
Percentage of vowels in consonant–vowel (CV) or vowel–consonant–vowel (VCV) syllables produced with the velopharynx closed throughout or during part of the vowel by children with cleft palate (CP) and typically developing (TD) children at 12, 14, and 18 months of age.
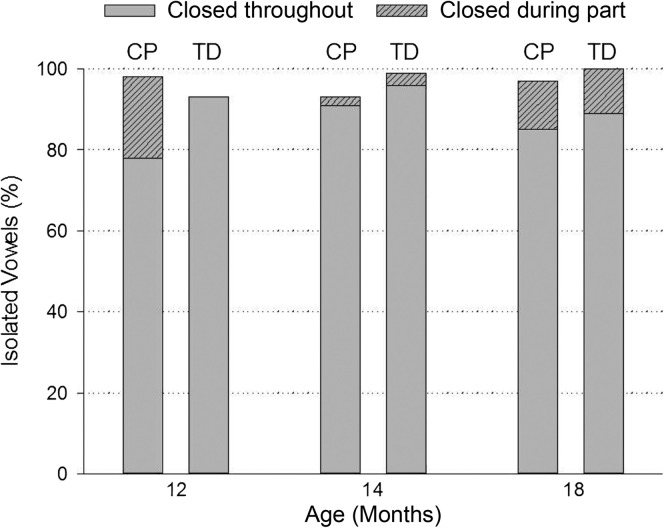
Figure 7 shows the mean percentage of isolated vowels produced with the velopharynx closed throughout or during part of the vowel by both groups of children across the three ages. At 12 months of age, five TD children produced 93% of isolated vowels with closure throughout the entire vowel. In comparison, six children with CP produced 79% of isolated vowels with closure throughout the entire vowel. In other words, TD children had approximately 3.9 times (95% CI [0.52, 176.00]) greater odds to have VP closure for isolated vowels at 12 months of age than children with CP. These results, however, were not statistically significant (see Table 2, p = .227). Both groups of children produced greater than 90% of isolated vowels with closure throughout the entire vowel at 14 months of age. At 18 months of age, both groups continued to produce similar percentages of isolated vowels with closure throughout the entire vowel although the overall percentages (85% for CP, 89% for TD) were slightly reduced compared with 14 months of age (91% for CP, 96% for TD). Last, Figure 7 also shows that children with CP exhibited high rates of VP closure at 12 months of age when only part of the vowel was considered.
Figure 7.
Percentage of isolated vowels produced with the velopharynx closed throughout or during part of the vowel by children with cleft palate (CP) and typically developing (TD) children at 12, 14, and 18 months of age.
Discussion
The purpose of the present preliminary analysis was to determine VP status of stop consonants and vowels produced by TD children and children with repaired CP at 12, 14, and 18 months of age and to determine if differences exist between the children at any of the ages.
TD Children
TD children in the present study produced oral stop consonants and vowels at 12 months of age with high consistency of VP closure. Oral stops were produced with especially high rates of VP closure that ranged from 95% to 100% across the children. The lowest percentage of stops produced with closure at any age was 87%, occurring at 14 months of age. At 12 months of age, isolated vowels and vowels in CV and VCV syllables were also produced with high percentages of VP closure at 93% and 98%, respectively. Isolated vowels may have been produced with less consistent VP closure due to the relative lack of oral air pressure—that is, velar elevation and closure may be facilitated by relatively high oral air pressure associated with oral stops, and, perhaps, vowels adjacent to oral stops. In addition, it has been reported that vowels, especially low vowels, are produced with reduced velar height (Bell-Berti & Krakow, 1990; Moll, 1962) and VP closure force (Kuehn & Moon, 1998). Regardless, the present findings suggest that young children possess the motor control to consistently achieve VP closure for production of stop consonants and vowels by at least 12 months of age if not sooner.
The relatively rare occurrence of a VP opening during production of oral stop consonants by some TD children may be explained by normal production variability and, perhaps, anatomy. Variability in various aspects of speech production is a hallmark of young children. Children tend to produce acoustic speech segments with greater durational variability compared with adults (Kent & Forner, 1980; Redford & Oh, 2015; Smith, 1992). Children also tend to exhibit greater variability of lip and jaw movements than adults (Green, Moore, Higashikawa, & Steeve, 2000; Sharkey & Folkins, 1985). Although little is known regarding variability of velar movements in young children, it is likely that inherent movement variability may have contributed to the present findings.
