Abstract
Purpose
The purpose of this article is to examine the ability of an acoustic measure, relative fundamental frequency (RFF), to distinguish between two subtypes of vocal hyperfunction (VH): phonotraumatic (PVH) and non-phonotraumatic (NPVH).
Method
RFF values were compared among control individuals with typical voices (N = 49), individuals with PVH (N = 54), and individuals with NPVH (N = 35).
Results
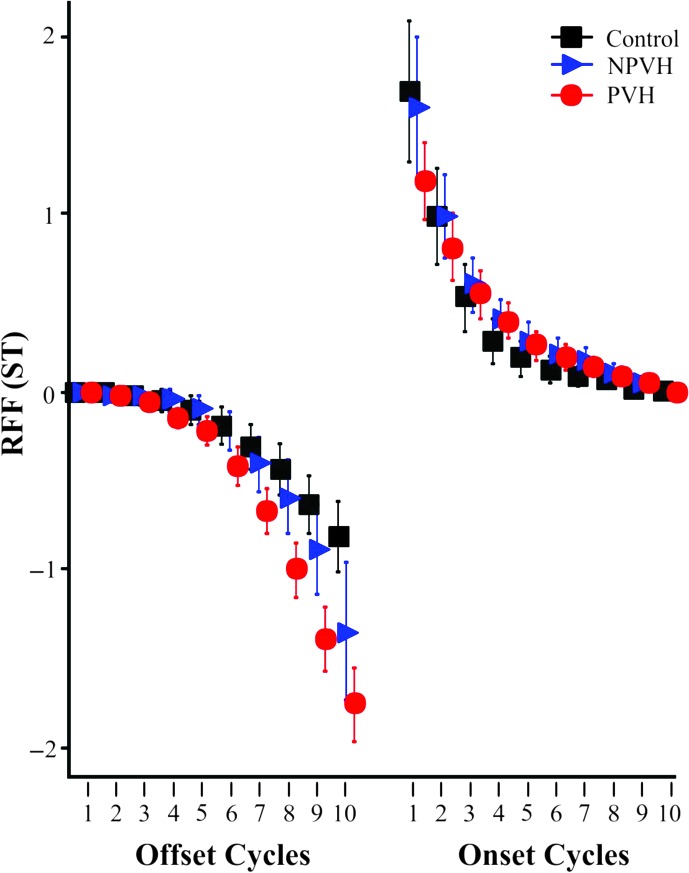
Offset Cycle 10 RFF differed significantly among all 3 groups with values progressively decreasing for controls, individuals with NPVH, and individuals with PVH. Individuals with PVH also had lower Offset Cycles 8 and 9 relative to the other 2 groups and lower RFF values for Offset Cycle 7 relative to controls. There was also a trend for lower Onset Cycle 1 RFF values for the PVH group compared with the NPVH group.
Conclusions
RFF values were significantly different between controls and individuals with VH and also between the two subtypes of VH. This study adds further support to the notion that the differences between these two subsets of VH may be functional as well as structural.
The clinical diagnosis of vocal hyperfunction (VH) is typically associated with the formation of vocal fold lesions due to chronic tissue trauma (e.g., vocal fold nodules) as well as chronic dysphonia and vocal fatigue in the absence of vocal fold tissue trauma or other conditions that could affect phonation, often referred to as primary muscle tension dysphonia (MTD; Roy & Bless, 2000; Van Houtte, Van Lierde, & Claeys, 2011). Hillman, Holmberg, Perkell, Walsh, and Vaughan (1989) proposed that these two manifestations of VH reflect different underlying pathophysiological mechanisms, which they originally referred to as adducted VH and nonadducted VH, and more recently suggested the terms phonotraumatic VH (PVH) and non-phonotraumatic VH (NPVH) be used to describe these two conditions (Mehta et al., 2015). In their view, both types of VH involve increased, and potentially less well-regulated, laryngeal muscle tension (Hillman et al., 1989), which results in heightened longitudinal (anterior–posterior) vocal fold tension and increased aerodynamic forces required to produce phonation. Individuals with PVH are additionally thought to demonstrate heightened transverse (medial–lateral) vocal fold tension, stemming from tight approximation of the vocal folds during phonation, which, in combination with increased aerodynamic parameters (e.g., transglottal pressure, peak-to-peak flow, and maximum flow declination rate), leads to higher vocal fold collision forces and tissue trauma. The increased aerodynamic parameters have been shown to be maintained following surgical intervention, suggesting that their elevation was not present solely due to the presence of vocal fold lesions (Hillman et al., 1989). In NPVH, an apparent imbalance between adductory and abductory forces precludes tight approximation of the vocal folds, thus actually reducing the potential for trauma to vocal fold tissue (reflected in a lack of increase in peak-to-peak flow and maximum flow declination rate) even though heightened aerodynamic forces (e.g., transglottal pressure and average glottal air flow rates) are necessarily used to produce phonation (Hillman et al., 1989).
Standard clinical voice assessment can usually differentiate between voice disorders that are associated with phonotrauma versus those presenting solely as MTD, primarily on the basis of using laryngeal videostroboscopy to ascertain if there are signs of tissue trauma on the medial surfaces of the vocal folds. However, beyond simply identifying which of these two conditions are present, there is currently a lack of capability to objectively characterize and differentiate the pathophysiological mechanisms that potentially underlie these two manifestations of VH.
