Abstract
Purpose
We present the first study of echolalia in deaf, signing children with autism spectrum disorder (ASD). We investigate the nature and prevalence of sign echolalia in native-signing children with ASD, the relationship between sign echolalia and receptive language, and potential modality differences between sign and speech.
Method
Seventeen deaf children with ASD and 18 typically developing (TD) deaf children were video-recorded in a series of tasks. Data were coded for type of signs produced (spontaneous, elicited, echo, or nonecho repetition). Echoes were coded as pure or partial, and timing and reduplication of echoes were coded.
Results
Seven of the 17 deaf children with ASD produced signed echoes, but none of the TD deaf children did. The echoic children had significantly lower receptive language scores than did both the nonechoic children with ASD and the TD children. Modality differences also were found in terms of the directionality, timing, and reduplication of echoes.
Conclusions
Deaf children with ASD sometimes echo signs, just as hearing children with ASD sometimes echo words, and TD deaf children and those with ASD do so at similar stages of linguistic development, when comprehension is relatively low. The sign language modality might provide a powerful new framework for analyzing the purpose and function of echolalia in deaf children with ASD.
Echolalia is the phenomenon whereby children repeat or echo the utterances of others. It occurs in some typically developing (TD) children (Volkmar, Paul, Klin, & Cohen, 2005), in individuals with a variety of disorders such as specific language impairment (Gallagher & Craig, 1984; Roberts, 2014), intellectual disability (Bishop, 1989; Cantwell & Baker, 1978; Darley, 1964), Tourette syndrome (Ganos, Ogrzal, Schnitzler, & Münchau, 2012), aphasia (Benson, 1996; Davis, 2007; Pick, 1924), epilepsy (Ganos et al., 2012), stroke (Suzuki, Itoh, Hayashi, Kouno, & Takeda, 2009), closed head injury (Levin, 1982), and in children with blindness (Fay, 1973). However, echolalia is perhaps best known for its occurrence in individuals with autism spectrum disorder (ASD).
In his groundbreaking work documenting the phenomenon of autism, Kanner (1943) noted that some children with autism echo their conversational partner's previous utterance verbatim. Since 1943, many authors (e.g., Paccia & Curcio, 1982; Prizant, 1983; Rydell & Mirenda, 1994; Schuler, 1979; Sterponi & Shankey, 2014; Wootton, 1999) have documented echolalia in the speech of a notable percentage of children with ASD: up to 75% of verbal children with ASD (Rutter & Lockyer, 1967) and 100% of children with ASD 37–54 months of age (Roberts, 2014). It is generally thought that the overall lower receptive language abilities of children with ASD play a role in echolalia (Boucher, 2003; Cantwell, Baker, & Rutter, 1978; Fay & Schuler, 1980; Howlin, 1981; Roberts, 1989; Schreibman & Carr, 1978); children with ASD produce less echolalic language as language skills increase (McEvoy, Loveland, & Landry, 1988; Roberts, 1989). Traditionally, echolalia has been viewed as meaningless, noncommunicative, and self-stimulating (e.g., Fay & Schuler, 1980; Lovaas, 1977; Schreibman & Carr, 1978; Wootton, 1999), although echoes can be used as a resource in conversation to achieve specific ends such as affirming a prior utterance, taking a conversational turn, labeling objects, or making a request (Prizant & Duchan, 1981; Prizant & Rydell, 1984; Rydell & Mirenda, 1994; Sterponi & Shankey, 2014).
Echolalia has often been classified into two categories based on when the echo occurs: immediate versus delayed. Immediate echolalia is the more common type and occurs when a child repeats his interlocutor's utterance immediately after it is produced (Wevrick, 1986). Delayed echolalia is when a child repeats an utterance or a segment from a television show or other media (also known as scripting; Silla-Zaleski & Vesloski, 2010) but with a significant time interval between the initial production of the utterance and its repetition (Simon, 1975). Like immediate echolalia, delayed echolalia has been variously interpreted as meaningless and automatic (e.g., Wolff & Chess, 1965) or as meaningful and functional (Prizant & Rydell, 1984).
Echoes can also be produced with modifications to the original utterance. These mitigated echoes are still considered echoes insofar as they are conversationally inappropriate repetitions of the previous utterance, yet they are qualitatively different from pure echoes. Pick (1924), who first documented mitigated echolalia in patients with aphasia, suggested that such modifications were indicative of the start of the patient's recovery. Fay (1967) later defined mitigated echolalia as the production of echoes that contained words that were either different from or supplemental to the original utterance; such changes can include pronominal substitutions, changes in prosody, semantic substitutions, expansions, or combinations of each of these (Bebko, 1990). Inasmuch as they add linguistic content not included in the original utterance, mitigated echoes may be a bridge from pure echolalia to more creative and productive use of language. Children who produce mitigated echoes have more well-developed language than do children who produce pure echoes (Fay & Butler, 1968; Fay & Coleman, 1977; Shapiro, Roberts, & Fish, 1970), and children's echolalia tends to become more mitigated over time (Roberts, 2014).
The use of manual signs has often been advocated as an alternative or augmentative channel of communication for children for whom spoken language is difficult, including many children with ASD. The literature on the success of such an approach is mixed, although some authors (e.g., Bonvillian & Nelson, 1976) have argued that in certain minimally verbal hearing children with ASD training with signs can succeed when speech training has failed. Bebko (1990) argued that cross-modal (i.e., sign) language training can be a valuable resource in teaching functional communication skills to hearing children who are echolalic. A review of eight studies on the use of signs to teach communication skills to hearing children with ASD (Schwartz & Nye, 2006) indicated a moderate overall treatment effect, although the authors were careful to note that these studies did not provide sufficient information to permit replication and were not generalizable because of the single-subject design of seven of the eight studies. However, regardless of whether such training is effective, the use of signs is common in clinical practice.
Despite the argument that signs are accessible to children with ASD, such children often show deficits in gesture development, particularly in prelinguistic pointing gestures (Özçalışkan, Adamson, & Dimitrova, 2015) and in gestures stemming from joint attention (Mundy, Sigman, & Kasari, 1990). Deficits in gesture use are considered part of the diagnostic criteria for ASD (American Psychiatric Association, 2013). Although signed languages are clearly languages in their own right (Klima & Bellugi, 1979; Stokoe, 1960), with structure at the phonological (e.g., Brentari, 1998; Sandler, 1989), morphological (e.g., Aronoff, Meir, & Sandler, 2000, 2005), and syntactic (e.g., Liddell, 1980; Neidle, Kegl, MacLaughlin, Bahan, & Lee, 2000) levels, they are executed in the same modality as gesture, and the distinction between the two is not always clear. It has recently been proposed that—like speakers who gesture while they talk—signers gesture while they sign; that is, imagistic and nondiscrete gestural elements accompany discrete linguistic elements (Goldin-Meadow & Brentari, 2015). It is thus difficult to state with certainty whether a given sign is purely linguistic or a combination of linguistic and gestural elements. This is a particular problem when we consider communicative signals used by both deaf and hearing people (such as pointing and handwaving).
Despite the relative abundance of research on sign language training in hearing children, there are few reports on the use of sign language by deaf individuals with ASD even though 1 in 59 American deaf children has an ASD diagnosis (Szymanski, Brice, Lam, & Hotto, 2012); a lack of validated instruments for identifying ASD in such children could lead to underdetection (Mood & Shield, 2014) or perhaps misdiagnosis. In a rare report, Jure, Rapin, and Tuchman (1991) described 46 children diagnosed as deaf and autistic. Twenty-one of the children used sign language, and five produced sign language echoes. In a case study, Poizner, Klima, and Bellugi (1990) described a native-signing adult Deaf woman with ASD whose signing consisted almost entirely of immediate sign echoes. These two reports suggest that echolalia is not specific to speech and hearing but rather is a modality-general phenomenon that reflects the gestalt, rote, and nonanalytical way that some individuals with ASD use language. Unfortunately, neither report contains sufficient detail to characterize the phenomenon in an in-depth way.
