Abstract
Pathological cardiac hypertrophy used to be elucidated by biomechanical, stretch-sensitive or neurohumoral mechanisms. However, a series of hints have indicated that hypertrophy process simulates senescence program. However, further evidence need to be pursued. To verify this hypothesis and examine whether cardiac senescence is a novel mechanism of hypertrophy induced by isoproterenol, 2-month-old male Sprague Dawley rats were subjected to isoproterenol infusion (0.25mg/kg/day) for 7 days by subcutaneous injection). Key characteristics of senescence (senescence-associated β-galactosidase activity, lipofuscin, expression of cyclin-dependent kinase inhibitors) were examined in cardiac hypertrophy model. Senescence-like phenotype, such as increased senescence-associated β-galactosidase activity, accumulation of lipofuscin and high levels of cyclin-dependent kinase inhibitors (e.g. p16, p19, p21 and p53) was found along the process of cardiac hypertrophy. Cardiac-specific transcription factor GATA4 increased in isoproterenol-treated cardiomyocytes as well. We further found that myocardial hypertrophy could be inhibited by resveratrol, an anti-aging compound, in a dose-dependent manner. Our results showed for the first time that cardiac senescence is involved in the process of pathological cardiac hypertrophy induced by isoproterenol.
Introduction
Pathological cardiac hypertrophy is the cellular response to biomechanical or neurohumoral stimuli. The defining features of hypertrophy are increased cardiomyocyte size, enhanced protein synthesis and reinduction of the so-called fetal gene program. Although hypertrophy has traditionally been considered as an adaptive response required to sustain cardiac output, in the long term, hypertrophy predisposes individuals to heart failure, arrhythmia and sudden death [1, 2]. Despite the recent advances in understanding the molecular and cellular processes that contribute to cardiac hypertrophy [2–4], there remains large unknown and the need for further investigation.
Cellular senescence was first introduced by Hayflick and Moorhead [5] to describe the permanent form of cellular proliferative arrest. Senescent cells are characterized by phenotypic changes[5–9]; for example, increased cell size, enhanced senescence-associated β-galactosidase (SA-β-gal) activity at pH 6 and high levels of cyclin-dependent kinase inhibitors (CDKIs), e.g.p16INK4a, p21, p53 et, which block the cell cycle. The mammalian heart has long been considered a quiescent organ. Although there are a few studies suggesting that cardiomyocytes can divide at a low rate under certain conditions [10, 11], it is widely believed that the majority of cardiomyocytes, if not all of them, are out of cell cycle shortly after born. Therefore, the question that has been raised is whether cardiomyocytes can undergo senescence. Previous studies[12, 13] have revealed that cardiomyocytes from old mice show certain senescence-associated properties, including high SA-β-gal activity, increased CDKIs expression, accumulated lipofuscin and decreased telomerase activity. Senescence-like features have also been reported for post-mitotic neurons from old C57Bl/6 mice [14] and adipocytes of mice on a high-fat diet [15], suggesting that post-mitotic cell senescence might be a broader phenomenon.
Aging is an independent risk factor of cardiovascular diseases. Hearts of aged mice and human showed hypertrophy and fibrosis [12, 16, 17]. Besides, cellular senescence and cardiac hypertrophy share certain features [1, 18]: an increase in cardiomyocyte size and enhanced protein synthesis. In addition, activation of β-adrenergic receptor (β-AR) signaling is one of the most important pathophysiological mechanisms of cardiac hypertrophy. Interestingly, recent researches established a role for β-AR signaling in mammalian longevity. Yan et al. reported that mice lacking ADCY5, encoding type 5-adenylyl cyclase (AC5) which activates the signaling transduction of β-AR, are stress resistant and have experienced a 30% increase in median lifespan [19]. On the other hand, transgenic mice engineered to overexpress β2-AR in cardiac tissue have reduced lifespan. In support, enhanced production of β2-AR caused by genetic variants is inversely associated with human lifespan [20]. Based on the fact that cardiac senescence and hypertrophy share defining features and signaling pathways, the aim of our study is to find out whether cardiac senescence is involved in the process of pathological cardiac hypertrophy and what could be the specific biomarkers for evaluating cardiac aging.
Materials and methods
Animals
2-month-old (300-350g) and 24-month-old (700-850g) male Sprague Dawley rats were purchased from the Animal Center of Tianqin, Changsha. Rats were raised in SPF environment at room temperature (25±2)°C and provided with a standard diet and water in compliance with the Institutional Animal Care and Use Committee of Peking University Health Science Center.
Isoproterenol-induced cardiac hypertrophy model
Cardiac hypertrophy induced by isoproterenol (ISO) was generated as previously described [21, 22]. ISO (0.25mg/kg/day) was administered to 2-month-old rats for 7 days by subcutaneous injection). Identical volume of saline was administered to age-matched rats as control. All protocols were approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center.
Echocardiography
24h after the last administration, trans-thoracic echocardiography was performed on rats with 2.0% isoflurane using a Vevo 770 ultrasound machine (VisualSonics, Toronto, Canada) with a 30 MHz probe (RMV707B). Two-dimensional echocardiography was captured by a short axis view at the level of the papillary muscles for the largest LV diameter. The diastolic left ventricular posterior wall thickness (LVPW;d) was measured from M-mode tracings. The average of three consecutive cardiac cycles was taken for each parameter. Echocardiography procedures were operated in accordance with the guideline of American Society of Echocardiography.
Histology analysis
For histological analysis, part of heart tissues were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, then dehydrated and embedded in paraffin for preparation of 5-μm histological sections. Rehydrated slides were stained with haematoxylin and eosin (HE) staining to evaluate cardiomyocyte area and picric-sirius red staining to measure fibrosis area. Another part of heart tissues were fixed in 20% sucrose solution at 4°C overnight and embedded in O.C.T. compound for preparation of 8-μm frozen sections. Detection of senescent cells was determined in cells and frozen sections with the senescence β-galactosidase staining kit (GenMed Scientifics Inc.U.S.A) and lipofuscin staining kit (GenMed Scientifics Inc.U.S.A). Samples were analyzed by two independent investigators in a blind fashion.
