Abstract
The prevalence of overweight (body mass index [BMI], 25 to 29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) have increased dramatically in the United States. Because increasing BMI is associated with the development of multiple different cancer types, including most GI cancers, providers will frequently encounter patients with GI cancer who are overweight or obese. Mounting evidence associates overweight and/or obesity with worsened prognosis in multiple GI cancers, including esophageal, gastric, hepatocellular, pancreatic, and colorectal. However, these data are observational and may be subject to bias and/or confounding. Furthermore, in some cancer types, the associations between BMI and outcomes is not linear, where overweight and class I obese patients may have an improvement in outcome. This report provides a brief highlight of existing studies that have linked overweight and/or obesity to prognosis in GI cancer; provides recommendations on best management practices; and discusses limitations, controversies, and future directions in this rapidly evolving area. There are multiple areas of promise that warrant continued investigation: What are the comparative contributions of energy balance, including weight, dietary patterns, and physical activity on cancer prognosis? What are the specific physiologic pathways that mediate the relationship between energy balance and prognosis? What is the relationship between low muscle mass (sarcopenia) or sarcopenic obesity and cancer prognosis? Are there subsets of patients for whom purposefully altering energy balance would be deleterious to prognosis? This area is rich with opportunities to understand how states of energy (im)balance can be favorably altered to promote healthy survivorship.
INTRODUCTION
The prevalence of overweight and obesity, defined using body mass index (BMI; Table 1), has reached epidemic levels in the United States, with more than two in three adults considered to be overweight or obese.1 Up to one in five deaths in the United States are associated with overweight or obesity, with the most common causes of death including ischemic heart disease, stroke, kidney disease, diabetes, and cancer.2 Each year > 291,000 men and women are diagnosed with GI cancer in the United States.3 Overweight and obesity are associated with an increased risk of developing several GI cancers, including esophageal, gastric, hepatocellular, pancreatic, and colorectal.4,5 Consequently, GI oncology providers frequently encounter patients who are overweight or obese.
Table 1.
WHO Classification of Body Mass Index

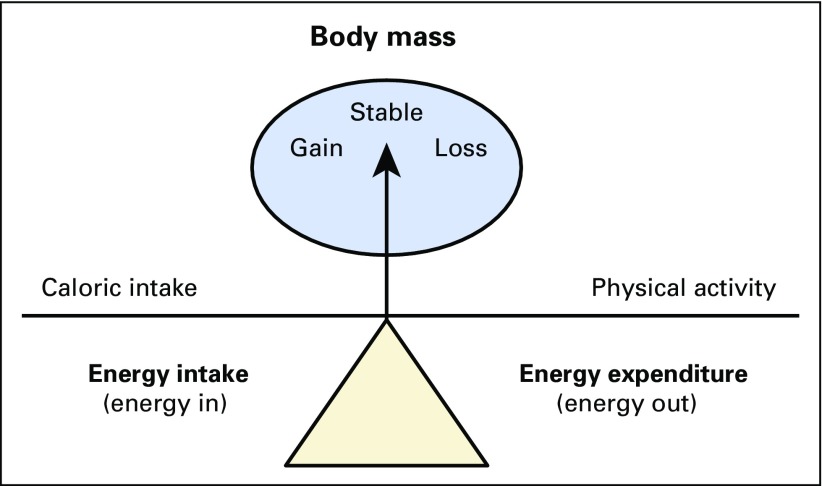
Overweight and obesity are the result of chronic energy imbalance (Fig 1).6 When caloric intake exceeds caloric expenditure, excess energy is stored in the form of adipose tissue, and subsequently body mass is increased. Adipose tissue was once believed to be an inert physiologic buffer to store excess energy. However, adipose tissue is now recognized as an active endocrine organ that promotes multiple physiologic changes that influence disease risk.7 There is a growing interest in the oncology community to understand how overweight and obesity may influence prognosis among patients diagnosed with cancer.6,8,9
Fig 1.
A simplified model of modifiable factors related to energy balance. Adapted from Demark-Wahnefried et al.6
The purpose of this report is to provide a brief highlight of existing studies that have linked overweight and obesity to prognosis in individual GI cancer sites; provide recommendations on best management practices; and discuss limitations, controversies, and future directions in this rapidly evolving area. Although there have been studies that examine the importance of overweight and/or obesity before diagnosis, we elected to focus on studies that measured overweight and/or obesity at the time of (or after) cancer diagnosis, because this is the period during which patients may be able to purposefully alter lifestyle behaviors to influence energy balance and weight management and the time during which oncology providers have the most frequent contact with patients.