In the last several years, a novel research population—native–signing children with ASD—has been described in a series of articles (Shield & Meier, 2012; Shield, Meier, & Tager-Flusberg, 2015; Shield, Pyers, Martin, & Tager-Flusberg, 2016). These authors report on children of Deaf parents who are exposed to American Sign Language (ASL) from birth (hence their status as “native” signers). Their exposure to signing is thus rich and lifelong unlike the vast majority of deaf children who are born to hearing parents (Mitchell & Karchmer, 2004) and who may not be exposed to signing, a fact which in itself can lead to cognitive delay (Schick, de Villiers, de Villiers, & Hoffmeister, 2007). The identification of this population of deaf children of Deaf parents has led to intriguing questions about the nature of autistic language. Signing children with ASD exhibit language characteristics similar to those of hearing, speaking children with ASD, for example, a preference for names over pronouns (Shield et al., 2015). Shield and colleagues also found that a minority of deaf children with ASD produced few or no signs at all despite lifelong exposure to ASL from their Deaf parents, just as a minority of hearing children with ASD are minimally verbal (Tager-Flusberg & Kasari, 2013).
In contrast, the fact that signs are produced manually and perceived visually may lead to linguistic behaviors that have no clear analog in speech; one example is the palm orientation reversals that have been identified in the signing of deaf children with ASD (Shield & Meier, 2012). These reversals take a phonological feature of the sign (the direction the palm faces) and change it from inward to outward or vice versa. Hearing, speaking children with ASD have never been found to produce a class of phonological reversals (e.g., the substitution of voiced phonemes for voiceless ones) nor would there be reason to expect such errors because the hypothesized cause of the palm orientation reversals lies in differences in how children with ASD imitate the visually perceptible body movements of others (self–other mapping; Rogers & Pennington, 1991). Thus, even if we consider ASD to have the same etiology in deaf and hearing children, the particular linguistic manifestations may be different in signing.
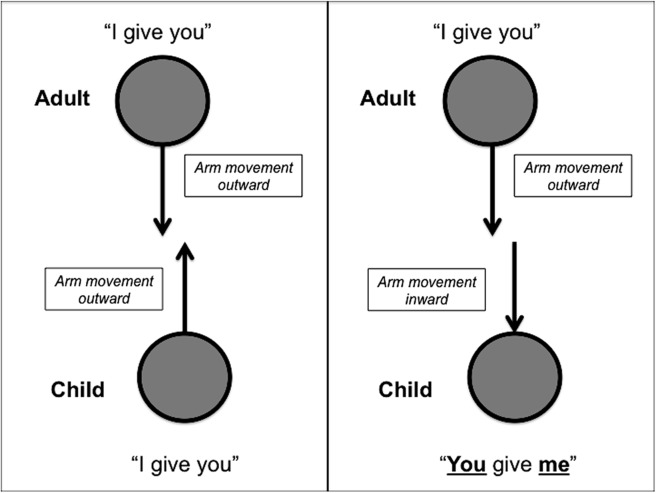
Signs are typically described in terms of the parameters of hand configuration (handshape and palm orientation), movement, and location (Stokoe, 1960). Of particular interest for the purpose of this article is the parameter of movement; arm movements through space can be imitated or echoed in several different ways. Assuming the adult and child are facing each other, as is typical, children may faithfully replicate movements as they are produced by the adult (a pure echo; e.g., a sign produced with movement outward from the adult's body toward the child is reproduced by the child with an analogous movement outward from the child's body toward the adult) or by omitting or changing the direction of movement in some way (a partial echo; i.e., the adult's sign is reproduced by the child either (a) without movement or (b) with a movement in any direction other than outward from the child's body toward the adult). We call such echoes partial rather than mitigated because we do not assume that they reflect a communicative breakthrough. In particular, previous research has shown that deaf and hearing children with ASD sometimes produce signs or imitate gestures as they appear from their own perspective (Ohta, 1987; Shield & Meier, 2012), resulting in reversed palm orientation and/or movement. When deaf children with ASD produce echoes that fit this pattern, some signing children with ASD might produce partial echoes in which the movement of signs is changed to reflect the absolute directionality of movement (see Figure 1) rather than movement that is relative to each signer. Such a change in movement may reflect the child's imitation style rather than an intention to contribute new information to the utterance.
Figure 1.
A pure echo (left) in which the directionality of the sign relative to the signer is maintained and a partial echo (right) in which the absolute directionality of the sign is maintained.
Direction of movement has linguistic value in sign, but this value varies as a function of lexical type. For lexical items such as nouns and plain verbs (which do not inflect), the direction of movement is lexically specified and does not vary; changing the movement direction would render the sign ill-formed. For a second class of verbs, agreement or inflecting verbs, the direction of movement indicates the subject and object of the verb (Padden, 1988). For example, the sign i-give-you starts at the signer's torso and moves outward toward the addressee, whereas you-give-me starts at a distal location away from the signer and then moves inward toward the signer's own body. Thus, partial echoes of this type of sign that preserve the absolute movement direction of the model will result in a shift in the interpretation of subject and object (see Figure 1). A third class of verbs known as spatial verbs (Padden, 1988) differ from the inflecting verbs described above in that the use of space does not designate subject and object but rather spatial referents. For example, a signer can indicate that he or she wants the interlocutor to look in a specific direction by pointing the sign look (using a “V” handshape with the index and middle fingers extended) in the direction of the location that the signer wants the interlocutor to look. For signs of this type, a change in the directionality of movement could result in the loss of this spatial information.
A deeper investigation of echolalia in signing children with ASD is warranted for two main reasons. First, there is a paucity of research on this population, even though ASD is diagnosed at a higher rate in deaf children (1 in 59 American children; Szymanski et al., 2012) than in the general population (1 in 68 children; Centers for Disease Control and Prevention, 2014). Second, such a study can address important questions about the nature of echolalia, specifically:
Does echolalia occur in signing children, and if so, how often?
What is the relationship between echolalia and overall language abilities?
What do sign echoes look like, and are there modality differences between signed and spoken echoes?
In this article, we investigate sign language echolalia in a sample of native-signing deaf children with an ASD diagnosis. We do so to better understand what echolalia is, why it occurs, and how language modality may affect the form of echoes that children produce.
Method
Participants
Children were recruited to participate in the study in two rounds over the course of several years. The goal of the larger project was not to investigate echolalia per se but to examine linguistic and cognitive development in deaf, signing children with ASD. In the pilot study, children were recruited through schools for the deaf in several states; in the main study, children were recruited via a video in ASL posted on social media (https://youtu.be/VeWmb6jLOgg), and research visits were conducted at the child's home or school. All children were raised in households in which ASL was the primary language. Only children born to two Deaf parents were tested; therefore, we were sure that children were raised in a rich signing environment. Parental hearing status and ASL use at home were confirmed via a parent survey and by direct observation when home visits were made.
All children in the ASD group had received an educational or medical diagnosis of ASD. In the pilot study, we did not verify these diagnoses; in the main study, diagnosis was confirmed via the Autism Diagnostic Observation Schedule–Second Edition (ADOS-2; Lord et al., 2012). The ADOS-2 was administered in ASL by two administrators who had attained research reliability on the instrument and were proficient in ASL. The ADOS-2 has not been officially translated into ASL or validated for use with deaf children; thus, this test was administered with modifications to best suit the sign modality according to a description published elsewhere (Shield et al., 2015). Thus, although we feel confident about the classifications of the children in our sample, our use of the ADOS-2 for signing children is not a standard use of the instrument. Although the diagnoses of all children recruited in the main study and reported here were confirmed via the ADOS-2, we also included one child (Child O) recruited in the pilot study who was not given the ADOS-2; thus, her ASD diagnosis was not independently confirmed. We included her because the focus of this study was the phenomenon of signing echolalia regardless of its source.
A total of 17 deaf children with an ASD diagnosis were observed: two in the pilot study and 15 in the main study. 1 One child (Child C) was observed twice, at ages 8;11 (years;months) and 12;7.
