Abstract
Purpose
The vocal auditory-motor control of individuals with hyperfunctional voice disorders was examined using a sensorimotor adaptation paradigm.
Method
Nine individuals with hyperfunctional voice disorders and 9 individuals with typical voices produced sustained vowels over 160 trials in 2 separate conditions: (a) while experiencing gradual upward perturbations in the fundamental frequency (fo) of their auditory feedback (shift-up) and (b) under no auditory perturbation (control). The shift-up condition consisted of 4 ordered (fixed) phases: baseline (no perturbation), ramp (gradual increases in heard fo), hold (a consistently higher heard fo), and after-effect (no perturbation). Adaptive responses were defined as the difference in produced fo during control and shift-up conditions.
Results
Adaptive responses were significantly different between groups. Individuals with typical voices generally showed compensatory adaptive responses, with decreased fo during the ramp and hold phases. Conversely, many individuals with hyperfunctional voice disorders instead displayed the opposite effect by following the direction of the perturbation. When fo was experimentally increased, speakers further increased their fo.
Conclusion
Results indicate that some individuals diagnosed with hyperfunctional voice disorders have disrupted auditory-motor control, suggesting atypical neurological function. These findings may eventually allow for the development of new interventions for hyperfunctional voice disorders.
Hyperfunctional voice disorders are those associated with vocal hyperfunction, inefficient and/or inappropriate phonatory behaviors. Hyperfunctional voice disorders include the common diagnoses of muscle tension dysphonia (voice disorders in the absence of known structural or neurological dysfunction) and benign fibrovascular lesions of the vocal folds thought to arise and/or persist through interactions with hyperfunctional vocal behaviors (e.g., vocal fold nodules and polyps). Although common, these disorders are ambiguous in nature, likely because of imprecise and subjective evaluation techniques and heterogeneity within the population. Hyperfunctional voice disorders are characterized historically by excessive laryngeal and paralaryngeal tension (e.g., Aronson, 1990; Dworkin, Meleca, & Abkarian, 2000; Morrison, Rammage, Belisle, Pullan, & Nichol, 1983; Roy, 2008). The clinical symptoms can include a high laryngeal position, supraglottic compression, vocal fry, low pitch, strained and/or breathy voice quality, medial compression of the vocal folds (i.e., hyperadduction) and/or an exaggerated posterior glottic opening, high laryngeal resistance, and abnormal speech breathing (Cavallo, Dakow, Schaeffer, & Wall, 2002; Gillespie, Gartner-Schmidt, Rubinstein, & Abbott, 2013; Lowell, Barkmeier-Kraemer, Hoit, & Story, 2008; Morrison, 1997; Morrison et al., 1983; Morrison, Rammage, & Emami, 1999; Zheng et al., 2012). Although these disorders are extremely common, little is known about their pathophysiology. In addition to poor vocal hygiene and other voice-use factors (Altman, Atkinson, & Lazarus, 2005; Van Houtte, Van Lierde, & Claeys, 2011), psychological factors (Roy, Bless, & Heisey, 2000a; Van Houtte et al., 2011), autonomic nervous system dysfunction related to stress management (Demmink-Geertman & Dejonckere, 2002; Helou, Wang, Ashmore, Rosen, & Abbott, 2013), and personality traits (Ng, Lo, Lim, Goh, & Kanagalingam, 2013; Roy & Bless, 2000; Roy, Bless, & Heisey, 2000b) have been implicated. However, there is no unifying framework for the etiology of hyperfunctional voice disorders or the contribution of these potential factors to their development.
