Abstract
Introduction:
This paper presents mobile application implementing a decision support system for acid-base disorder diagnosis and treatment recommendation.
Material and methods:
The application was developed using the official integrated development environment for the Android platform (to maximize availability and minimize investments in specialized hardware) called Android Studio.
Results:
The application identifies disorder, based on the blood gas analysis, evaluates whether the disorder has been compensated, and based on additional input related to electrolyte imbalance, provides recommendations for treatment.
Conclusion:
The application is a tool in the hands of the user, which provides assistance during acid-base disorders treatment. The application will assist the physician in clinical practice and is focused on the treatment in intensive care.
Keywords: clinical decision support systems, acid-base disorder diagnosis, acid-base disorder treatment
1. INTRODUCTION
Application for diagnosing acid-base balance and recommending treatments falls under the category of decision support systems.
Decision support systems (DSS) are systems with primary function to gather and interpret information which can influence and improve the process of decision making in organizations. These are interactive, computer based systems which use models and information to aid problem solving and decision making.
Information technologies have found use in almost all branches of healthcare. Advances in medicine in recent decades are in significant correlation with the advances in the information technology and modern information technologies and have enabled faster, more reliable and comprehensive data collection (1). Electronic medical records, electronic prescriptions, diagnostic tools, digital image processing are just some of the examples. Additionally, health workers are increasingly using online repositories such as PubMed, Google Scholar and specialized tools like Find Zebra to aid them in the process of finding answers for complex clinical problems. This paper focuses on a subfield of the DSS, called clinical decision support systems. The clinical decision support systems utilize established clinical knowledge and updated patient information with the aim of improving patient care (2). The focus is to improve patient-doctor interaction starting with the initial consultations up to diagnosis and patient-progress monitoring. Time constraints imposed on physicians contribute not only to the number of physician errors but also to postponement of final diagnosis (3). The consequences are in damage caused to the patient and financial costs to the institutions with average costs for hospital due to malpractice lawsuits in the US are 300,000 USD per lawsuit (4). Research conducted in 2009 concludes that 32% of physician errors were caused by insufficient amount of time devoted to the assessment of the patient’s condition (5).
Clinical decision support systems vary in type and complexity. Systems can be passive, with user explicitly creates a request for support, semi-active: observation systems which execute automatically but information is presented to user only upon request, and active systems which are automatically activated, executing and pre-senting information without waiting for explicit request from the end-user. All of these systems are implemented to accommodate health care workers across various specters of expertise and specialization. As an example, software intended for use in medical student population is designed differently than software aimed at experienced specialists. The basic example of a clinical decision support system checks the input data entered by a health worker and checks whether the value is within the reference range. System of medium complexity includes prognostic calculators and automated guidance in clinical practice. Prognostic calculators are used for automated diagnosis, based on clinical scoring systems. An example of the system is the multiform glioblastom calculator based on scoring system of the The European Organisation for Research and Treatment of Cancer (EORTC) (6).
Due to powerful computing resources and widespread use of smartphones another field is emerging in clinical support systems in the form of mobile applications. These applications use the underlying hardware of common smartphones. Delivery is easy and fast, due to developed infrastructure of official mobile app stores. It is estimated that the Apple App Store has over 14 000 applications in the category for medicine (6). The number is somewhat lower for the Google Play (5000 applications) (6). Most of the applications are distributed without any charge. Aiming to maximize availability and minimize investments in specialized hardware this paper proposes a clinical decision support system for acid-base balance diagnosis and treatment recommendation implemented for the smartphones with the Android operating system.
2. ACID-BASE STATUS
The normal variation in acid-base balance implies fluctuation in hydrogen ions in extracellular fluid within narrow limits from pH 7.36 (44nmol/L) to 7.44 (36nmol/L) (7, 8). Acid-base status of trans-cellular space is regulated utilizing active transfer of acids and bases outside of cellular space. Pathological conditions with an increased concentration of hydrogen ions are called acidosis. Conversely, states with reduced concentrations of hydrogen ions are called alkalosis. Acidosis and alkalosis are terms associated with systemic disorders in concentration of hydrogen ions. Disturbances in the concentration of hydrogen ions in blood, are called acidemia and alkalemia. In order to keep acid-base status within physiological range, organism engages powerful regulatory mechanisms: buffer systems, lungs, kidneys and skeletal system. Buffer systems act immediately, but have relatively small capacity. Respiratory system is activated after several hours. Most powerful buffer capacity lies in the kidneys and skeletal system, but they also require most time to activate. Buffer systems act within extracellular and intracellular fluid. Most important extracellular system is the bicarbonate buffer system. On the other hand, the most important intracellular system is the protein and phosphate buffer system (8). Considering their ability to remove carbon dioxide from the organism, lungs can have a significant impact on acid-base status. If lung ventilation doubles, pH blood value will increase (alkalosis). Conversely, the reduction in lung ventilation will decrease pH value (acidosis). The total buffering power of respiratory system is almost twice the power of all chemical buffer systems combined.