A control group of 18 TD deaf children exposed natively to ASL by their Deaf parents was also tested. These children were screened for ASD using the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003). All fell below the clinical threshold score of 11, indicating few potential ASD symptoms; the group mean was 2.39 (SD = 2.35, range = 0–7), which was significantly lower than that of the ASD group (M = 12.94, SD = 6.9, range = 4–31), t(32) = 6.12, p < .0001. Thus, none of the children included in the TD group were considered at risk for ASD. 2 For children in the ASD group who scored under threshold on the SCQ, ASD diagnosis was confirmed using the ADOS-2.
All children recruited into the main study were administered tests of nonverbal intelligence and receptive language abilities. Intellectual ability was measured using the Test of Nonverbal Intelligence–Fourth Edition (TONI-4; Brown, Sherbenou, & Johnsen, 2010). ASL comprehension was measured using the ASL Receptive Skills Test (Enns, Zimmer, Boudreault, Rabu, & Broszeit, 2013), which measures children's understanding of ASL grammar and is appropriate for use with children ages 3 to 13. We report the characteristics of the two groups of participants in Table 1. The two groups were not significantly different for chronological age, t(33) = 0.04, p = .97 (ns), or mental age, t(32) = 1.42, p = .17 (ns). However, the TD children's standard language score (M = 108.7, SD = 6.3, range = 91–116) was significantly higher, t(32) = 6.26, p < .0001, than that of the ASD children (M = 88.2, SD = 12.2, range = 70–113).
Table 1.
Chronological age, nonverbal intelligence (NVIQ), ASL comprehension, and SCQ scores of the TD and ASD groups.
| Group | N | Age, M (SD) | NVIQ, M (SD) | ASL RST, M (SD) | SCQ, M (SD) |
|---|---|---|---|---|---|
| ASD | 17 M, 5 F | 9.27 (2.80) | 96.88 (8.74) a | 88.19 (12.23) a | 12.94 (6.88) |
| TD | 18 M, 10 F | 9.31 (1.77) | 101.56 (10.30) | 108.72 (6.29) | 2.39 (2.35) |
Note. ASL = American Sign Language; SCQ = Social Communication Questionnaire; TD = typically developing; ASD = autism spectrum disorder; M = male; F = female; RST = Receptive Skills Test.
Mean scores do not include one child tested in the pilot study for whom no follow-up was conducted in the main study, because no intelligence or language tests were administered in the pilot study.
Procedure
In the pilot study, children were observed in one session lasting 10–15 minutes. During this session, the experimenter (the first author, a hearing man fluent in ASL) administered a number of experimental tasks designed to elicit specific ASL structures. These tasks included a fingerspelling task, a visual perspective taking task, a lexical elicitation task using pictures, and a task testing the children's ability to learn a novel sign.
In the main study, which took place 4 years later, children with ASD were observed at home or at school in three 1-hr sessions, and the TD children were observed in two 1-hr sessions. The ADOS-2 was administered (with modifications for deaf signing children) in the first session to the ASD group only by a research-reliable ADOS administrator fluent in ASL. In the second and third sessions (first and second sessions for the TD group), all children were tested at home or at school on a battery of cognitive and linguistic tasks, including tasks testing theory of mind, visual perspective taking, fingerspelling, mental rotation, gesture imitation, pronouns, and comprehension of the verb agreement and classifier constructions of ASL.
Coding
Taped data collection sessions were first watched for evidence of any echoed signs by a proficient signer expert in ASD and research reliable on the ADOS-2. The ADOS-2 scoring sheets (which contain an item for scoring echoed language) were consulted for any evidence of echolalia that had been noted by the test administrator. When one or more echoes were observed, the data were coded using ELAN, software for coding multimodal language data (Wittenburg, Brugman, Russel, Klassmann, & Sloetjes, 2006). For data collected in the pilot study, the entire testing session was coded. For data collected in the main study, the ADOS-2 session and one of the experimental tasks (the visual perspective taking task) were coded. The visual perspective taking task was chosen for coding because it was prefaced by a set of signed instructions by the examiner, yielding more opportunities for echoing, whereas most of the other tasks had minimal signed instructions. A second coder recoded 10% of the data to establish interrater agreement. All signs produced by the children were coded for spontaneity, directionality, reduplication, and timing.
Spontaneity of Children's Productions
Each sign was assigned one of four possible values: spontaneous, elicited, echoed, or nonechoed repetition. Signs were coded as spontaneous when they were produced without prompting by the examiner. Signs were coded as elicited when they were produced in response to questions or prompts from the examiner. Signs were coded as echoes when the child repeated the examiner's previous sign within the next conversational turn and the repetition was judged by the rater to be conversationally inappropriate. Signs were coded as nonechoed repetitions when they were repetitions of the examiner's previous sign but were part of a routine (e.g., counting along with the examiner) or were confirmations or corrections of a sign (production of a corrected sign form following correction by an examiner or repetition of a newly learned sign). We thus adopted relatively conservative criteria for the coding of signs as echoes; mere repetition of the adult's previous sign was not enough for an utterance to be coded as an echo.
Directionality
Echoes were coded for their directionality of movement. Echoes that maintained the directionality of signs relative to the signer were coded as pure echoes, and echoes exhibiting changes to the directionality of the adult sign were coded as partial echoes. For example, when the child imitated outward movement from the adult signer as outward movement from the child, a pure echo was coded. In contrast, when the child imitated outward movement from the adult as inward movement to the child, a partial echo was coded (see Figure 1). We coded for directionality in light of previous work showing that some children with ASD change the direction of movement or palm orientation when imitating gestures or signs (Ohta, 1987; Shield & Meier, 2012) and because such movement changes could result in changes in an echo's interpretation.
Reduplication
We coded the number of reduplicated movements or cycles exhibited in children's echoes and compared them with the number of cycles exhibited in the adult's utterance. In previous work, deaf children (Meier, Mauk, Cheek, & Moreland, 2008) and Deaf mothers (Holzrichter & Meier, 2000) sometimes add movement cycles to signs. In a preliminary look at our data, we noted instances in which children added movement cycles as they echoed. Thus, we systematically coded this reduplication.
Timing
After noting instances in which the child's echo began before the adult had ceased signing, we decided to code for the temporal relationship between the adult's sign and the child's echo. The onset and offset of the child's signs were coded in relation to the adult's previous utterances. Echoes were coded as overlapping with the examiner's sign when the first purposeful handshape of the child occurred while the examiner was still producing the sign being echoed. Here, purposeful handshape is defined as the target handshape for the sign, regardless of the location of the hand itself.
Results
A careful review of the recordings revealed that none of the TD children produced any sign echoes, so none of their data were coded further. Of the 17 deaf children with ASD, seven showed evidence of manual echolalia. For these seven echoic children, 214 min plus 41 s of video-recorded assessments were coded in ELAN, yielding a total of 566 signs. Of those, we observed 146 spontaneous signs (25.8% of the sample), 146 echoes (25.8%), 226 elicited signs (39.93%), and 48 nonechoed repetitions (8.48%). Interrater agreement on 10% of the data (21 minutes of three participants) was .85 for sign type; Cohen's κ = .8, indicating excellent agreement. Table 2 summarizes the sign production of the seven echoic children.
Table 2.