Common evaluation techniques for VH are visual inspection via laryngoscopy; auditory-perceptual assessment, such as the Consensus Auditory-Perceptual Evaluation of Voice (Kempster, Gerratt, Verdolini Abbott, Barkmeier-Kraemer, & Hillman, 2009); and manual palpation of the larynx. However, these techniques are subjective and prone to difficulties with reliability. For example, supraglottal compression is often attributed to individuals with VH; however, it is also present in individuals with healthy voices (Behrman, Dahl, Abramson, & Schutte, 2003; Pemberton et al., 1993; Sama, Carding, Price, Kelly, & Wilson, 2001; Stager et al., 2001; Stager, Bielamowicz, Regnell, Gupta, & Barkmeier, 2000; Stager, Neubert, Miller, Regnell, & Bielamowicz, 2003). Furthermore, even expert clinicians show weak interrater agreement for visual estimates of tension (Milstein, 1999; Stepp, Heaton, Jetté, Burns, & Hillman, 2010). Auditory-perceptual measures are often considered the “gold standard” for judgments of vocal quality (Kreiman, Gerratt, Kempster, Erman, & Berke, 1993); however, judgments of vocal quality have variable inter- and intra-rater reliability (Eadie et al., 2010; Schaeffer & Sidavi, 2011; Wuyts, De Bodt, & Van de Heyning, 1999). Last, although assessment of the tension of the neck strap musculature through manual palpation is a common clinical technique (e.g., Roy & Bless, 1998), clinical palpation scales also have variable interrater reliability (Angsuwarangsee & Morrison, 2002; Stepp, Heaton, et al., 2011). None of these common clinical methods are capable of quantitatively assessing VH or the potential functional differences between PVH and NPVH. Such capabilities could improve the clinical management of hyperfunctional voice disorders by providing objective metrics upon which to base treatment decisions (e.g., decisions about when to end voice therapy on the basis of a quantified reduction in VH).
The relative fundamental frequency (RFF) of vocalic cycles before and after voiceless consonants is an acoustic measure that shows promise in detecting functional changes in voice. RFF measures the duration of the 10 vocalic cycles preceding the voiceless consonant (offset cycles) and the 10 vocalic cycles following the voiceless consonant (onset cycles). The inverse of the duration of each cycle is converted into semitones (ST) relative to the fundamental frequency close to the center of each of the voiced phonemes before and after the voiceless consonant. In previous studies, the RFF values of the vocalic cycle closest to the voiceless consonant for both the preceding (Offset Cycle 10) and the subsequent (Onset Cycle 1) voiced phonemes were lower in individuals with VH relative to those with healthy voices (Lien et al., 2015; Stepp, Hillman, & Heaton, 2010a; Stepp, Sawin, & Eadie, 2012). Previous work examining differences in RFF values demonstrated that these values remain unchanged in individuals who have received surgery to remove vocal nodules or vocal polyps (e.g., structural change; Stepp, Hillman, et al., 2010a). RFF values were, however, shown to increase in individuals with VH after a successful course of voice therapy (i.e., functional change; Stepp, Merchant, Heaton, & Hillman, 2011), suggesting that these changes in RFF values may be due to functional differences in voice use rather than representative of structural differences. These results support the use of RFF to examine the potential functional differences between these two subtypes of VH. In fact, one study examined if RFF values were different among individuals with vocal nodules, polyps, and MTD. Although statistically significant effects among the groups were not found, results indicated that there was a trend for RFF values to be lower in individuals with nodules and polyps than in individuals with MTD (Stepp, Hillman, et al., 2010a). One possible explanation for the lack of significant differences found in this study is that only three RFF tokens were analyzed from each speaker. Subsequent work has indicated that in order for RFF to be reliable, a minimum of six RFF tokens is required (Eadie & Stepp, 2013).
Thus the current study examined RFF values in a large subset of individuals with either healthy voices or VH, both PVH and NPVH, using nine RFF tokens per speaker. On the basis of previous work (Lien et al., 2015; Stepp, Hillman, et al., 2010a; Stepp et al., 2012), we expected individuals with healthy voices to have higher RFF values than both VH groups. In addition, due to the hypothesized functional differences for the two subtypes of VH (Hillman et al., 1989), we hypothesized that RFF values would differ between individuals with PVH and individuals with NPVH.
Method
Participants
All participants completed written consent. Consent was completed in compliance with either the Boston University Institutional Review Board or the Massachusetts General Hospital Institutional Review Board.
Control
The control group comprised 49 adults (M = 39.2 years, SD = 18.3 years; 37 women, 12 men), all of whom reported no prior history of voice, speech, language, or hearing disorders. Fifty-one percent of control participants were examined endoscopically to verify normal vocal status. The remaining 49% of control participants were not examined endoscopically; therefore, the presence of VH cannot be definitively ruled out. However, all members of the control group had voice quality consistent with vocally normal productions as deemed perceptually by a certified speech-language pathologist.
Vocal Hyperfunction (VH)
The VH group comprised 89 adult participants (72 women, 17 men), all of whom were diagnosed with a voice disorder associated with VH by a board-certified laryngologist. All of these individuals had vocal disturbances for 3 months or greater in duration, suggestive of a chronic voice disorder. During diagnostic evaluation, the vocal folds were visualized with a flexible or rigid endoscope with a stroboscopic light source. The VH group was further divided into two subsets: PVH and NPVH. The PVH group consisted of 54 participants (M = 31.1 years, SD = 14.7 years; 47 women, seven men), all of whom were diagnosed with organic vocal fold lesions (see Table 1). The remaining 35 participants (M = 41.7 years, SD = 13.7 years; 25 women, 10 men) were diagnosed as having primary MTD and were classified as the NPVH group.
Table 1.