Notwithstanding these other potential factors, key similarities between acoustic, perceptual, and physiological aspects of voice production in individuals with hyperfunctional voice disorders and those with hearing loss inspired our hypothesis that auditory-motor integration may be disrupted in hyperfunctional voice disorders. Like individuals with hyperfunctional voice disorders, speakers with hearing loss use higher laryngeal resistance (Higgins, Carney, & Schulte, 1994), and their voices are frequently described as strained and breathy (Arends, Povel, Van Os, & Speth, 1990; Forner & Hixon, 1977; Higgins et al., 1994; Read, 1989). These symptoms are speculated to be caused by laryngeal dysregulation and/or incoordination (Forner & Hixon, 1977; Itoh, Horii, Daniloff, & Binnie, 1982; Lane, Perkell, Svirsky, & Webster, 1991; Metz, Whitehead, & Whitehead, 1984). Furthermore, like individuals with hyperfunctional voice disorders, speakers with hearing loss also display deviations in speech breathing (Forner & Hixon, 1977; Perkell et al., 2000). The similarities in the symptoms of dysphonia in individuals with hearing loss and individuals with hyperfunctional voice disorders suggest potential similarities in their mechanisms. Thus, in this study, we hypothesized that speakers with hyperfunctional voice disorders may have an impairment of their auditory-motor control. Disordered sensorimotor integration, with inappropriate updating and maintaining of feedforward vocal control based on auditory feedback, might explain the development and persistence of hyperfunctional behaviors. For instance, initial entrance into a cycle of dysphonia may be precipitated by disruptions in typical voice, whether organic (e.g., an upper respiratory infection or high voice-use situation) or psychological (e.g., emotional stress). In most speakers, these acute changes to voice are short lived. However, such acute changes in an individual with disordered auditory-motor integration might persist past the original disruption due to maladaptive reactions. Therefore, here we investigated the potential auditory-motor basis of hyperfunctional voice disorders through a classic fundamental frequency (fo) perturbation experiment in which fo was gradually modified over time to determine adaptive responses, providing information about feedforward and feedback mechanisms of speech motor control.
The speech sensorimotor system uses discrepancies between expected and actual sensory feedback to update motor programs. Gradual modification of speakers' feedback is a paradigm known as sensorimotor adaptation, which has been employed by a number of research groups over the years. These types of experiments have been shown to yield a robust, consistent response in which speakers compensate over time for the gradually increased manipulations in fo: If fo is experimentally increased, speakers respond by lowering their fo over time (Jones & Keough, 2008; Jones & Munhall, 2000, 2002, 2005; Keough & Jones, 2009; Patel, Niziolek, Reilly, & Guenther, 2011). As evidenced by short-term maintenance of the response after removal of the shift, speakers seem to temporarily update (i.e., adapt) their motor programs. We hypothesized that individuals with hyperfunctional voice disorders would show abnormal responses to this type of gradual auditory perturbation.
Method
Participants
Nine individuals with hyperfunctional voice disorders aged 20 to 39 years (eight women, one man) and nine individuals with typical voices aged 18 to 30 years (five women, four men) were included in this study. Individuals with voice disorders were diagnosed by a laryngologist based on comprehensive voice evaluation procedures that included videolaryngostroboscopy and perceptual assessments; none reported any history of other hearing or language disorders. Four participants with hyperfunctional voice disorders had phonotraumatic lesions (i.e., nodules, polyps). One of these participants had undergone surgical excision of the nodules approximately 12 years prior to her participation in this study. The overall severity of the dysphonia of each speaker with a hyperfunctional voice disorder was rated by a certified speech-language pathologist using the Consensus Auditory-Perceptual Evaluation of Voice (Kempster, Gerratt, Verdolini Abbott, Barkmeier-Kraemer, & Hillman, 2009) based on the sustained vowel samples produced during the baseline phase of the experiment (see below). The mean overall severity for the group was 29.8, with a range from 12.0 to 68.1. Individuals with typical voices reported no history of voice, speech, language, or hearing disorders. Additional participants were recruited for this study, but their data were unable to be included because of the inability to complete study procedures, difficulty in tracking fo due to severe roughness and/or diplophonia, and unstable fo across the baseline phase of the control condition (likely related to apparent distraction from the task).