REVIEW OF OVERWEIGHT AND OBESITY AND INDIVIDUAL GI CANCERS
Esophageal Cancer
Overweight and/or obesity are associated with disease-free survival and overall survival among patients with esophageal adenocarcinoma and squamous cell carcinoma in some,10,11 but not all, studies.12 The association between BMI and survival among patients with esophageal adenocarcinoma is modified by smoking status.10 Among 236 never-smokers with stage I to III esophageal adenocarcinoma, obesity at the time of esophagectomy was independently associated with two-fold higher risk of experiencing disease recurrence or death when compared with normal weight (hazard ratio [HR], 2.03; 95% CI, 1.30 to 3.18; P = .002). Among 542 past and current smokers, obesity was not associated with disease recurrence or death (HR, 1.00; 95% CI, 0.76 to 1.33; P = .94). Among 243 patients with stage I to III squamous cell carcinoma, overweight or obesity at the time of esophagectomy was independently associated with a three-fold higher risk of experiencing disease recurrence or death when compared with normal weight (HR, 2.94; 95% CI, 1.13 to 7.6; P = .027).11 Several studies have reported that overweight and obese patients are more often diagnosed with esophageal adenocarcinoma (v squamous cell).12,13 It is unclear if this pattern is causal (the presence of overweight or obesity increases the propensity to develop one histologic type of esophageal cancer v another, such as with gastroesophageal reflux disease or Barrett esophagus as a precursor to esophageal adenocarcinoma13) or is the result of greater prediagnosis and treatment-related weight loss among patients with squamous cell esophageal cancer.11 Collectively, these data indicate that overweight and obesity at the time of diagnosis is an adverse prognostic characteristic among patients with esophageal cancer.
Gastric Cancer
Overweight and obesity are associated with disease-free survival and overall survival among patients with gastric cancer in some,14-16 but not all, studies.17 The adverse prognostic effect of overweight and/or obesity may vary by primary tumor characteristics (T stage) and regional lymph node involvement (N stage). Among 216 patients with pT2/T3 tumors, overweight and obesity at the time of gastrectomy were independently associated with a shorter 5-year survival rate when compared with normal weight (37.8% v 58.5%; P = .03).14 No difference in survival was observed after the inclusion of patients with pT1 and pT4 tumors (49.1% v 63.4%; P = .09). Similar adverse associations with overweight and obesity have been reported in patients with stage II gastric cancer but not earlier (stage I) or later (stage III or IV) disease.15,16 Among 84 patients with stage II or III gastric cancer, higher intraperitoneal fat thickness (the distance between the anterior peritoneum and retroperitoneum at the umbilicus) quantified using computed tomography at the time of receiving neoadjuvant chemotherapy was independently associated with a three-fold increase in the risk of disease recurrence or death (HR, 3.28; 95% CI, 1.55 to 6.93; P = .002), whereas BMI was not associated with disease outcomes in this sample (P = .56).18 This study highlights the potential limitations of BMI to fully characterize adiposity.19 Collectively, these data indicate that overweight and obesity are adverse prognostic characteristics among patients with gastric cancer.
Pancreatic Cancer
Obesity is associated with disease-free survival and overall survival among patients with pancreatic cancer.20-23 Among 285 patients with stage I or II pancreatic cancer who underwent pancreatectomy, class II or III obesity (BMI ≥ 35 kg/m2) at the time of diagnosis was independently associated with a 1.7-fold increase in the risk of disease recurrence or death compared with a BMI ≤ 35 kg/m2 (HR, 1.65; 95% CI, 1.65 to 2.69; P = .045).20 Class II or III obesity is also associated with poorer overall survival in patients with locally advanced and metastatic pancreatic cancer compared with normal weight.22 Studies using computed tomography have demonstrated that excess intra-abdominal adiposity and low skeletal muscle mass are associated with poor prognosis among patients with pancreatic cancer.24,25 Among 484 patients with pancreatic cancer undergoing palliative chemotherapy, sarcopenia (a low skeletal muscle area from computed tomography in the lumbar [L3] region) was independently associated with overall survival (HR, 1.72; 95% CI, 1.30 to 2.28; P < .001).25 Collectively, these data indicate that obesity, particularly class II or III obesity, and sarcopenia are adverse prognostic characteristics among patients with pancreatic cancer.
