Abstract
Currently, there is a lack of surveys that report the occurrence of gastrointestinal parasites in the white-headed capuchin monkey (Cebus albifrons). We therefore assessed the presence and richness (= number of different parasite genera) of parasites in C. albifrons in wildlife refuges (n = 11) and in a free-ranging group near a human village (n = 15) in the Ecuadorian Amazon. In the 78 samples collected (median of 3 samples per animal), we identified a total of 6 genera of gastrointestinal parasites, representing protozoa, nematodes, acanthocephalans and cestodes. We observed a high prevalence (84%) across the 26 individuals, with the most prevalent parasite being Strongyloides sp. (76.9%), followed by Hymenolepis sp. (38.5%) and Prosthenorchis elegans (11.5%). We found Entamoeba histolytica/dispar/moskovskii/nuttalli and Capillaria sp. in only a minority of the animals (3.8%). In addition, we observed unidentified strongyles in approximately one-third of the animals (34.6%). We found a total of 6 parasite genera for the adult age group, which showed higher parasite richness than the subadult age group (5) and the juvenile age group (3). Faecal egg/cyst counts were not significantly different between captive and free-ranging individuals or between sexes or age groups. The free-ranging group had a higher prevalence than the captive group; however, this difference was not significant. The only genus common to captive and free-ranging individuals was Strongyloides sp. The high prevalence of gastrointestinal parasites and the presence of Strongyloides in both populations support results from previous studies in Cebus species. This high prevalence could be related to the high degree of humidity in the region. For the free-ranging group, additional studies are required to gain insights into the differences in parasite prevalence and intensity between age and sex groups. Additionally, our study demonstrated that a serial sampling of each individual increases the test sensitivity.
Keywords: Cebus albifrons, Prevalence, Parasite richness, Faecal egg/cyst counts, Amazonian Ecuador
Graphical abstract
Highlights
-
•
This study brings new information about primate parasites to Ecuador.
-
•
We analyse the prevalence, richness and FECC among the two habitats, sex and age.
-
•
Adults harbour more genera of parasites than subadults and juveniles.
-
•
Sampling effort was essential to find a good number of parasites genera.
-
•
E. histolytica/dispar and P. elegans were found only in captive individuals.
1. Introduction
Gastrointestinal parasites are commonly found in non-human primates (NHPs) in both captive and wild populations. Over the last decades, wildlife is commonly infected with gastrointestinal parasites (Ghandour et al., 1995, Eckert et al., 2006, Bezjian et al., 2008). However, habitat fragmentation is considered as impacting primate conservation because it could increase parasite richness and parasite load, affecting primate host ecology (Vitazkova and Wade, 2007), including host survival and population density (Chapman et al., 2005), reproduction (Beltran-Bech and Richard, 2014) and behaviour (Nunn and Altizer, 2006, Wren et al., 2010). Parasites are also considered to be a threat to public health (Daszak et al., 2000, Gillespie et al., 2008), as NHPs, either captive or free-ranging, are reservoirs of many human pathogens (Chapman et al., 2006, Vitazkova and Wade, 2006, Friant, 2007, Johnson-Delaney, 2009). Habitat fragmentation, along with bushmeat hunting and tourism, results in altered interfaces between animals and people (Homsy, 1999, Wolfe et al., 2005) that can facilitate the transmission of parasites from NHPs to humans and vice versa (Munene et al., 1998, Muriuki et al., 1998, Nizeyi et al., 2002, Gillespie and Chapman, 2006, Goldberg et al., 2008, Kowalewski et al., 2011). Parasitological studies conducted in NHPs revealed that primates with less human contact have a lower prevalence and intensity of parasites compared to groups with more human contact (McGrew et al., 1989). However, the type of parasite varies according to habitat. For example, the presence in NHPs of certain human parasites (e.g., Giardia duodenalis) is found at sites with more intense human contact (Vitazkova and Wade, 2006). In captive NHPs destined for the animal trade or as pets, the lack of relevant knowledge among pet owners, zoo keepers and veterinarians (Renquist and Whitney, 1987) may affect cross-species parasite transmission. A recent study demonstrated possible zoonotic transmission of Entamoeba nuttalli between NHPs and zoo keepers in 5 zoos (Levecke et al., 2015). Thus, it is important to study parasites in NHPs with different levels of contact with humans.
Most parasitological studies have been focused on Old World NHPs (Lilly et al., 2002, Hahn et al., 2003, Ocaido et al., 2003, Legesse and Erko, 2004, Gillespie et al., 2005, Muehlenbein, 2005, Chapman et al., 2006, Friant, 2007, Hopkins and Nunn, 2010, Howells et al., 2011). When studies did include New World NHPs, they were primarily Alouatta sp. (Stoner and González-Di Pierro, 2005, Cristóbal-Azkarate et al., 2010, Kowalewski et al., 2011, Milozzi et al., 2012, Behie et al., 2014, Maldonado-López et al., 2014). To broaden the scope of research, we chose to conduct our study on a Cebus species. The white-fronted Capuchin, Cebus albifrons, is distributed in several countries of South America. In Ecuador, there are two subspecies (Cebus albifrons and Cebus albifrons aequatorialis). The former species, the subject of our study, is found in the western Amazon. Its conservation status is of Least Concern (de la Torre et al., 2008) and it is also listed in Appendix II of CITES (Convention on the International Trade in Endangered Species of Wild Fauna and Flora). Unfortunately, there are only a few studies on the ecology, behaviour and health of this species (Defler, 1979, Pozo, 2004, Derby, 2008, Cervera and Donati, 2012). Cebus albifrons is a middle-sized primate found in high density biomass regions in the forest (Tello, 2003). Habitat fragmentation and hunting for the pet trade represent major threats for its populations (Nuñez-Iturri and Howe, 2007).
