Abstract
Aim:
To examine the morphologic variations of occipital sulci patterns in patients with schizophrenia and migraine haeadache regarding gender and laterality as well as damage of visual pathway in patients with schizophrenia using magnetic resonance imagining (MRI) and visual evoked potentials (VEPs).
Methods:
This study included 80 patients and brain scans and visual evoked potential responses recorded over the occipital cortex were performed to analyze the occipital region of both hemispheres. Average total volumes of both hemispheres and average values of amplitude of the healthy population for the comparasion were used.
Results:
There was statistically significant difference between subjects considering gender (p=0.012). Parameters of parieto-occipital fissure (p=0.0314) showed statistically significant positive correlation with P100 amplitude (p=0.05), body of the calcarine sulcus (p=0.0213) had significant positive correlation with P100 amplitude (p=0.04), retro calcarine sulcus (p=0.0516) and P100 amplitude (p=0.03) showed statistically significant difference only of left hemisphere in male patients with schizophrenia with shallower depth of the sulcus and P100 amplitude reduction.
Conclusion:
Schizophrenia is associated with the volume changes of the occipital lobe. Comparative analysis of morphologic differences in the gray matter of occipital lobe using MRI and VEPs revealed changes especially of the left hemisphere (shallower depth of sulcus and P100 amplitude reduction) only among a group of male patients.
Keywords: morphologic differences, visual pathway, neuroradiology, neurophysiology
1. INTRODUCTION
Schizophrenia is a neurodevelopmental disorder featuring complex aberrations in the structure, wiring, and chemistry of multiple neuronal systems. Dysfunctional neuronal networks are involved in the pathophysiology of the disease and releted to symptom dimensions. Neurodevelopmental disturbances and additional neurodegenerative processes in subgroups of patients are hypothesised to be underlying pathophysiological mechanisms (1, 2). Synaptic pathology, oligodendrocyte dysfunction and decreased neurogenesis may lead to disconnection syndrome as a basis of symptoms and cognitive deficits. On the functional level, deficits are evident in symptom dimensions, a subtle loss of volumes in specific brain regions, and in the activation pattern of neuronal networks (3). Imaging studies suggest that the subtle morphological correlates of schizophrenia outlined above sit beside localized alterations in the morphology and molecular composition of specific neuronal, synaptic and glial populations. Recent work has also shown basic sensory processing deficits which are particularly prominent in the visual system and may be related to cognitive deficits and outcome (4). A primary approach to analyzing integrity of visual processing is use of visual evoked potentials (VEPs).
The VEPs provide an objective measure of brain function, as early visual processing dysfunction in schizophrenia, with preferential deficits being found in the magnocellular pathway, though parvocellular deficits have been found as well (5).
Understanding the nature of sensory processing deficits may provide insight into mechanisms of pathology in schizophrenia, impaired signal amplification, and could lead to treatment strategies including sensory processing rehabilitation that may improve outcome (6). Preferential magnocellular dysfunction may provide a substrate for dorsal stream dysfunction as well as higher level cognition deficits and outcome (7).
The aim of the study was to determine the morphologic differences in the brain structures of occipital region regarding laterality and P100 amplitude between patients (in the Clinical Center University of Sarajevo, in three diferent clinical departments) with schizophrenia and patients with migraine headache using MRI and VEPs. Structural deficits in occipital cortex, particularly in optic radiations, and their relationship to early visual processing deficits, document the importance of subcortical as well as cortical dysfunction in schizophrenia. These findings may provide understanding the nature of sensory processing deficits and pathophysiology of schizophrenia.
2. MATERIALS AND METHODS
Patients and study design
This prospective, comparative study included 80 subjects (31 males and 49 females), 21-67 years old, diagnosed with schizophrenia and migraine headache, in the period from 2011 to 2015. All subjects signed a written informed consent and the Ethics Committee of the University Clinical Center University of Sarajevo gave an ethical consent to perform the study. It was conducted at the Department of Psychiatry, Department of Neurology, Department of Radiology Clinical Center University of Sarajevo.