Hepatocellular Carcinoma
Overweight and obesity are associated with time to recurrence, disease-free survival, and overall survival among patients with hepatocellular carcinoma.26-28 Among 159 patients with hepatocellular carcinoma who underwent liver transplantation, overweight and obesity were associated with doubling in the incidence of recurrent disease (16% v 8%; P < .05) and shortened time to recurrence (approximately 10 months v approximately 24 months; P < .05) compared with normal weight, respectively.26 Overweight and obesity are also associated with significantly lower 5-year survival rates in patients who undergo repeat hepatectomy for recurrent hepatocellular carcinoma (51.9% v 92.0%; P < .05).28 Several studies have reported that intra-abdominal adiposity quantified using computed tomography is independently associated with time to recurrence (HR, 1.08 per 10 cm2; P = .036),29 and overall survival (HR, 1.35; 95% CI, 1.09 to 1.66; P = .005).30 The association between overweight and obesity with poor prognosis in patients with hepatocellular carcinoma has been hypothesized to be influenced by the increased incidence of microvascular invasion among overweight and obese patients.27,31 Sarcopenia (HR, 1.52; 95% CI, 1.18 to 1.96; P < .001), intramuscular fat deposits (HR, 1.34; 95% CI, 1.05 to 1.71; P = .02), and intra-abdominal fat (HR, 1.35; 95% CI, 1.09 to 1.66; P = .005) quantified using computed tomography are associated with overall survival in patients with hepatocellular carcinoma.30 Collectively, these data indicate that overweight, obesity, and sarcopenia are adverse prognostic characteristic among patients with hepatocellular carcinoma.
Colorectal Cancer
Class II and III obesity (BMI ≥ 35 kg/m2) is associated with disease-free survival and overall survival among patients with nonmetastatic colorectal cancer.32-35 The relationship between BMI and outcomes in colon cancer is often J-shaped (Fig 2), such that patients who are underweight (BMI < 18.5 kg/m2) or with class II or III obesity (BMI ≥ 35 kg/m2) have poorer prognosis compared with those with a BMI > 18.5 to < 35 kg/m2. Furthermore, there is an obesity paradox in colon cancer, such that patients who are overweight (BMI, 25.0 to 29.9) tend to have superior outcomes compared with those of a normal weight (as depicted in Fig 2). The explanation for this paradox is not clear. Proposed explanations of this observation include a true biologic effect (tumors in such patients may be less aggressive and/or more responsive to therapy or patients may have better physiologic reserve to tolerate therapy), differences in body composition with favorable components more prominent with a certain level of increased BMI,37 or methodological issues (BMI not being the best measure of adiposity, unmeasured or accounted for confounders, and selection biases resulting from conditioning on a variable that is associated with both BMI and cancer outcomes [eg, a collider bias]).38-40
Fig 2.
J-shaped relationship between body mass index and time to recurrence in patients with stage III colon cancer. Dashed lines represent 95% CI. Adapted from Renfro et al.36
The relationship between obesity and disease recurrence or death among patients with colorectal cancer is modified by sex (Pinteraction = .013).32,34 Among 25,291 patients diagnosed with stage II or III colon cancer participating in adjuvant chemotherapy trials, men with class II or III obesity at the time of trial enrollment were 16% more likely to experience disease recurrence or death compared with normal weight (HR, 1.16; 95% CI, 1.01 to 1.33; P = .03). Among women, class II or III obesity was not associated with disease recurrence or death (HR, 1.06; 95% CI, 0.93 to 1.21; P = .35). A similar pattern has been observed among patients with stage III rectal cancer, such that men with obesity were 61% more likely to experience local disease recurrence as compared with normal weight (HR, 1.61; 95% CI, 1.00 to 2.59; P = .06), whereas no relationship was observed among women (HR, 1.01; 95% CI, 0.57 to 1.81; P = .80).34 Differences in the storage of excess adiposity may explain the effect modification of sex between obesity and prognosis among patients with colorectal cancer. Women often store excess adiposity on the lower extremities, whereas men often store excess adiposity in the abdominal region.41 This hypothesis is strengthened by the observation that intra-abdominal adiposity quantified using computed tomography42,43 or waist circumference44,45 is independently associated with disease recurrence and death among patients with colorectal cancer. Several polymorphisms associated with obesity-related genes may influence recurrence among colon cancer,46 and metabolomic and transcriptomic signaling of intra-abdominal adipose tissue may differ by disease stage.47 In addition to all-cause and cancer-specific mortality, obesity is associated with an increased risk of cardiovascular-specific mortality among patients with nonmetastatic colorectal cancer (HR, 1.68; 95% CI, 1.07 to 2.65; P = .019).48,49