NHP parasite diversity is not very well known for Cebus albifrons. For free-ranging capuchins, only a few reports are found in the literature (Dunn and Lambrecht, 1963, Ewing et al., 1968, Phillips et al., 2004). In Ecuador, there is only one study, that of lymphatic filariasis in the coastal region (García-Solorza and Levi-Castillo, 1954). For captive capuchins, a single study has been reported from Peru (Guerrero et al., 2012). There are, however, published studies on the gastrointestinal parasites in other species of Cebus (see Appendix for more details).
To understand how parasitism is affected by host-intrinsic and -extrinsic factors, we correlated parasite prevalence, parasite richness (number of parasite genera), and faecal egg/cyst count with sampling effort, host sex and age, and between captive and free-ranging groups of capuchins with different levels of contact with humans. This study also creates baseline data for parasites of this species.
2. Methods
2.1. Study site and study groups
This study was done in Tena (Napo) and Puyo (Pastaza), two main cities in Western Amazon. The monthly mean precipitation is 324 mm for Puyo and 407 mm for Tena. The daily mean temperature for Puyo is 21.1 °C and is 23.8 °C for Tena, according to the Instituto Nacional de Meteorología e Hidrología (INAMHI, 2014). We examined gastrointestinal parasites in two populations, one captive and one free-ranging. The captive population was studied in three wildlife refuges in Puyo. Most NHPs from wildlife refuges have been donated by families or confiscated by the police during roadside checks. The average size of the enclosures (n = 5) in these wildlife refuges was 76.5 m2, resulting in an average animal density of 0.15 per m2. Prior to this study, deworming was not established as a standard procedure. These animals had frequent contact with the animal caretakers and tourists, who regularly fed them. The free-ranging population lived in the small town of Misahualli (Tena, Napo) (1° 2′ 7.0″ S, 77° 39′ 59.4″ W). The home range of this group is approximately 2.04 ha. The home range (range in which animals were seen in 95% of the observations) was calculated based on following the group for a 3-month period (from March to May in 2012) and recording the group position every hour with a GPS Garmin eTrex 30 (Garmin International Inc., Olathe, KS, USA). The data were mapped and analysed using ArcView 3.2 with the “animal movement” extension using the 95% fixed Kernel method (Hooge and Eichenlaub, 2000).
2.1.1. Sampling
Between March 2011 and May 2013, we collected a total of 78 faecal samples from a total of 26 habituated individuals, 11 captives and 15 free-ranging (see Table 1). For the free-ranging group, individuals were followed daily from 8 a.m. to 6 p.m. for three months (March–May). All animals were individually identified to facilitate serial sampling (3 samples per animal taken at least 24 h apart to increase the test diagnostic sensitivity) (Stoner, 1996). We collected the samples immediately after defecation (Chapman et al., 2012) to avoid a possible contamination by parasites in the environment (mean time between defecation and sample collection = 2.4 min). We stored the faecal samples in 50 ml Falcon tubes in 10% phosphate buffered formalin at 4 °C to prevent eggs from hatching as well as to prevent deterioration of the parasite eggs and cysts. We labelled Falcon tubes with the animal ID, time and date.
Table 1.
Study population of Cebus albifrons sampled in the Amazonian region in Ecuador.
| Captive Population N = 11 |
Free-Ranging Group N = 15 |
|
|---|---|---|
| Age | ||
| Adults | 7 | 5 |
| Subadults | 2 | 7 |
| Juveniles | 2 | 3 |
| Sex | ||
| Males | 11 | 11 |
| Females | 0 | 4 |
2.2. Faecal analysis
We analysed all samples at the International Centre of Zoonoses at the Central University of Ecuador. We processed one gram of stool using a formol-ether concentration method for each sample. Briefly, we filtered 1 g of sample through a gauze mesh into a sterilized urine sample collection cup with 10 ml of distilled water. The filtered solution was then centrifuged for 1 min at 426 g. After the supernatant was discarded, the sediment was suspended in 5 ml of 10% formalin. A total of 1.5 ml of diethyl ether was added. The suspension was thoroughly mixed by shaking it. The suspension was centrifuged for 2 min at 239 g. One drop of the concentrate was transferred to a microscope slide, which then was covered by a 22 mm × 22 mm coverslip. A second drop was transferred to a separate slide. Before dropping the coverslip, a drop of Lugol's iodine was added to facilitate the detection of protozoa. Parasites were identified on the basis of egg colour, shape and contents using a standard light microscope and 100X – 400X magnification. The identification of parasites was based on Flynn, 1973, Thienpont et al., 1986 and Hasegawa et al. (2009).