Diagnoses in the phonotraumatic vocal hyperfunction group (N = 54).
| Diagnosis | Number of Participants |
|---|---|
| Vocal fold nodules (unilateral or bilateral) | 41 |
| Vocal fold polyp (unilateral or bilateral) | 11 |
| Vocal fold nodule (unilateral) and vocal fold polyp (unilateral) | 1 |
| Vocal fold scarring (unilateral) and vocal fold nodule (unilateral) | 1 |
Audio Recording Procedure
All signals were acquired digitally and recorded for offline analysis. Participants were recorded in either (a) a quiet space at Boston Medical Center using a dynamic headset microphone (model WH20XLR; Shure, Niles, IL) sampled at 44.1 kHz with 16-bit resolution, (b) a sound-treated room at Boston University using the same headset microphone and sampling rate, or (c) a sound-treated room at Massachusetts General Hospital using a Sennheiser MKE104 lavalier microphone (Sennheiser, Wedemark, Germany) sampled at 20 kHz and 16-bit resolution. During all recordings, an experimenter first modeled nine utterances for the participant before the participant produced the utterances. Each utterance consisted of a vowel–voiceless consonant–vowel instance with the center voiceless consonant /f/ (i.e., /ɑfɑ ɑfɑ ɑfɑ/, /ifi ifi ifi/, and /ufu ufu ufu/), yielding nine vowel–voiceless consonant–vowel productions. These stimuli were selected as they yielded the lowest intraspeaker variability in the stimuli set explored in a previous study (Lien, Gattuccio, & Stepp, 2014). Participants were asked to produce the RFF utterances in their typical pitch and loudness. The use of nine utterances in contrast to the three utterances used in a previous study (Stepp, Hillman, et al., 2010a) allowed the calculation of a more valid estimate of RFF (Eadie & Stepp, 2013). If any stimulus was misarticulated or glottalized, the experimenter instructed the participant to repeat the stimulus.
Acoustic Analysis
RFF values were calculated using an automated MATLAB program (Lien, 2015; MATLAB, 2013). In brief, the algorithm calculated the period and associated fundamental frequency (F0) of the 10 vocalic cycles of the vowel preceding the voiceless consonant (offset) and the 10 vocalic cycles of the vowel following the voiceless consonant (onset). F0 values for offset cycles were converted to ST using the F0 of Offset Cycle 1 as reference, and F0 values for onset cycles were converted to ST using the F0 of Onset Cycle 10 as reference. These reference frequencies were selected as they are closest to the center of the vowel and therefore are most representative of a steady-state portion of the vowel prior to any transitions. See Lien (2015) for full details about the automation algorithm and its validation.
Statistical Analysis
Two separate two-way analysis of variance (ANOVA) models—one for offset RFF values and one for onset RFF values—were used to examine within-group (Vocal Cycle), between-groups (Control, PVH, NPVH), and interaction effects. Effect sizes for the factors were calculated using a squared partial curvilinear correlation (ηp 2). Tukey's post hoc analyses were conducted with a corrected α level of 0.05. Cohen's d effect sizes were also calculated to further assess statistically significant pairwise group differences. Cohen's d values were designated as either small (0.2–0.3), medium (approximately 0.5), or large (> 0.8) effect sizes (Witte & Witte, 2010). All statistical analyses were conducted in Minitab (2012).
Receiver operating characteristic (ROC) curves were generated using RFF values from Offset Cycle 10 and Onset Cycle 1 to examine their effectiveness of distinguishing between groups. The ROC curves were created using a step size of 0.001 ST. The true positive rate (sensitivity) and the true negative rate (specificity) for thresholds of RFF values between the minimum and maximum observed values in the sample (ranging from −5.41 ST to 0.67 ST for Offset Cycle 10 and −3.12 ST to 4.46 ST for Onset Cycle 1) were calculated and used to produce the ROC curves.
Due to interest in determining the best sensitivity threshold, sensitivity and specificity values were utilized to calculate the maximum positive likelihood ratio (LR+) and the associated negative likelihood ratio (LR−). LRs provide information regarding the confidence with which an RFF value across a certain threshold identifies membership to a particular group. In comparisons between the control and the VH groups, the LR+ is the confidence that an RFF value indicates a member of a VH group. For comparisons between the PVH and NPVH groups, an LR+ is the confidence that the RFF value indicates a member of the PVH group. In addition to LR+ and LR−, area under the curve (AUC) values were calculated for each ROC curve. These AUCs provided further information regarding the effectiveness of RFF values in discriminating between any two given groups.
Results
Offset RFF
Due to a lack of usable offset cycles, one individual in the PVH group was excluded from the offset analyses. Table 2 displays the results of the two-way ANOVA on offset RFF values. There were statistically significant main effects of both Group and Vocal Cycle as well as a significant interaction of Vocal Cycle × Group. Follow-up Tukey's post hoc analyses were conducted on the interaction of Vocal Cycle × Group. Table 3 reports Cohen's d values for the statistically significant differences of each offset cycle (p adj < .05). The PVH group had significantly lower RFF values than both the control group and the NPVH group for Offset Cycles 8–10 and significantly lower RFF values than the control group for Offset Cycle 7 (p adj < .05; see Figure 1). Cohen's d values for the difference between the PVH group and the control group for Offset Cycles 8–10 were all large (range: 1.08–1.30) whereas the Cohen's d value for Offset Cycle 7 was medium-to-large (0.80). Cohen's d values for the difference in Offset Cycles 8–10 between the PVH and NPVH groups were all medium to medium-to-large (0.42–0.74). Only Offset Cycle 10 RFF values were statistically lower in the NPVH group than in the control group (p adj < .05, Cohen's d = 0.56, medium effect size).
Table 2.
Results of two-way analysis of variance on offset relative fundamental frequency values.
| Effect | df | ηp 2 | F | p |
|---|---|---|---|---|
| Group (control, PVH, NPVH) | 2 | .09 | 68.03 | < .01* |
| Vocal cycle | 9 | .51 | 146.48 | < .01* |
| Vocal cycle × group | 18 | .10 | 7.81 | < .01* |
Note. PVH = phonotraumatic vocal hyperfunction; NPVH = non-phonotraumatic vocal hyperfunction.
Significant at p < .05.
Table 3.
Cohen's d effect sizes for statistically significant group differences on offset relative fundamental frequency values from Tukey's post hoc tests.
| Offset cycles | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| Control versus non-phonotraumatic VH | — | — | — | 0.56 |
| Control versus phonotraumatic VH | 0.80 | 1.08 | 1.25 | 1.30 |
| Non-phonotraumatic VH versus phonotraumatic VH | — | 0.72 | 0.74 | 0.42 |
Note. Em dashes indicate not significant (p adj > .05); VH = vocal hyperfunction.
