Abstract
Introduction
When gastric cancer or carcinoid patients have coexisting diseases such as duodenal adenoma, FAP, or Crohn's disease, periodic observation of the duodenum is necessary.
Methods
Between August 2013 and April 2015, we performed four consecutive laparoscopic total gastrectomies with double tract reconstruction for duodenal surveillance. Three of the patients were diagnosed with gastric cancer, while the remaining patient was diagnosed with gastric carcinoid.
Results
No deaths occurred, and three of the patients showed no early complications. One patient with Crohn's disease developed anastomotic leakage, but it was successfully managed conservatively. On endoscopy three to seven months later, we were able to observe the duodenum via jejunal anastomosis in all of the patients.
Discussion
Roux-en-Y reconstruction is one of the options after laparoscopic total gastrectomy. However, given that periodical endoscopic examinations of the duodenum are strongly recommended after surgery, double-tract reconstruction may be preferable in these patients.
Conclusion
Although more detailed data are required, double-tract reconstruction may be the best choice for patients requiring total gastrectomy with regular check-up of the duodenum.
Keywords: Laparoscopic total gastrectomy, Double tract reconstruction, Duodenal surveillance
Highlights
-
•
When gastric cancer patients have coexisting duodenal diseases, periodic observation of the duodenum is necessary.
-
•
Given that postoperative endoscopic examinations of the duodenum are needed after total gastrectomy, double-tract reconstruction may be preferable.
-
•
This is the first report of total gastrectomy with double-tract reconstruction in gastric cancer patients with FAP.
-
•
Double-tract reconstruction following total gastrectomy may be technically feasible for duodenal surveillance.
1. Introduction
Laparoscopic gastrectomy has been established as a minimally invasive treatment in gastric surgery, especially early gastric cancer [1], [2]. While distal gastrectomy is performed for gastric cancer located in the lower or middle third of the stomach, total or proximal gastrectomy is performed for lesions located in the upper third. Total gastrectomy is particularly essential for treating multiple cancers. Although Roux-en-Y reconstruction is one of the options after laparoscopic total gastrectomy for gastric cancer, a different reconstruction method should be considered for patients with comorbidities who require regular duodenal checkup after surgery. Other available surgery routes include the jejunal interposition method and the double tract method. We herein describe double tract reconstruction following total gastrectomy for duodenal surveillance. This work has been reported in line with the PROCESS criteria [3].
2. Methods
2.1. Patients
Between August 2013 and April 2015, we performed four consecutive laparoscopic total gastrectomies with double tract reconstruction at Chiba University Hospital, Japan. Three of the patients were diagnosed with gastric cancer, while the remaining patient was diagnosed with gastric carcinoid. Staging was performed using Japanese classification of gastric carcinoma [4].
2.2. Procedure
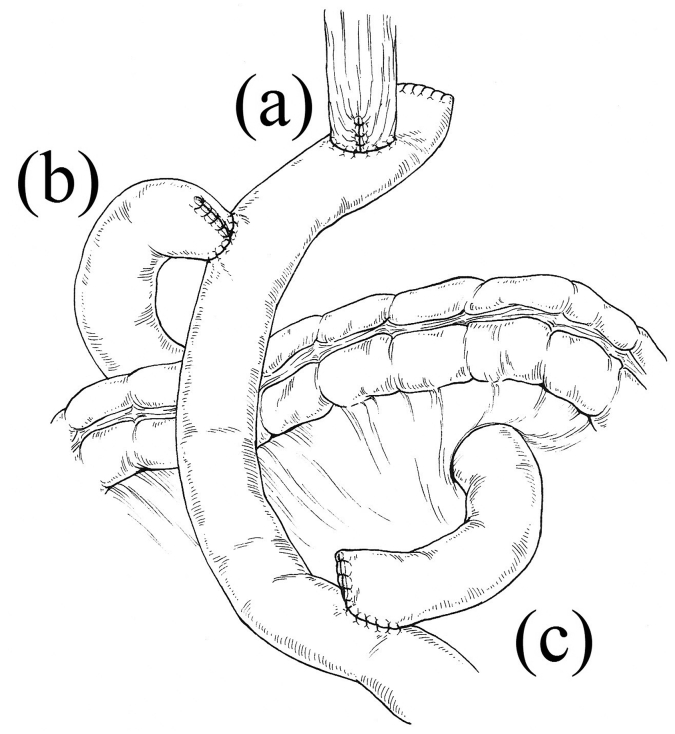
After laparoscopic total gastrectomy with D1+ lymphadenectomy performed in accordance with the Japanese Gastric Cancer treatment guidelines [5], the jejunum was divided approximately 25 cm distal to the Treitz ligament. The distal jejunal limb was then directly brought up to the esophageal stump. Esophagojejunostomy was performed in a side-to-side (overlap) fashion [6] using a linear stapler (Endo GIA™ 45; Covidien, Mansfield, MA, USA) (Patients 1 and 4), or end-to-side fashion using a circular stapler [7] (EEA™ OrVil™ 21-mm device; Covidien) (Patients 2 and 3). Side-to-side jejunoduodenostomy was then performed using a modified “delta-shaped anastomosis” [8] technique. With this technique, a V-shaped anastomosis was made between the posterior side of the duodenum and the jejunal limb, approximately 15 cm distal to the esophagojejunal anastomosis, using a 45-mm linear stapler (Endo GIA™ 45; Covidien). The entry hole of the stapler was closed using the same 45-mm linear stapler. Side-to-side jejunojejunostomy was then made using a 60-mm linear stapler (Endo GIA™ 45; Covidien), approximately 30 cm distal to the jejunoduodenostomy (Fig. 1).