Protein analysis
Total protein was extracted from frozen heart, resolved and electrotransferred as described. Antibodies used for western blot were as follows: anti-p16 (Abcam), anti-p19 (MBL International Corporation), anti-p21 (MBL International Corporation), anti-p53 (MBL International Corporation), anti-GATA4 (Santa Cruz Biotechnology), and anti-eif5 (Santa Cruz Biotechnology).
Quantitative RT-qPCR
Total RNA was extracted from heart tissue using Trizol® Reagent (Invitrogen), and cDNA was synthesized using M-MLV reverse transcriptase (Takara Bio) and random primers (Takara Bio) according to manufacturer instructions. Quantitative PCR was performed in triplicate using the Gene Expression Assay (Applied Biosystems) on an Applied Biosystems Fast 7500 machine with GAPDH as an endogenous normalization control. Primer sequences are available upon request.
Cell culture and treatment
Neonatal cardiomyocytes (NRCM) from 1- or 2-day-old SD rats were isolated as described previously. Cardiomyocytes were incubated in DMEM supplemented with 10% FBS for 36 h at 37°C. After subjected to serum free culture for 12h, NRCM were pre-incubated with 10−6, 10−5 and 10−4 mol/L resveratrol for 30min. Then 10-6mol/L ISO was added to the culture medium containing resveratrol for 48h [23, 24]. To visualize cardiomyocyte borders, fixed cells were incubated in wheat germ agglutinin conjugated to Alexa Fluor 488 (Invitrogen) at 1 mg/ml in PBS. Nuclei were stained blue by DAPI. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center University.
Statistical analysis
GraphPad Prism Software was used for data analysis. All data are expressed as the mean±SEM. Paired data were evaluated using Student’s t-test. Differences were considered statistically significant at P<0.05.
Results
The establishment of pathological cardiac hypertrophy model
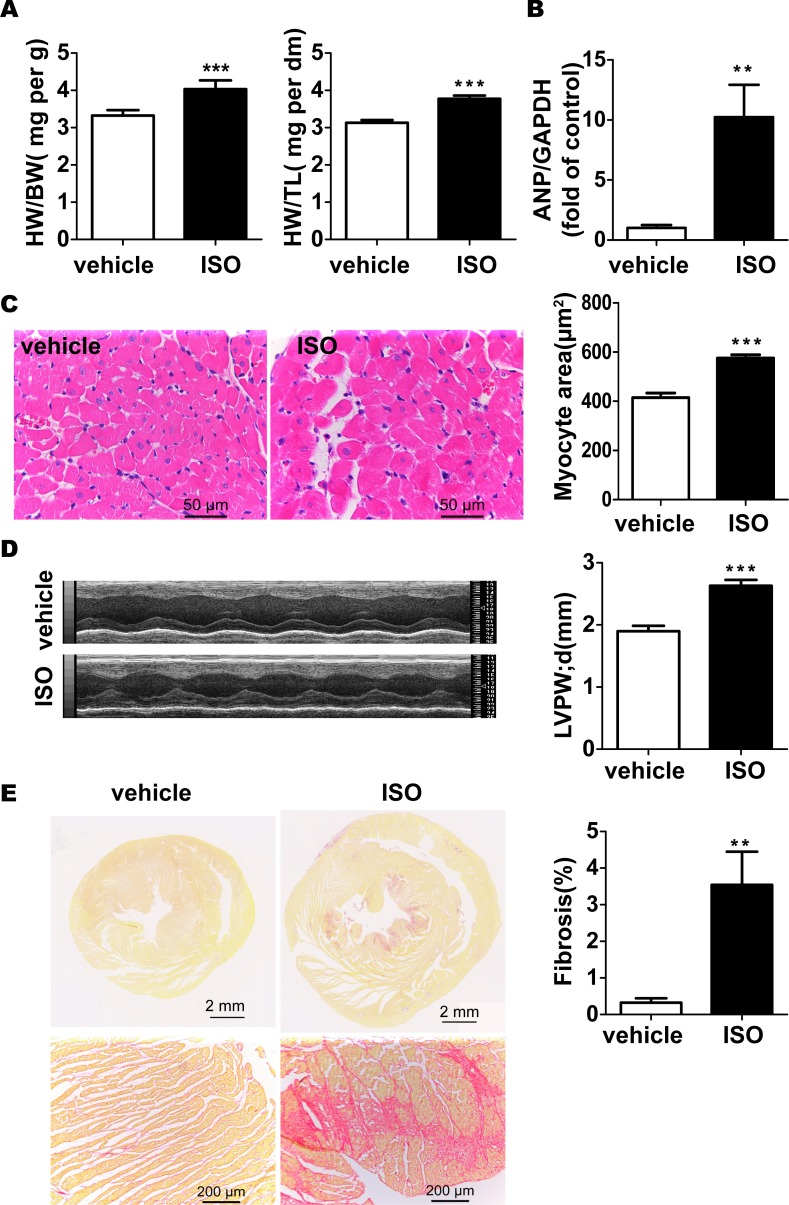
To analyze whether cardiac senescence is involved in the process of pathological cardiac hypertrophy, we first induced hypertrophy in 2-month-old rat hearts by infusion with ISO. Canonical hypertrophic markers, such as heart-to-body-weight (HW/BW) and heart-weight-to-tibia-length (HW/TL) ratio, cardiomyocytes size and expression level of atrial natriuretic peptide (ANP), was selected to assess cardiac hypertrophy model. After subcutaneous injection of ISO for 7 days, SD rats had higher HW/BW (Fig 1A) and HW/TL (Fig 1A) ratio compared with controls. This data was further confirmed by HE staining, which showed enlarged cardiomyocytes area. Moreover, these hearts had markedly thicker ventricular wall and expressed higher level of ANP than controls (Fig 1B–1D). Fibrosis, as the main distinction between pathological and physiological cardiac hypertrophy, is also found marked increase in rats upon ISO treatment (Fig 1E). Taken together, these results indicate that the model of pathological cardiac hypertrophy was successfully established by ISO in rats.