2.3. Statistical analysis
We calculated the prevalence as the number of infected individuals with a particular parasite divided by the total number of individuals. Parasite richness is defined as the number of parasite genera found in one individual. For each individual, we counted the number of parasite genera observed across the 3 samples. We counted the number of eggs and cysts in a total of 6 slides (2 slides per sample x 3 samples per animal). We used the total number of eggs and cysts counted in one individual as a surrogate for the intensity of infection (Stoner, 1996, Gillespie, 2006). We used several statistical tests to analyse the results: 1) Fisher's exact test to determine significance of differences in parasite prevalence between the groups and sexes; 2) Chi Square test to assess differences in prevalence in each age group; 3) Mann-Whitney U test to assess differences in parasite richness and intensity (as estimated by egg and cyst counts); and 4) Kruskal-Wallace test to assess differences in parasite richness and age groups. Finally, we studied the impact of serial sampling on the qualitative (absence/presence) and the quantitative (faecal egg/cyst counts) assessment of gastrointestinal parasite infections. The McNemar test was used to assess whether increasing the sampling effort increases the prevalence. Similarly, we applied the Wilcoxon signed rank test to verify whether the parasite richness and intensity of infection change when one, two or three consecutive samples were examined. All statistical tests were done using Statistica (Dell Statistica, Tulsa, OK, USA). A significance level of alpha <0.05 was set for all statistical analyses.
3. Results
3.1. Parasite richness, prevalence, and intensity of infection
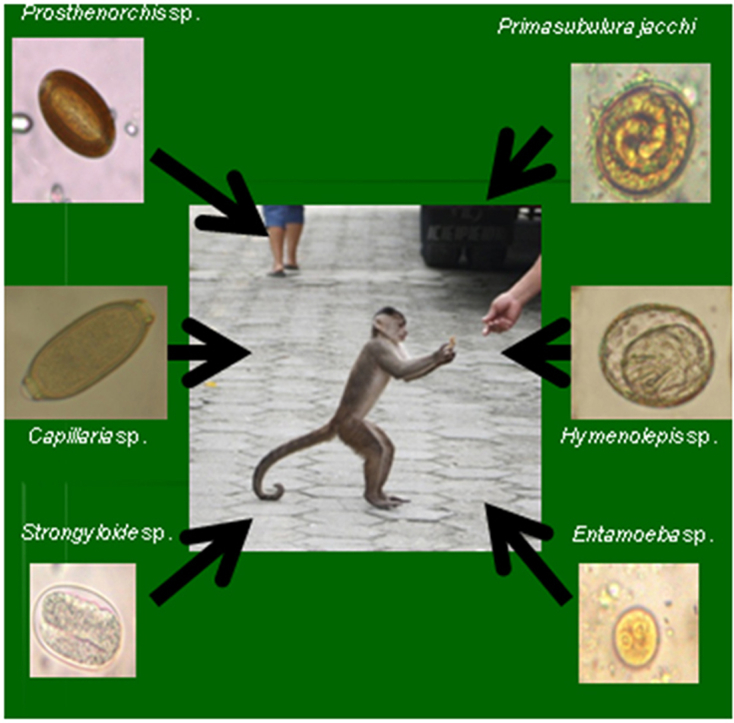
We detected a total of 6 parasite genera across the 26 animals, including representatives of protozoa (Entamoeba histolytica/dispar/moskovskii/nuttalli), nematodes (Capillaria sp., Strongyloides sp., and unidentified Strongyles), Acanthocephalans (Prosthenorchis elegans) and cestodes (Hymenolepis sp.) (Fig. 1). Of the 26 individuals tested, 22 (84%) harboured at least one parasite genus. Parasite prevalence in free-ranging NHPs was greater than in captive animals (15/15 vs. 7/11, P = 0.02), whereas sexes (males: 18/22, vs. females: 4/4, P = 0.49) and age groups (juveniles: 4/5 vs. subadults: 9/9 vs. adults: 9/12, ϰ2 = 2.57, p = 0.277) were equally frequently parasitized. Free-ranging animals harboured more parasite genera (4) than those living in captivity (3) (U = 137.00, P < 0.05), and males (6) more than females (2) (not significantly different, U = 54.5, P = 0.5). Adults (6) harboured more parasite genera than juveniles (3) and subadults (5) (H = 7.247, P = 0.03).
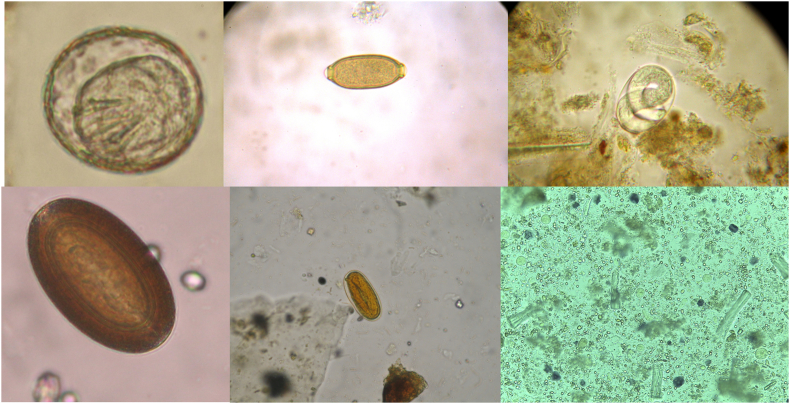
Fig. 1.
From left to right in each row: Hymenolepis sp., Capillaria sp., Strongyloides sp., Prosthenorchis elegans, Strongyle (unidentified), Entamoeba histolytica/dispar/moskovskii/nuttalli. (40x).