The study sample was divided into two groups:
S group included 40 patients with schizophrenia (21 males and 19 females)) who were on the hospital treatment and under antipsychotic drugs, diagnosed with schizophrenia according to ICD-10 criteria (8), the average age was 41.50 years (SD±10.44; range 22-67).
M group included 40 patients with migraine headache (10 males and 30 females), hospitalized at the Department of Neurology, diagnosed with migraine headache criteria (9), the average age was 38.50 years (SD±6.59; range 30-53).
Magnetic resonance imaging (MRI) scans of occipital lobe of 80 human brains (the left and the right hemispheres) were examined at the Department of Radiology, Clinical Center University of Sarajevo. The sulci (regions) of interest of the occipital lobe (coronal sections and descriptive analysis) through the magnetic resonance imaging volume of a single patient were identified (ROI): parieto-occipital fissure (POF), body of the calcarine sulcus (BCS), retrocalcarine sulcus (RCS) according to gender.
Visual Evoked Potentials (VEPs), as a measurement of the electrical signal, recorded at the scalp over the occipital cortex of 80 human brains in response to light stimulus, were examined at the Department of Neurology, Clinical Center University of Sarajevo.
The VEPs were used primarily to measure the functional integrity of the visual pathways from retina via the optic nerves to the visual cortex of the brain and recorded from occipital scalp overlying the calcarine fissure.
3. METHODS
Neuroradiology method-magnetic resonance imaging (MRI).
The MRI scans were perfomed on a Siemens 3T supraconducting magnet system to get very strong and homogeneous field T2TSE3D-RST-TRA (Avanto, Siemens, Erlangen, Germany). It was applied the relaxation time T2 (TR=750/TE114) with sequences of turbo spin echo (TSE) in transverse planes and layer thickness of 0.6 mm, T1 sequence (voxel resolution: 1mm×1mm×1.25mm, TI: 20ms, TD: 500ms, TR: 9.7ms, TE: 4.0ms, FLIP: 10, Matrix: 256×256, Rect. FOV: 7/8, Partitions: 128, Time=13 min and 12 seconds). For the purpose of group analysis sulcus depth (mm), t-statistical map was generated for each hemisphere by application of t>2.66 (p<0.01, with the rate of freedom of 61). Two statistical methods, group analysis of size and interhemispheric symmetry, were used in order to test significant differences of sulcus depth between groups. Volumes of the sulcal paterns of the occipital region (cc) of both the left and the right hemispheres for each patient were investigated to provide a quantitative description of the variability of the location of a given brain structure.
The image data were resampled onto a standard grid with cubical voxels 1 mm wide. The gray-matter voxels extending for 1 mm on either side of the banks of the sulcus were included in the set of voxels constituting the sulcus. We identified occipital sulci and marked their corresponding gray matter voxel on magnetic resonance images around POF, BCS and RCS. Average total volumes (cubic centimeters, cc) of both the left and the right hemispheres of the healthy population for the comparasion were as follows: POF 24.0, BCS 11.2, RCS 2.7.
Neurophysiological method - Visual evoked potentials (VEPs).
Patients were subjected to examination by Visual evoked potentials (VEPs) and patterns for psychophysical and electrophysiological experiments were generated using a Medelec Synergy, Version 10.1 (Oxford Instruments Medical, United Kingdom). The pattern used was alternate pattern, each evoked potential recorded right and left eyes was recorded and processed, then the evoked potential was recorded from both eyes, and processed to calculate P100 amplitude. For all experimental runs, the stimulus consisted of a checkerboard pattern with equal numbers of light and dark checks (16 black and 16 white, size 2x2 cm). Luminance was 50 cd/m2, Michelson contrast = 80%. Each check subtended a visual angle of 0.65° both horizontally and vertically, while the checkerboard as a whole subtended visual angles of 5.25° vertically and horizontally. In all experimental runs the checkerboard was presented in the center of a monitor with a gray background. The stimulation of the entire field of view with both eyes, then the whole field of view individually for left and right and for the halves of the visual fields both eyes. Eye that was not watching was covered. Average values of amplitude (the entire eye field both eyes) of the healthy population for the comparison were as follows - P100 amplitude (µV): entire field of view (both eyes) 3.3, left eye: 3.4, right eye 3.4, right field of view (left eye) 3.7, right eye 3.8, left field of view (left eye) 3.1, right eye 3.1.