BMI is associated with progression-free survival and overall survival among patients with metastatic colorectal cancer.50 Among 21,149 patients participating in first-line chemotherapy trials, the relationship between BMI and outcome was L-shaped. The risk of progression or death was highest among patients who were underweight (BMI < 18.5 kg/m2); risk then nadirs at 28 kg/m2 and plateaus at body mass indices > 28 kg/m2. It is plausible that this relationship depicts reverse causality, such that patients who are underweight may have more extensive disease.51 A further explanation may be related to body composition, where sarcopenia has been associated with inferior outcomes in patients with metastatic colorectal cancer.52 In this pooled analysis, the effect of BMI did not differ according to treatment with targeted versus nontargeted therapy. However, several reports have suggested that higher intra-abdominal adiposity may be associated with fewer responders according to Response Evaluation Criteria in Solid Tumors (RECIST; 111.9 ± 12 cm2 in responders v 210.8 ± 58 cm2 in nonresponders; P = .03)53 and accelerated time to progression (9 v 14 months; P < .001)54 among patients with metastatic colorectal cancer who are treated with bevacizumab. The reason for these observations is not clear. Excess intra-abdominal adiposity is associated with elevated levels of serum vascular endothelial growth factor,55 which may impair the efficacy of bevacizumab to sufficiently affect the vascular endothelial growth factor pathway to slow tumor growth; however, this hypothesis has not been confirmed.
BEST MANAGEMENT PRACTICES FOR THE GI ONCOLOGY PROVIDER
Many patients with GI cancer who are overweight or obese have comorbid health conditions such as cardiovascular and cerebrovascular disease, diabetes, and metabolic syndrome at the time of diagnosis, which may influence treatment decision making. Although surgery may be more complex in overweight/obese patients,56 with few exceptions, primarily at the extremes, postoperative morbidity rates and incidence of complications are often similar to those of normal-weight patients.57 Full weight-based systemic therapy should be used when treating obese patients,58 because rates of toxicity are similar or lower among obese patients compared with normal-weight patients.33,34
GI oncology providers are uniquely positioned to offer guidance about weight management and energy balance to patients. Patients often view oncologists as decision makers for their health, and the oncologist recommendation is possibly the biggest catalyst to initiate behavior change.61 Patients are likely to remember recommendations about weight management and energy balance from their oncologist if there is the perception that the provider values such behaviors. A diagnosis of cancer is often viewed as a teachable moment, when patients may be more amenable to adopting recommendations about weight management and energy balance.61 Although there are currently no randomized studies that demonstrate purposeful alterations in energy balance factors, including weight loss, physical activity, or diet modification, affect cancer outcomes, there are consistent observational data regarding factors that influence energy balance, including physical activity and diet, which may be discussed with patients. Whether altering these factors will improve outcomes and whether changing body composition is more important than consideration of weight change is not known. Furthermore, the value of altering particular energy balance factors likely will differ by cancer type. However, purposeful alterations in energy balance–related behaviors improve cardiovascular and metabolic risk factors (hypertension, impaired fasting glucose, visceral obesity) and improve a variety of patient-reported outcomes, including physical function and overall quality of life.8,62-64 On the basis of available data, it is reasonable that GI oncology providers encourage consumption of a balanced and healthy diet, at least weight maintenance and possibly weight loss in obese patients, physical activity, and reduction of sedentary behaviors (eg, television and computer use, prolonged sitting). These recommendations are consistent with clinical practice guidelines for patients with cancer.8,62-64 GI oncology providers should consider making the following recommendations to patients:
Recommendations for Diet
Consume a diet pattern that is high in vegetables, fruits, and whole grains,62 and avoid a western pattern diet that is characterized by frequent consumption of red and processed meats, sugar desserts and sugar-sweetened beverages, and refined grains.65
The use of meal replacement products, including packaged entrees and shakes,68 or referral to commercial weight loss programs may be may useful to promote initial weight loss for survivors of disease sites where overweight or obesity are associated with outcomes.69
At this time there is limited evidence to suggest the consumption (or avoidance) of specific dietary constituents.62
Recommendations for Physical Activity
Participate in regular physical activity toward of the volume goal of a minimum of 150 minutes per week of moderate-intensity aerobic activity.62,63 However, any volume of physical activity that a patient can do should be considered better than sedentary behavior.63
Many patients will elect to use walking as their primary modality of activity. For these patients, a walking cadence of 100 steps per minute is consistent with moderate intensity for most adults.70 Activity should be accumulated in bouts of ≥ 10 minutes; the use of a pedometer is encouraged to quantify step counts, and 1,000 steps in 10 minutes or 3,000 steps in 30 minutes is a useful mnemonic to guide patients.