The prevalence of each of the different genera across the two groups, the sex and the age is reported in Table 2.
Table 2.
Prevalence and egg/cyst count of 6 parasite genera detected in Cebus albifrons in the Ecuadorian Amazon by group, sex and age.
| Parasite Genera |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cestode |
Nematoda |
Acantocephala |
Protozoa |
||||||||||
| Number of animals |
Hymenolepis sp. |
Strongyloides sp. |
Strongyles (unidentified) |
Capillaria sp. |
Prosthenorchis elegans |
Entamoeba histolytica/dispar/moskovskii/nuttalli |
|||||||
| Prevalence | Mean Egg Count |
Prevalence | Mean Egg Count |
Prevalence | Mean Egg Count |
Prevalence | Mean Egg Count |
Prevalence | Mean Egg Count |
Prevalence | Mean Cyst Count |
||
| Group | |||||||||||||
| Captive | 11 | 0 | 0 | 6 (54.5) | 50.8 | 0 | 0 | 0 | 0 | 3 (27.7) | 156 | 1 (9.1) | 3 |
| Free- Ranging | 15 | 10 (66.6)* | 4.3 | 14 (93.3)* | 9.7 | 9 (60.0)* | 5.6 | 2 (13.3) | 3 | 0 | 0 | 0 | 0 |
| Sex | |||||||||||||
| Males | 22 | 10 (45.4) | 4.3 | 17 (77.3) | 23.3 | 7 (31.8) | 3.1 | 2 (9.1) | 3 | 3 (13.6) | 156 | 1 (4.0) | 3 |
| Females | 4 | 0 | 0 | 3 (75.0) | 14.6 | 2 (50.0) | 15.5 | 0 | 0 | 0 | 0 | 0 | |
| Age | |||||||||||||
| Adult | 12 | 2 (16.6) | 9 | 8 (66.6) | 15.7 | 2 (16.6) | 4.5 | 1 (8.33) | 3 | 1 (8.3) | 17 | 1 (8.33) | 3 |
| Subadult | 9 | 6 (66.6) | 11.8 | 8 (88.8) | 5.2 | 4 (44.4) | 2.6 | 1 (11.1) | 1 | 2 (22.2) | 226.5 | 0 | 0 |
| Juveniles | 5 | 2 (40.0) | 11 | 4 (80.0) | 68.25 | 3 (60.0) | 11.3 | 0 | 0 | 0 | 0 | 0 | 0 |
*(p < 0.05).
The most prevalent parasite was Strongyloides sp. (20/26), followed by Hymenolepis sp. (10/26). Capillaria sp. (2/26) and Entamoeba histolytica/dispar/moskovskii/nuttalli (1/26) were only found in a minority of the animals. Important differences in parasitic infection were observed between the captive animals (n = 11) and free-ranging ones (n = 15). Entamoeba histolytica/dispar/moskovskii/nuttalli and Prosthenorchis elegans were only observed in the captive NHP, whereas Hymenolepis sp., Capillaria sp. and unidentified strongyles were only observed in the free-ranging animals. Strongyloides sp. was observed in both groups but was more prevalent in free-ranging animals (P = 0.032). Similar differences across the sex and age groups can be noted. Strongyloides sp. (P = 0.676) and Strongyles (unidentified) (P = 0.504) were found across both sexes, while all other parasites were exclusively found in males. Across the three age groups, only Strongyloides (ϰ2 = 1.4, P = 0.481), Strongyles (unidentified) (ϰ2 = 4.4, P = 0.105), and Hymenolepis (ϰ2 = 5.4, P = 0.06) were observed. Capillaria spp. (ϰ2 = 0.5, P = 0.751) and Prosthenorchis elegans (ϰ2 = 1.7, P = 0.411) were not found in juveniles. Entamoeba histolytica/dispar/moskovskii/nuttalli was only observed in adult animals (ϰ2 = 1.2, P = 0.545).
The mean egg counts ranged from 3 to 156. Prosthenorchis elegans had the highest egg output (156) followed by Strongyloides sp. (22) (Table 2). Faecal egg and cyst counts were not significantly different between sexes and age groups for all parasite genera. Significant differences in egg counts were only observed between captive (0) and free-ranging animals for Hymenolepis sp. (4.3) (U = 137.5, P < 0.05) and Strongyles (unidentified) (5.6) (U = 137.5, P < 0.05).