Fig. 1.
A schematic diagram of double-tract reconstruction. Three anastomoses were made: (a) side-to-side or end-to-side esophagojejunal anastomosis, (b) side-to-side jejunoduodenal anastomosis, and (c) side-to-side jejunojejunal anastomosis.
3. Results
Four cases were reconstructed via the double-tract method following laparoscopic total gastrectomy with D1+ lymphadenectomy. The clinicopathological backgrounds of the four patients are shown in Table 1. The mean age was 66.8 years, and the mean duration of hospital stay was 20.3 days. The mean operative time was 464.3 min, and the mean blood loss was 137.5 ml.
Table 1.
Patient characteristics.
| Case no. |
1 |
2 |
3 |
4 |
| Sex | Male | Male | Female | Male |
| Age, yr | 61 | 82 | 72 | 49 |
| Comorbidity | FAP | Duodenal adenoma | Crohn's disease | Duodenal SMT |
| Diagnosis | Gastric cancer | Gastric cancer | Gastric cancer | Gastric carcinoid |
| Tumor location/macroscopic findings | U, Less, 5 mm, IIc | ML, Less, 100 mm, IIa | U, Less, IIa, 10 mm | UML, multiple, 2 mm |
| M, Less, 15 mm, IIc | L, Gre, 15 mm, IIc | M, Less, IIa, 15 mm | ||
| Clinical TNM classification/stage | cT1aN0M0, cStage IA | cT1bN0M0, cStage IA | cT1aN0M0, cStage IA | cT1aN0M0, cStage IA |
| cT1bN0M0, cStage IA | cT1aN0M0, cStage IA | cT1aN0M0, cStage IA | ||
| Pathology | M, Ant, IIc, tub1, pT1a | M, Less, Type 4, por2, pT2 | U, Less, IIa, tub1, pT1a | UM, triple |
| U, Less, IIb, tub1, pT1a | L, Ant, Iic, tub2, pT1a | U, Less, IIa, tub1, pT1a | Neuroendocrine tumor G1, pT1a | |
| U, Less, IIb, tub1, pT1a | pN1 | pN0 | pN0 | |
| L, LessAnt, status post ESD, tub1 | ||||
| pN0 | ||||
| Operative time, min | 468 | 500 | 400 | 489 |
| Blood loss, ml | 220 | 5 | 125 | 200 |
| Postoperative hospital stay, day | 16 | 13 | 28 | 11 |
| Follow-up time, month | 31 | 48 | 45 | 24 |
| Postoperative endoscopic examination timing, month | 5, 17, 29 | 3, 27 | 3, 36 | 7 |
| Recurrence | No | No | No | No |
| Reflux esophagitis | No | No | No | No |
| New manifestations of duodenal disease | No | No | No | No |
FAP, familial adenomatous polyposis; SMT, submucosal tumor.
No deaths occurred, and three of the patients showed no early complications. One patient with Crohn's disease (Patient 3) developed anastomotic leakage on Day 6 after surgery, but it was successfully managed conservatively. The patients resumed oral food intake by Day 22 after the surgery. Some late complications were observed in all of the patients, as follows: diarrhea (Patients 1, 2, 3) and weight loss of more than 5 kg (Patients 1, 2, 4).
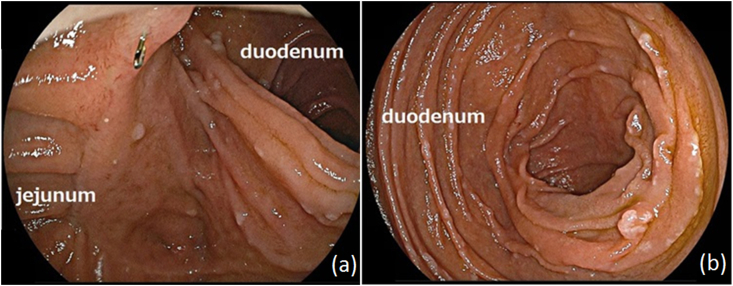
All of the patients underwent gastroendoscopy three to seven months after the surgery, and the duodenum was able to be inspected via duodenojejunal anastomosis in all of them (Fig. 2). No signs of reflux esophagitis, recurrence, or new manifestations of duodenal diseases were revealed during regular follow-up up to a mean of 37 months after surgery.