Figure 1.
Relative fundamental frequency (RFF) means and 95% confidence intervals in semitones (ST) for participants with phonotraumatic vocal hyperfunction (PVH; red circles) and non-phonotraumatic vocal hyperfunction (NPVH; blue triangles) and controls (black squares). Offset Cycle 10 values are significantly different among all three groups. The PVH group had lower RFF values for Offset Cycles 8 and 9 relative to both groups and lower RFF values for Offset Cycle 7 relative to the control group. In addition, Onset Cycle 1 RFF is significantly different between the PVH and control groups and trends toward significant differences between the PVH and NPVH groups.
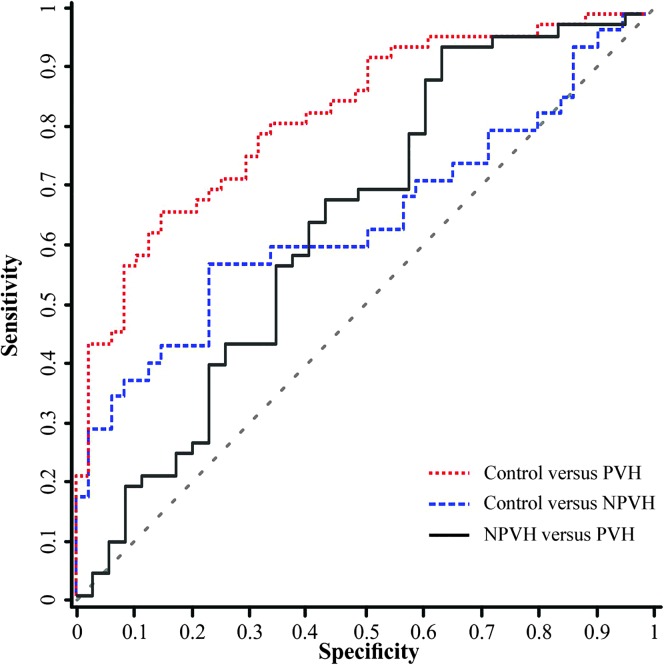
On the basis of previous work (Stepp, Hillman, et al., 2010a; Stepp, Merchant, et al., 2011; Stepp et al., 2012) and results of the post hoc testing indicating that Offset Cycle 10 was statistically different among all three groups, additional analyses were conducted on Offset Cycle 10 RFF values. RFF values in the PVH group for Offset Cycle 10 (M = −1.76 ST) were significantly lower than in the NPVH group (M = −1.35 ST), and both groups were significantly lower than the control group (M = −0.82 ST). Figure 2 displays the three ROC curves generated to examine the effectiveness of using Offset Cycle 10 RFF to differentiate among the three groups. Offset 10 RFF ROC analysis for the control group versus the PVH group revealed an AUC of 0.80, a maximum LR+ of 20.8 (occurring at −1.92 ST with 0.43 sensitivity and 0.98 specificity) with an associated LR− of 0.58. Offset Cycle 10 RFF ROC analysis for the NPVH group versus the PVH group revealed an AUC of 0.61, a maximum LR+ of 2.20 (occurring at −2.33 ST with 0.19 sensitivity and 0.91 specificity) with an associated LR− of 0.89. Offset Cycle 10 RFF values for the control group versus the NPVH group revealed an AUC of 0.63, a maximum LR+ of 13.7 (occurring at −1.92 ST with 0.29 sensitivity and 0.98 specificity) with an associated LR− of 0.73.
Figure 2.
Receiver operating characteristic curve for the difference in Offset Cycle 10 relative fundamental frequency values between control and phonotraumatic vocal hyperfunction (PVH; red dotted line), control, and non-phonotraumatic vocal hyperfunction (NPVH; blue dashed line), and NPVH and PVH (black solid line). Chance is indicated by the dashed diagonal gray line.
Onset RFF
Table 4 displays the results of a two-way ANOVA on onset RFF values. There was a statistically significant main effect of Vocal Cycle and a significant interaction of Vocal Cycle × Group, but no significant main effect of Group. Follow-up Tukey's post hoc analyses were conducted on the interaction of Vocal Cycle × Group. Onset Cycle 1 RFF for the PVH group (M = 1.18 ST) was significantly lower than for the control group (M = 1.68 ST, p adj < .05, medium effect size of Cohen's d = 0.44) and trended toward significantly lower RFF values than the NPVH group (M = 1.59 ST, p adj = .066). There were no other significant group differences among any of the other nine onset cycles.
Table 4.
Results of mixed-design analysis of variance on onset relative fundamental frequency values.
| Effect | df | ηp 2 | F | p |
|---|---|---|---|---|
| Group (control, PVH, NPVH) | 2 | .003 | 2.34 | .10 |
| Vocal cycle | 9 | .440 | 115.95 | < .01* |
| Vocal cycle × group | 18 | .020 | 1.71 | .03* |
Note. PVH = phonotraumatic vocal hyperfunction; NPVH = non-phonotraumatic vocal hyperfunction.
Significant at p < .05.
One ROC curve was generated to examine the effectiveness of using Onset Cycle 1 RFF to differentiate between PVH and controls, which were significantly different on Onset Cycle 1 RFF. Onset Cycle 1 RFF ROC analysis revealed an AUC of 0.60, a maximum LR+ of 1.60 (occurring at 0.96 ST with 0.43 sensitivity and 0.73 specificity) with an associated LR− of 0.78.