Higher volumes of regular physical activity (250 to 300 minutes per week) may be required for the prevention of weight regain.71
Patients with physical impairments that may serve as a barrier or make it unsafe to engage in physical activity61 should be referred to trained rehabilitation health care professionals.72
LIMITATIONS, CONTROVERSIES, AND FUTURE DIRECTIONS
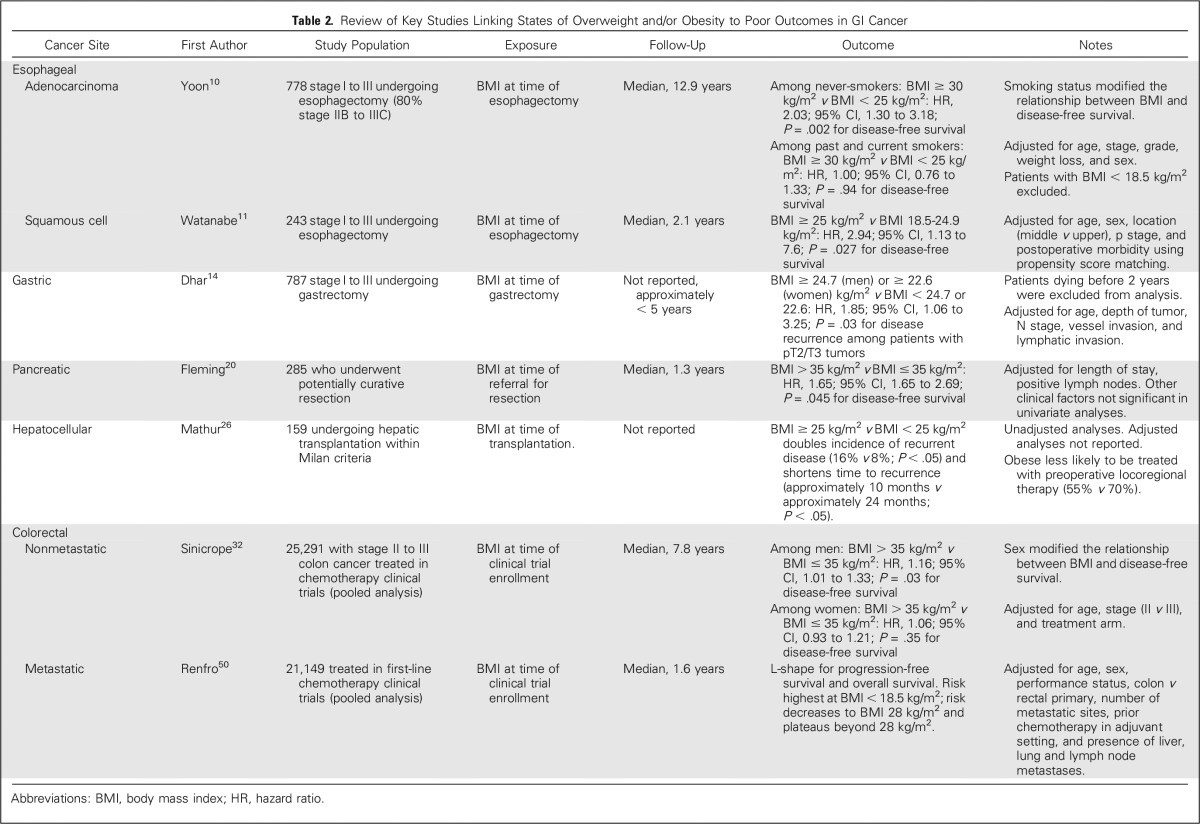
Overweight and/or obesity are associated with prognosis in multiple GI cancers (Table 2). Much of the evidence describing the association between overweight or obesity and prognosis has been reported within the past decade. Given the infancy of this area of investigation, studies conducted have several methodological limitations. The majority of studies have been retrospective analyses of medical records that define overweight or obesity using BMI. More recently, studies have leveraged computed tomography imaging, which is often implemented in preoperative staging and long-term surveillance of many GI cancers. The use of computed tomography allows for the quantification of intra-abdominal adipose tissue and skeletal muscle, which may provide additional specificity about tissue composition compared with that of BMI alone. There is emerging evidence that low levels of muscle mass (ie, sarcopenia) may affect prognosis among various GI cancers. Similar to adipose tissue, skeletal muscles possess potent endocrine properties that regulate inflammation, fat oxidation, and glucose homeostasis.73 Several of these biologic processes have been hypothesized to mediate the relationship between body composition and cancer progression.74 Many of the studies included in this review conducted statistical analyses that accounted for known prognostic factors, such as age, sex, cancer stage, and other tumor- or treatment-related characteristics. Few reports accounted for other prognostic factors that may influence the relationship between overweight or obesity and prognosis, such as smoking history, the presence of comorbid health conditions (diabetes and others), performance status, medication use, physical activity, and diet or alcohol consumption. As demonstrated in the example of esophageal adenocarcinoma, smoking status modified the relationship between obesity and prognosis after esophagectomy.10 Therefore, the inclusion of these important covariates will help to better characterize the relationship between overweight or obesity and prognosis.