Number (proportion) of signs coded for each child producing echoes.
| Child | Age (years;months) | Number of signs coded | Duration of video coded (min:s) | Echoes | Elicited signs | Nonecho repetitions | Spontaneous signs |
|---|---|---|---|---|---|---|---|
| A | 5;1 | 97 | 19:13 | 5 (.05) | 21 (.22) | 6 (.06) | 65 (.67) |
| I | 5;3 | 65 | 32:08 | 2 (.03) | 7 (.11) | 6 (.09) | 50 (.77) |
| V | 7;1 | 39 | 38:52 | 6 (.15) | 20 (.51) | 6 (.15) | 7 (.18) |
| L | 9;5 | 89 | 30:11 | 68 (.76) | 6 (.07) | 5 (.06) | 10 (.11) |
| R | 9;8 | 7 | 30:26 | 1 (.14) | 0 | 0 | 6 (.86) |
| O | 11;9 | 188 | 15:47 | 39 (.21) | 122 (.65) | 20 (.11) | 7 (.04) |
| C1 a | 8;11 | 58 | 11:22 | 25 (.43) | 28 (.48) | 5 (.09) | 0 |
| C2 | 12;7 | 23 | 36:52 | 0 | 22 (.96) | 0 | 1 (.04) |
| Total | 566 | 214:41 | 146 (.26) | 226 (.40) | 48 (.08) | 146 (.26) | |
Child C was tested in both the pilot study (C1) and the main study (C2), at an interval of 3 years and 8 months.
We compared the results for the seven echoic children with ASD with those for the 10 nonechoic children with ASD on five dimensions: age, nonverbal intelligence, receptive language, and ADOS-2 and SCQ raw scores (see Table 3). We found that the echoic children had lower receptive ASL skills (M SS = 79.3, SD = 8.5) than did the nonechoic children with ASD (M SS = 93.5, SD = 11.22), t(14) = 2.66, p = .02. The echo-producing children were also nonsignificantly younger (M = 8.69 years, SD = 2.98 years) than the nonechoic children (M = 9.68 years, SD = 2.75 years), t(15) = 0.71, p = .49 (ns). There were no significant differences between the groups in terms of nonverbal intelligence, t(14) = 0.13, p = .90 (ns), ASD severity as indicated by ADOS-2 total score, t(14) = 0.49, p = .63 (ns), or SCQ score, t(14) = 0.39, p = .70 (ns).
Table 3.
Comparison of the children with ASD who echoed and children with ASD who did not echo on the basis of age, nonverbal intelligence (NVIQ), ASL comprehension, and two measures of ASD.
| Child group | N | Age, M (SD) | NVIQ, M (SD) | ASL RST, M (SD) | ADOS-2, M (SD) | SCQ, M (SD) |
|---|---|---|---|---|---|---|
| Echoers | 7 a | 8.69 (2.98) | 96.50 (12.49) | 79.33 (8.48) | 12.83 (6.43) | 13.83 (9.41) |
| Nonechoers | 10 | 9.68 (2.75) | 97.10 (6.37) | 93.50 (11.22) | 11.50 (4.58) | 12.40 (5.38) |
Note. ASD = autism spectrum disorder; ASL = American Sign Language; RST = Receptive Skills Test; ADOS-2 = Autism Diagnostic Observation Schedule–Second Edition; SCQ = Social Communication Questionnaire.
One child in the echo group was tested in the pilot study only, before measures of intelligence, language, and ASD were collected. For this child, only age is included in the calculations.
We also matched the echoic children for age and nonverbal intelligence with six TD children from the control group and found that the echoic children had significantly lower ASL comprehension than did the age- and IQ-matched TD children, t(10) = 7.2, p < .0001 (see Table 4).
Table 4.
Comparison of the deaf children with ASD who echoed and TD deaf children on the basis of age, nonverbal intelligence (NVIQ), and ASL comprehension
| Child group | N | Age, M (SD) | NVIQ, M (SD) | ASL RST, M (SD) |
|---|---|---|---|---|
| TD | 6 | 8.74 (2.02) | 97.67 (12.24) | 108.67 (5.35) |
| ASD echoers | 6 | 8.18 (2.92) | 96.50 (12.49) | 79.33 (8.48) |
Note. ASD = autism spectrum disorder; TD = typically developing; ASL = American Sign Language; RST = Receptive Skills Test.
We also analyzed the kinds of signs that children echoed. The 146 echoes consisted of 57 verbs (39.0%), 42 nouns (28.8%), 19 pronouns (13.0%), 12 adjectives (8.2%), four question words (2.7%), and 12 signs categorized as “other” (8.2%). The “other” category included items such as greetings (bye-bye), adverbs (now), and discourse markers such as #ok. 3 All of the echoed pronouns were echoes of the first-person pronoun me, and all were echoed as me, except for one token echoed as you, which is discussed in the next section.
Directionality
We analyzed the extent to which echoes were exact repetitions of the examiner's signs or whether the children changed the directionality of the signs. Of the 146 echoes, 133 (91.1%) maintained the same articulation as the original sign (pure echoes) and the other 13 echoes (8.9%) included a modification to the original sign (partial echoes). The 13 partial echoes were produced by three of the echoic children: Children O and L each produced five partial echoes, and Child C produced three. Eleven of these echoes were verbs, one was a noun (turtle), and one was a pronoun (you). For a detailed description of each partial echo, see the Appendix. We did not find changes in directionality in the spontaneous signs, elicited signs, or nonechoed repetitions.
We also found one instance of a pure echo of a pronoun combined with a partial echo of a directional verb. In this instance, Child C echoed the signed phrase you, you-copy-me in the following manner: you (pure echo) i-copy-you (partial echo).
Reduplication
We analyzed whether the signed echoes exhibited increased movement cycles with respect to the adult sign model. Seventeen of 146 echoed signs (11.6%), which were produced by three of the children with ASD, exhibited increased movement cycles compared with the input sign. The average adult sign in these examples exhibited 1.7 (SD = 0.7) cycles, and the average child echo exhibited 4.9 (SD = 2.4) cycles, a difference of 3.2 (SD = 2.0) cycles per sign. To illustrate one example, the sign more consists of the two hands, each in an O-configuration, moving to contact at the fingertips; in one instance the examiner brought her fingertips together twice, while in his echo Child L brought his fingertips together six times; see Table 5.
Table 5.
Echoes exhibiting increased movement cycles compared with the adult form.
| Child | Sign | Number of cycles produced |
Difference a | |
|---|---|---|---|---|
| Examiner | Subject | |||
| V | show | 2 | 6 | 4 |
| L | more | 2 | 6 | 4 |
| L | funny | 2 | 3 | 1 |
| O | show | 1 | 3 | 2 |
| O | game | 1 | 2 | 1 |
| O | doll | 2 | 9 | 7 |
| O | name | 2 | 6 | 4 |
| O | picture | 1 | 6 | 5 |
| O | match | 1 | 2 | 1 |
| O | show | 1 | 2 | 1 |
| O | picture | 1 | 3 | 2 |
| O | show | 1 | 3 | 2 |
| O | word | 3 | 9 | 6 |
| O | fingerspell | 3 | 8 | 5 |
| O | invent | 2 | 4 | 2 |
| O | copy | 2 | 6 | 4 |
| O | your-turn | 1 | 4 | 3 |
| M (SD) | 1.7 (0.7) | 4.9 (2.4) | 3.2 (2.0) | |
Number of cycles executed by the child in excess of the adult form.
In addition to these reduplicated echoes, all seven echoic children produced increased movement cycles on at least one nonechoic sign, for a total of 32 reduplicated nonechoes (18 spontaneous signs, 13 elicited signs, and one nonecho repetition). In total, the echoic children produced multiple or increased movement cycles on 49 of the 566 (8.7%) signs they produced, although only the echoes and nonechoed repetitions can be compared with the adult's immediate prior production.
Timing
We analyzed two aspects of the timing of signed echoes: (a) delay: how quickly the child initiated the echo after the onset of the adult's sign and (b) overlap: whether the child's sign was initiated before the adult had finished signing. The average delay between the onset of the examiner's sign and the child's echo was 1.11 s (SD = 1.06 s). Of the 146 echoes, 75 (51.4%) overlapped with the examiner's signs. Four of the seven echoing children produced echoes that overlapped with the examiner's sign; 32 of Child L's 68 echoes (47.1%) overlapped with the examiner's original sign, 17 of Child C's 25 echoes (68%) overlapped; 24 of Child O's 39 echoes (61.5%) overlapped; and two of Child V's six echoes (33.3%) overlapped. The average duration of overlap for these 75 echoes was 0.62 s (SD = 0.53 s). We also checked whether the nonechoed repetitions overlapped with the adult's previous signs; 26 of 48 (54.2%) nonechoed repetitions overlapped with the adult's previous sign, and the average overlap on these repetitions was 1.74 s (SD = 1.49 s).