3.2. Impact of sampling effort on parasite richness, prevalence and intensity of infection
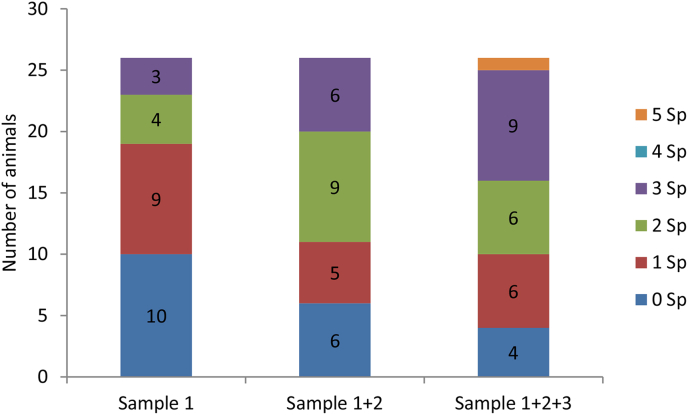
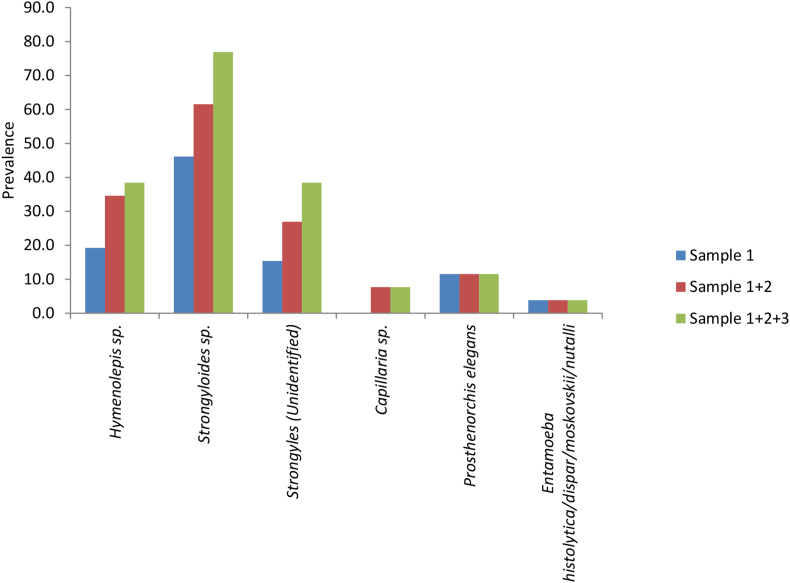
Overall, more parasites were detected when more samples were examined, both in terms of parasite richness (Fig. 2) and prevalence (Fig. 3). For example, when only one sample was examined, 3 parasite genera were found in 3 out of 26 individuals. When examining the second and third samples, 6 and 9 individuals were found, respectively, with 3 parasite genera (Z = −3.1, P < 0.05). An increase in prevalence was observed in general (N = 26, P = 0.031), and specifically for two parasites, Strongyloides (1 sample: 12/26 vs. 2 samples: 16/26 vs. 3 samples: 20/26, P = 0.008) and Strongyles (unidentified) (1 sample: 4/26 vs. 2 samples: 7/26 vs. 3 samples: 10/26, P = 0.031). For Hymenolepis spp. (1 sample: 5/26 vs. 2 samples: 9/26 vs. 3 samples: 10/26, P = 0.063) and the remaining 3 parasite genera, no difference in prevalence was observed.
Fig. 2.
Cumulative richness from the first sample to the third sample for the 26 animals sampled.
Fig. 3.
Cumulative prevalence of parasites from each sample.
3.2.1. Faecal egg and cyst count
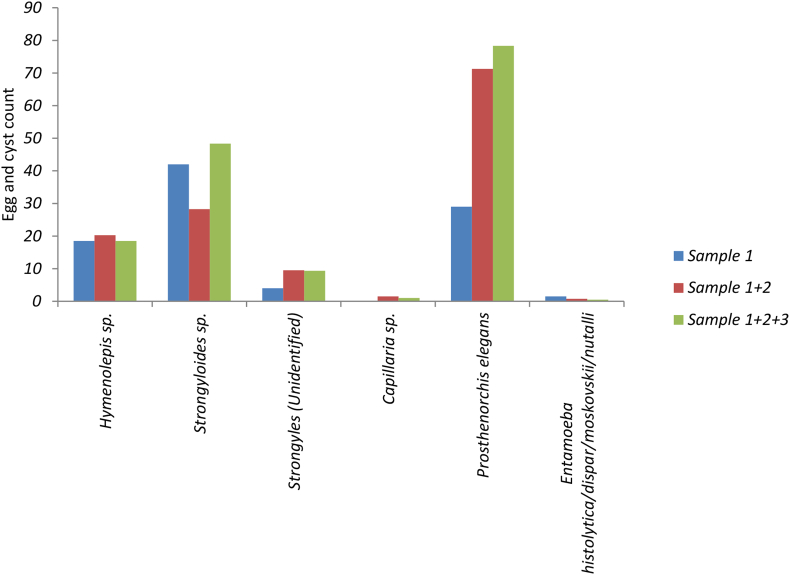
The faecal egg count did not increase significantly with serial sampling for Strongyloides sp. (Z = −1.8, p = 0.068), Hymenolepis sp. (Z = −0.1, p = 0.919) and Strongyles (unidentified) (Z = −1.8, p = 0.062). In the case of the other parasites, only a few individuals (1–3) tested positive and counts did not increase significantly. For example, for Prosthenorchis elegans, the number of eggs increased across the three samples, but not significantly (Z = −1.6, p = 0.109) (Fig. 4).
Fig. 4.
Cumulative egg and cyst count of parasites from each sample.