Discussion
The transition from a vowel into a voiceless consonant (offset) and the voiceless consonant into a vowel (onset) demonstrated differences among healthy controls, individuals with NPVH, and individuals with PVH. In examination of the vowel offset, individuals with NPVH had significantly lower Offset Cycle 10 RFF values than healthy controls with no other differences noted in the RFF of cycles that were further from the voiceless consonant. RFF values from individuals with PVH were significantly different at an earlier time point. RFF values of Offset Cycles 8–10 leading into the voiceless consonant were all significantly decreased in individuals with PVH relative to both individuals with NPVH and healthy controls, and RFF values of Offset Cycle 7 were significantly decreased relative to the control group. In addition, when examining the vowel onset, individuals with PVH had significantly lower RFF values at the first cycle of the vowel (Onset Cycle 1) relative to the control group and trended toward lower RFF values relative to the NPVH group. This study supports the hypothesis that RFF values have the potential to distinguish between the voices of individuals with PVH and individuals with NPVH. Findings from this study are consistent with the trend of lower RFF values found previously in individuals with PVH than with individuals in NPVH (Stepp, Hillman, et al., 2010a).
Individuals with VH and healthy controls have been shown to have differences in RFF values (Lien et al., 2015; Stepp, Hillman, et al., 2010a; Stepp et al., 2012). Stepp, Merchant, et al. (2011) have previously proposed a model incorporating a simple summation of vocal fold tension, aerodynamic factors, and abductory factors to explain these differences. The authors proposed that the lowered RFF noted in individuals with VH could be accounted for by a simple reduction in the effects of tension, presumably due to higher baseline laryngeal tension in individuals with VH. However, this model in its current state may not be adequate to explain the apparent ability of RFF to distinguish between the PVH and NPVH groups or explain the differences seen in offset RFF and onset RFF. The previous model might still apply if the RFF measure is simply reflecting a difference in the overall level of VH. This would mean that patients with vocal fold lesions are displaying a higher degree of the type of VH that RFF is sensitive to than MTD patients. If this is the case, one could speculate that higher levels of VH might develop in PVH patients because of their need to compensate for the presence of lesions (reactive or secondary VH) in addition to the degree of VH that was associated with the onset of their voice disorder. However, we believe that it is also possible that the difference in RFF between PVH and NPVH reflects differences in the underlying pathophysiological mechanisms (e.g., longitudinal and transverse vocal fold tension) associated with these two conditions. Therefore, below we offer some updates to the previously proposed model to encompass the nuances of the differences seen in the RFF values of individuals with PVH and NPVH as a function of offset and onset RFF.
Modification to Previously Hypothesized Mechanisms for RFF Changes
Offset RFF
Longitudinal Vocal Fold Tension. Previous researchers have suggested that individuals with healthy voices have increases in their vocal fold tension preceding production of a voiceless consonant in order to cease vocal fold vibrations (Halle & Stevens, 1971; Stevens, 1977). Of note, there is no consensus regarding the physiological mechanisms for increasing longitudinal vocal fold tension. Some studies report an increase in cricothyroid activity (Jaiswal, 2011; Stevens, 1977), another suggested a stiffening of the vocal body via constriction of the vocalis muscle (Hirano, 1974), and others proposed that vocal fold stiffness is a product of vertical stretching that occurs from an increase in laryngeal height (Sonninen, 1968; Stevens, 1977). In addition to an increase in longitudinal vocal fold tension during vowel offset, both intraoral and subglottal pressures have been shown to increase (Löfqvist, Koenig, & McGowan, 1995; Löfqvist & McGowan, 1992), and pressure across the glottis (defined as the subglottal pressure minus the intraoral pressure; Ladefoged, 1967) decreases. Ladefoged (1967) noted that this decrease in pressure across the glottis was accompanied by a decrease in F0. However, the reduction in F0 he observed experimentally was smaller than expected, which he suggested was due to the increase in longitudinal vocal fold tension counteracting the effect on F0 from decreased pressure across the glottis (Ladefoged, 1967).
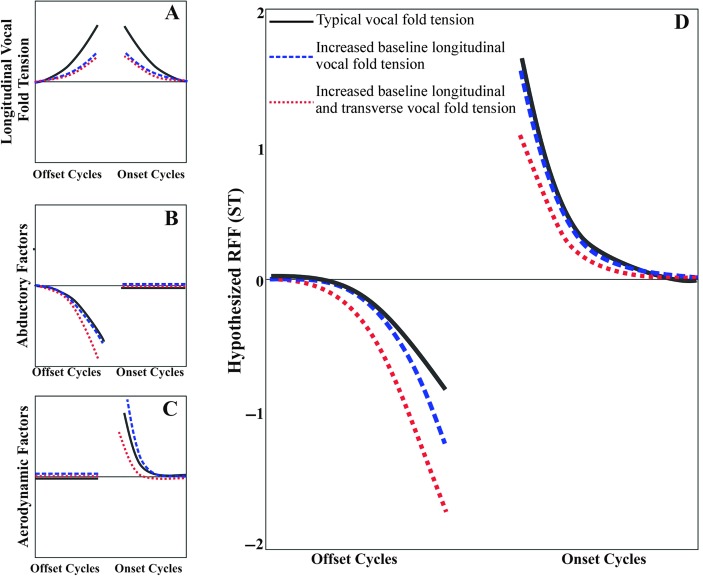
Individuals with VH have been suggested to have increased baseline longitudinal vocal fold tension (Hillman et al., 1989). Therefore, at this transition from a vowel to a voiceless consonant, which may require increased longitudinal vocal fold tension, increased baseline tension may affect the ability of individuals with VH to further increase their longitudinal vocal fold tension. The proposed result is a reduction in the increase in longitudinal vocal fold tension in both the PVH and NPVH groups as compared with vocally healthy controls. These groups are unable to mitigate the effects of decreased pressure across the glottis (Ladefoged, 1967) and therefore have a decrease in F0 as compared with healthy controls (See Figure 3, panel A).
Figure 3.
Hypothesized effects on relative fundamental frequency (RFF) in semitones (ST) of longitudinal vocal fold tension (panel A), abductory factors (panel B), and aerodynamic factors (panel C) for individuals with typical vocal fold tension (black solid line), increased baseline longitudinal tension (blue dashed line), and increased baseline longitudinal and transverse tension (red dotted line). The hypothesized summed effect of panels A, B, and C is depicted in panel D, the hypothesized RFF for all offset and onset vocal cycles.