Procedure
Participants were seated comfortably in front of a computer monitor, on which stimulus cues were displayed. The participant wore either a Shure omnidirectional MX153 subminiature earset microphone or a Shure headset WH20QTR microphone positioned at a fixed distance of approximately 7 cm from the mouth at a 45° angle. The microphone signal was amplified via an RME Quadmic II microphone preamplifier and digitized via a MOTU UltraLite-MK3 or MOTU Microbook hybrid soundcard. Auditory feedback to the participant of his or her own speech was delivered through a pair of Sennheiser HD 280 Pro headphones, which provide attenuation of air-conducted sound by approximately 32 dB. The auditory feedback through the headphones was set to be amplified by at least 5 dB relative to the sound level at the microphone. When relevant, the fo of voicing and associated harmonics were transformed using Audapter (Cai, Boucek, Ghosh, Guenther, & Perkell, 2008), a MATLAB software package for configurable real-time manipulation of acoustic parameters. The total processing delay was < 45 ms. Trial initiation and Audapter manipulations were controlled by a custom MATLAB program. Both the perturbed and unperturbed signals were recorded digitally at 16 kHz.
There were two conditions in the experiment: a shift-up and a control. Participants participated in the two conditions (each 30 min) with the order counterbalanced. Each condition consisted of 160 consecutive 11-s trials, including a 3-s production of a sustained /ɑ/. In the shift-up condition, participants first produced 20 utterances while receiving typical (unperturbed) feedback, referred to as the baseline. In the following 60 trials (referred to as the ramp), the fo of their auditory feedback was increased by 1.6 cents 1 for each successive trial, reaching a total level of +100 cents of perturbation by the end of the ramp. This was followed by the hold, consisting of 40 trials with the feedback maintained at 100 cents above the participants' true fo. Finally, participants performed 20 trials in which the feedback was again unperturbed (the after-effect). Perturbations, when applied, were throughout the entire period of voicing. In the control condition, all 160 trials were completed without any fo perturbation. The participants did not receive any information about differences between the two conditions. The responses of one participant with a hyperfunctional voice disorder during the last eight trials of the hold were not recorded because of temporary equipment failure; however, they continued to receive appropriate auditory feedback. The individual trace of this participant indicates this period via a dotted line in Figure 1.
Figure 1.
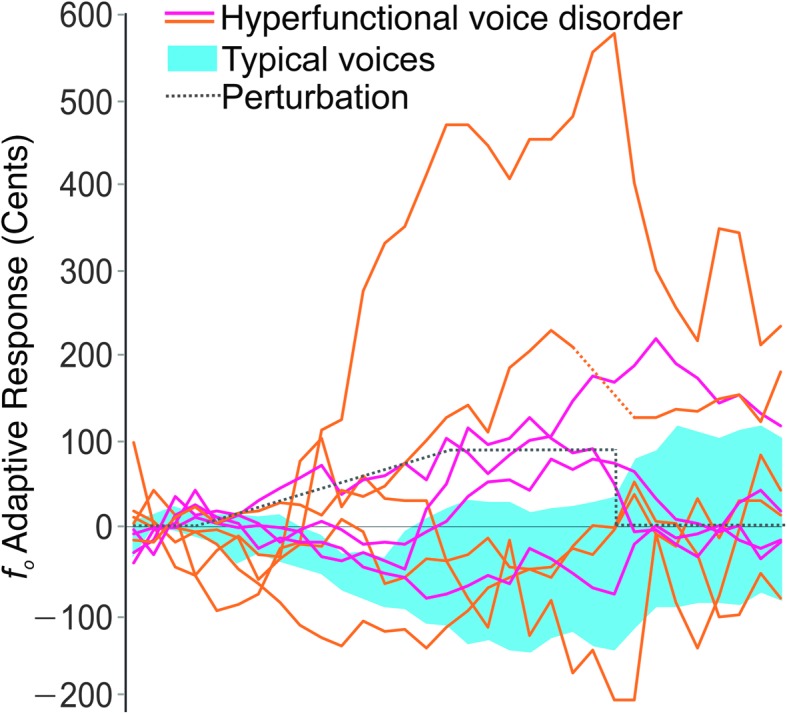
An adaptive shift-up perturbation was applied to the fo of auditory feedback (black dotted line), with a maximum perturbation of 100 cents. The 95% confidence intervals of the adaptive responses in the speakers with typical voices (n = 9; in blue) are negative, compensating for the direction of the perturbation. Individual adaptive responses of nine speakers with hyperfunctional voice disorders (in orange for individuals with accompanying vocal fold lesions and in magenta for those without) are more variable, with many speakers showing a distinct following response (positive changes, in the direction of the perturbation). Adaptive responses are plotted as the mean across five-trial blocks.