4. Discussion
4.1. Parasite genera found (prevalence and zoonotic potential of each genus)
We identified 6 parasite genera in our surveys that contribute to the parasite ecology of C. albifrons, with Strongyloides sp. and Hymenolepis sp. being the most prevalent. NHP locomotion on the ground increases the possibility of Strongyloides transmission when the filariform larvae developed in the soil come in contact with the skin. Studies of terrestrial NHPs found a high prevalence of Strongyloides sp. of 44% and 60.7% (Ocaido et al., 2003, Bezjian et al., 2008) in baboons and 81.2% in captive orangutans with ground-dwelling behaviour (Mul et al., 2007). Cebus albifrons, however, is an arboreal primate. Specific information on C. albifrons in their natural habitat is lacking, but C. capucinus was reported to spend only 5% of their time on the ground (Rose, 1994). Both the captive and the free-ranging groups in our study spent an average of 70% of their observed time on the ground (Ramirez-W. and V.-Curicama, unpublished data). Thus, the high prevalence of Strongyloides found in our study may be due to the predominantly terrestrial locomotion. One study in the Tambopata area of Peru showed a lower prevalence (18%) of Strongyloides in C. albifrons (Phillips et al., 2004) than in our population; however, the Tambopata group was 40 km away from any human settlements, and ground-dwelling behaviour was not reported for this group. A long-term study would help us correlate Strongyloides sp. prevalence and the time spent on the ground for both the captive and the free-ranging groups. Given its high level of infection, the free-ranging group of C. albifrons may nevertheless represent a threat to other communities they encounter. For the captive group, the high prevalence of Strongyloides sp. might be associated with several factors: high densities in the cages, which causes a constant re-infection, and lack of environmental enrichment, both of which can result in animals spending more time on the ground, which, as described above, can increase the possibility of transmission. In captive C. apella, Strongyloides sp. was also the most prevalent (Santa Cruz et al., 2000). Even though we did not identify the species of Strongyloides in our samples, Strongyloides cebus is the only Strongyloides to infect neotropical primates (Little, 1966) and has been observed in Cebus species (Guerrero et al., 2012) and in Lagothrix cana (Tenorio Mati et al., 2013). In marmosets (Callithrix penicillata) inoculated subcutaneously with third stage infective larvae of Strongyloides stercoralis, the parasite established and was maintained for up to approximately 10 months (Tenorio Mati et al., 2014). According to Darling (1911), however, the only way of infection would be through the oral mucosa because S. stercoralis does not infect Cebus through the skin. As NHPs are social and mouth contact between individuals is common (e.g., during grooming), the oral mucosa might be an infection route. The prevalence of Strongyles (unidentified) may be due to our inability to identify these species because there is a morphological similarity in the eggs of certain strongyles genera, such as Oesophagostomum sp., Necator sp. and Ancylostoma sp., and neither coprocultures nor molecular techniques were performed in order to differentiate those genera (Little, 1981).
Cestodes are known to infect neotropical primates (Pacheco et al., 2003, Müller, 2007, Parr et al., 2013b). Hymenolepis has an indirect life cycle associated with rats and arthropods, including beetles (Evans et al., 1998) and cockroaches (Johnson-Delaney, 2009), as intermediate hosts and which are present in human settlements (Michaud et al., 2003). Hymenolepis sp. was only present in the free-ranging group. Montenegro (2011) found Hymenolepis sp. in C. flavius, with a prevalence of 16.7%, which is lower than in our study; however, those animals were in a private reserve away from human settlements. The diet of Cebus albifrons is very generalized (Terborgh, 1983), including arthropods. We believe this is one of the reasons for a high prevalence of Hymenolepis sp. in the free-ranging group. Hymenolepis nana and Hymenolepis diminuta, two cestodes that infect humans (PAHO, 2003), might also infect Cebus. In the case of Hymenolepis nana, this species has a direct lifecycle, thus increasing the prevalence of Hymenolepis sp. Hymenolepis cebidarum also infects cebids (Dunn, 1963). Unfortunately, there is not enough published information about this species. Pacheco et al. (2003) found and measured several eggs of Hymenolepis sp. in free-ranging Callicebus nigrifons. They concluded that these animals were infected with several species of Hymenolepis that could only be identified at necropsy.
Prosthenorchis elegans was at a high prevalence in this study and was found only in captive individuals. The reason probably is that it is transmitted by cockroaches and other insects, specifically found in high density captive facilities of wildlife refuges because of the food accessibility, and can become a parasite reservoir for NHPs. Prosthenorchis sp. has been reported to infect C. capucinus (Yamashita, 1963, Parr et al., 2013b). Prosthenorchis elegans has been reported in C. albifrons (Garner et al., 1967, Cárdenas-Callirgos et al., 2010) and from other neotropical primates, both captive (Wolff et al., 1990, Berrocal and Naranjo, 1999, Pérez García et al., 2007, Pissinatti et al., 2007, Pérez et al., 2008) and free-ranging (Berrocal and Naranjo, 1999, Phillips et al., 2004, Pinto et al., 2008, Müller et al., 2010, Wenz et al., 2010, Tavela et al., 2013). However, most of these studies are case reports in which surgical interventions are usually described, but there are no available data on the prevalence and the intensity of infection. Only Monteiro et al. (2010) report the prevalence of parasites in two neotropical primates, one similar to those found in this study. This parasite is sometimes of medical importance in captive NHPs, causing death (Arrojo, 2002, Guerrero et al., 2012, Perez et al., 2013). Currently there are no successful medical treatments available, although surgical removal of the parasites has been reported in other host species (Wolff et al., 1990, Pérez et al., 2008).
The other parasites with a low prevalence in this study were Capillaria sp. and Entamoeba histolytica/dispar/moskovskii/nuttalli. Capillaria sp. was reported to infect Cebus capucinus (Foster and Johnson, 1939) and Alouatta palliata (Helenbrook et al., 2015) with a prevalence of 38%.