Renal system buffering effect is in conserving filtered bicarbonates, regulating titratable acidity and forming ammoniac ion. Skeletal system has a role in correcting chronic disorders of acid-base status.
Metabolic acidosis (pH<7.35, reduced level of bicarbonate < 24mmol/L, compensatory hyperventilation- reduced pCO2) occurs as a result of increase in the amount of hydrogen ions and due to loss of bicarbonate ions (increased intake of acid, increased synthesis of volatile acids, the accumulation of metabolic acids, chronic renal failure, distal renal tubular acidosis, diarrhea, malignant processes, proximal renal tubular acidosis, Fanconi syndrome, Wilson’s disease) (8). Base deficit is the basis of metabolic acidosis, and is due to the above mentioned etiological factors. At the expense of reduction of plasma bicarbonate concentration (used to buffer the hydrogen ions), there is an increase in the concentration of other anions (in order to achieve and maintain electro neutrality).
The anion may be chloride (Cl-) or other anion of a metabolic product acid. Based on this, we distinguish: hyperchloremic metabolic acidosis (kidney disarrangements - interstitial nephritis with uremia, hydronephrosis, proximal and distal renal tubular acidosis, gastrointestinal causes - diarrhea, uretero-enterostomy) metabolic acidosis with anion deficit (renal failure, diabetic ketoacidosis, L-lactacidemia, ingestion of salicylates) and their combination (L-lactacidemia) (9). Anion gap higher then 15 mmol/L indicates metabolic acidosis, and greater then 25 mmol/L confirms metabolic acidosis. Furthermore, based on the value of the anion gap it can be determined whether acidosis is with elevated or normal anion gap (Equation 1).
Equation 1:
Anion gap = [Na+]–[Cl-]–[HCO3-] [mmol/L]
The primary cause of respiratory acidosis pH < 7.35, elevated pCO2, compensatory decrease levels of bicarbonate < 24 mmol/L, is an acute or chronic respiratory system insufficiency as: acute airways obstruction, acute restriction lung disease, acute circulatory insufficiency, depression of the respiratory center, neurological diseases - poliomyelitis, polyneuropathy, myasthenia gravis, chronic obstructive pul- monary disease, chronic inhibition of respiratory center of the chest wall diseases, diseases of the lung parenchyma. Metabolic alkalosis pH > 7.45, serum bicarbonate levels > 24mmol/L, compensatory hypoventilation - increased pCO2; occurs due to loss of acid outside of cellular liquids as due to loss of HCl by vomiting, hypokalemia, hypercalcemia, use of diuretics not saving potassium - furosemide, ethacrynic acid; and because of the influences that increase the concentration of bicarbonate in the extracellular fluid as milky-alkali syndrome, Conn’s disease, Liddle’s syndrome, the therapeutic use of the mineralocorticoid, the application of organic acids, post hypercapnic condition (8).
Metabolic alkalosis can be divided into those that are salt dependent - Cl- responsible as contraction alkalosis, vomiting, use of diuretics, antacids entry; and to those not dependant of salt - Cl- resistant as when sodium chloride is not used in treatment and potassium chloride is used, presence of chloride in the urine (8, 9).
Respiratory alkalosis pH>7.45, hyperventilation - decreased pCO2, compensatory reduction in the level of bicarbonate < 24mmol/L is such a disturbance of acid-base balance in which increased alveolar ventilation causes a decrease in pCO2, and consequently reduce the level of bicarbonate, and increasing blood pH values. It is divided into acute as hyperventilation due to anxiety, high temperatures, salicylate poisoning, sepsis; and chronic as brain tumors, encephalitis, liver cirrhosis, coma, pregnancy (8). In everyday practice, complex forms of acid-base status disorders, with combintion of these conditions, are not rare.