Data Analyses
An autocorrelation method via Praat (Boersma & Weenink, 2008) scripts was used to estimate the mean fo over each trial. When fo mistracking occurred (e.g., because of noise such as tongue clicks or instances of glottal fry), the mean fo was obtained manually in Praat by excluding the noise or by adjusting the pitch range settings when the fo mistracking changed the mean estimate more than 2 Hz. The fo of each trial was converted to cents relative to the mean fo during the baseline trials, thus normalizing for individual variation in mean fo. The mean fo values in cents during the shift-up conditions were normalized by subtracting the fo values of the control condition to determine the resulting adaptive responses.
A two-way mixed-model analysis of variance was performed on the adaptive responses to assess the effect of group (random, between-subjects; individuals with typical voices, individuals with hyperfunctional voice disorders), phase (within-subject; baseline, ramp, hold, after-effect), and their interaction. Factor effect sizes were quantified using the squared partial curvilinear correlation, ηp 2. Tukey simultaneous tests were applied to compare groups as a function of phase. In individuals with hyperfunctional voice disorders, average adaptive responses during the hold phase were compared with the ratings of their overall severity of dysphonia using a Pearson's product–moment correlation coefficient.
Results
Consistent with previous studies (Jones & Munhall, 2000, 2005), typical speakers generally showed clear compensatory adaptive responses, with decreased fo during the ramp and hold phases. Conversely, many individuals with hyperfunctional voice disorders did not show this typical adaptive response. Instead, many speakers displayed the opposite effect by following the direction of the perturbation (e.g., when fo was experimentally increased, the speakers responded by further increasing their fo); this is qualitatively apparent in Figure 1. Overall, the adaptive responses showed a significant (p < .001, ηp 2 = .08) interaction between group (individuals with typical voices, individuals with hyperfunctional voice disorders) and phase (Figure 2). As expected, there was not a difference between group responses during the baseline phase (p adj > .05). However, beginning during the ramp, speakers with typical voices displayed compensatory (negative) responses, whereas the average response of individuals with hyperfunctional voice disorders was positive. During the hold phase, although the speakers with typical voices showed an average compensatory response of −55.6 cents (SD = 102.9 cents), the average response of speakers with hyperfunctional voice disorders was 72.5 cents (SD = 176.7 cents). This difference was maintained in the after-effect phase, in which the average response of the speakers with typical voices essentially returned to baseline (M = 5.5 cents, SD = 123.1 cents), whereas the hyperfunctional voice disorder group largely maintained their increased fo values (M = 57.3 cents, SD = 119.1 cents).
Figure 2.