Entamoeba histolytica/dispar/moskovskii/nuttalli is transmitted directly through a faecal – oral route (Taylor et al., 2013). The presence of this species complex may suggest a zoonotic transmission; however, because they are morphologically indistinguishable, only molecular characterization can detect transmission between humans and wildlife (VanderWaal et al., 2014). This species complex has not been previously reported in C. albifrons. However, E. histolytica has been found in the New World NHPs Ateles belzebuth hybridus, Leontopithecus chrysomelas, and Saguinus oedipus (Verweij et al., 2003), although differentiation from E. nuttalli was not possible at that time, and Alouatta caraya and Lagothrix lagotricha (Regan et al., 2014), as well as the Old World NHPs Papio hamadryas, Cercopithecus aethiops, and Pan troglodytes verus (Ghandour et al., 1995, Legesse and Erko, 2004, Levecke et al., 2010, Howells et al., 2011). The prevalence of E. histolytica in captive New World NHPs at the Twycross Zoo is low (11.8% for Alouatta caraya and 16.7% for Lagothrix lagotricha) (Regan et al., 2014). The low prevalence in our study is not necessarily a cause for public health concern, because we have not identified the Entamoeba of this species complex, but it is important to know if NHP populations can host naturally acquired E. histolytica (the human variant) infections. Several human and non-human genotypes have been identified in captive animals (Verweij et al., 2003, Tachibana et al., 2007); thus, caution is warranted and the use of molecular tools to identify the human and the NHP strains (Tachibana et al., 2013) and to identify the possible transmission between humans and NHP or vice versa is important (Levecke et al., 2015).
4.2. Parasite richness data
The parasite richness we found (6) is similar to the other two studies of capuchins in Peru, 5 parasites for the Pucallpa Zoo (Paratriotaenia oedipomidatis, Trichostrongylidae, Oxyuriodea, Crytosporidium spp, and Balantidium coli) (Guerrero et al., 2012), and 3 for the Tambopata group (Ascaris sp. , Trichuris trichiura and Schistosoma mansoni) (Phillips et al., 2004). None of these genera were found in our study. In our study, significantly higher parasite richness is found in the free-ranging group than in the captive group. The high contact between tourists and NHPs observed during a behavioural study (Ramirez-W., unpublished data) might be associated with this parasite richness. Rates of contact with people can be associated with oral and faecal transmission routes. For example, diapers left by tourists can come in contact with NHPs. Additionally, the monkeys drink from a creek contaminated by faeces coming from the houses nearby, likely with E. histolytica/dispar/moskovskii/nuttalli and other parasites.
4.3. The relationships of prevalence and richness with habitat, sex and age
Unfortunately, seasonality was impossible to analyse because these two regions (Puyo and Tena) have the highest humidity and precipitation rates in Ecuador, and there is no variation in patterns of climate data throughout the year. However, humidity has been demonstrated to influence the prevalence of some parasites (Stuart et al., 1990, Wren et al., 2015). For example, a humid environment contributes to the development of larvae from Strongyloides (Elsheikha and Khan, 2011, Armon and Cheruti, 2012), and may partly explain the high prevalence of Strongyloides reported here for Puyo and Tena.
Both groups are, or were, in contact with humans, and other studies have demonstrated that this may increase the possibility of parasite transmission (Chapman et al., 2005, Kowalewski and Gillespie, 2009). The free-ranging group from Misahualli has similar conditions to the Cebus capucinus group from the Manuel Antonio National Park, where monkeys eat food provided by tourists (Chinchilla et al., 2007). Captive NHPs eat food from the ground, and wild NHPs also eat food from the ground and soak food in water found in tree holes, water that could be contaminated by infective parasite ova and larvae (Stoner, 1996). Additionally, NHPs are social (Defler, 1979) which makes parasite transmission easier (Côté and Poulin, 1995). Primate locomotion on the ground increases the possibility of parasite transmission when larvae are in contact with the skin, as is the case for Strongyloides sp., or when cysts are ingested; for example, Entamoeba sp. The high prevalence of Hymenolepis in the free-ranging group might be associated with randomly dispersed defecation and frequent ground dwelling behaviour. Despites the low prevalence of eggs of both Hymenolepis nana and H. diminuta in the soil (Kumar Rai et al., 2000), a frequent ground dwelling behaviour could enhance the probability of contamination when individuals walk from one tree to another.
Parasite prevalence in the captive group might be linked to contaminated water and human excrement exposure, to the small area where the captive individuals are held and to constant exposure to infection. For the free-ranging group, the home range area is small and they are fed by tourists and locals. These conditions are similar to those in captivity, with similar risks of parasite transmission related to the poor habitat quality (Wolfe et al., 2005, Goldberg et al., 2008, Rwego et al., 2008). Nunn and Tae-Won Dokey (2006) found a positive correlation between parasite richness and range use. Crowding in a small area results in the repeated use of this area and a high risk of faecal contamination. Monkeys in these conditions can come into contact with directly transmitted parasites such as Entamoeba sp. cysts, and they also have a greater probability of encountering intermediate hosts of indirectly transmitted parasites (e.g., Hymenolepis diminuta, Prosthenorchis elegans). Moreover, there is a positive correlation between parasite richness and host density (Altizer et al., 2003). NHPs crowding in a small area might have a greater chance of reinfection by directly transmitted parasites (e.g., Strongyles). In Costa Rica, home range sizes for a Cebus group are greater (Parr et al., 2013a) and parasite richness is lower than in the Misahualli Group. Unfortunately, the other study of capuchins in Manuel Antonio, Costa Rica, does not report data on home range size (Chinchilla et al., 2007) (Table 3).