Relative to anatomy, young children typically achieve velar–adenoidal rather than velar–pharyngeal contact to achieve closure (Peterson-Falzone, Hardin-Jones, & Karnell, 2001; Zajac & Vallino, 2016). Given that adenoid tissue may be irregular in shape and size in young children, it is possible that closure may not occur at times due to inconsistent contact between soft tissues (e.g., see Figure 8-6, Zajac & Vallino, 2016, p. 211). Therefore, even in the presence of normal velar movement, complete closure may not occur at times due to structural irregularity of adenoids.
Last, it should be noted that all stop consonants identified for NRP coding were in either CV or VCV syllables. Stops that terminated a syllable (i.e., the final stop in a CVC syllable) or that occurred adjacent to a nasal consonant (i.e., “man go”) were not included for analysis. This was done to eliminate potential effects of velar lowering associated with utterance offset and coarticulation, respectively.
Children With CP
Some children with repaired CP did not achieve consistent VP closure for stop consonants and vowels in CV and VCV syllables until 14 months of age. Of the three children with CP who showed this pattern (Participants 12, 14, and 16), one had palatal surgery at 10 months of age, and two had palatal surgery at 11 months of age. These children, therefore, did not achieve consistent VP closure until 3 to 4 months following palate repair. All three of these children continued to show consistent VP closure at 18 months of age, indicating that they had structurally adequate VP mechanisms.
Although the reason for a gradual increase of VP closure for some children with repaired CP is not entirely clear, it may be due to several mutually nonexclusive factors. First, all three children were producing oral stops with inconsistent closure prior to 14 months of age. This suggests that some type of feedback and learning may have occurred, perhaps involving intraoral air pressure and/or acoustic cues associated with the stop burst. Stoel-Gammon (1998) highlighted the importance of practice and feedback that infants need to establish links “between one's own oral-motor movements and the acoustic signal that results” (p. 97). Such a link between velar movement and the acoustic signal may not have been firmly established for some of the children with CP at 12 months of age given their fairly recent palatal surgery. Second, it is also possible that some children experienced a growth spurt in VP structures that facilitated closure. Both the hard and soft palates show relatively accelerated growth from birth to 18 months of age (Vorperian et al., 2005). As indicated previously, young children typically achieve velar–adenoidal closure. There is also rapid growth of adenoids during the first year of life and early childhood (Handelman & Osborne, 1976; Jaw, Sheu, Liu, & Lin, 1999). Increased consistency of VP closure for some children with repaired CP, therefore, may have been due to a growth spurt of either the palate and/or adenoids. Last, there may have been differences in the healing rate of the soft palate for some of the children. It is possible that it took longer for sutures to dissolve and soft tissue to become more flexible for the children who showed a delay in VP closure.
Clinical Implications
NRP is a relatively easy diagnostic technique that is generally well tolerated by young children. As part of the larger study, we have found that approximately 70% of young children between 12 and 18 months of age will tolerate wearing the nasal cannula while speaking. The nasal cannula does not occlude the nostrils, so there is minimal effect on typical speech production. Although all infants cannot tolerate the technique, there are limited alternatives to obtaining physiologic data on VP function in young children.
On the basis of the present findings, if a child with repaired CP does not exhibit consistent VP closure—perhaps on 85% of oral stop consonants—by 14 months of age, this may serve as a red flag for VP insufficiency. Krochmal et al. (2013), for example, reported on five children with repaired cleft palate who exhibited VP closure on less than 85% of stops at 2 years of age. Four of the children were noted to have questionable VP function on the basis of clinical speech assessments, and two of the children received secondary palatal surgery within 2 years. Krochmal et al. further noted that NRP agreement with clinical assessments increased to 100% when the criterion of stops with VP closure was reduced to 75%.
Limitations of the Study
Several limitations of the study need to be noted. First, the sample size for both TD children and children with repaired CP was relatively small. Additional research with greater numbers of children is needed to confirm the present findings and refine potential NRP cutoffs relative to clinical assessments. Second, perceptual assessments of the children by blinded listeners were not done. Given that the NRP technique does not provide estimates of VP port area and may be sensitive to relatively small gap sizes, the perceptual significance of oral stops being coded as VP open is not known. Despite these limitations, NRP monitoring has potential value as an early marker of VP insufficiency for children with repaired cleft palate.