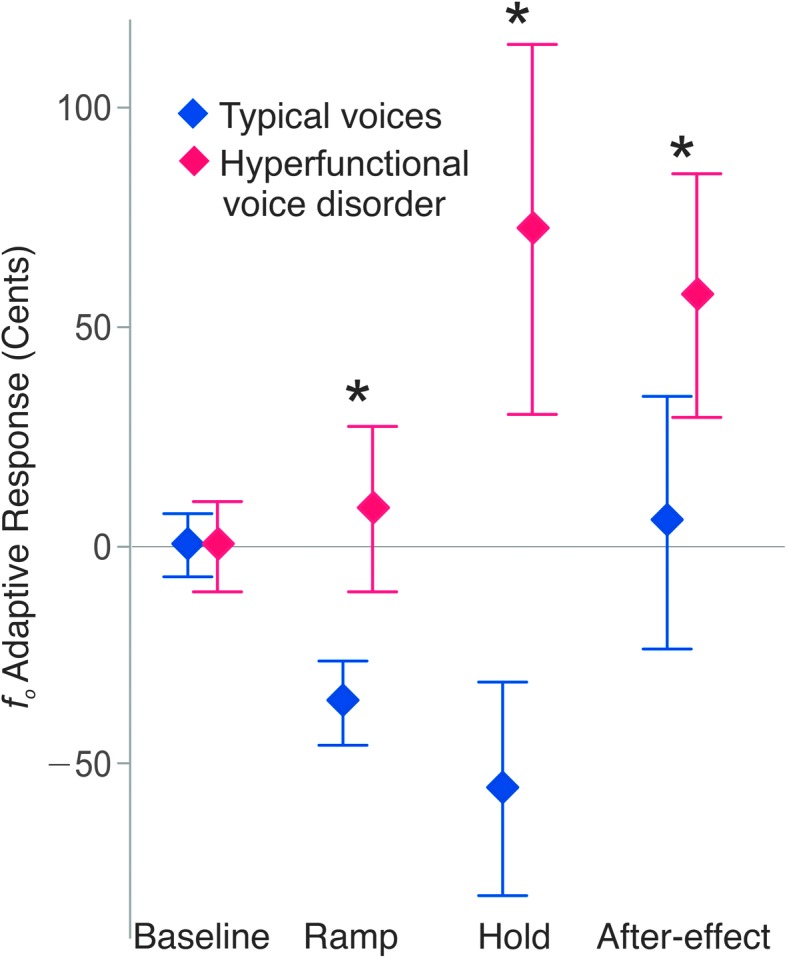
Average adaptive responses of each group as a function of experimental phase. Error bars: 95% confidence intervals. Speakers with hyperfunctional voice disorders had significantly higher adaptive responses (p adj ≤ .001) than speakers with typical voices.
Although four individuals with hyperfunctional voice disorders did show typical compensatory responses (Figure 1), no usual clinical descriptors explained this variation. For instance, as illustrated in Figure 1, the presence of laryngeal lesions was not predictive of abnormal adaptive responses. Furthermore, comparison of average adaptive responses during the hold phase with ratings of the overall severity of dysphonia yielded a nonsignificant Pearson's product–moment correlation coefficient (r = .07). One particular participant had an extremely large, positive adaptive response; however, group reanalysis without this individual's data yielded qualitatively similar statistical results, with a statistically significant interaction between group and phase (p < .001, ηp 2 = .07).
Discussion
Based on these sensorimotor adaptation experimental results, we suggest that some individuals with hyperfunctional voice disorders have disordered auditory-motor integration, with inappropriate updating and maintaining of feedforward vocal control based on auditory feedback. Entrance into the cycle of dysphonia may be precipitated by disruptions in typical voice, whether organic (e.g., an upper respiratory infection or high voice-use situation) or psychological (e.g., situation of emotional stress), but it persists past the original disruption because of this disordered auditory-motor adaptation. This core speech motor control disorder may still interact with the other previously noted factors such as vocal hygiene (Van Houtte et al., 2011), psychological factors (Van Houtte et al., 2011), and reactions to stressors (Demmink-Geertman & Dejonckere, 2002; Helou et al., 2013).
These results mirror previous examinations into a far less prevalent voice disorder known as spasmodic dysphonia (SD), which once had hypothesized psychogenic mechanisms. SD results in a strained and/or breathy voice during speech production, is characterized by muscle spasms (Nash & Ludlow, 1996), and has a higher prevalence in women (Schweinfurth, Billante, & Courey, 2002). In the 1950s, SD was widely believed to be caused “by psychoneurosis from either occupational stress or emotional trauma” (Arnold, 1959, p. 161, as cited in Aronson, 1990), a belief that persisted for decades thereafter (Aminoff, Dedo, & Izdebski, 1978). However, it is now known that SD is a focal dystonia of the larynx, a motor control disorder characterized by abnormal processing of somatosensory feedback (Termsarasab et al., 2016), which continues to lead to research aimed at improving its assessment and treatment. Thus, despite the limited sample size in the current study, the results suggest that much more research is needed to fully delineate the true etiology of hyperfunctional voice disorders to develop unbiased and effective assessments and interventions.