Fig 1. Pathological cardiac hypertrophy induction.
(A) HW/BW and HW/TL ratio in ISO-treated rats and controls (B) The gene expression of ANP was examined with the use of quantitative RT-PCR. (C) Cardiomyocyte area was evaluated by H&E staining and quantified. (D) Wall thickness was measured from M-mode tracings as described in the methods and calculated. (E) The percent area of myocardial fibrosis was examined by picric-sirius red staining. Data are means ± SEM (n = 6; **P < 0.01, ***P < 0.001 vs. vehicle group).
Increased number of SA-β-gal positive cardiomyocytes and amount of lipofuscin in ISO-treated cells
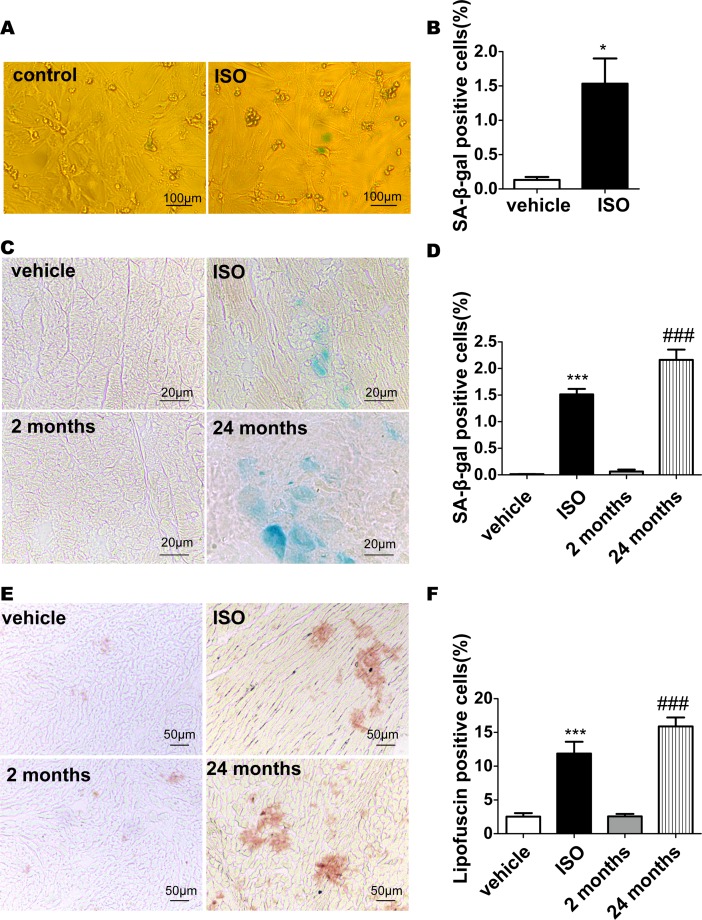
To verify our hypothesis that whether senescence is involved in the hypertrophy process, key characteristics of senescent cells were examined in the cardiac hypertrophy model described above. We first examined SA-β-gal activity in cultured neonatal rat cardiomyocytes incubated with 10−6 mol/L ISO for 48h. The percentage of SA-β-gal positive cells increased significantly compared with that of the control cells (control: 0.133%±0.043%; ISO: 1.533%±0.367%; n = 3, p<0.05, Fig 2A and 2B). We further detected SA-β-gal activity in tissue sections from ISO induced hypertrophy rat model and found the positive staining cardiomyocytes were rarely observed in the myocardium of vehicle group, whereas the ratio of SA-β-gal positive cardiomyocytes was significantly increased upon ISO treatment (vehicle: 0.013%±0.005%; ISO: 1.514%±0.101%, n = 6, p<0.001; Fig 2C and 2D). As the parallel positive control, SA-β-gal staining was also assessed in old rats without any treatment (Fig 2C). SA-β-gal positive cells in young (2-month-old) rats comprised 0.064%±0.036%, in aged (24-month-old) rats comprised 2.163%± 0.193% (n = 6; P < 0.001; Fig 2D) which is comparable to the cardiomyocytes in ISO-induced hypertrophy model. These results indicate that the standard aging biomarker, SA-β-gal activity, is enhanced in ISO-treated cardiomyocytes.
Fig 2. Increased number of senescence associated β-galactosidase (SA-β-gal)-positive cardiomyocytes and amount of lipofuscin in aging and treated cells.
(A) Cardiomyocytes were incubated with 10−5 mol/L ISO for 48h. Cells were then stained for the presence of SA-β-gal as described in the Methods. Data are means ± SEM (n = 3; *P < 0.05 vs. vehicle). (B) The percentage of SA-β-gal-positive cells was calculated. (C) Frozen heart tissue sections were analyzed for SA-β-gal staining, and (D) the number of SA-gal-positive cells was counted. (E), Frozen heart tissue sections were analyzed for lipofuscin and (F) the percentage of lipofuscin-positive cells was calculated. Data are means ± SEM (n = 6; ***P < 0.001 vs.vehicle group; ###P < 0.001 vs. 2 months.).
Another highlight for cellular senescence is the intracellular accumulation of lipofuscin, especially for post-mitotic cells which are not capable to degrade or get rid of it. Lipofuscin is known as an autofluorescent, nondegradable, and polymeric substance composed primarily of cross-linked protein and lipid residues [25, 26]. Previous studies showed that cardiomyocytes of aged hearts accumulate a large amount of lipofuscin due to imbalance between protein damage and clearance of damaged proteins[25]. To investigate whether cardiac hypertrophy model induced by ISO exhibited a senescent phenotype, the amount of lipofuscin was examined. Compared with age-matched (2-month-old) vehicle group, frozen sections of rats treated with ISO accumulated a large amount of lipofuscin (vehicle: 2.533%±0.533%; ISO:11.880%±1.747%; n = 6, p<0.001, Fig 2E and 2F). This phenomenon was also observed in lipofuscin staining of aged (24-month-old) rats as the positive control (2-month-old rats: 2.583%±0.354%; 24-month-old:15.900%±1.309%, n = 6; p<0.001; Fig 2E and 2F). These results indicate that lipofuscin, another senescent marker, accumulated in ISO-treated cardiomyocytes.