Complex forms may be additive, as a combination of resipratory and metabolic acidosis; example in diabetes that lasts for a long time - a combination of diabetic ketoacidosis and in uremic acidosis due to diabetic nephropathy - severe academia (10, 11, 12, 13). Respiratory and metabolic alkaloses are usually seen in patients on mechanical ventilation and nasogastric suction - heavy alkalemia.
3. MOBILE APPLICATION
The application was developed using the official integrated development environment for the Android platform called Android Studio, which was at time of writing in version 1.3.
Activites package contains application activities that includes activity for input of parameters from a blood test, activity for displaying diagnosed disorders and entering additional parameters and an activity for treatment recommendations. In addition, the customized software keyboard was created as a separate activity. The model package contains a class that encapsulates blood result data (BloodResult) and two packages disorders and electrolytes that model disorders of the acid-base status and electrolyte disturbances.
Disorders package models various types of acid-base status disorders and their relationships. Each disorder was inherited from an abstract class Disorder. This class has only two abstract methods: getTreatment and getName. Input parameters of the method getTreatment are objects of type BloodResults and body mass, which are relevant for determining the treatment. Each class that inherits the class Disorder implements the getTreatment method separetely (which is in accordance with the domain of the problem, since the treatment varies between disorders).
Not only is the treatment different for each disorder, but there are variations within individual disorders. Specifically for treatment of certain disorders, it is necessary to know the cause that led to the disorder for treatment recommendation to be correct. For example, in metabolic acidosis, treatment is different for metabolic acidosis arising from the accumulation of acids in comparison to metabolic acidosis formed due to the loss of bicarbonate ions. Bearing in mind the above, for the recommendation of metabolic acidosis treatment, anion gap should be calculated.
To determine the disorder, the parameters from a blood test are needed. Within utils package there is Diagnostics class that contains an algorithm for determining the disorder Comparison range of key parameters required for diagnosis (pH, partial pressure of carbon dioxide and the amount of bicarbonate) is different for venous, arterial and capillary blood. For this reason the constructor of Diagnostics class receives these ranges. The class has a method called calculate Disorder which takes blood analysis as input (an object of type BloodResult) and as a result returns the disorder (an object of type Disorder).
The algorithm will determine which disorder of the acid-base status it is and whether it is offset by a secondary disorder. For example, acidosis is often accompanied by hyperkalemia and alkalosis is usually accompanied by hypokalemia. Besides potassium, significant values are also values of sodium, calcium and magnesium.
If the values are outside the normal range, the end user is promted to enter their respective values. Entered data is then used to extend treatment recommendations with recommendations for treating electrolyte disorder in addition to acid-base status disorder.
Electrolytes package contains classes that model these electrolytes. In this regard, four classes of Sodium, Calcium, Magnesium and Potassium have been created, inherited from an abstract class Electrolyte.
Each child class implements a method that returns the value of the concentration, a method that returns the name of the disorder (the method is passed Context of the activity that calls it since the names of the disorder are within the string resources of the application), and a method that returns treatment for the disorder.
In clinical practice, besides pH level, for determining acid-base status of the patient the following parameters are used:
PaCO2 - - presents partial pressure of carbon dioxide in blood and it is the most sensitive indicator of lung function. This value is indicator of concentration of carbon dioxide in mixture of alveolar gases and is based on Daltons law which states that every gas in mixture of gases has partial pressure which is directly proportional to its concentration. Pressure is presented in kilo pascal or torr unit. Decrease in PaCO2 is called hypocapnia and points to hyperventilation (forced and fast breathing), while an increase in PaCO2 is called hypercapnia and points to hypoventilation (breathing disorder that is characterized with decreased concentration of air that comes into the lungs during inspiration). Normal partial pressure of CO2 is within range of 4.7 kPa–5.9 kPa (10).
PaO2 - - represents partial pressure of oxygen in the blood and is an indicator of the adequacy of gas exchange. The oxygen in the arterial blood is transferred in two forms: as dissolved and as bound to hemoglobin of the red blood cells. Decreased PaO2 in blood is called hypoxemia. The reference value of PaO2 is within range 9.3–13.3 kPa (10).
Value of HCO3- bicarbonate ions in the blood.
The base excess - Base excess is a calculated value, which estimates the level of metabolic disorders regardless of the value of the partial pressure of CO2. It is defined as the amount of strong acid (or base), expressed in mmol/ L, which is necessary to bring the pH of 100% oxygenated blood brought by titration to the value of 7.4 at a temperature of 37°C and CO2 partial pressure of 40 mmHg. The base excess 2 mg/dL indicates a metabolic alkalosis (8, 9).