Table 2.
Review of Key Studies Linking States of Overweight and/or Obesity to Poor Outcomes in GI Cancer
A significant controversy in the field is that it is unknown if the relationship between overweight and/or obesity and prognosis in GI cancer is causal. All of the evidence conducted in this area has been observational and is susceptible to confounding and bias. If the relationship between overweight or obesity and prognosis in GI cancer is causal, two scenarios are plausible.75 In scenario one, overweight or obesity may have a fixed (nonreversible) biologic effect that influences cancer development, making weight status useful as a prognostic biomarker; in scenario two, overweight or obesity may have a dynamic (reversible) biologic effect on cancer that can be favorably altered with weight loss, making weight status useful as a biomarker predictive of treatment benefit.76 There is growing mechanistic evidence that overweight and obesity induce alterations in proinflammatory cytokines, lipid metabolites, adipokines, and insulin and insulin growth factor signaling pathways.74 Each of these alterations may independently or additively influence drug resistance and disease recurrence or progression. Conversely, if the relationship between overweight or obesity and prognosis in GI cancer is noncausal, it may be the result of several forms of bias or confounding. Selection bias is one such example, such that patients included in observational studies systemically differ from the intended population. Several studies included in this review have attempted to address this issue by sampling patients consecutively (all patients treated for a certain cancer between two time periods). A second form of selection bias may be that patients with overweight or obesity systematically present later in the natural course of the disease. Among patients with colon cancer, overweight and obesity are associated with increased risk of presenting with T3 or T4 tumors and N1 or N2 lymph node staging.77 The use of restriction or stratification by disease stage in the statistical analysis may help to isolate the effects of overweight and obesity; such approaches have been implemented in patients with gastric, pancreatic, and colorectal cancers. Last, the relationship between overweight or obesity and prognosis may be confounded by unknown or unmeasured variables.
There are multiple areas of promise that warrant continued investigation.6 Overweight and obesity are the result of chronic energy imbalance—too much energy consumed and too little energy expended. The comparative contributions of weight, dietary patterns, and physical activity on cancer progression are unknown.6 Therefore, the key provocative question is whether and when weight loss is needed to alter disease outcomes versus modifying individual energy balance components such as dietary patterns (calorically similar but nutritionally different) or energy expenditure (via physical activity) and/or altering body composition. For example, participation in postdiagnosis of physical activity is associated with lower rates of disease recurrence and death among patients with stage III colon cancer, independent of BMI.78 In the absence of weight loss, participation in physical activity is associated with a variety of changes in body composition, including increases in skeletal muscle mass, reductions in intra-abdominal adiposity, and improvements in various inflammatory and metabolic markers.79 There is a need to conduct interventional studies that aim to gather important data regarding feasibility, safety, and the effects of altering and sustaining behavioral changes on intermediate biomarkers. These studies will help to clarify the comparative contributions of individual energy balance–related factors and refine key design aspects to be used in definitive phase III trials with disease end points.