Expression of CDKIs and GATA4 increased in ISO-treated cells
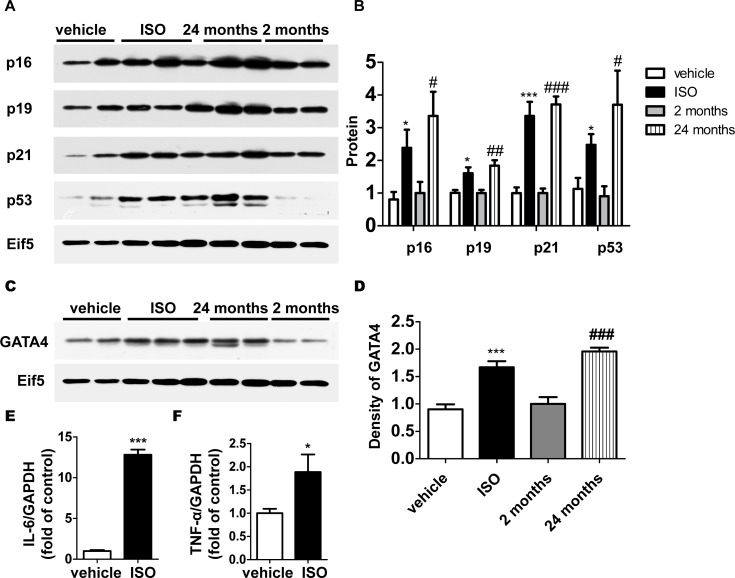
Increased level of CDKIs, which block the cell cycle is one of the most classic mechanisms of mitotic cell senescence [7, 8, 27, 28]. Since Diana Jurk et al[14] for the first time testified that post-mitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response in vivo, the role of CDKIs in post-mitotic cell senescence has got more and more attention. The gene expression of CDKIs was evaluated to confirm the involvement of cardiac senescence in hypertrophy process. The results of western blot demonstrated that gene expression of p16, p19 and p21 significantly increased in ISO-treated rats compared with that of age-matched (2-month-old) vehicle group, as well as p53 (Fig 3A and 3B). Higher protein level of CDKIs in aged (24-month-old) rats as positive control was consistent with previous reports [12, 13, 29]. The similar expression pattern of high expressed CDKIs in cardiomyocyte not only showed the senescent phenotype, but also indicated that CDKIs might have some other potential function beyond the cell-cycle regulation in hypertrophy.
Fig 3. Expression of CDKIs and GATA4 increased in ISO-treated cells.
(A) The protein level of cell cycle inhibitors and Eif5 (loading control) was examined by western blotting. (B) The protein level was quantified by densitometry. (C) The protein level of GATA4 and Eif5 was examined by western blotting and (D) quantified by densitometry. The gene expression of SASP factors (E) and (F) was examined with the use of quantitative RT-PCR. Data are means ± SEM (n = 6; *P < 0.05, ***P < 0.001 vs. vehicle; # P < 0.05, ## P < 0.01,###P < 0.001 vs. 2 months. The mean value for 2-month-old rats was expressed as 1 unit).
Besides applying canonical aging biomarkers, we are trying to find some cardiac-specific hints. GATA4 plays a key role in cardiac specification, development and function. Perturbation of transcription factor expression and regulation disrupts normal heart structure and function [30–32]. The recent research described that GATA4 is stabilized in cells undergoing senescence and in turn activates the transcription factor NF-κB to initiate the SASP and facilitate senescence. Therefore, GATA4 might be considered as a positive senescence regulator [33]. To examine whether GATA4 functions in cardiac senescence, western blot was performed to detect the expression of GATA4 in cardiomyocytes. The results showed the expression of GATA4 was up-regulated in hearts of aged (24-month-old) rats compared with 2-month-old rats (Fig 3C and 3D). This phenomenon was also observed in ISO-treated cardiomyocytes (Fig 3C and 3D). SASP genes were also examined by realtime PCR. The mRNA level of interleukin-6(IL-6) and tumor necrosis factor-α (TNF-α) both increased in ISO-treated group (Fig 3E and 3F). These results not only present the similar phenotype between hypertrophy and cardiac senescence, but also indicate that the transcription factor GATA4 might be actively involved in heart aging and hypertrophy. The novel function of GATA4 in aging need to be further explored.