The current study is limited in its ability to provide generalizable and definitive information about the nature of hyperfunctional voice disorders. The sample studied was relatively small, and unsurprisingly, the responses of individuals with hyperfunctional voice disorders were not homogeneous. There may be distinct subtypes of hyperfunctional voice disorders, and the disordered auditory-motor integration mechanism we propose may reflect etiology only in a subset. Furthermore, given the variability in responses, a consideration for data interpretation is the variability in amplification of the auditory feedback (e.g., related to the frequency response of the headphones, level differences between left and right headphones, manipulation of amplitude in Audapter during pitch perturbation, estimated gain correction factor for perturbed signals, processing delay, and variability in speaker productions from trial to trial). Future studies with larger sample sizes, more exhaustive perceptual and instrumental voice assessment, more comprehensive case histories and demographics, and increased control over trial-to-trial auditory feedback are necessary. It is also worth noting that the tasks employed consisted of phonation over two 30-min conditions, which could have differentially resulted in fatigue for speakers with hyperfunctional voice disorders. However, no participants mentioned fatigue, likely because of the minimal actual phonation time during the experiment (approximately 3 s per each of the 160 trials per condition).
Finally, there was a mismatch between the vocal motor control task used in this study and the primary symptomology in hyperfunctional voice disorders. Although individuals with hyperfunctional voice disorders can present with abnormal fo, the primary deviances are those of voice quality. However, voice quality is a multifactorial and complex percept with no robust acoustic correlates, limiting its usefulness in controlled perceptual and production experiments. Thus, we chose fo perturbations for this first sensorimotor adaptation study in hyperfunctional voice disorders to provide increased experimental control and more robust results. However, given that analysis of adaptive responses required relatively periodic voice waveforms, our sample was necessarily limited to mild-to-moderate voices. Individuals with hyperfunctional voice disorders can present with relatively severe voices, which may have affected our findings. Future work to elucidate acoustic measures of voice quality that are reliably related to listener perceptions and are amenable to real-time perturbation will be needed to adapt this work to the realm of voice quality.
Conclusion
The results here are the first evidence that some individuals diagnosed with hyperfunctional voice disorders demonstrate signs of a motor speech disorder. The improper processing of auditory feedback is neurological in nature. Generalization of these findings may justify a radical shift in the clinical treatment of hyperfunctional voice disorders and may eventually allow for the development of new therapies.
Acknowledgments
This work was supported by National Institutes of Health Grants DC015570 and DC004663 from the National Institute on Deafness and Other Communication Disorders. We thank T. Streeter for assistance with equipment calibration, F. H. Guenther for helpful discussions, and R. E. Hillman, J. Van Stan, J. T. Baxter, V. S. McKenna, and V. Ramsumair for patient referrals.
Funding Statement
This work was supported by National Institutes of Health Grants DC015570 and DC004663 from the National Institute on Deafness and Other Communication Disorders.
Footnote
A cent is a logarithmic unit of measure of frequency, like the semitone. 100 cents are equivalent to 1 semitone.