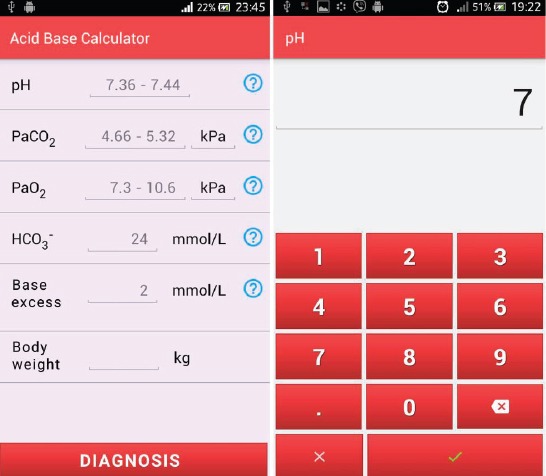
An additional parameter is the body mass that represents relevant information in treatment recommendations. The values of the partial pressure of carbon dioxide and oxygen can be entered in kilo pascals or in millimeters of mercury scale. The display for input of parameters is also the applications’ home screen and it is shown in Figure 1. The principles of good design interaction as key parameters include (14):
Figure 1.
Disorder diagnosis and treatment recommendation
a) Visibility;
b) Logic;
c) Consistency;
d) Convenient for the purpose;
e) Feedback;
f) Limitations.
Application interface is designed in accordance with the templates provided for the Android platform, so that all screens applications rely on predefined and recommended templates. Consistency is secured using one master layout for all screens of application. Restrictions refer to entry fields for which there is validation in terms of restrictions on the number and types of characters. To enter parameters, a separate screen is created that represents a form of customized software keyboard that contains only digits and separator in the form of dot. In this way it is ensured that the user enters only numeric values. Additionally, a limit on the size of the numbers to be entered is introduced. This limit is different for all parameters. Also, to access the screen with the diagnosis, the user must enter all the required parameters. Otherwise, the user is shown Android Toast message notifying that all parameters are required. This message represents the type of feedback that informs the user about the error that occurred. In design of interaction, metaphors were also being used. Within view of diagnosis, sections are divided into layout that recall the vertically arranged sheets of paper. This metaphor visually separates sections and symbolizes theses on paper, as a way of summarizing information. This view is in line with the new principles proposed by Google for Android applications.
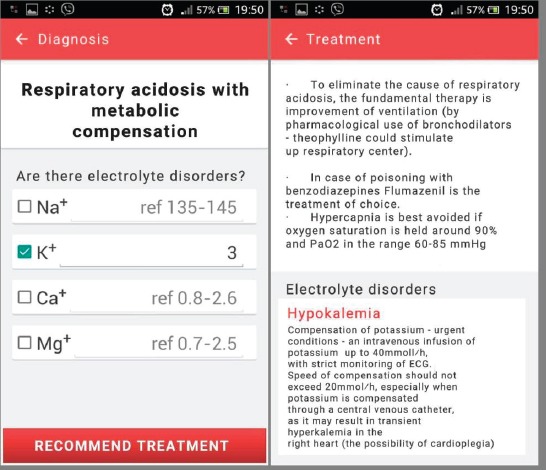
Home screen, as presented in Figure 1, prompts the user to enter values obtained from blood gas analysis along with patient body mass. For each value, a normal reference range is show along with help link that describes the meaning of each parameter. For parameter input, a separate software keyboard was created. For entering partial pressures of CO2 and O2 user can choose the unit of measure (kilo Pascal or millimeters of mercury scale). Pressing the Diagnosis will open the screen shown in Figure 1, where the diagnosis of the disorder is given. Since disorders in acid-base status are accompanied by electrolyte disorders, user is prompted to also enter this data, if values are out of normal range.
After pressing the Recommend treatment button, the final screen is shown where treatment recommendations are given. In the example shown in Figure 2, treatment recommendations are given for respiratory acidosis with metabolic compensation (Figure 2). Since the disorder caused a decrease in potassium levels, treatment recommendation for hypopotassemia is also given.
Figure 2.
Application home screen and value input screen
4. MAIN RESULTS
The main objective of this research was development of a mobile clinical decision support system implemented as an Android application. The mobile application would give the smartphone a role of an access terminal for service diagnostics. Research has shown that the number of applications in the field of medicine on leading mobile platforms is rather low. However a growing trend in using such applications is noticed. Additional focus of the work was to demonstrate that the use of formal methods, in collaboration with medical experts, resulted in a mobile application implementing accurate medical device, with the advantage of high availability and relatively low cost (15).