Resveratrol prevents pathological cardiac hypertrophy induced by ISO
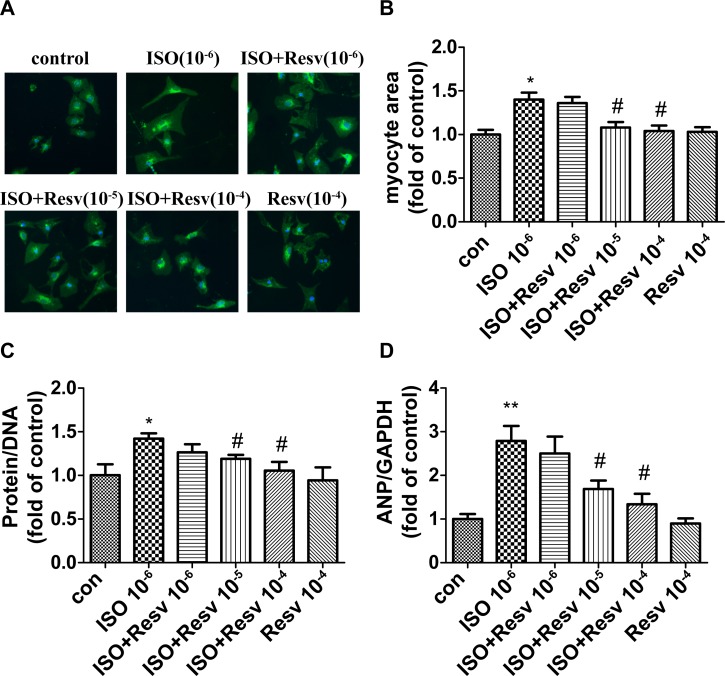
Resveratrol, a phytoalexin, obtained from grape skin possesses diverse biochemical and physiological properties, including antioxidant, antiplatelet, and anti-inflammatory properties as well as a wide range of health benefits ranging from chemoprevention to extending life span[34, 35]. One of the highlights is that resveratrol can mimic the effect of caloric restriction and anti-aging. In order to further confirm the involvement of cardiac senescence in the process of hypertrophy, resveratrol, a widely recognized anti-aging regent was used to verify whether hypertrophy could be intervened upon ISO treatment. It showed that cardiomyocytes treated by ISO possesses the enhanced SA-β-gal activity, while the positive staining percentage decreased in the cardiomyocytes treated by resveratrol in a dose-dependent manner (S1 Fig). In addition, cardiomyocytes stimulated by ISO exhibited typical features of myocyte hypertrophy: enlarged cell area, a higher protein /DNA ratio and highly expressed ANP. While all these phenotypes mentioned above (Cell area, protein/DNA ratio and expression level of ANP) were attenuated by resveratrol in a dose-dependent manner (Fig 4), which suggested cardiomyocyte hypertrophy could be inhibited by resveratrol, thus indicating the senescence mechanism might be involved in this pathological process.
Fig 4. Resveratrol prevents pathological cardiac hypertrophy induced by ISO.
Cardiomyocyte area was evaluated by (A) wheat germ agglutinin (WGA) staining and (B) quantified. (C) The ratio of protein/DNA was quantitated. (D) The gene expression of ANP was examined with the use of quantitative RT-PCR. Data are means ± SEM. (n = 3,*P < 0.05, **P < 0.01 vs. control; #P < 0.05 vs. ISO group. The mean value for control was expressed as 1 unit).
Discussion
Cellular senescence has been considered as the intrinsic mechanism for tumor suppression. Recently, the emerging evidence shows that senescence progress might be involved in multiple physiological and pathological process besides tumor, such as atherosclerosis and inflammation. Therefore, senescence could be a wider mechanism rather than its phenotype. Moreover, whether senescence also exists in post-mitotic cells such as cardiomyocytes still remains debate. Our present results show for the first time that cardiac senescence phenotype occurs in ISO-induced pathological cardiac hypertrophy by analysis of a wide range of senescence markers. The similar results were also reported in angiotensin II-induced cardiac hypertrophy model and dilated cardiomyopathy caused by cardiac-specific Bmi1 deletion [36] manifested by the increased ratio of SA-β-gal positive cells. It suggested that not only the cardiac senescence does exist in heart but also is involved in multiple hypertrophy models. Increased accumulation of lipofuscin in heart is one of the most consistent features of aging across species of mammals, which is also seen in ISO-treated rats. The mechanism by which lipofuscin accumulates in ISO-induced cardiac hypertrophy model is currently unclear. It was reported that β-AR stimulation provokes cardiac oxidative stress. Especially, in the chronic phase of ISO infusion, ROS may participate in cardiac remodeling, especially in respect to wall stiffness, based on fibrogenesis [37, 38]. Besides, myocyte autophagy was decreased during cardiac hypertrophy, which was associated with progressively increased cardiac oxidative stress [39, 40]. Thus, increased oxidative stress and low autophagy activity lead to the disturbances of proteostasis and an impairment of the proteasomal system which is resulted in the accumulation of highly cross-linked undegradable aggregates such as lipofuscin as the final consequence. Lipofuscin may account in part for the cardiac dysfunction in aged rats and ISO-treated rats due to deleterious effects of lipofuscin on cardiac cellular function. There are several researches showed that post-mitotic cell senescence is associated with activation of inhibitory cell cycle regulators [12, 13]. It was reported that p21 gene expression increased significantly in angiotensin II-induced cardiac hypertrophy [36]. In our study, along the process of hypertrophy, the expression of p21 and p16 increased, as noted in other studies [13]. Notably, p21 is a necessary signal transducer between DNA damage response and senescence-like phenotype in neurons, as in senescing fibroblasts[14]. Increased expression of p21 in ISO-treated cardiomyocytes may due to DNA damage response caused by oxidative stress in the process of cardiac hypertrophy.
Myocyte hypertrophy was suppressed by resveratrol in a dose-dependent manner, which suggested hypertrophy could be inhibited by intervening aging process. In support of our hypothesis, other anti-aging regents, such as rapamycin[41, 42] and metformin[43], are proven to prevents cardiac hypertrophy induced by various pathological stimulants[44–47]. Rapamycin attenuated cardiac hypertrophy mainly by three pathways (a) AKT/mTOR/S6 kinase signaling, which is important in the regulation of protein synthesis[44, 48], (b) promoting autophagy through a mechanism involving the modulation of Noxa and Beclin-1 expression by the MEK/ERK signaling pathway[49] or (c) inhibiting NF-κB activation[50]. Recent studies demonstrated that the anti-hypertrophic effects of metformin are associated with AMPK activation and prevention of mitochondrial dysfunction through the SIRT1/eNOS/p53 pathway [47, 51, 52]. Collecting all these evidence mentioned above, it suggests that targeting anti-aging pathway might become an effective strategy in intervening hypertrophy.
In addition, our study challenges the conventional opinion that senescence is only defined in proliferating cells. From this point of view, we might need to revisit the concept of senescence. The widely accepted category of senescence is divided into replicative senescence and premature senescence. In this study, 2–3 month old rats were used to induce myocardial hypertrophy, which exhibited senescence-like features. Such changes did not completely simulate physiological aging process. The most notable difference in our study is that cardiac systolic function did not show the difference between 2-month and 24-month old rats in physiological aging as reported[12], while cardiac systolic function enhanced after 7 days ISO treatment in pathological aging (S2 Fig). This phenomenon may be partially related to the compensation in the long process of physiological aging. While, enhanced cardiac systolic function might due to positive inotropic action of ISO at the early time point (day 7) of administration. According to our study, cardiac systolic function declined at the late time point upon administration (day 14) in C57BL/6 mice (unpublished data). Meanwhile, there is no clear definition for heart aging so far. Since the widely accepted category of senescence is divided into replicative senescence and premature senescence, likewise, cardiac senescence might be subdivided into myocardial physiological aging and pathological aging. Nevertheless, the further investigation on mechanism of cardiac senescence is necessary.