Statistical analysis
The research task was to define the differences between patients with schizophrenia and patients with migraine headache according to morphology of the brain regions using MRI and VEPs of both groups. For the purposes of correlation and associative analysis multivariate analysis of variance, Pearsons correlation coefficient and Point-biserial correlation was applyed using χ2 test, T-test of independent samples, T-test of paired samples, Kolmogorov-Smirnov test and Levene`s test for equality of variances. Statistically significant differences were considered those in which the p value was less than 0.05 (p<0.05).
4. RESULTS
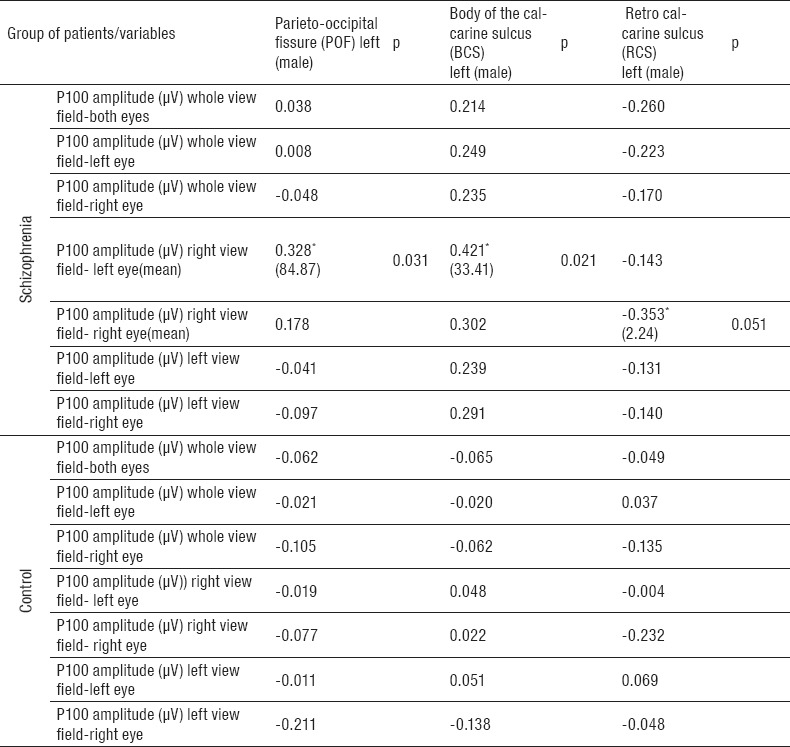
The study was conducted on a group of 80 subjects divided into two groups: among 40 patients with schizophrenia 21 (52.5%) were males and 19 (47.5%) females; in the control group 10 (25.0%) patients were males and 30 (75.0%) females (p=0.012). Average age of patients with schizophrenia was 41.50±10.43 years, and of controls 38.50±9.48 years (Table 1). The morphological variation of the sulci of the occipital region and P100 amplitude of the human brain was examined in 80 patients (patients with schizophrenia and controls) using magnetic resonance imaging and visual evoked potentials. Significant differences between the groups were registered of occipital lobe with some specific regions (left hemisphere) and P100 amplitude (left side) only in male patients with schizophrenia. Patterns of parieto-occipital fissure (m=22.490; SD=84.872; p=0.0314) showed statistically significant positive correlation with P100 amplitude (m=2.83; SD=0.412; p=0.05) regarding gender only in male