Transverse Vocal Fold Tension. In addition to the elevated longitudinal tension that is hypothesized to be present in both PVH and NPVH, transverse vocal fold tension may interact with abductory factors to modulate offset RFF values in the PVH group. In the transition from a vowel to a voiceless consonant, abduction precedes the start of the voiceless consonant (Fukui & Hirose, 1983). In his examination of healthy young and older adults, Watson (1998) theorized that the decrease in F0 noted in older adults during abduction was attributable to decreased vocal fold contact area as the vocal folds abduct, resulting in a longer vibratory period. Kunduk, Yan, McWhorter, and Bless (2006) noted during high-speed video evaluation that an older adult participant took longer to cease vocal fold vibration than a younger adult participant, similar to Watson's theory. Although not age-related, similar patterns of abduction may have occurred in individuals with PVH due to their tightly approximated vocal folds (Hillman et al., 1989) or increased transverse vocal fold tension. This may have subsequently increased the effect of abduction due to the presence of longer vibratory periods that may have occurred during the transition into the voiceless consonant (see Figure 3, panel B), resulting in lower RFF values for Offset Cycles 7–10 in the PVH group (see Figure 3, panel D).
Onset RFF
Longitudinal Vocal Fold Tension. Similar to offset RFF, individuals with healthy voices have been suggested to have increases in their vocal fold tension during and following production of a voiceless consonant in order to cease vocal fold vibrations (Halle & Stevens, 1971; Löfqvist, Baer, McGarr, & Story, 1989; Stevens, 1977). The stiffness present during the voiceless consonant to prevent voicing is hypothesized to be carried over into the following vowel, resulting in increased F0 (Halle & Stevens, 1971). Löfqvist et al. (1989) further supported this hypothesis, noting that increased longitudinal vocal fold tension, caused by increased cricothyroid activity during a voiceless consonant, was positively correlated with the fundamental frequency during the following vowel. The increased baseline longitudinal vocal fold tension that has been proposed in individuals with both PVH and NPVH may have reduced their ability to increase longitudinal vocal fold tension following the production of the voiceless consonant (see Figure 3, panel A).
Transverse Vocal Fold Tension. In addition to increases in peak and minimum airflow (Löfqvist et al., 1995; Löfqvist & McGowan, 1992), the increased pressure across the glottis during the transition from a voiceless consonant to a vowel is hypothesized to further increase the F0 at the start of the vowel (Ladefoged, 1967). As individuals with PVH have increased closing velocities and tightly approximated vocal folds (Hillman et al., 1989), the time over which these aerodynamic forces are active may be shorter compared with healthy individuals and individuals with NPVH. This reduction in the effects of the aerodynamic forces may reduce the increase in F0 during the onset of the vowel, which is consistent with what was seen in individuals with PVH in this data set (see Figure 3, panel C). Individuals with NPVH may have the opposite effect: Their vocal folds may not be completely adducted during the initiation of vocal fold vibration. Although differences in Onset Cycle 1 RFF values between the NPVH and PVH groups did not reach significance, the trend suggests that there may be differences between the two groups. To be specific, the increased glottal gap size in individuals with NPVH in comparison to individuals with PVH may result in the aerodynamic forces being active over a longer period of time, subsequently having a larger effect and thereby increasing the F0 of the Onset Cycle 1 RFF values (see Figure 3, panels C and D).
Summary and Clinical Implications
Results from this work suggest that Offset Cycle 10 may provide a window into the functional differences between these groups and further supplement the evaluation process for individuals with voice disorders. ROC analysis on Offset Cycle 10 RFF for both the PVH compared with control groups and PVH compared with NPVH groups had high LR+ values (20.8 and 13.7, respectively). However, the low sensitivity values reinforce the variability in presentation of individuals with VH, in both the PVH and NPVH varieties. Future work will explore further this variability to continue to categorize and understand the compositions of these potential subgroups of VH.
This work adds to the growing body of literature suggesting that PVH and NPVH may not only be structurally different, but may also be functionally different. The significant differences between the VH subgroups noted in this study, which were absent in a previous study (Stepp, Hillman, et al., 2010a), may represent an improvement in RFF methodology. For example, Stepp, Hillman, et al. (2010a), examined RFF values from three RFF instances in running speech. The current study, however, examined RFF values from nine uniform utterances with a center phoneme of /f/. These stimuli have been shown to provide decreased within-speaker variability relative to RFF estimates from running speech (Lien et al., 2014). In addition, as previous work indicated that at least six RFF utterances are needed to be reliable (Eadie & Stepp, 2013), the use of nine utterances in this study likely further contributed to more reliable RFF estimates in each speaker. Last, the use of an automated algorithm to calculate RFF values likely provided a more objective estimate of RFF values than previous manual calculations. These methodological improvements in sum likely contributed to the differences between the current study and Stepp, Hillman, et al.'s previous work (2010a).
Limitations and Future Directions
The current study proposed an updated model for hypothesized RFF differences; however, the basis of the model relies on data from studies with small sample sizes and previously proposed hypotheses regarding vocal behavior during phonation onset and offset. The lack of large-scale empirical evidence to support the current hypothesis is a limitation of this work. In addition, the modified model offers only one proposed interaction of factors, potentially disregarding other rationales for the differences among the PVH, NPVH, and control groups. More direct empirical evaluation is essential to examine the proposed functional differences between the two subgroups of VH; however, the current results may provide a framework for future hypothesis. Future studies are warranted to examine these three groups with more direct evaluation of the hypothesized mechanisms for their functional differences. To be specific, concurrent high-speed videoendoscopic imaging with the acquisition of the acoustic signal will provide information regarding the vocal fold kinematics and glottal configuration. In addition, examining the relationship between RFF values and other measures of vocal fold stiffness (McKenna, Heller Murray, Lien, & Stepp, 2016; Stepp, Hillman, & Heaton, 2010b) may provide further information regarding the proposed stiffness and kinematics of the vocal folds. Future use of modeling could allow for systematic testing of the acoustic ramifications of changes in vocal fold stiffness in proposed models of healthy individuals, individuals with PVH, and individuals with NPVH.
