Abstract
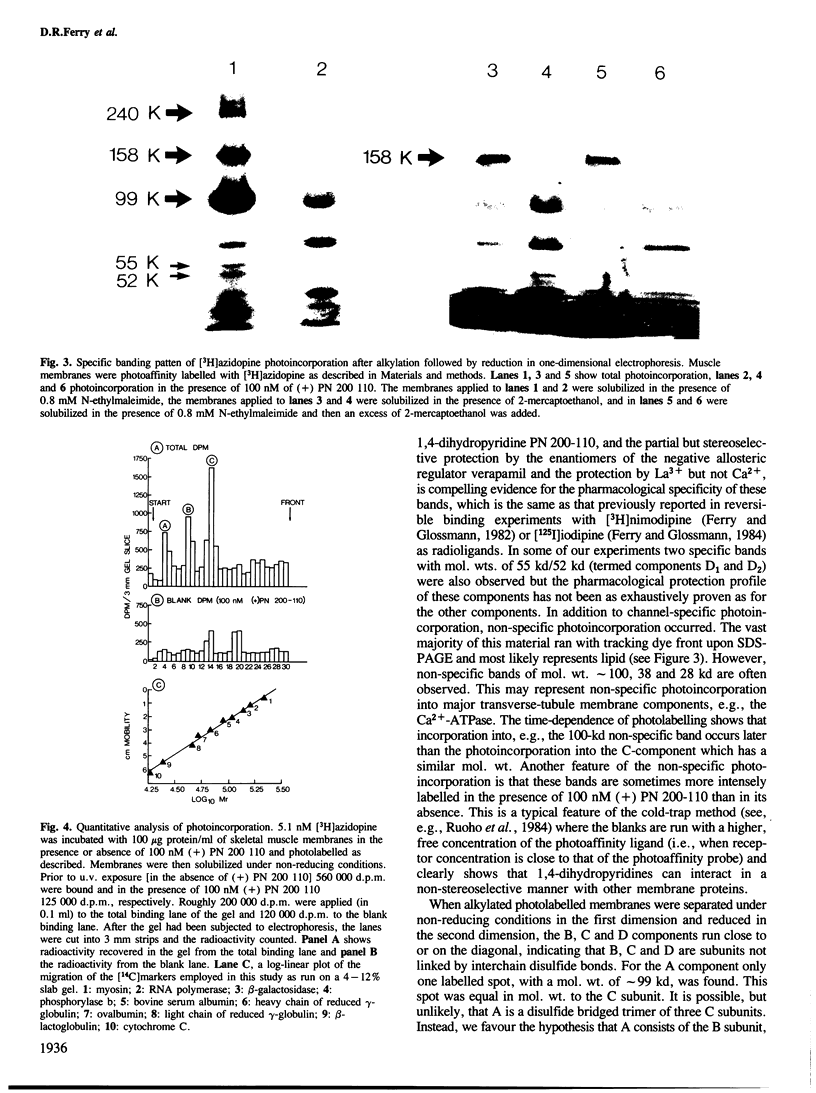
The arylazide 1,4-dihydropyridine, [3H]azidopine, binds with high affinity to calcium channels in partially purified guinea-pig skeletal muscle transverse tubule membranes. Upon brief exposure to u.v. light, [3H]azidopine incorporates covalently into transverse tubule membrane proteins, as judged by SDS-PAGE. After alkylation of sulfhydryl groups with N-ethylmaleimide three specifically labelled bands of mol wts. 240 kd, 158 kd and 99 kd are always observed with fluorography after one-dimensional SDS-PAGE. Two other specific bands with mol. wts. of 52 kd and 55 kd, respectively, were sometimes observed. Two-dimensional SDS-PAGE (non-reduced but alkylated in the first dimension and reduced in the second dimension) revealed that the 240-kd band after reduction migrates with a mol. wt. of 99 kd. The 158-kd and 99-kd bands do not change in mobility. It is suggested that [3H]azidopine binds in such a way that the arylazide moiety of the ligand comes into contact with at least three calcium channel components: the A component of mol. wt. 240 kd, the B component of mol. wt. 158 kd and a C component of mol. wt. 99 kd. B and C are non-covalently bonded subunits of the channel, whereas A could be a heterodimer consisting of B and C, linked by disulfide bonds. Subunits of smaller mol. wt. may be also part of the ionic pore. Photolabelling of transverse tubule membranes after high energy irradiation with 10 MeV electrons supports this interpretation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Hidaka H. Potentiation in various agonists-induced contractions of rabbit mesenteric artery by sulfhydryl reagents. Jpn J Pharmacol. 1983 Feb;33(1):145–154. doi: 10.1254/jjp.33.145. [DOI] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Norman R. I., Lazdunski M. Purification of the dihydropyridine receptor of the voltage-dependent Ca2+ channel from skeletal muscle transverse tubules using (+) [3H]PN 200-110. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1357–1366. doi: 10.1016/0006-291x(84)91241-5. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., Lipshutz G. M., Denney G. H. Direct photoaffinity labeling of the high affinity nitrendipine-binding site in subcellular membrane fractions isolated from canine myocardium. J Biol Chem. 1984 May 10;259(9):5384–5387. [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Glossmann H. 125I-iodipine, a new high affinity ligand for the putative calcium channel. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):186–189. doi: 10.1007/BF00506200. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Glossmann H. Identification of putative calcium channels in skeletal muscle microsomes. FEBS Lett. 1982 Nov 8;148(2):331–337. doi: 10.1016/0014-5793(82)80835-1. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Glossmann H. Calcium channels: evidence for oligomeric nature by target size analysis. EMBO J. 1983;2(10):1729–1732. doi: 10.1002/j.1460-2075.1983.tb01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Glossmann H. Putative calcium channel molecular weight determination by target size analysis. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):292–297. doi: 10.1007/BF00512466. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Rombush M., Goll A., Glossmann H. Photoaffinity labelling of Ca2+ channels with [3H]azidopine. FEBS Lett. 1984 Apr 9;169(1):112–118. doi: 10.1016/0014-5793(84)80299-9. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Boschek C. B. Purification of the putative calcium channel from skeletal muscle with the aid of [3H]-nimodipine binding. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):1–11. doi: 10.1007/BF00498821. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Solubilization and partial purification of putative calcium channels labelled with [3H]-nimodipine. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):279–291. doi: 10.1007/BF00512465. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Glossmann H. Target size analysis and molecular properties of Ca2+ channels labelled with [3H]verapamil. Eur J Biochem. 1984 May 15;141(1):177–186. doi: 10.1111/j.1432-1033.1984.tb08172.x. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Glossmann H. Target size analysis of skeletal muscle Ca2+ channels. Positive allosteric heterotropic regulation by d-cis-diltiazem is associated with apparent channel oligomer dissociation. FEBS Lett. 1983 Jun 27;157(1):63–69. doi: 10.1016/0014-5793(83)81117-x. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Striessnig J., Schober M., Glossmann H. (-)-[3H]Desmethoxyverapamil, a novel Ca2+ channel probe. Binding characteristics and target size analysis of its receptor in skeletal muscle. FEBS Lett. 1984 Oct 29;176(2):371–377. doi: 10.1016/0014-5793(84)81199-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D. A., Garcia M. C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982 Jul 15;298(5871):292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- Halbach S., Schönsteiner G., Ebner F., Reiter M. The effects of p-chloromercuriphenylsulfonic acid (PCMBS) on force of contraction of mammalian myocardium and on ATP hydrolysis by sarcolemmal ATPase. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):121–129. doi: 10.1007/BF00508836. [DOI] [PubMed] [Google Scholar]
- Hall C., Ruoho A. Ouabain-binding-site photoaffinity probes that label both subunits of Na+,K+-ATPase. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4529–4533. doi: 10.1073/pnas.77.8.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P., Triggle D. J., Venter J. C. Nitrendipine and isoproterenol induce phosphorylation of a 42,000 dalton protein that co-migrates with the affinity labeled calcium channel regulatory subunit. Biochem Biophys Res Commun. 1984 Jun 29;121(3):890–898. doi: 10.1016/0006-291x(84)90761-7. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Pinter A., Lieman-Hurwitz J., Fleissner E. The nature of the association between the murine leukemia virus envelope proteins. Virology. 1978 Dec;91(2):345–351. doi: 10.1016/0042-6822(78)90382-3. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Robb R. J., Terhorst C., Strominger J. L. Submit and disulfide structure of monomeric and dimeric forms of detergent-soluble HLA antigens. J Biol Chem. 1977 Jul 10;252(13):4694–4700. [PubMed] [Google Scholar]
- Striessnig J., Zernig G., Glossmann H. Human red-blood-cell Ca2+-antagonist binding sites. Evidence for an unusual receptor coupled to the nucleoside transporter. Eur J Biochem. 1985 Jul 1;150(1):67–77. doi: 10.1111/j.1432-1033.1985.tb08989.x. [DOI] [PubMed] [Google Scholar]
- Woody A. Y., Vader C. R., Woody R. W., Haley B. E. Photoaffinity labeling of DNA-dependent RNA polymerase from Escherichia coli with 8-azidoadenosine 5'-triphosphate. Biochemistry. 1984 Jun 19;23(13):2843–2848. doi: 10.1021/bi00308a001. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]