Diagnosis and treatment of disorders of acid-base balance with high level of accuracy requires a significant amount of time. Although the number of input parameters is not high, the number of diseases that occur as well as specific treatment for each significantly complicates the task. This the time that physicians in clinical practice usually cannot afford. During development, the biggest challenge was a detailed exploration of the problem domain, with the aim of mapping the collected information in a suitable algorithm.
Domain knowledge for interpreting the key parameters of the system was acquired from the experts from the field of medicine. During the evaluation of the system, the authors identified possibilities for improvements as parameterization of the algorithms for diagnosing and treatment. Reference values of the input parameters are different for arterial, venous and capillary blood samples. Capillary blood samples are most commonly used due to simplicity of its collection.
5. CONCLUSION
The application is a tool in the hands of the user, which provides assistance during acid-base disorders treatment. Research into similar applications has shown that there are mobile applications that define the primary disorder. However, this application would be a pioneer in recommending the treatment, taking into account the current state of the patient, medical history and concurrent diseases (takes into account the possible existence of electrolyte imbalance). The application will assist the physician in clinical practice and is focused on the treatment in intensive care. Distribution via Google Play would make it easily accessible and convenient.
REFERENCES
- 1.Masic I, Begic E. Information Technology - a Tool for Development of the Teaching Process at the Faculty of Medicine, University of Sarajevo. Acta Inform Med. 2015;23(2):108–112. doi: 10.5455/aim.2015.23.108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A Roadmap for National Action on Clinical Decision Support. Journal of the American Medical Informatics Association: JAMIA. 2007;14(2):141–5. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009 Nov 9;169(20):1881–7. doi: 10.1001/archinternmed.2009.333. [DOI] [PubMed] [Google Scholar]
- 4.Singh H, Graber M. Reducing Diagnostic Error Through Medical Home-Based Primary Care Reform. JAMA. 2010;304(4):463–4. doi: 10.1001/jama.2010.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaelsen SR, Christensen IJ, Grunnet K, Stockhausen MT, Broholm H, Kosteljanetz M, et al. Clinical variables serve as prognostic factors in a model for survival from glioblastoma multiforme: an observational study of a cohort of consecutive non-selected patients from a single institution. BMC Cancer. 2013 Sep 3;13:402. doi: 10.1186/1471-2407-13-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-Pérez B, de la Torre-Díez I, López-Coronado M, Sainz-de-Abajo B, Robles M, García-Gómez JM. Mobile clinical decision support systems and applications: a literature and commercial review. J Med Syst. 2014 Jan;38(1):4. doi: 10.1007/s10916-013-0004-y. [DOI] [PubMed] [Google Scholar]
- 7.Adrogué HJ, Gennari FJ, Galla JH, Madias NE. Assessing acid-base disorders. Kidney Int. 2009 Dec;76(12):1239–47. doi: 10.1038/ki.2009.359. [DOI] [PubMed] [Google Scholar]
- 8.Gamulin S, Marusic M, Kovac Z. Patofiziologija. 7th ed. Zagreb, Croatia: Medicinska naklada; 2011. pp. 315–32. [Google Scholar]
- 9.Simonovic-Zivancevic S. Opsta patoloska fiziologija. Kragujevac, Serbia: University of Kragujevac; 2006. pp. 310–47. [Google Scholar]
- 10.Jukic M, Gasparovic V, Husedzinovic I. Intenzivna njega. Zagreb, Croatia: Medicinska naklada; 2006. pp. 368–73. [Google Scholar]
- 11.Hall EJ. Guyton and Hall Textbook of Medical Physiology. 12th ed. Philadelphia, USA: Saunders/Elsevier; 2011. pp. 379–85. [Google Scholar]
- 12.Narins RG, Emmett M. Simple and mixed acid-base disorders: a practical approach. Medicine (Baltimore) 1980 May;59(3):161–87. doi: 10.1097/00005792-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014 Oct 9;371(15):1434–45. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 14.Preece J, Rogers Y, Sharp H. Interaction Design. New York, NY, USA: John Wiley and Sons Inc; 2002. pp. 368–73. [Google Scholar]
- 15.Masic I, Begic E. Mobile Clinical Decision Support Systems in Our Hands - Great Potential but also a Concern. Stud Health Technol Inform. 2016;226:63–6. [PubMed] [Google Scholar]