In the current study, classification of disordered participants as having either PVH or NPVH was based solely on whether or not videostroboscopic examination revealed vocal fold lesions that are associated with phonotrauma. It could be argued that this classification scheme may not be valid because it does not describe two different manifestations of VH but rather different points in the progression of the same condition. In other words, participants with NPVH may have simply been assessed before they developed phonotrauma, and the reported differences between the two groups (PVH vs. NPVH) are due primarily to the presence of vocal fold lesions in the PVH group. However, this seems highly unlikely because all of the participants in the NPVH group had been experiencing chronic vocal symptoms associated with MTD for at least 3 months (most of them much longer) prior to being assessed, which seems of adequate duration to produce some signs of phonotrauma if that were going to eventually happen. In addition, the persistence of non-normal acoustic (e.g., RFF; Stepp, Hillman, et al., 2010a) and aerodynamic (Hillman et al., 1989) measures in PVH patients following surgical removal of the lesions and prior to voice therapy adds further evidence for the existence of underlying hyperfunctional mechanisms that are not purely attributable to the presence of lesions.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC012651 awarded to Cara E. Stepp and Grant DC011588 awarded to Robert E. Hillman. The article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Thanks to Victoria McKenna for perceptual ratings of vocal quality; Defne Abur, Christina Stevens, Alexandria Martinson, and Laura Enflo for assistance with audio data recording; and Melissa Cooke, Amanda Fryd, and Molly Bresnahan for help with audio recording segmentation.
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC012651 awarded to Cara E. Stepp and Grant DC011588 awarded to Robert E. Hillman.
References
- Angsuwarangsee T., & Morrison M. (2002). Extrinsic laryngeal muscular tension in patients with voice disorders. Journal of Voice, 16, 333–343. [DOI] [PubMed] [Google Scholar]
- Behrman A., Dahl L. D., Abramson A. L., & Schutte H. K. (2003). Anterior-posterior and medial compression of the supraglottis: Signs of nonorganic dysphonia or normal postures? Journal of Voice, 17, 403–410. [DOI] [PubMed] [Google Scholar]
- Eadie T. L., Kapsner M., Rosenzweig J., Waugh P., Hillel A., & Merati A. (2010). The role of experience on judgments of dysphonia. Journal of Voice, 24, 564–573. [DOI] [PubMed] [Google Scholar]
- Eadie T. L., & Stepp C. E. (2013). Acoustic correlate of vocal effort in spasmodic dysphonia. Annals of Otology, Rhinology & Laryngology, 122, 169–176. [DOI] [PubMed] [Google Scholar]
- Fukui N., & Hirose H. (1983). Laryngeal adjustments in Danish voiceless obstruent production. Annual Bulletin, Research Institute of Logopedics and Phoniatrics, 17, 61–71. [Google Scholar]
- Halle M., & Stevens K. (1971). A note on laryngeal features. In Quarterly Progress Report 101 (pp. 198–212). Cambridge, MA: Research Laboratory of Electronics MIT. [Google Scholar]
- Hillman R. E., Holmberg E. B., Perkell J. S., Walsh M., & Vaughan C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech and Hearing Research, 32, 373–392. [DOI] [PubMed] [Google Scholar]
- Hirano M. (1974). Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatrica et Logopaedica, 26, 89–94. [DOI] [PubMed] [Google Scholar]
- Jaiswal S. (2011). Cricothyroid muscle activity at voicing transitions. Unpublished doctoral dissertation, University of Iowa, Iowa City. [Google Scholar]
- Kempster G. B., Gerratt B. R., Verdolini Abbott K., Barkmeier-Kraemer J., & Hillman R. E. (2009). Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. American Journal of Speech-Language Pathology, 18, 124–132. [DOI] [PubMed] [Google Scholar]
- Kreiman J., Gerratt B. R., Kempster G. B., Erman A., & Berke G. S. (1993). Perceptual evaluation of voice quality: Review, tutorial, and a framework for future research. Journal of Speech and Hearing Research, 36, 21–40. [DOI] [PubMed] [Google Scholar]
- Kunduk M., Yan Y., McWhorter A. J., & Bless D. (2006). Investigation of voice initiation and voice offset characteristics with high-speed digital imaging. Logopedics Phoniatrics Vocology, 31, 139–144. [DOI] [PubMed] [Google Scholar]
- Ladefoged P. (1967). Three areas of experimental phonetics. London, England: Oxford University Press. [Google Scholar]
- Lien Y.-A. S. (2015). Optimization and automation of relative fundamental frequency for objective assessment of vocal hyperfunction. Unpublished doctoral dissertation, Boston University, MA. [Google Scholar]
- Lien Y.-A. S., Calabrese C. R., Michener C. M., Heller Murray E. S., Van Stan J. H., Mehta D. D., … Stepp C. E. (2015). Voice relative fundamental frequency via neck-skin acceleration in individuals with voice disorders. Journal of Speech, Language, and Hearing Research, 58, 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien Y.-A. S., Gattuccio C. I., & Stepp C. E. (2014). Effects of phonetic context on relative fundamental frequency. Journal of Speech, Language, and Hearing Research, 57, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfqvist A., Baer T., McGarr N. S., & Story R. S. (1989). The cricothyroid muscle in voicing control. The Journal of the Acoustical Society of America, 85, 1314–1321. [DOI] [PubMed] [Google Scholar]
- Löfqvist A., Koenig L. L., & McGowan R. S. (1995). Vocal tract aerodynamics in /aCa/ utterances: Measurements. Speech Communication, 16, 49–66. [Google Scholar]