Discussion
Incidence of Echolalia in Signing Children With ASD
We present the first study to examine whether deaf children with ASD who are exposed to a sign language from birth echo signs. A subset of the deaf, native-signing children—seven of 17 (41%)—produced signed echoes. The prevalence of sign language echolalia in our sample is somewhat higher than that in the one previous report of sign language echolalia, in which five of 21 (24%) signing children with ASD echoed signs (Jure et al., 1991), although small sample sizes in both studies limit our ability to draw conclusions about the overall prevalence of the phenomenon. We add to Jure et al.'s earlier report by demonstrating that sign echolalia is not a result of late or impoverished exposure to a signed language since all of the children in our sample had Deaf, signing parents. We have found that a significant minority of signing children echo signs, even when they have been brought up in the richest possible signing environment from the day they were born. We confirm, as suggested by Jure et al., that echolalia is a feature of language in ASD and is not a by-product of speech or hearing. Rather, it is an aspect of how some children with ASD produce language, be it signed or spoken. We cannot determine at this time whether sign echolalia occurs at the same rate as speech echolalia. Reports on hearing children with ASD have suggested that 75%–100% of such children produce echoes, especially before age 5 years (Roberts, 2014; Rutter & Lockyer, 1967). The children in our sample were older, and future work should investigate younger signing children with ASD to address this question.
The children with ASD who produced sign language echoes had significantly lower receptive language skills than did the nonechoic children with ASD; their receptive language scores were also significantly lower than the age- and IQ-matched TD controls. Neither age, nor intelligence, nor ASD severity were related to echolalia. Thus, echolalia appears to occur at a stage in both sign and speech development when language comprehension is low.
We observed one child (C) in both the pilot and main studies. In the pilot, 25 of his 58 signs (43.1%) were echoes, but 3 years and 8 months later he no longer produced any echoes in a much longer language sample. Although the information for this child represents our only longitudinal data point, we believe this finding supports the idea that echolalia decreases as linguistic competence increases.
Mitigated Echolalia
In line with previous research on spoken language echolalia, we find evidence in our data of echoes both with and without modification (i.e., partial and pure echoes). Mitigated echoes have been interpreted as a step forward from purely echoic language toward more creative and productive speech, especially when pronouns are appropriately modified (Bebko, 1990; Fay, 1979). We find several instances that fit this pattern; three agreement verbs and one pronoun were partially echoed with a 180° reversal of movement, yielding perfectly interpretable, situationally correct meanings (see the Appendix). For example, when the examiner signed i-show-you, the child's echo (with inward movement toward his own body) could be interpreted as you-show-me.
However, other echoes in our data set are inconsistent with the hypothesis that changes in movement reflect a communicative breakthrough. First, the deletion of movement in a second token of the verb i-show-you and the change in movement direction in the verb you-copy-me resulted in echoes with no clear subject or object. Similarly, the echo of the verb ask (in which the examiner produced the verb in the direction of the kitchen, where the mother was located, meaning “ask your mother”) was produced with movement toward the left side of the child's body and away from the kitchen, with consequent loss of the object referent. Likewise, the spatial verb look was rendered uninterpretable when echoed with a change in direction; the examiner directed the sign toward the object on the table between him and the child (meaning “look at the object on the table”), whereas the child echoed with the sign pointing away from the object on the table. The noun turtle and plain verb blow-out were rendered ill-formed when echoed with a change in movement, because the direction of movement of these signs is lexically specified and cannot be changed.
Thus, we find it unlikely that all of the partial echoes in our sample are mitigated echoes that represent an advance over purely echoic language. Instead, some of the partial echoes in our data seem to be the result of imitation by the child without comprehension. The significant difference in receptive language comprehension between the echoic and nonechoic children with ASD supports this hypothesis, and the three children who produced the partial echoes were among the lowest scoring children in the entire sample (note that Child O participated in only the pilot study and thus was not tested for receptive language).
The interesting effect of language modality is evident. Different sign types require different imitation strategies. Plain verbs and nouns never change their movement direction, so children must learn to produce them with the same movement produced by the adult model, regardless of differences in perspective. Thus, children must come to understand that changes in movement to such signs render them ill-formed and uninterpretable. By contrast, agreement verbs change movement direction depending on subject and object (e.g., i-show-you vs. you-show-me), so children must come to understand that changes in movement do not render signs uninterpretable but rather change the semantic content of the utterance. Thus, the ability to distinguish between these different lexical types and use movement appropriately for linguistic purposes requires relatively advanced morphosyntactic competence. Given the variety of errors found in the data and the low receptive language scores of the echoic children, we find it doubtful that these children possessed this knowledge about different lexical types in ASL. Our conclusions are tentative given the limited data set. However, the different imitation possibilities in the sign modality broaden our ability to investigate how children with ASD echo and imitate. Future work with signing children should specifically test hypotheses about the nature of such errors.
Differences Between Signed and Spoken Echoes
We documented 19 instances of echoed pronouns, all echoes of the first-person pronoun me. Eighteen of these pronouns were pure echoes (me) and one was a partial echo (you). These echoes are reminiscent of the pronoun reversals associated with ASD (i.e., using the first-person pronoun I or me to indicate the interlocutor). Again, we find it unlikely that the children intended to refer in these instances. First, it is much more typical for children with ASD to use second-person pronouns for self-reference than first-person pronouns for reference to others, probably because the child is most often addressed with the second-person pronoun you (Dale & Crain-Thoreson, 1993; Evans & Demuth, 2012; Kanner, 1943). Second, the echolalic contexts in which all but one of these pronouns appeared suggest noncomprehension by the children. Thus, the evidence for true spontaneous pronoun reversals in the signing of children with ASD is very thin. The very few documented instances of pronoun reversals in sign (e.g., Petitto, 1987) were produced by much younger TD deaf children who apparently interpreted the indexical pronouns as lexical signs (i.e., as names).
Two other potential differences were found between signed and spoken echoes. Children produced an increased number of sign cycles compared with the adult model, and children produced echoes that overlapped temporally with the adult sign. We know of no analogy for either phenomenon in speech; hearing children with ASD do not tend to reduplicate syllables of the words they are echoing (though reduplication does occur very early on in typical phonological development; Ferguson, 1983) nor do they echo words at the same time that their interlocutors are still in the process of producing them.
With regard to reduplicated movement cycles in the children's echoes (and other signs), reduplication of monosyllabic 4 manual signs can also occur in typical development. For example, the sign more (as described above) may be repeated for an unspecified number of movement cycles (somewhat analogous to drawing out a vowel in speech, as in mooooooore). Very young TD deaf children often produce multiple cycles of manual movements when babbling or signing (Meier et al., 2008), and Deaf mothers sometimes increase cyclicity when signing to young children (Holzrichter & Meier, 2000). Both echoic and nonechoic children with ASD produced reduplicated sign movements, whether spontaneous or echoed. Thus, while we note this unique feature of sign language development, reduplication in sign is not limited to echolalia. However, the tendency of children with ASD to perseverate in their movements (a core symptom) may lead to increased reduplication of movement cycles in signing. More work in the future is needed to test the relationship between other perseverative behaviors in ASD and sign reduplication.
For the issue of temporal overlap, there appears to be a clear modality difference between signing and speech. We know of no reports of speech echolalia in which children echo words before the word or phrase has been completely produced by the children's interlocutors, possibly for several reasons. First, the visual-gestural modality of signing makes it possible to produce signs at the same time that another person is still signing without creating interference, whereas speaking at the same time that another person is speaking creates disruptive sensory interference. The children in our sample overlapped with the adult on a significant proportion of both echoes and nonechoed repetitions, suggesting that overlap is not just a property of sign echolalia. However, it is possible that the adult examiners in the study, who were not native signers, could have been signing more slowly than usual, whether because of their nonnative abilities or in an attempt to facilitate the children's comprehension. Signs are generally produced about twice as slowly as words because of the relatively massive size of the sign articulators (Klima & Bellugi, 1979), providing more opportunity for temporal overlap. Temporal overlap could thus be more likely in signing than in speech, although future work should directly compare the two.