Effective biomarkers are crucial to assess aspects of aging. SA-β-gal staining, expression of CDKIs and SASP factors, lipofuscin and telomere foci are included in the markers which have been applied in cardiac senescence [12, 13, 53]. Although each of these markers is related to senescence, the effectiveness varies. For example, the percentage of SA-β-gal staining in aged heart is extremely low compared with other tissues in the same rat (S3 Fig), such as liver, due to the tremendous difference in the base level of SA-β-galactosidase in each specific tissues [8]. In addition, most of SASP factors are acute phase reactants that exhibit a marked change in expression in response to viral infection and other intercurrent illness unrelated to aging[8]. Therefore, it is valuable to explore organ-specific even cell-specific senescence biomarkers other than conventional markers for the cardiac senescence. Previous study [33, 54] reported that the key transcriptional factor during heart development, GATA4, plays an important role in senescence by activating the transcription factor NF-κB to initiate the SASP and facilitate senescence phenotype. Moreover, there is a significant spatial correlation between GATA4 and p16INK4a in oligodendrocytes, pyramidal neurons, and astrocytes from older humans, further supporting the role of GATA4 in senescence during human aging. In our study, GATA4 is upregulated in ISO-induced hypertrophy model and 24-month old rats, which could serve as an indicator for the heart aging. Although the specificity of GATA4 in heart aging is still need to be further investigated, it somewhat directs the future research in exploring the markers to identify the aging and hypertrophy.
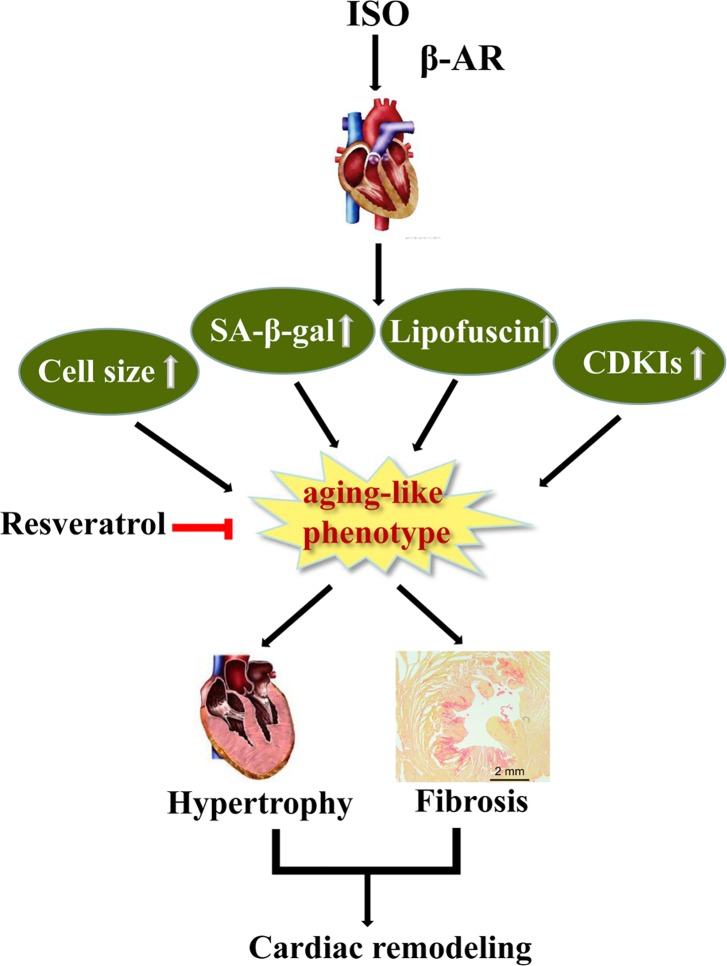
Aging is an important risk factor of cardiovascular diseases such as hypertension, cardiac hypertrophy and heart failure. In our study, we also detected several parameters showing hearts of aging rats present cardiac hypertrophy (S4 Fig). Accordingly, senescence mechanism might contribute to promoting a certain diseases progress. Thus, our study might provide a possible prospect that anti-aging might be a powerful strategy for treating cardiovascular diseases, at least for pathological hypertrophy (Fig 5). Indeed, along the same lines, several studies showed anti-aging reagent does help in certain types of diseases, such as rapamycin was proven to prevents cardiac hypertrophy induced by various pathological stimulants [44, 48–50]. The more precise relationship between hypertrophy and senescence still need to be further dissected, and the intensive mechanism is still required to be further explored as well, such as the impact of microenvironment between fibroblast and cardiomyocyte, the sequential and/or interplay effect between these two types of cell in heart during the aging process. These findings will ultimately shed light on the cardiac aging and related diseases.
Fig 5. A working model for the senescence mediated β-AR induced cardiac hypertrophy.
Supporting information
(A) Cardiomyocytes were stained for the presence of SA-β-gal as described in the Methods. (B) The percentage of SA-β-gal-positive cells was calculated. Data are means ± SEM (n = 3; *P < 0.05 vs.control group,# P < 0.05 vs.ISO group).
(TIF)
(A) Ejection fraction and (B) fractional shortening in 2-month-old and 24-month-old rats; (C) ejection fraction and (D) fractional shortening in ISO-treated rats and controls. Data are means ± SEM, n = 6, **P < 0.01 vs. vehicle group.
(TIF)
Frozen sections of heart, liver, spleen, lung and kidney from young (2-month-old) and old (24-month-old) rats were analyzed for SA-β-gal staining (n = 6).