patients with schizophrenia only on the left side, without differences on the right side in the same group based on parameters of MRI (m=22.403; SD=85.513), and VEP analysis (m=3.43; SD=0.271) (Table 2). In parameters of body of the calcarine sulcus (m=9.713; SD=33.411; p=0.0213) regarding gender statistically significant positive correlation with P100 amplitude (m=1.40; SD=1,272; p=0.04) was noticed only on the left side in male patients with schizophrenia without differences on the right based on parameters of MRI (m=10.218; SD=5.977), and VEP analysis (m=3.48; SD=0.271) (Table 2). Statistically significant positive correlation in parameters of retrocalcarine sulcus (m=2.883; SD=2.244; p=0.0516), and P100 amplitude (m=2.71; SD=1.442; p=0.03) regarding gender was noted only in male patients with schizophrenia, with no differences on the right side of these patients based on parameters of MRI (m=2.035; SD=6.587) and VEP analysis (m=3.36; SD=0.200) (Table 2). In schizophrenia group, all investigated parameters only on the left side, showed statistically significant differences in comparation with the right side of the same group. All correlations of occipital sulci patterns and P100 amplitude (whole view field of the left eye, whole view fields of both eyes, whole view field of right eye and left view field of left eye) were positive. Negative correlations were found in the control group with no statistically significant differences (Table 3).
Table 1.
Age distribution of subjects
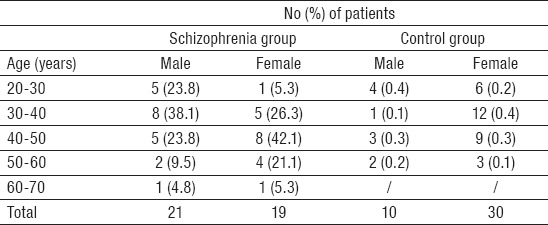
Table 2.
Morphologic structures of occipital lobe and P100 amplitude according to gender
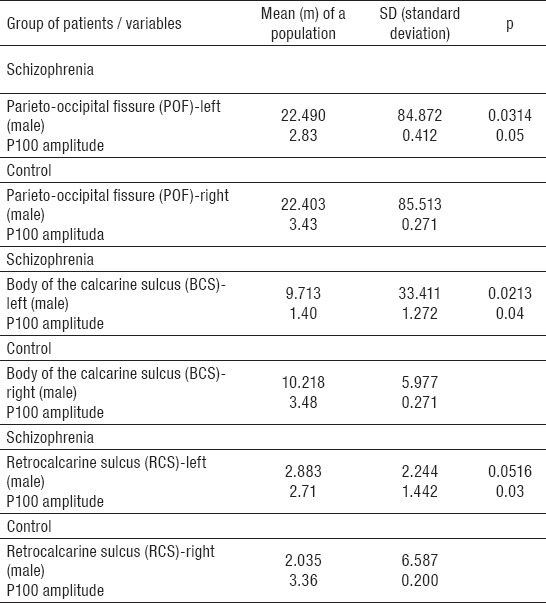
Table 3.