Implications for Theory and Practice
Our results clearly show that some deaf children are echolalic in signing, despite rich and early exposure to ASL. This finding highlights the fact that children who echo language do so regardless of the modality in which the language is expressed, and they do so at similar stages in linguistic development, when comprehension is low. Because the children in our sample are part of the tiny minority of deaf children who have Deaf parents, we hypothesize that sign echolalia could be more common among deaf children of hearing parents, who make up 90%–95% of the deaf pediatric population (Mitchell & Karchmer, 2004). These children are likely to have more linguistic and cognitive difficulties than are deaf children of Deaf parents (Schick et al., 2007) because of a relatively impoverished home language environment.
Although we cannot directly comment on whether signed communication should be used either as an alternative to speech or as a bridge to spoken language, we find no compelling reason to think that signing is a more accessible method of communication for hearing children whose speech consists largely of echoes. Language is language, and a mature use of language requires the flexible and creative manipulation of linguistic symbols—words or signs—rather than their rote, gestalt production. However, although we cannot say with certainty whether sign language training could be of benefit to some hearing children through practice with communicative symbols in a different modality, we certainly advocate the use of signing with children for whom the speech signal is not readily accessed, that is, children who are deaf or hard of hearing. However, this study presents little reason to expect that children who are echolalic in speech would not also show the same patterns in signing. Given the paucity of research in this area, future work should investigate how some echolalic signing children develop more creative language over time, as did Child C in our sample.
We also do not know whether signing children employ immediate or delayed sign echoes communicatively, as some echolalic hearing children do with spoken language echoes, for example to affirm a prior utterance, take a conversational turn, label objects, or make a request (Prizant & Duchan, 1981; Prizant & Rydell, 1984; Rydell & Mirenda, 1994; Sterponi & Shankey, 2014). A study of delayed echoes in such children could be particularly important for understanding how such a communicative style might manifest and be understood in deaf, signing children. We investigated only immediate sign echoes.
From a theoretical perspective, this study underscores the fact that language is an amodal brain process that leads to a similar phenomenon in signing and speech. We find strong evidence for echolalia being a largely modality–independent process that is a characteristic of the language of some children on the autism spectrum, particularly those with low receptive language—regardless of whether they speak or sign. Despite this underlying similarity, the channel through which language is expressed (the hands or the vocal tract) has an effect on the form of the echoes. We have highlighted three such modality differences here: directionality, reduplication, and timing. The modality differences between signing and speech could also affect the incidence of echolalia, although we do not currently claim to know whether this is the case.
Limitations of the Current Study
Small sample sizes are always an issue when dealing with such a rare population as native-signing children with ASD. Although our sample of 17 such children is quite large given the circumstances, we cannot be certain that the prevalence of echolalia that we found in our sample is reflective of the entire population of such children. We also cannot know whether socioeconomic status plays a role in our sample because this information was not collected. On average, families with deaf children are socioeconomically disadvantaged compared with the general population (Boss, Niparko, Gaskin, & Levinson, 2011), and this fact may negatively affect linguistic and cognitive outcomes (Macaulay & Ford, 2013).
This study was not designed to study echolalia per se; the purpose of both the pilot and main studies was to investigate questions related to perspective taking. The analysis of echoes was thus conducted post hoc, after we noticed the presence of these echoes. Thus, we relied on naturalistic rather than experimental methods. In future work, tasks should be designed to experimentally test hypotheses of interest, such as the difference between echoes of inflecting and spatial verbs that we have discussed. In this way, we will be more certain whether the echoes observed are examples of mitigation or of imitation without comprehension.
A further limitation relates to imitation. Children with ASD often show differences (e.g., Ohta, 1987; Rogers & Pennington, 1991) and deficits (Williams, Whiten, & Singh, 2004) in the imitation of manual gestures. We did not test the imitation abilities of the children in our sample, and difficulties with imitation could be responsible for some of the unique echoes described. Thus, we urge caution in the interpretation of our data.
Although we went to great lengths to verify the diagnosis of ASD in the main study, we were unable to confirm the diagnosis of one child who participated in the pilot study (Child O). This participant produced the greatest number of echoes described here. We reiterate that our primary focus was on the phenomenon of sign echolalia, regardless of the syndrome that may explain its occurrence.
Conclusion
This is the first study of sign language echolalia in deaf children exposed to ASL from birth by their Deaf parents. Deaf children with ASD sometimes echo signs, just as hearing children with ASD sometimes echo words, and both deaf and hearing children do so at similar stages of linguistic development, when comprehension is relatively low. However, the visual-gestural modality of signing differs in several key ways from the vocal-auditory modality of speech, and differences were found in the directionality, reduplication, and timing of signed echoes. In particular, the various possibilities for imitation in signing shed new light on the possible motivations for echolalia and on the nature of language and communication in children with ASD in general. Studies of deaf children with ASD (and other types of disorders) help clarify the nature of these disorders by helping researchers understand which phenomena are truly caused by deficits in language development and which are merely by-products of the vocal-auditory modality of speech.
Acknowledgments
This research was begun while the first author was a postdoctoral research fellow in the Department of Psychological and Brain Sciences at Boston University. Financial support was provided by the National Institute on Deafness and Other Communication Disorders (Grant F32DC011219 to the first author) and the Autism Science Foundation (Grant REG 14-04 to the first author). We thank H. Tager-Flusberg for research support, T. Sampson for research assistance, C. Enns for use of the ASL Receptive Skills Test, and D. Mood and S. Butler Koestler for ADOS-2 administration.
Appendix
Partial Echoes Produced by Three Children
| Sign type | Adult sign gloss a | Description of adult sign | Child echo gloss a | Description of child echo | Child | Number of instances |
|---|---|---|---|---|---|---|
| Inflecting verb | your-turn | Outward movement from adult to child | my-turn | Movement from right to left across child's body followed by inward movement from adult toward child | O | 1 |
| you-copy-me | Outward movement from adult to child | copy | Movement from right to left across child's body | O | 1 | |
| you-copy-me | Outward movement from adult toward child's body | i-copy-you | Inward movement from adult toward child's body | O | 1 | |
| i-show-you | Outward movement from adult toward child's body | you-show-me | Inward movement from adult toward child's body | O | 1 | |
| i-show-you | Outward movement from adult toward child's body | show | Static; no movement | O | 1 | |
| ask mom | Movement toward examiner's left shoulder (in direction of kitchen, where mother was) | ask | Movement toward child's left shoulder, away from location of mother | L | 1 | |
| Spatial verb | i look x | Fingers outward toward child | i look y | Fingers inward toward child | L | 1 |
| i look x | Fingers outward toward child and middle of table | i look y | Fingers facing left side of child | C | 1 | |
| Plain verb | blow-out | Movement outward from examiner's body toward child's body | [unintelligible] | Movement toward child's left side and inward | L | 3 |
| Pronoun | me | Index finger pointed at adult | you [ill-formed] | Fingers pointed at adult | O | 1 |
| Noun | turtle | Thumb facing outward toward child's body | turtle [ill-formed] | Thumb facing left side of child's body | C | 1 |
Subscript letters x and y refer to different spatial loci (typically in the space in front of the signer), as is conventional in the sign language literature.
Funding Statement
This research was begun while the first author was a postdoctoral research fellow in the Department of Psychological and Brain Sciences at Boston University. Financial support was provided by the National Institute on Deafness and Other Communication Disorders (Grant F32DC011219 to the first author) and the Autism Science Foundation (Grant REG 14-04 to the first author).
Footnotes
The 15 deaf children with ASD included in the main study and the 18 TD children have been described in two other published studies (Shield et al., 2015, 2016).
Because of a limited budget, the children in the TD group were not given the ADOS-2 to rule out ASD classification.