References
- Altman K. W., Atkinson C., & Lazarus C. (2005). Current and emerging concepts in muscle tension dysphonia: A 30-month review. Journal of Voice, 19, 261–267. [DOI] [PubMed] [Google Scholar]
- Aminoff M., Dedo H., & Izdebski K. (1978). Clinical aspects of spasmodic dysphonia. Journal of Neurology, Neurosurgery & Psychiatry, 41, 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends N., Povel D. J., Van Os E., & Speth L. (1990). Predicting voice quality of deaf speakers on the basis of glottal characteristics. Journal of Speech and Hearing Research, 33, 116–122. [DOI] [PubMed] [Google Scholar]
- Arnold G. E. (1959). Spastic dysphonia: I. Changing interpretations of a persistent affliction. Logos, 2(3), 3–14. [Google Scholar]
- Aronson A. E. (1990). Clinical voice disorders: An interdisciplinary approach (3rd ed.). New York, NY: Thieme, Inc. [Google Scholar]
- Boersma P., & Weenink D. (2008). Praat: Doing phonetics by computer (Version 5.0.20). Retrieved from http://www.praat.org/
- Cai S., Boucek M., Ghosh S. S., Guenther F. H., & Perkell J. S. (2008). A system for online dynamic perturbation of formant frequencies and results from perturbation of the Mandarin Triphthong /iau/. Paper presented at the 8th International Seminar on Speech Production, Strasbourg, France. [Google Scholar]
- Cavallo S. A., Dakow C., Schaeffer N., & Wall M. (2002). Speech breathing behavior in normal and moderately to severely dysphonic subjects during connected speech. Journal of Medical Speech-Language Pathology, 10(1), 1–18. [Google Scholar]
- Demmink-Geertman L., & Dejonckere P. H. (2002). Nonorganic habitual dysphonia and autonomic dysfunction. Journal of Voice, 16, 549–559. [DOI] [PubMed] [Google Scholar]
- Dworkin J. P., Meleca R. J., & Abkarian G. G. (2000). Muscle tension dysphonia. Current Opinion in Otolaryngology & Head and Neck Surgery, 8, 169–173. [Google Scholar]
- Forner L. L., & Hixon T. J. (1977). Respiratory kinematics in profoundly hearing-impaired speakers. Journal of Speech and Hearing Research, 20, 373–408. [DOI] [PubMed] [Google Scholar]
- Gillespie A. I., Gartner-Schmidt J., Rubinstein E. N., & Abbott K. V. (2013). Aerodynamic profiles of women with muscle tension dysphonia/aphonia. Journal of Speech, Language, and Hearing Research, 56, 481–488. [DOI] [PubMed] [Google Scholar]
- Helou L. B., Wang W., Ashmore R. C., Rosen C. A., & Abbott K. V. (2013). Intrinsic laryngeal muscle activity in response to autonomic nervous system activation. Laryngoscope, 123, 2756–2765. [DOI] [PubMed] [Google Scholar]
- Higgins M. B., Carney A. E., & Schulte L. (1994). Physiological assessment of speech and voice production of adults with hearing loss. Journal of Speech and Hearing Research, 37, 510–521. [DOI] [PubMed] [Google Scholar]
- Itoh M., Horii Y., Daniloff R. G., & Binnie C. A. (1982). Selected aerodynamic characteristics of deaf individuals during various speech and nonspeech tasks. Folia Phoniatrica, 34, 191–209. [DOI] [PubMed] [Google Scholar]
- Jones J. A., & Keough D. (2008). Auditory-motor mapping for pitch control in singers and nonsingers. Experimental Brain Research, 190, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. A., & Munhall K. G. (2000). Perceptual calibration of F0 production: Evidence from feedback perturbation. Journal of the Acoustical Society of America, 108(3 Pt. 1), 1246–1251. [DOI] [PubMed] [Google Scholar]
- Jones J. A., & Munhall K. G. (2002). The role of auditory feedback during phonation: Studies of Mandarin tone production. Journal of Phonetics, 30, 303–320. [Google Scholar]
- Jones J. A., & Munhall K. G. (2005). Remapping auditory-motor representations in voice production. Current Biology, 15, 1768–1772. [DOI] [PubMed] [Google Scholar]
- Kempster G. B., Gerratt B. R., Verdolini Abbott K., Barkmeier-Kraemer J., & Hillman R. E. (2009). Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. American Journal of Speech Language Pathology, 18, 124–132. [DOI] [PubMed] [Google Scholar]