Correlation of P100 amplitude with MRI. *Statistical correlation on 0.05
5. DISCUSSION
Schizophrenia is a serious and chronic brain disorder whose underlying neuropathology has proven difficult to identify. As distributed structural circuits of cortical and subcortical areas serve normal brain functions, disrupted communication within and between brain regions may be the core pathology of schizophrenia. Altered connectivity and brain network topology has been described in the brains of schizophrenia patients. Widespread alterations in connectivity are present in the brains of chronic schizophrenia patients and there is some evidence that patterns of reduced connectivity cut across the different stages of the disorder, including those with an increased risk of developing the illness. Structural connectomic studies have identified altered connectivity within sub-networks in chronic patients (10, 11, 12). These studies provide insight into structural connectivity based brain correlates of schizophrenia. There are associations between brain imaging and psychopathological findings and also between the progression of neuroradiological and psychopathological changes (13-18). This observation supports the alternative view that a variety of different pathoplastic factors generate multiple dimensions of variation in brain structure and function in schizophrenia. In schizophrenia there is a range of structural brain changes with severe neurophysiological deficits in brain information processing at perceptual levels (19-24). Structural deficits in occipital cortex, particularly in optic radiations, and their relationship to early visual processing deficits, document the importance of subcortical as well as cortical dysfunction in schizophrenia. Magnetic resonance imaging (MRI) and VEP studies also suggest that many of the structural brain abnormalities in schizophrenia occur in „areas that are normally sexually dimorphic”. These results may suggest that normal sexual dimorphism may help to protect schizophrenic women from some of the more severe cognitive consequences of brain abnormalities associated with the disorder (25). Our research conducted using MRI and VEPs revealed changes in the occipital lobe, especially of the left hemisphere (shallower depth of sulcus and P100 amplitude reduction) among a group of male patients with schizophrenia with significant differences in the area of the parieto-occipital fissure, body of the calcarine sulcus, and the retro calcarine sulcus (26, 27). This results were consistent with a study that compared the morphology of the cerebral cortex (gyri and sulci depth) of the occipital lobe and differences in visual structures of P100 amplitude in patients with schizophrenia (28, 29). Visual evoked potential measured in our study has shown that P100 (amplitude reduction) was only on the left eye in patients with schizophrenia. Our findings are consistent with studies that examined the morphology of the cortical surface, and which recorded a reduction of the gyrus index in both cerebral hemispheres of 3%-4.5%, but it was more pronounced in the area of the left side (30), which is consistent with the findings of five studies conducted in relation to the volume of the occipital lobes in schizophrenia (31). In our study a descriptive comparative analysis of morphological variations were correlated with asymmetry of the left hemisphere with a statistically significant P100 amplitude reduction on the left eye only in male subjects (32).
6. CONCLUSION
The neurodevelopmental hypothesis of schizophrenia provided a valuable framework that allowed a condition that usually presents with frank disorder in adolescence or early adulthood to be understood at least in part as a consequence of events occurring early in development. The leading hypothesis for the aetiology of schizophrenia is related to disturbance in normal brain development. The principal assumption is that normal brain development is disrupted in specific ways at critical periods and the resulting lesion produces the symptoms of schizophrenia only through interaction with the normal maturation processes in the brain, which occur in late adolescence or early adulthood. Schizophrenia is associated with the volume changes of the occipital lobe, and findings of our study support the view that schizophrenia is associated with impairment of early visual stream processing. Recent findings suggest that we now need to go further and view the functional psychoses as members of a group of related and overlapping syndromes that result in part from a combination of genetic and environmental effects on brain development and that are associated with specific and general impairments of cognitive function.
REFERENCES
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. doi:10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia. Mol Psychiatry. 2012;17:1228–38. doi: 10.1038/mp.2012.23. doi:10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitiş O, Eker MC, Zengin B, Akyılmaz DA, Yalvaç D, Ozdemir HI, Işman Haznedaroğlu D, Bilgi MM, Gönül AS. The disrupted connection between cerebral hemispheres in schizophrenia patients: a diffusion tensor imagining study. Turk Psychiatry Derg. 2011;22:213–21. doi:10.1111/j.1440-1819.2011.02293.x. [PubMed] [Google Scholar]