As is conventional in the signing literature, a fingerspelled sign is denoted with the pound sign (#).
Although a strict analogy between sign and spoken language phonology is difficult to make because of the difference in modality, most scholars agree that a syllable in sign language consists of a hand movement and a location or a movement between two locations (Perlmutter, 1992; Sandler, 1989). Unlike in spoken languages, most signs are monosyllabic (Brentari, 1998), although multisyllabic signs are certainly possible, especially in compound signs.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Aronoff M., Meir I., & Sandler W. (2000). The universal and the particular in sign language morphology. University of Maryland Working Papers in Linguistics, 10, 1–24. [Google Scholar]
- Aronoff M., Meir I., & Sandler W. (2005). The paradox of sign language morphology. Language, 81, 301–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko J. M. (1990). Echolalia, mitigation, and autism: Indicators from child characteristics for the use of sign language and other augmentative language systems. Sign Language Studies, 66, 61–78. [Google Scholar]
- Benson D. F. (1996). Aphasia: A clinical perspective. New York, NY: Oxford University Press. [Google Scholar]
- Bishop D. V. M. (1989). Autism, Asperger's syndrome, and semantic-pragmatic disorder: Where are the boundaries? British Journal of Disorders of Communication, 24, 107–121. [DOI] [PubMed] [Google Scholar]
- Bonvillian J. D., & Nelson K. E. (1976). Sign language acquisition in a mute autistic boy. Journal of Speech and Hearing Disorders, 41, 339–347. [DOI] [PubMed] [Google Scholar]
- Boss E. F., Niparko J. K., Gaskin D. J., & Levinson K. L. (2011). Socioeconomic disparities for hearing-impaired children in the United States. Laryngoscope, 121, 860–866. https://doi.org/10.1002/lary.21460 [DOI] [PubMed] [Google Scholar]
- Boucher J. (2003). Language development in autism. International Journal of Pediatric Otorhinolaryngology, 67, S159–S163. [DOI] [PubMed] [Google Scholar]
- Brentari D. (1998). A prosodic model of sign language phonology. Cambridge, MA: MIT Press. [Google Scholar]
- Brown L., Sherbenou R. J., & Johnsen S. K. (2010). Test of Nonverbal Intelligence–Fourth Edition (TONI-4). Austin, TX: Pro-Ed. [Google Scholar]
- Cantwell D., Baker L., & Rutter M. (1978). A comparative study of infantile autism and specific developmental receptive language disorder—IV. Analysis of syntax and language function. Journal of Child Psychology and Psychiatry, 19, 351–362. [DOI] [PubMed] [Google Scholar]
- Cantwell D. P., & Baker L. (1978). Imitations and echoes in autistic and dysphasic children. Journal of the American Academy of Child Psychiatry, 17, 614–624. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report, 63, 1–21. [PubMed] [Google Scholar]
- Dale P. S., & Crain-Thoreson C. (1993). Pronoun reversals: Who, when, and why? Journal of Child Language, 20, 573–589. [DOI] [PubMed] [Google Scholar]
- Darley F. L. (1964). Diagnosis and appraisal of communication disorders. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Davis G. A. (2007). Aphasiology: Disorders and clinical practice. Boston, MA: Allyn & Bacon. [Google Scholar]
- Enns C. J., Zimmer K., Boudreault P., Rabu S., & Broszeit C. (2013). American Sign Language: Receptive Skills Test. Winnipeg, Manitoba, Canada: Northern Signs Research. [Google Scholar]
- Evans K. E., & Demuth K. (2012). Individual differences in pronoun reversal: Evidence from two longitudinal case studies. Journal of Child Language, 39, 162–191. [DOI] [PubMed] [Google Scholar]
- Fay W. H. (1967). Mitigated echolalia of children. Journal of Speech, Language, and Hearing Research, 10, 305–310. [DOI] [PubMed] [Google Scholar]
- Fay W. H. (1973). On the echolalia of the blind and of the autistic child. Journal of Speech and Hearing Disorders, 38, 478–489. [DOI] [PubMed] [Google Scholar]
- Fay W. H. (1979). Personal pronouns and the autistic child. Journal of Autism and Developmental Disorders, 9, 247–260. [DOI] [PubMed] [Google Scholar]
- Fay W. H., & Butler B. (1968). Echolalia, IQ, and the developmental dichotomy of speech and language systems. Journal of Speech, Language, and Hearing Research, 11, 365–371. [DOI] [PubMed] [Google Scholar]
- Fay W. H., & Coleman R. (1977). A human sound transducer: Temporal capabilities of a profoundly echolalic child. Brain and Language, 4, 396–402. [DOI] [PubMed] [Google Scholar]
- Fay W. H., & Schuler A. (1980). Emerging language in autistic children. Baltimore, MD: University Park Press. [Google Scholar]
- Ferguson C. A. (1983). Reduplication in child phonology. Journal of Child Language, 10 https://doi.org/10.1017/S0305000900005274 [DOI] [PubMed] [Google Scholar]
- Gallagher T. M., & Craig H. K. (1984). Pragmatic assessment: Analysis of a highly frequent repeated utterance. Journal of Speech and Hearing Disorders, 49, 368–377. [DOI] [PubMed] [Google Scholar]
- Ganos C., Ogrzal T., Schnitzler A., & Münchau A. (2012). The pathophysiology of echopraxia/echolalia: Relevance to Gilles De La Tourette syndrome. Movement Disorders, 27, 1222–1229. [DOI] [PubMed] [Google Scholar]
- Goldin-Meadow S., & Brentari D. (2015). Gesture, sign and language: The coming of age of sign language and gesture studies. Behavioral and Brain Sciences, 1–82. https://doi.org/10.1017/S0140525X15001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzrichter A. S., & Meier R. P. (2000). Child-directed signing in American Sign Language. In Chamberlain C., Morford J. P., & Mayberry R. I. (Eds.), Language acquisition by eye (pp. 25–40). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Howlin P. A. (1981). The effectiveness of operant language training with autistic children. Journal of Autism and Developmental Disorders, 11, 89–105. [DOI] [PubMed] [Google Scholar]
- Jure R., Rapin I., & Tuchman R. (1991). Hearing-impaired autistic children. Developmental Medicine and Child Neurology, 33, 1062–1072. [DOI] [PubMed] [Google Scholar]
- Kanner L. (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250. [PubMed] [Google Scholar]
- Klima E. S., & Bellugi U. (1979). The signs of language. Cambridge, MA: Harvard University Press. [Google Scholar]
- Levin H. S. (1982). Neurobehavioral consequences of closed head injury. New York, NY: Oxford University Press. [Google Scholar]
- Liddell S. K. (1980). American Sign Language syntax. The Hague, the Netherlands: Mouton. [Google Scholar]
- Lord C., Rutter M., DiLavore P. C., Risi S., Gotham K., & Bishop S. L. (2012). Autism Diagnostic Observation Schedule–Second Edition (ADOS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Lovaas O. I. (1977). The autistic child: Language development through behavior modification. New York, NY: Irvington. [Google Scholar]
- Macaulay C. E., & Ford R. M. (2013). Family influences on the cognitive development of profoundly deaf children: Exploring the effects of socioeconomic status and siblings. Journal of Deaf Studies and Deaf Education, 18, 545–562. https://doi.org/10.1093/deafed/ent019 [DOI] [PubMed] [Google Scholar]
- McEvoy R. E., Loveland K. A., & Landry S. H. (1988). The functions of immediate echolalia in autistic children: A developmental perspective. Journal of Autism and Developmental Disorders, 18, 657–668. [DOI] [PubMed] [Google Scholar]
- Meier R. P., Mauk C. E., Cheek A., & Moreland C. J. (2008). The form of children's early signs: Iconic or motoric determinants? Language Learning & Development, 4, 63–98. [Google Scholar]
- Mitchell R. E., & Karchmer M. A. (2004). Chasing the mythical ten percent: Parental hearing status of deaf and hard of hearing students in the United States. Sign Language Studies, 4, 138–163. [Google Scholar]