Acknowledgments
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Grant R01DE022566. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Reuben Adatorwovor, Jacqueline Dorry, Katie Garcia, Katlyn Latimer, Kathleen McGrath, Daniela Vivaldi, and Rachel Ungaro for assistance with various aspects of data collection and analysis.
Appendix
A standardized set of toys was used to elicit the following words containing oral stops in word initial and final position.
| Initial | Final |
|---|---|
| puppy | pop |
| pop | up |
| baby | bib |
| tub | tub |
| tummy | goat |
| duck | cat |
| Dora | bed |
| dog | head |
| car | duck |
| cow | book |
| goat | dog |
| go | pig |
Note. Only stops in word initial position were analyzed for the present study. Italics indicate targeted stop.
Funding Statement
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Grant R01DE022566.
References
- Ali L., Hoffman P. R., Kramer M. B., & Daniloff R. G. (1983). Aerodynamic measures of coarticulation in /VNC/ sound sequences. Perceptual and Motor Skills, 56, 479–483. [DOI] [PubMed] [Google Scholar]
- Bell-Berti F., & Krakow R. A. (1990). Anticipatory velar lowering: A co-production account. In Haskins Laboratories Status Report on Speech Research: SR-103/104 (pp. 21–38). New Haven, CT: Haskins Laboratories. [Google Scholar]
- Bosma J. F., Truby H. M., & Lind J. (1965). Cry motions of the newborn infant [monograph]. Acta Paediatrica Scandinavica, 163, 63–91. [Google Scholar]
- Chapman K. L. (2008). Speech and language of children with cleft palate. In Moller K. T. & Glaze L. E. (Eds.), Cleft lip and palate: Interdisciplinary issues and treatment (2nd ed., pp. 243–292). Austin, TX: Pro-Ed. [Google Scholar]
- Chapman K. L., Hardin-Jones M., & Halter K. A. (2003). The relationship between early speech and later speech and language performance for children with cleft lip and palate. Clinical Linguistics & Phonetics, 17, 173–197. [DOI] [PubMed] [Google Scholar]
- Green J. R., Moore C. A., Higashikawa M., & Steeve R. W. (2000). The physiologic development of speech motor control: Lip and jaw coordination. Journal of Speech, Language, and Hearing Research, 43, 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelman C. S., & Osborne G. S. (1976). Growth of the nasopharynx and adenoid development from one to eighteen years. Angle Orthodontist, 46, 243–259. [DOI] [PubMed] [Google Scholar]
- Jaw T. S., Sheu R. S., Liu G. C., & Lin W. C. (1999). Development of adenoids: A study by measurement with MR images. Kaohsiung Journal of Medical Sciences, 15, 12–18. [PubMed] [Google Scholar]
- Jones C. E., Chapman K. L., & Hardin-Jones M. A. (2003). Speech development of children with cleft palate before and after palatal surgery. The Cleft Palate—Craniofacial Journal, 40, 19–31. [DOI] [PubMed] [Google Scholar]
- Kent R. D., & Forner L. L. (1980). Speech segment duration in sentence recitations by children and adults. Journal of Phonetics, 8, 157–168. [Google Scholar]
- Kent R. D., & Miolo G. (1995). Phonetic abilities in the first year of life. In Fletcher P. & MacWhinney B. (Eds.), The handbook of child language (pp. 303–334). Oxford, United Kingdom: Blackwell. [Google Scholar]
- Kent R. D., & Murray A. D. (1982). Acoustic features of infant vocalic utterances at 3, 6, and 9 months. The Journal of the Acoustical Society of America, 72, 353–365. [DOI] [PubMed] [Google Scholar]
- Krochmal D. J., Zajac D. J., Alhudaib O. M., Emodi O., & van Aalst J. A. (2013). The assessment of velopharyngeal function using nasal ram pressure testing following palatoplasty. The Cleft Palate—Craniofacial Journal, 50, 542–547. [DOI] [PubMed] [Google Scholar]
- Kuehn D. P., & Moon J. B. (1998). Velopharyngeal closure force and levator veli palatini activation levels in varying phonetic contexts. Journal of Speech, Language, and Hearing Research, 41, 51–62. [DOI] [PubMed] [Google Scholar]
- Mehta D. R., & Patel N. R. (1995). Exact logistic regression, theory and examples. Statistics in Medicine, 14, 2143–2160. [DOI] [PubMed] [Google Scholar]