Another area of critical importance is unraveling the biologic mechanisms that mediate the relationship between energy balance and prognosis. This knowledge will help to refine the development of interventions and identify which patients are most likely to benefit. Large-scale clinical trials of chemotherapy, targeted therapy, radiation, and other related interventions should measure BMI and other energy balance–related variables such as waist circumference, physical activity, dietary intake, and biologic markers, as feasible. Clinical trials offer an excellent resource to embed energy balance and correlative science companion measures, because the study populations are often large and well defined (homogeneous), with complete treatment-related information and excellent long-term outcome data collection. The relationship between sarcopenia or sarcopenic obesity (low skeletal muscle in the presence of a high BMI) and cancer outcomes and the biologic mechanisms that mediate this relationship is a promising area that warrants additional investigation. Finally, an important area of investigation is which patients with GI cancer should (v should not) alter their current states of energy balance and if it is stage dependent. For example, in colorectal cancer, there is evidence that class II or III obesity is associated with a worsened prognosis in the adjuvant setting but a more favorable prognosis in the metastatic setting. Conversely, in pancreatic cancer, class II or III obesity seems to have a consistent adverse effect on prognosis in resectable, locally advanced, and metastatic disease.
In conclusion, GI oncology providers are likely to treat a high proportion of patients who are overweight or obese at the time of diagnosis. There is emerging evidence from observational studies that overweight and/or obesity is an adverse prognostic characteristic among various GI cancers. GI oncology providers are uniquely positioned to help encourage healthy lifestyle practices related to energy balance that promote weight management and healthy body composition. Additional data from prospective studies and randomized trials are urgently needed. These additional data will allow for more definitive guidance to patients with GI cancer.
Footnotes
Supported by National Cancer Institute Grants No. U54CA155850, R21CA182767, F31CA192560, and R25CA092203 (J.C.B.); and U54CA155626, R01CA149222, R01CA169141, and R01CA118553 (J.A.M.).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Justin C. Brown
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Obesity and Energy Balance in GI Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Justin C. Brown
No relationship to disclose
Jeffrey A. Meyerhardt
No relationship to disclose
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Masters RK, Reither EN, Powers DA, et al. The impact of obesity on US mortality levels: The importance of age and cohort factors in population estimates. Am J Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ligibel JA, Alfano CM, Hershman D, et al. Recommendations for obesity clinical trials in cancer survivors: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33:3961–3967. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 10.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4561–4567. doi: 10.1200/JCO.2011.37.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Ishimoto T, Baba Y, et al. Prognostic impact of body mass index in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2013;20:3984–3991. doi: 10.1245/s10434-013-3073-8. [DOI] [PubMed] [Google Scholar]
- 12.Blom RL, Lagarde SM, Klinkenbijl JH, et al. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol. 2012;19:766–771. doi: 10.1245/s10434-011-2103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer. 2010;116:5619–5627. doi: 10.1002/cncr.25745. [DOI] [PubMed] [Google Scholar]
- 14.Dhar DK, Kubota H, Tachibana M, et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 15.Moriwaki Y, Kunisaki C, Kobayashi S, et al. Does body mass index (BMI) influence morbidity and long-term survival in gastric cancer patients after gastrectomy? Hepatogastroenterology. 2003;50:284–288. [PubMed] [Google Scholar]
- 16.Ojima T, Iwahashi M, Nakamori M, et al. Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy: An analysis of 689 consecutive cases managed by a single center. Arch Surg. 2009;144:351–358. doi: 10.1001/archsurg.2009.20. discussion 358. [DOI] [PubMed] [Google Scholar]
- 17.Inagawa S, Adachi S, Oda T, et al. Effect of fat volume on postoperative complications and survival rate after D2 dissection for gastric cancer. Gastric Cancer. 2000;3:141–144. doi: 10.1007/pl00011708. [DOI] [PubMed] [Google Scholar]
- 18.Li XT, Tang L, Chen Y, et al. Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. J Cancer Res Clin Oncol. 2015;141:1237–1247. doi: 10.1007/s00432-014-1893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: Where is the ‘beef’? Int J Obes. 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- 20.Fleming JB, Gonzalez RJ, Petzel MQ, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 21.Mathur A, Luberice K, Paul H, et al. Increasing body mass index portends abbreviated survival following pancreatoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2015;209:969–973. doi: 10.1016/j.amjsurg.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 22.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelucchi C, Galeone C, Polesel J, et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43:47–52. doi: 10.1097/MPA.0b013e3182a7c74b. [DOI] [PubMed] [Google Scholar]