- Mood D., & Shield A. (2014). Clinical use of the Autism Diagnostic Observation Schedule–Second Edition with children who are deaf. Seminars in Speech and Language, 35, 288–300. https://doi.org/10.1055/s-0034-1389101 [DOI] [PubMed] [Google Scholar]
- Mundy P., Sigman M., & Kasari C. (1990). A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders, 20, 115–128. [DOI] [PubMed] [Google Scholar]
- Neidle C., Kegl J., MacLaughlin D., Bahan B., & Lee R. G. (2000). The syntax of American Sign Language. Cambridge, MA: MIT Press. [Google Scholar]
- Ohta M. (1987). Cognitive disorders of infantile autism: A study employing the WISC, spatial relationship conceptualization, and gesture imitations. Journal of Autism and Developmental Disorders, 17, 45–62. [DOI] [PubMed] [Google Scholar]
- Özçalışkan Ş., Adamson L. B., & Dimitrova N. (2015). Early deictic but not other gestures predict later vocabulary in both typical development and autism. Autism, 20, 754–763. https://doi.org/10.1177/1362361315605921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccia J. M., & Curcio F. (1982). Language processing and forms of immediate echolalia in autistic children. Journal of Speech and Hearing Research, 25, 42–47. [DOI] [PubMed] [Google Scholar]
- Padden C. (1988). Interaction of morphology and syntax in American Sign Language. New York, NY: Garland Press. [Google Scholar]
- Perlmutter D. M. (1992). Sonority and syllable structure in American Sign Language. Linguistic Inquiry, 23, 407–442. [Google Scholar]
- Petitto L. A. (1987). On the autonomy of language and gesture: Evidence from the acquisition of personal pronouns in American Sign Language. Cognition, 27, 1–52. [DOI] [PubMed] [Google Scholar]
- Pick A. (1924). On the pathology of echographia. Brain, 47, 417–429. [Google Scholar]
- Poizner H., Klima E. S., & Bellugi U. (1990). What the hands reveal about the brain. Cambridge, MA: MIT Press. [Google Scholar]
- Prizant B. M. (1983). Language acquisition and communicative behavior in autism: Toward an understanding of the “whole” of it. Journal of Speech and Hearing Disorders, 48, 296–307. [DOI] [PubMed] [Google Scholar]
- Prizant B. M., & Duchan J. F. (1981). The functions of immediate echolalia in autistic children. Journal of Speech and Hearing Disorders, 46, 241–249. [DOI] [PubMed] [Google Scholar]
- Prizant B. M., & Rydell P. J. (1984). Analysis of functions of delayed echolalia in autistic children. Journal of Speech and Hearing Research, 27, 183–192. [DOI] [PubMed] [Google Scholar]
- Roberts J. M. (1989). Echolalia and comprehension in autistic children. Journal of Autism and Developmental Disorders, 19, 271–281. [DOI] [PubMed] [Google Scholar]
- Roberts J. M. A. (2014). Echolalia and language development in children with autism. In Arciuli J. & Brock J. (Eds.), Communication in autism (pp. 53–74). Amsterdam, the Netherlands: Benjamins. [Google Scholar]
- Rogers S. J., & Pennington B. F. (1991). A theoretical approach to the deficits in infantile autism. Development and Psychopathology, 3, 137–162. [Google Scholar]
- Rutter M., Bailey A., & Lord C. (2003). Social communication questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rutter M., & Lockyer L. (1967). A five to fifteen year follow-up study of infantile psychosis. I. Description of sample. British Journal of Psychiatry, 113, 1169–1182. [DOI] [PubMed] [Google Scholar]
- Rydell P. J., & Mirenda P. (1994). Effects of high and low constraint utterances on the production of immediate and delayed echolalia in young children with autism. Journal of Autism and Developmental Disorders, 24, 719–735. [DOI] [PubMed] [Google Scholar]
- Sandler W. (1989). Phonological representation of the sign: Linearity and nonlinearity in American Sign Language. Dordrecht, the Netherlands: de Gruyter. [Google Scholar]
- Schick B., de Villiers P., de Villiers J., & Hoffmeister R. (2007). Language and theory of mind: A study of deaf children. Child Development, 78, 376–396. [DOI] [PubMed] [Google Scholar]
- Schreibman L., & Carr E. G. (1978). Elimination of echolalic responding to questions through the training of a generalized verbal response. Journal of Applied Behavior Analysis, 11, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler A. L. (1979). Echolalia: issues and clinical applications. Journal of Speech and Hearing Disorders, 44, 411–434. [DOI] [PubMed] [Google Scholar]
- Schwartz J. B., & Nye C. (2006). Improving communication for children with autism: does sign language work? EBP Briefs, 1(2), 1–17. [Google Scholar]
- Shapiro T., Roberts A., & Fish B. (1970). Imitation and echoing in young schizophrenic children. Journal of the American Academy of Child Psychiatry, 9, 548–567. [DOI] [PubMed] [Google Scholar]
- Shield A., & Meier R. P. (2012). Palm reversal errors in native-signing children with autism. Journal of Communication Disorders, 45, 439–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield A., Meier R. P., & Tager-Flusberg H. (2015). The use of sign language pronouns by native-signing children with autism. Journal of Autism and Developmental Disorders, 45, 2128–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield A., Pyers J., Martin A., & Tager-Flusberg H. (2016). Relations between language and cognition in native-signing children with autism spectrum disorder. Autism Research, 9, 1304–1315. https://doi.org/10.1002/aur.1621 [DOI] [PubMed] [Google Scholar]
- Silla-Zaleski V. A., & Vesloski M. J. (2010). Using DRO, behavioral momentum, and self-regulation to reduce scripting by an adolescent with autism. Journal of Speech and Language Pathology, Applied Behavior Analysis, 5, 80–87. [Google Scholar]
- Simon N. (1975). Echolalic speech in childhood autism. Consideration of possible underlying loci of brain damage. Archives of General Psychiatry, 32, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Sterponi L., & Shankey J. (2014). Rethinking echolalia: Repetition as interactional resource in the communication of a child with autism. Journal of Child Language, 41, 275–304. [DOI] [PubMed] [Google Scholar]
- Stokoe W. C. (1960). Dictionary of American Sign Language on linguistic principles. Burtonsville, MD: Linstok. [Google Scholar]
- Suzuki T., Itoh S., Hayashi M., Kouno M., & Takeda K. (2009). Hyperlexia and ambient echolalia in a case of cerebral infarction of the left anterior cingulate cortex and corpus callosum. Neurocase, 15, 384–389. [DOI] [PubMed] [Google Scholar]
- Szymanski C. A., Brice P. J., Lam K. H., & Hotto S. A. (2012). Deaf children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 2027–2037. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H., & Kasari C. (2013). Minimally verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Research, 6, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar F. R., Paul R., Klin A., & Cohen D. J. (2005). Handbook of autism and pervasive developmental disorders, diagnosis, development, neurobiology, and behavior. Hoboken, NJ: Wiley. [Google Scholar]
- Wevrick P. (1986). The role of echolalia in children with various disorders: An overview and treatment considerations. Human Communication Canada, 10(3), 25–29. [Google Scholar]
- Williams J. H., Whiten A., & Singh T. (2004). A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders, 34, 285–299. [DOI] [PubMed] [Google Scholar]
- Wittenburg P., Brugman H., Russel A., Klassmann A., & Sloetjes H. (2006). ELAN: A professional framework for multimodality research. In Proceedings of the 5th International Conference on Language Resources and Evaluation (LREC 2006) (pp. 1556–1559). Paris, France: European Language Resources Association. [Google Scholar]
- Wolff S., & Chess S. (1965). An analysis of the language of fourteen schizophrenic children. Journal of Child Psychology and Psychiatry, 6, 29–41. [DOI] [PubMed] [Google Scholar]
- Wootton A. J. (1999). An investigation of delayed echoing in a child with autism. First Language, 19, 359–381. [Google Scholar]