Fig. 2.
The findings of an endoscopic examination five months after the operation. (a) A jejunoduodenal anastomosis without stenosis. (b) We were able to observe the duodenum.
4. Discussion
Roux-en-Y reconstruction is one of the typical options after laparoscopic total gastrectomy for gastric cancer. However, observing the duodenum with a gastroendoscope can be quite difficult after this reconstruction. Given that periodical endoscopic examinations of the duodenum are strongly recommended after surgery in patients with comorbidities such as duodenal adenoma, FAP, or Crohn's disease, other reconstruction options may be preferable in these patients.
We employed a double-tract reconstruction method after laparoscopic total gastrectomy for this purpose in our series of patients, since this method requires only a minor modification to the Roux-en-Y reconstruction procedure. A delta-shaped intracorporeal anastomosis technique, developed for B-1 reconstruction after distal gastrectomy [8], was applied for this modification.
The benefits of double-tract reconstruction after total gastrectomy have been described as (1) its technical simplicity; (2) the preserved passage of food though the duodenum; and (3) the lack of any need to create a duodenal stump, one of the risk sites of rupture [9]. In addition, double-tract reconstruction also allows much easier endoscopic access to the duodenum after surgery than Roux-en-Y reconstruction.
Double-tract reconstruction after laparoscopic proximal gastrectomy using intracorporeal delta-shaped anastomosis has been also described by Jun Hong et al. [10] and shown to be technically feasible and safe. The length of the alimentary limb between esophagojejunostomy to gastrojejunostomy has been recommended to be 15–20 cm to achieve a satisfactory gastroendoscopic follow-up of the duodenum. We secured a similar length (approximately 15 cm) between esophagojejunostomy and duodenojejunostomy in our series, as the surgery did not induce too much tension or relaxation of the reconstructed alimentary limb. As such, postoperative endoscopic check-ups of the duodenum were performed without any difficulty in all four of the present cases. However, long-term follow-up is necessary to determine whether this length between esophagojejunostomy and duodenojejunostomy does not cause bile reflux without residual stomach.
5. Conclusion
To our knowledge, this is the first report of total gastrectomy with double-tract reconstruction in gastric cancer patients with FAP and Crohn's disease. Given that all of the patients were successfully treated under laparoscopic guidance, we feel that double-tract reconstruction may be the best choice for patients requiring total gastrectomy with regular check-up of the duodenum after surgery. However, such cases are believed to be relatively rare; therefore, more data are needed before any hard recommendations are made.
Ethical approval
Ethics committee application has not been made because of the article.
Sources of funding
The authors have none to declare.
Author contribution
HH, NH, KH and RO made, analyzed, and interpreted our patient's imaging examinations. HH and NH demonstrated surgery for our patients. The manuscript was prepared by RO under the supervision of HH, NH, HG, KH, and HM. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Registration of research studies
The authors have none to declare.
Guarantor
Ryota Otsuka, Hideki Hayashi.
Consent
Written informed consent was obtained from patients for publication of this case series and accompanying images.
References
- 1.Katai H., Mizusawa J., Katayama H., Takagi M., Yoshikawa T., Fukagawa T. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2016;20:699–708. doi: 10.1007/s10120-016-0646-9. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y., Huang C., Sun Y., Su X., Cao H., Hu J. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J. Clin. Oncol. 2016;34:1350–1357. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 3.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P. PROCESS Group. Preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer A Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 6.Inaba K., Satoh S., Ishida Y., Taniguchi K., Isogaki J., Kanaya S. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J. Am. Coll. Surg. 2010;211:e25–e29. doi: 10.1016/j.jamcollsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Sakuramoto S., Kikuchi S., Futawatari N., Moriya H., Katada N., Yamashita K. Technique of esophagojejunostomy using transoral placement of the pretilted anvil head after laparoscopic gastrectomy for gastric cancer. Surgery. 2010;147:742–747. doi: 10.1016/j.surg.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya S., Gomi T., Momoi H., Tamaki N., Isobe H., Katayama T. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J. Am. Coll. Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara Y., Kusunoki M., Nakagawa K., Tanaka T., Hatada T., Yamamura T. Evaluation of J-pouch reconstruction after total gastrectomy: rho-double tract vs. J-pouch double tract. Dig. Surg. 2000;17:475–481. doi: 10.1159/000051943. discussion 81–82. [DOI] [PubMed] [Google Scholar]
- 10.Hong J., Qian L., Wang Y.P., Wang J., Hua L.C., Hao H.K. A novel method of delta-shaped intracorporeal double-tract reconstruction in totally laparoscopic proximal gastrectomy. Surg. Endosc. 2016 Jun;30(6):2396–2403. doi: 10.1007/s00464-015-4490-5. [DOI] [PubMed] [Google Scholar]