- Keough D., & Jones J. A. (2009). The sensitivity of auditory-motor representations to subtle changes in auditory feedback while singing. Journal of the Acoustical Society of America, 126, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H., Perkell J., Svirsky M., & Webster J. (1991). Changes in speech breathing following cochlear implant in postlingually deafened adults. Journal of Speech and Hearing Research, 34, 526–533. [DOI] [PubMed] [Google Scholar]
- Lowell S. Y., Barkmeier-Kraemer J. M., Hoit J. D., & Story B. H. (2008). Respiratory and laryngeal function during spontaneous speaking in teachers with voice disorders. Journal of Speech, Language, and Hearing Research, 51, 333–349. [DOI] [PubMed] [Google Scholar]
- Metz D. E., Whitehead R. L., & Whitehead B. H. (1984). Mechanics of vocal fold vibration and laryngeal articulatory gestures produced by hearing-impaired speakers. Journal of Speech and Hearing Research, 27, 62–69. [DOI] [PubMed] [Google Scholar]
- Morrison M. (1997). Pattern recognition in muscle misuse voice disorders: How I do it. Journal of Voice, 11, 108–114. [DOI] [PubMed] [Google Scholar]
- Morrison M. D., Rammage L. A., Belisle G. M., Pullan C. B., & Nichol H. (1983). Muscular tension dysphonia. Journal of Otolaryngology, 12, 302–306. [PubMed] [Google Scholar]
- Morrison M., Rammage L., & Emami A. J. (1999). The irritable larynx syndrome. Journal of Voice, 13, 447–455. [DOI] [PubMed] [Google Scholar]
- Nash E. A., & Ludlow C. L. (1996). Laryngeal muscle activity during speech breaks in adductor spasmodic dysphonia. Laryngoscope, 106, 484–489. [DOI] [PubMed] [Google Scholar]
- Ng J. H., Lo S., Lim F., Goh S., & Kanagalingam J. (2013). Association between anxiety, type A personality, and treatment outcome of dysphonia due to benign causes. Otolaryngology–Head and Neck Surgery, 148, 96–102. [DOI] [PubMed] [Google Scholar]
- Patel R., Niziolek C., Reilly K., & Guenther F. H. (2011). Prosodic adaptations to pitch perturbation in running speech. Journal of Speech, Language, and Hearing Research, 54, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkell J. S., Guenther F. H., Lane H., Matthies M. L., Perrier P., Vick J., … Zandipour M. (2000). A theory of speech motor control and supporting data from speakers with normal hearing and with profound hearing loss. Journal of Phonetics, 28, 233–272. [Google Scholar]
- Read T. E. (1989). Improvement in speech production following use of the UCH/RNID cochlear implant. Journal of Laryngology and Otology. Supplement, 18, 45–49. [PubMed] [Google Scholar]
- Roy N. (2008). Assessment and treatment of musculoskeletal tension in hyperfunctional voice disorders. International Journal of Speech-Language Pathology, 10, 195–209. [DOI] [PubMed] [Google Scholar]
- Roy N., & Bless D. M. (2000). Personality traits and psychological factors in voice pathology: A foundation for future research. Journal of Speech, Language, and Hearing Research, 43, 737–748. [DOI] [PubMed] [Google Scholar]
- Roy N., Bless D. M., & Heisey D. (2000a). Personality and voice disorders: A multitrait-multidisorder analysis. Journal of Voice, 14, 521–548. [DOI] [PubMed] [Google Scholar]
- Roy N., Bless D. M., & Heisey D. (2000b). Personality and voice disorders: A superfactor trait analysis. Journal of Speech, Language, and Hearing Research, 43, 749–768. [DOI] [PubMed] [Google Scholar]
- Schweinfurth J. M., Billante M., & Courey M. S. (2002). Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope, 112, 220–223. [DOI] [PubMed] [Google Scholar]
- Termsarasab P., Ramdhani R. A., Battistella G., Rubien-Thomas E., Choy M., Farwell I. M., … Simonyan K. (2016). Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neuroimage: Clinical, 10, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtte E., Van Lierde K., & Claeys S. (2011). Pathophysiology and treatment of muscle tension dysphonia: A review of the current knowledge. Journal of Voice, 25, 202–207. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Q., Zhang B. R., Su W. Y., Gong J., Yuan M. Q., Ding Y. L., & Rao S. Q. (2012). Laryngeal aerodynamic analysis in assisting with the diagnosis of muscle tension dysphonia. Journal of Voice, 26, 177–181. [DOI] [PubMed] [Google Scholar]