- Löfqvist A., & McGowan R. S. (1992). Influence of consonantal environment on voice source aerodynamics. Journal of Phonetics, 20, 93–110. [Google Scholar]
- MATLAB. (2013). Matlab R2013a (Version 8.1.0.604) [Computer software]. Natick, MA: The Mathworks. [Google Scholar]
- McKenna V. S., Heller Murray E. S., Lien Y. S., & Stepp C. E. (2016). The relationship between relative fundamental frequency and a kinematic estimate of laryngeal stiffness in healthy adults. Journal of Speech, Language, and Hearing Research, 58, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. D., Van Stan J. H., Zañartu M., Ghassemi M., Guttag J. V., Espinoza V. M., … Hillman R. E. (2015). Using ambulatory voice monitoring to investigate common voice disorders: Research update. Frontiers in Bioengineering and Biotechnology, 3, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. F. (1999). Laryngeal function associated with changes in lung volume during voice and speech production in normal speaking women. Unpublished doctoral dissertation, University of Arizona, Tucson. [Google Scholar]
- Minitab. (2012). Minitab Statistical Software (Version 16.2.3) [Computer software]. State College, PA: Author. [Google Scholar]
- Pemberton C., Russell A., Priestley J., Havas T., Hooper J., & Clark P. (1993). Characteristics of normal larynges under flexible fiberscopic and stroboscopic examination: An Australian perspective. Journal of Voice, 7, 382–389. [DOI] [PubMed] [Google Scholar]
- Roy N., & Bless D. M. (1998). Manual circumlaryngeal techniques in the assessment and treatment of voice disorders. Current Opinion in Otolaryngology & Head and Neck Surgery, 6, 151–155. [Google Scholar]
- Roy N., & Bless D. M. (2000). Personality traits and psychological factors in voice pathology: A foundation for future research. Journal of Speech, Language, and Hearing Research, 43, 737–748. [DOI] [PubMed] [Google Scholar]
- Sama A., Carding P., Price S., Kelly P., & Wilson J. (2001). The clinical features of functional dysphonia. Laryngoscope, 111, 458–463. [DOI] [PubMed] [Google Scholar]
- Schaeffer N., & Sidavi A. (2011). Toward a more quantitative measure to assess severity of dysphonia posttherapy: Preliminary observations. Journal of Voice, 25, E159–E165. [DOI] [PubMed] [Google Scholar]
- Sonninen A. (1968). The external frame function in the control of pitch in the human voice. Annals of the New York Academy of Sciences, 155, 68–90. [Google Scholar]
- Stager S. V., Bielamowicz S., Gupta A., Marullo S., Regnell J. R., & Barkmeier J. (2001). Quantification of static and dynamic supraglottic activity. Journal of Speech, Language, and Hearing Research, 44, 1245–1256. [DOI] [PubMed] [Google Scholar]
- Stager S. V., Bielamowicz S. A., Regnell J. R., Gupta A., & Barkmeier J. M. (2000). Supraglottic activity: Evidence of vocal hyperfunction or laryngeal articulation? Journal of Speech, Language, and Hearing Research, 43, 229–238. [DOI] [PubMed] [Google Scholar]
- Stager S. V., Neubert R., Miller S., Regnell J. R., & Bielamowicz S. A. (2003). Incidence of supraglottic activity in males and females: A preliminary report. Journal of Voice, 17, 395–402. [DOI] [PubMed] [Google Scholar]
- Stepp C. E., Heaton J. T., Braden M. N., Jetté M. E., Stadelman-Cohen T. K., & Hillman R. E. (2011). Comparison of neck tension palpation rating systems with surface electromyographic and acoustic measures in vocal hyperfunction. Journal of Voice, 25, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp C. E., Heaton J. T., Jetté M. E., Burns J. A., & Hillman R. E. (2010). Neck surface electromyography as a measure of vocal hyperfunction before and after injection laryngoplasty. Annals of Otology, Rhinology & Laryngology, 119, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp C. E., Hillman R. E., & Heaton J. T. (2010a). The impact of vocal hyperfunction on relative fundamental frequency during voicing offset and onset. Journal of Speech, Language, and Hearing Research, 53, 1220–1226. [DOI] [PubMed] [Google Scholar]
- Stepp C. E., Hillman R. E., & Heaton J. T. (2010b). A virtual trajectory model predicts differences in vocal fold kinematics in individuals with vocal hyperfunction. The Journal of the Acoustical Society of America, 127, 3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp C. E., Merchant G. R., Heaton J. T., & Hillman R. E. (2011). Effects of voice therapy on relative fundamental frequency during voicing offset and onset in patients with vocal hyperfunction. Journal of Speech, Language, and Hearing Research, 54, 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp C. E., Sawin D. E., & Eadie T. L. (2012). The relationship between perception of vocal effort and relative fundamental frequency during voicing offset and onset. Journal of Speech, Language, and Hearing Research, 55, 1887–1896. [DOI] [PubMed] [Google Scholar]
- Stevens K. N. (1977). Physics of laryngeal behavior and larynx modes. Phonetica, 34, 264–279. [DOI] [PubMed] [Google Scholar]
- Van Houtte E., Van Lierde K., & Claeys S. (2011). Pathophysiology and treatment of muscle tension dysphonia: A review of the current knowledge. Journal of Voice, 25, 202–207. [DOI] [PubMed] [Google Scholar]
- Watson B. C. (1998). Fundamental frequency during phonetically governed devoicing in normal young and aged speakers. The Journal of the Acoustical Society of America, 103, 3642–3647. [DOI] [PubMed] [Google Scholar]
- Witte R. S., & Witte J. S. (2010). Statistics (9th ed.). Hoboken, NJ: Wiley. [Google Scholar]
- Wuyts F. L., De Bodt M. S., & Van de Heyning P. H. (1999). Is the reliability of a visual analog scale higher than an ordinal scale? An experiment with the GRBAS scale for the perceptual evaluation of dysphonia. Journal of Voice, 13, 508–517. [DOI] [PubMed] [Google Scholar]