- 4.Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin. 2013;26:72–87. doi: 10.1097/YCO.0b013e32835d9e6a. doi:10.1093/schbul/sbu076. [DOI] [PubMed] [Google Scholar]
- 5.Brenner CA, Lysaker PH, Wilt MA, O’Donnell BF. Visual processing and neuropsychological function in schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111:125–36. doi: 10.1016/s0165-1781(02)00139-7. doi:http://dx.doi.org/10.1016/S0165-1781(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 6.Connolly JF, Gruzelier H, Manchanda R, Hirsch SR. Visual evoked potentials in schizophrenia. Brit J Psychiat. 1983;142:152–5. doi: 10.1192/bjp.142.2.152. [DOI] [PubMed] [Google Scholar]
- 7.Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. doi:http://dx.doi.org/10.1016/S0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd Ed. Oxford: Blackwell Publishing 2004. (The International Headache Society); 2004. [Google Scholar]
- 10.Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–9. doi: 10.1016/j.biopsych.2010.03.035. doi:http://dx.doi.org/10.1016/j.schres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–9. doi: 10.1016/j.biopsych.2010.08.022. doi:10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collin G, de Reus MA, Cahn W, Hulshoff Pol HE, Kahn RS, van den Heuvel MP. Disturbed grey matter coupling in schizophrenia. Eur Neuropsychopharmacol. 2013;23:46–54. doi: 10.1016/j.euroneuro.2012.09.001. doi:10.1016/j.euroneuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci. 2011;261:150–4. doi: 10.1007/s00406-011-0242-2. doi:10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohid H, Faizan M, Faizan U. Alterations of the occipital lobe in schizophrenia. Neurosciences (Riyadh) 2015;20:213–24. doi: 10.17712/nsj.2015.3.20140757. doi:10.17712/nsj.2015.3.20140757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler PD, De Santi LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. doi:http://dx.doi.org/10.1016/S0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 16.Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. doi:10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–27. doi: 10.1016/s0920-9964(02)00222-0. doi:http://dx.doi.org/10.1016/S0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 18.Doniger GM, Foxe JJ, Murrary MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–20. doi: 10.1001/archpsyc.59.11.1011. doi:10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 19.Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer B, Light GA, Braff DL. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:1113–19. doi: 10.1016/s0006-3223(02)01813-9. doi:http://dx.doi.org/10.1016/S0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- 20.Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:190–96. doi: 10.1176/jnp.14.2.190. doi:10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 21.Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159:1395–02. doi: 10.1176/appi.ajp.159.8.1395. doi:10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- 22.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–41. doi: 10.1016/s0920-9964(01)00405-4. doi:10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 23.Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15:339–49. doi: 10.31887/DCNS.2013.15.3/mrubinov. http://creativecommons.org/licenses/by-nc-nd/3.0/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davatzkikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–27. doi: 10.1001/archpsyc.62.11.1218. doi:10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 25.Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodriguez-Sánchez JM. Neuropsychological functioning and brain structure in schizophrenia. Int Rev Psychiatry. 2007;19:325–36. doi: 10.1080/09540260701486647. doi:10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lin L, Lin CP, Zhou Y, Chou KH, Lo Cy, Su TP, Jiang T. Abnormal topological organization of structural brain networks in schizophrenia. Schizoph Res. 2012;141:109–18. doi: 10.1016/j.schres.2012.08.021. doi:10.1016/j.schres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Collin G, Cahn W, Hulshoff Pol, Kahn RS. Disturbed grey matter coupling in schizophrenia. Eur Neuropsychopharmacol. 2013;23:46–54. doi: 10.1016/j.euroneuro.2012.09.001. doi:10.1016/j.euroneuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–33. doi: 10.1176/appi.ajp.158.7.1126. doi:10.1001/archpsyc.62.5.495. [DOI] [PubMed] [Google Scholar]
- 29.Phillips OR, Nuechterlein KH, Asarnow RF, Clark KA, Cabeen R, Yang Y, Woods RP, Toga AW, Narr KL. Mapping corticocortical structural integrity in schizophrenia and effects of genetic liability. Biol Psychiatry. 2011;70:680–9. doi: 10.1016/j.biopsych.2011.03.039. doi:10.1016/j.biopsych.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–36. doi: 10.1038/nrn3465. doi:10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66:781–95. doi: 10.1016/j.neuron.2010.04.036. doi:10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taş A, Cakir Gundogan F, Akgun H, Erdem U, Sobaci G. Spatial tuning function of pattern visual evoked potentials in multiple sclerosis patients without optic neuritis history. Med Glas (Zenica) 2013;10:99–205. [PubMed] [Google Scholar]