(TIF)
(A) HW/TL ratio in 2-month-old and 24-month-old rats. Cardiomyocyte area was evaluated by H&E staining(C) and quantified (B). The gene expression of β-MHC (D) and ANP (E) were examined with the use of quantitative RT-PCR. Data are means ± SEM, n = 6, # P < 0.05, ###P < 0.001 vs. 2 months.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the grants from the National Basic Research Program of China (grant number. 2014CBA02003) and the National Natural Science Foundation of China (grant numbers. 81672091, 81541142, 81471893, 81270157, 91539123, 81070078). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243 . [DOI] [PubMed] [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983 . [DOI] [PubMed] [Google Scholar]
- 3.Heineke J, Auger-Messier M, Correll RN, Xu J, Benard MJ, Yuan W, et al. CIB1 is a regulator of pathological cardiac hypertrophy. Nat Med. 2010;16(8):872–9. doi: 10.1038/nm.2181 ; PubMed Central PMCID: PMCPMC2917617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106(29):12103–8. doi: 10.1073/pnas.0811371106 ; PubMed Central PMCID: PMCPMC2715539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. ; PubMed Central PMCID: PMCPMC40985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. doi: 10.1016/j.cell.2005.02.003 . [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039 ; PubMed Central PMCID: PMCPMC3836174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorrentino JA, Sanoff HK, Sharpless NE. Defining the toxicology of aging. Trends Mol Med. 2014;20(7):375–84. doi: 10.1016/j.molmed.2014.04.004 ; PubMed Central PMCID: PMCPMC4082749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Xue L, Weng M, Sun Y, Zhang Z, Wang W, et al. Sp1 is essential for p16 expression in human diploid fibroblasts during senescence. PLoS One. 2007;2(1):e164 doi: 10.1371/journal.pone.0000164 ; PubMed Central PMCID: PMCPMC1764714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680 ; PubMed Central PMCID: PMCPMC2991140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6. doi: 10.1038/nature11682 ; PubMed Central PMCID: PMCPMC3548046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120(17):1695–703. doi: 10.1161/CIRCULATIONAHA.109.871137 . [DOI] [PubMed] [Google Scholar]
- 13.Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7(2):125–36. doi: 10.1111/j.1474-9726.2007.00358.x . [DOI] [PubMed] [Google Scholar]
- 14.Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11(6):996–1004. doi: 10.1111/j.1474-9726.2012.00870.x ; PubMed Central PMCID: PMCPMC3533793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–7. doi: 10.1038/nm.2014 . [DOI] [PubMed] [Google Scholar]
- 16.Linton PJ, Gurney M, Sengstock D, Mentzer RM Jr., Gottlieb RA. This old heart: Cardiac aging and autophagy. J Mol Cell Cardiol. 2015;83:44–54. doi: 10.1016/j.yjmcc.2014.12.017 ; PubMed Central PMCID: PMCPMC4459942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swynghedauw B, Besse S, Assayag P, Carre F, Chevalier B, Charlemagne D, et al. Molecular and cellular biology of the senescent hypertrophied and failing heart. Am J Cardiol. 1995;76(13):2D–7D. . [DOI] [PubMed] [Google Scholar]
- 18.Xing Y, Niu T, Wang W, Li J, Li S, Janicki JS, et al. Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS One. 2012;7(9):e44899 doi: 10.1371/journal.pone.0044899 ; PubMed Central PMCID: PMCPMC3444497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–58. doi: 10.1016/j.cell.2007.05.038 . [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Yang F, Xu K, Cao H, Zheng GY, Zhang Y, et al. Common genetic variants of the beta2-adrenergic receptor affect its translational efficiency and are associated with human longevity. Aging Cell. 2012;11(6):1094–101. doi: 10.1111/acel.12011 ; PubMed Central PMCID: PMCPMC3633790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Q, Yang C, Wu J, Lu H, Zheng X, Zhang Y, et al. Downregulation of beta-Adrenoceptors in Isoproterenol-Induced Cardiac Remodeling through HuR. PLoS One. 2016;11(4):e0152005 doi: 10.1371/journal.pone.0152005 ; PubMed Central PMCID: PMCPMC4818026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Chen CX, Gu WL, Gao JP, Wan Y, Lv J. [Effect of Chrysanthemum indicum on ventricular remodeling in rats]. Zhong Yao Cai. 2010;33(7):1112–5. . [PubMed] [Google Scholar]
- 23.Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015;10(2):e0115341 doi: 10.1371/journal.pone.0115341 ; PubMed Central PMCID: PMCPMC4315597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolinsky VW, Soltys CL, Rogan KJ, Chan AY, Nagendran J, Wang S, et al. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J Mol Med (Berl). 2015;93(4):413–25. doi: 10.1007/s00109-014-1220-8 . [DOI] [PubMed] [Google Scholar]
- 25.Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68(3):355–65. doi: 10.1016/j.cardiores.2005.08.014 . [DOI] [PubMed] [Google Scholar]
- 26.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–95. doi: 10.1016/j.cell.2011.07.030 . [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Wang H, Xue L, Zhang Z, Tong T. Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J Biol Chem. 2004;279(30):31524–32. doi: 10.1074/jbc.M400365200 . [DOI] [PubMed] [Google Scholar]
- 28.Li N, Li Q, Cao X, Zhao G, Xue L, Tong T. The tumor suppressor p33ING1b upregulates p16INK4a expression and induces cellular senescence. FEBS Lett. 2011;585(19):3106–12. doi: 10.1016/j.febslet.2011.08.044 . [DOI] [PubMed] [Google Scholar]
- 29.Bernhard D, Laufer G. The aging cardiomyocyte: a mini-review. Gerontology. 2008;54(1):24–31. doi: 10.1159/000113503 . [DOI] [PubMed] [Google Scholar]
- 30.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276(32):30245–53. doi: 10.1074/jbc.M102174200 . [DOI] [PubMed] [Google Scholar]