- Milenkovic P. H. (2005). TF32 Lab Automation [software]. Madison, WI. [Google Scholar]
- Moll K. L. (1962). Velopharyngeal closure on vowels. Journal of Speech and Hearing Research, 17, 30–77. [DOI] [PubMed] [Google Scholar]
- Oller D. K. (1986). Metaphonology and infant vocalizations. In Lindblom B. & Zetterstrom R. (Eds.), Precursors of early speech (pp. 21–35). New York, NY: Stockton Press. [Google Scholar]
- Peterson-Falzone S. J., Hardin-Jones M. A., & Karnell M. P. (2001). Cleft palate speech (3rd ed.). Saint Louis, MO: Mosby. [Google Scholar]
- Preisser J. S., & Koch G. G. (1997). Categorical data analysis in public health, Annual Review of Public Health, 18, 51–82. [DOI] [PubMed] [Google Scholar]
- Redford M. A., & Oh G. (2015). Fixed temporal patterns in children's speech despite variable vowel durations. In The Scottish Consortium for ICPhS 2015 (Ed.), Proceedings of the 18th International Congress of Phonetic Sciences. Glasgow, United Kingdom: The University of Glasgow. [PMC free article] [PubMed] [Google Scholar]
- Sasaki C. T., Levine P. A., Laitman J. T., & Crelin E. S. (1977). Postnatal descent of the epiglottis in man: A preliminary report. Archives of Otolaryngology—Head & Neck Surgery, 103, 169–171. [DOI] [PubMed] [Google Scholar]
- Sharkey S. G., & Folkins J. W. (1985). Variability of lip and jaw movements in children and adults: Implications for the development of speech motor control. Journal of Speech and Hearing Research, 28, 8–15. [DOI] [PubMed] [Google Scholar]
- Smith B. L. (1992). Relationships between duration and temporal variability in children's speech. The Journal of the Acoustical Society of America, 91, 2165–2174. [DOI] [PubMed] [Google Scholar]
- Stark R. E., & Nathanson S. N. (1974). Spontaneous cry in the newborn infant: Sounds and facial gestures. In: Bosma J. F. (Ed.), Fourth symposium on oral sensation and perception: Development in the fetus and infant (pp. 323–352). Bethesda, MD: U.S. Government Printing Office. [PubMed] [Google Scholar]
- Stoel-Gammon C. (1998). Role of babbling and phonology in early linguistic development. In Wetherby A. M., Warren S. F., & Reichle J. (Eds.), Transitions in prelinguistic communication (pp. 87–110). Baltimore, MD: Brookes. [Google Scholar]
- Thom S. A., Hoit J. D., Hixon T. J., & Smith A. E. (2006). Velopharyngeal function during vocalization in infants. The Cleft Palate–Craniofacial Journal, 43, 539–546. [DOI] [PubMed] [Google Scholar]
- Thompson A. E., & Hixon T. J. (1979). Nasal air flow during normal speech production. The Cleft Palate Journal, 16, 412–420. [PubMed] [Google Scholar]
- Vorperian H. K., Kent R. D., Lindstrom M. J., Kalina C. M., Gentry L. R., & Yandell B. S. (2005). Development of vocal tract length during early childhood: A magnetic resonance imaging study. The Journal of the Acoustical Society of America, 117, 338–350. [DOI] [PubMed] [Google Scholar]
- Wasz-Hockert O., Lind J., Vuorenkoski V., Partanen T., & Valanne E. (1968). The infant cry: A spectrographic and auditory analysis. London, United Kingdom: Spastics International Medical Publications. [Google Scholar]
- Zajac D. J. (2000). Pressure-flow characteristics of /m/ and /p/ production in speakers without cleft palate: Developmental findings. The Cleft Palate—Craniofacial Journal, 37, 468–477. [DOI] [PubMed] [Google Scholar]
- Zajac D. J., & Preisser J. (2016). Age and phonetic influences on velar flutter as a component of nasal turbulence in children with repaired cleft palate. The Cleft Palate—Craniofacial Journal, 53, 649–656. [DOI] [PubMed] [Google Scholar]
- Zajac D. J., & Vallino L. D. (2016). Evaluation and management of cleft lip and palate: A developmental perspective. San Diego, CA: Plural. [Google Scholar]
- Zajac D. J., van Aalst J. A., Vallino L. D., & Napoli J. A. (2011, April). Nasal ram pressure as an indicator of velopharyngeal closure during stop consonants in 2-year-olds following palate repair. Paper presented at the ACPA Annual Meeting, San Juan, Puerto Rico. [Google Scholar]