- 24.Balentine CJ, Enriquez J, Fisher W, et al. Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg. 2010;14:1832–1837. doi: 10.1007/s11605-010-1297-5. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10:e0139749. doi: 10.1371/journal.pone.0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathur A, Franco ES, Leone JP, et al. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB (Oxford) 2013;15:504–510. doi: 10.1111/j.1477-2574.2012.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel AB, Lim EA, Wang S, et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539–543. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utsunomiya T, Okamoto M, Kameyama T, et al. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58:839–844. doi: 10.1136/gut.2008.164053. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Siegel AB, Wang S, Jacobson JS, et al. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2010;28:1063–1069. doi: 10.3109/07357907.2010.483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119:1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 34.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: Findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 35.You JF, Tang R, Changchien CR, et al. Effect of body mass index on the outcome of patients with rectal cancer receiving curative anterior resection: Disparity between the upper and lower rectum. Ann Surg. 2009;249:783–787. doi: 10.1097/SLA.0b013e3181a3e52b. [DOI] [PubMed] [Google Scholar]
- 36. Renfro LA, Grothey A, Xue Y, et al. ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer. J Natl Cancer Inst 106:dju333, 2014 . [DOI] [PMC free article] [PubMed]
- 37.Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22:2663–2668. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez MC, Pastore CA, Orlandi SP, et al. Obesity paradox in cancer: New insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 39.Lajous M, Bijon A, Fagherazzi G, et al. Body mass index, diabetes, and mortality in French women: Explaining away a “paradox”. Epidemiology. 2014;25:10–14. doi: 10.1097/EDE.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banack HR, Kaufman JS. The obesity paradox: Understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102. doi: 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br J Nutr. 2008;99:931–940. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 42.Lee CS, Murphy DJ, McMahon C, et al. Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. J Gastrointest Cancer. 2015;46:243–250. doi: 10.1007/s12029-015-9709-0. [DOI] [PubMed] [Google Scholar]
- 43.Ballian N, Lubner MG, Munoz A, et al. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J Surg Oncol. 2012;105:365–370. doi: 10.1002/jso.22031. [DOI] [PubMed] [Google Scholar]
- 44.Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prizment AE, Flood A, Anderson KE, et al. Survival of women with colon cancer in relation to precancer anthropometric characteristics: The Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebio A, Gerger A, Matsusaka S, et al. Genetic variants within obesity-related genes are associated with tumor recurrence in patients with stages II/III colon cancer. Pharmacogenet Genomics. 2015;25:30–37. doi: 10.1097/FPC.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liesenfeld DB, Grapov D, Fahrmann JF, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: The ColoCare study. Am J Clin Nutr. 2015;102:433–443. doi: 10.3945/ajcn.114.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 49.Pelser C, Arem H, Pfeiffer RM, et al. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014;120:1540–1547. doi: 10.1002/cncr.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: Pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34:144–150. doi: 10.1200/JCO.2015.61.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieffers JR, Mourtzakis M, Hall KD, et al. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89:1173–1179. doi: 10.3945/ajcn.2008.27273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 53.Ghiringhelli F, Vincent J, Guiu B, et al. Bevacizumab plus FOLFIRI-3 in chemotherapy-refractory patients with metastatic colorectal cancer in the era of biotherapies. Invest New Drugs. 2012;30:758–764. doi: 10.1007/s10637-010-9575-3. [DOI] [PubMed] [Google Scholar]
- 54.Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 55.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, et al. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 56.Curet MJ. Special problems in laparoscopic surgery. Previous abdominal surgery, obesity, and pregnancy. Surg Clin North Am. 2000;80:1093–1110. doi: 10.1016/s0039-6109(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 57.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 58.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 59. Reference deleted.
- 60. Reference deleted.
- 61.Brown JC, Schmitz KH. The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc. 2014;46:2202–2209. doi: 10.1249/MSS.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 63.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 64.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: Healthy lifestyles, version 2.2014. J Natl Compr Canc Netw. 2014;12:1222–1237. doi: 10.6004/jnccn.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 66. Reference deleted.
- 67. Reference deleted.
- 68.Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: An updated systematic review. Ann Intern Med. 2015;162:501–512. doi: 10.7326/M14-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marshall SJ, Levy SS, Tudor-Locke CE, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36:410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 71.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 72.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 73.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 74.Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodwin PJ. Obesity and breast cancer outcomes: How much evidence is needed to change practice? J Clin Oncol. 2016;34:646–648. doi: 10.1200/JCO.2015.64.7503. [DOI] [PubMed] [Google Scholar]
- 76. Ballman KV. Biomarker: Predictive or prognostic? J Clin Oncol 33:3968-3971, 2015. [DOI] [PubMed]
- 77.Brändstedt J, Wangefjord S, Nodin B, et al. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: A cohort study. Biol Sex Differ. 2012;3:23. doi: 10.1186/2042-6410-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 79.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]