- 31.Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101(34):12573–8. doi: 10.1073/pnas.0400752101 ; PubMed Central PMCID: PMCPMC515098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103(39):14471–6. doi: 10.1073/pnas.0602543103 ; PubMed Central PMCID: PMCPMC1636702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349(6255):aaa5612 doi: 10.1126/science.aaa5612 ; PubMed Central PMCID: PMCPMC4942138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Wahab BA, Abdel-Wahab MM. Protective effect of resveratrol against chronic intermittent hypoxia-induced spatial memory deficits, hippocampal oxidative DNA damage and increased p47Phox NADPH oxidase expression in young rats. Behav Brain Res. 2016;305:65–75. doi: 10.1016/j.bbr.2016.02.030 . [DOI] [PubMed] [Google Scholar]
- 35.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misaka T, Suzuki S, Miyata M, Kobayashi A, Ishigami A, Shishido T, et al. Senescence marker protein 30 inhibits angiotensin II-induced cardiac hypertrophy and diastolic dysfunction. Biochem Biophys Res Commun. 2013;439(1):142–7. doi: 10.1016/j.bbrc.2013.08.002 . [DOI] [PubMed] [Google Scholar]
- 37.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, et al. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65(1):230–8. doi: 10.1016/j.cardiores.2004.08.013 . [DOI] [PubMed] [Google Scholar]
- 38.Zhang GX, Ohmori K, Nagai Y, Fujisawa Y, Nishiyama A, Abe Y, et al. Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. J Mol Cell Cardiol. 2007;42(4):804–11. doi: 10.1016/j.yjmcc.2007.01.012 . [DOI] [PubMed] [Google Scholar]
- 39.Li B, Chi RF, Qin FZ, Guo XF. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: association with oxidative stress. Exp Physiol. 2016;101(8):1050–63. doi: 10.1113/EP085586 . [DOI] [PubMed] [Google Scholar]
- 40.Nishida K, Otsu K. Autophagy during cardiac remodeling. J Mol Cell Cardiol. 2016;95:11–8. doi: 10.1016/j.yjmcc.2015.12.003 . [DOI] [PubMed] [Google Scholar]
- 41.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–6. doi: 10.4161/cc.10.24.18486 . [DOI] [PubMed] [Google Scholar]
- 42.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. doi: 10.1038/nature08221 ; PubMed Central PMCID: PMCPMC2786175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY). 2011;3(2):148–57. doi: 10.18632/aging.100273 ; PubMed Central PMCID: PMCPMC3082009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuzman JA, O'Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148(7):3477–84. doi: 10.1210/en.2007-0099 . [DOI] [PubMed] [Google Scholar]
- 45.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109(24):3050–5. doi: 10.1161/01.CIR.0000130641.08705.45 . [DOI] [PubMed] [Google Scholar]
- 46.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279(31):32771–9. doi: 10.1074/jbc.M403528200 . [DOI] [PubMed] [Google Scholar]
- 47.Zhang CX, Pan SN, Meng RS, Peng CQ, Xiong ZJ, Chen BL, et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin Exp Pharmacol Physiol. 2011;38(1):55–62. doi: 10.1111/j.1440-1681.2010.05461.x . [DOI] [PubMed] [Google Scholar]
- 48.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107(12):1664–70. doi: 10.1161/01.CIR.0000057979.36322.88 . [DOI] [PubMed] [Google Scholar]
- 49.Gu J, Hu W, Song ZP, Chen YG, Zhang DD, Wang CQ. Rapamycin Inhibits Cardiac Hypertrophy by Promoting Autophagy via the MEK/ERK/Beclin-1 Pathway. Front Physiol. 2016;7:104 doi: 10.3389/fphys.2016.00104 ; PubMed Central PMCID: PMCPMC4796007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Zeng S, Zou J, Chen Y, Yue Z, Gao Y, et al. Rapamycin attenuated cardiac hypertrophy induced by isoproterenol and maintained energy homeostasis via inhibiting NF-kappaB activation. Mediators Inflamm. 2014;2014:868753 doi: 10.1155/2014/868753 ; PubMed Central PMCID: PMCPMC4089551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol Sin. 2011;32(7):879–87. doi: 10.1038/aps.2010.229 ; PubMed Central PMCID: PMCPMC4003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med. 2014;18(4):709–20. doi: 10.1111/jcmm.12220 ; PubMed Central PMCID: PMCPMC3981893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kajstura J, Pertoldi B, Leri A, Beltrami CA, Deptala A, Darzynkiewicz Z, et al. Telomere shortening is an in vivo marker of myocyte replication and aging. Am J Pathol. 2000;156(3):813–9. doi: 10.1016/S0002-9440(10)64949-8 ; PubMed Central PMCID: PMCPMC1876843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao CX, Wei QX, Zhang YY, Wang WP, Xue LX, Yang F, et al. miR-200b targets GATA-4 during cell growth and differentiation. RNA Biol. 2013;10(4):465–80. doi: 10.4161/rna.24370 ; PubMed Central PMCID: PMCPMC3710353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Cardiomyocytes were stained for the presence of SA-β-gal as described in the Methods. (B) The percentage of SA-β-gal-positive cells was calculated. Data are means ± SEM (n = 3; *P < 0.05 vs.control group,# P < 0.05 vs.ISO group).
(TIF)
(A) Ejection fraction and (B) fractional shortening in 2-month-old and 24-month-old rats; (C) ejection fraction and (D) fractional shortening in ISO-treated rats and controls. Data are means ± SEM, n = 6, **P < 0.01 vs. vehicle group.
(TIF)
Frozen sections of heart, liver, spleen, lung and kidney from young (2-month-old) and old (24-month-old) rats were analyzed for SA-β-gal staining (n = 6).
(TIF)
(A) HW/TL ratio in 2-month-old and 24-month-old rats. Cardiomyocyte area was evaluated by H&E staining(C) and quantified (B). The gene expression of β-MHC (D) and ANP (E) were examined with the use of quantitative RT-PCR. Data are means ± SEM, n = 6, # P < 0.05, ###P < 0.001 vs. 2 months.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.