Table 3.
Home range area and density of the Misahualli Group and of a group of Cebus in Costa Rica and their PSR.
| Number of Individuals | Home Range (ha) | Density (indiv/ha) | PSR | |
|---|---|---|---|---|
| Ecuador | ||||
| Misahualli | 15 | 5.38 | 2.78 | 2.5 |
| Costa Rica | ||||
| Santa Rosa | ||||
| CP | 24–26 | 80 | 0.3 | 1.36 ± 0.76 |
| EX | 8–11 | 125 | 0.088 | 1.22 ± 0.94 |
| GN | 33–35 | 188 | 0.186 | 1.27 ± 0.84 |
| LV | 20–23 | 174 | 0.13 | 1.27 ± 0.84 |
Source: Costa Rica data adapted from Parr et al. (2013a). PSR = Parasite richness.
In the free-ranging group, a similar rate of parasite richness was found for both males and females. This result must be viewed with caution because more males were sampled, and Hymenolepis sp. was found only in males. Rose (1994) observed males of C. capucinus consuming more invertebrates than females, and therefore males might be more at risk of becoming infected given the lifecycle of H. diminuta. In rats experimentally infected with this species, the intensity of infection was higher in males than females, which could be attributed to the presence of testosterone, lowering the immune function (Addis, 1946).
The egg output shows another disparity regarding gastrointestinal parasites in males and females as reported by Wenz et al. (2010), who found a mean egg output per 100 μl of concentrated sediment for Strongyloides stercoralis of 2.5 for males and 1 for females. These figures are lower than the FEC figures found in our study. However, their methods differed from ours, and the groups mentioned in their study are arboreal. In our study, the egg output is higher in males, other than for the unidentified strongyles.
A high rate of contact between juveniles and subadults may not be a significant predictor for infections with parasites (MacIntosh et al., 2012, Gómez et al., 2013). Juveniles and subadults might be under more stress than adults because of their lower status and their immune function is not entirely developed (Müller-Graf et al., 1996). Moreover, in young baboons, it is thought that parasites, particularly nematodes, use a transmammary route of transmission (Müller-Graf et al., 1996). Contrary to these findings, our study showed there were no significant differences in prevalence with age or sex. Also in our study, parasite richness was higher in adults than subadults and juveniles. These results may be due to status in the group hierarchies. Adults have been in contact with the environment much longer and consume more food, increasing the risk of infection (Nunn et al., 2003).
4.4. Impact of sampling effort on parasite richness, prevalence and intensity of infection
In our study, as has been shown before (Muehlenbein, 2005), the number of parasite genera increases when three samples are taken. The detection of parasites depends on their biology, especially their fecundity and how often they release eggs. For example, Hymenolepis diminuta is a parasite that releases up to 250 000 eggs per day. This could be a reason why Hymenolepis was detected in the free-ranging group from sample 1 to sample 3 in infected individuals. In a study on Callicebus nigrifrons, Pacheco et al. (2003) sampled four individuals infected with Himenolepis sp. and found in three a regular pattern of egg numbers in their samples. Thus, serial sampling of identified hosts increases the accuracy of prevalence data. Milozzi et al. (2012) found a similar prevalence of parasites (86.8%) when sampling three times. Regarding the intensity of infection, our study did not find a significant increase in egg/cyst counts when sampling three times. This might be because samples were not taken with the same interval between them, nor were the samples taken at the same time each day. To see whether the parasite has a release pattern of eggs or not, this method should be applied on consecutive days at the same hour.
This study reports on the parasite richness as well as the prevalence and the egg/cyst count of gastrointestinal parasites found in 26 individuals. The parasite distribution in this study reflects the presence of new parasites for Cebus albifrons. The multiple samples and the formol-ether concentration method is an accurate method with high sensitivity for most parasites, except for Giardia sp., which is commonly found in captive groups (Levecke, 2010). However, FECC did not increase with sampling effort. These findings suggest an impact of the human population on the free-ranging group. The free-ranging group had an overall higher prevalence and parasite richness than the captive group. It is possible that the free-ranging-group might be a source of infection for Hymenolepis sp. and Capillaria sp. Further research is needed to observe whether our findings further relate to biodiversity conservation, primate behaviour, the life cycle of parasites, and human health, since each parasite is affected by a range of ecological factors, such as humidity, temperature, behaviour, density, arthropod consumption, and water sources. The captive population where we found the Entamoeba histolytica complex species needs a deeper screening to identify the human and the NHP strains. In addition, coprocultures and molecular techniques are necessary to differentiate unidentified strongyles. This study presents baseline data for future research on gastrointestinal parasites for Cebus albifrons in Ecuador.
Acknowledgements
We thank the Ecuadorian government, the Ministerio del Ambiente, for their support of our primate research (Research permit N° 004/2013-IC-FAU-DPAP/MAE). The research described here was undertaken following all Ecuadorian legislation. We also thank the University of Liège, Universidad Central del Ecuador and Universidad de las Fuerzas Armadas ESPE. We would like to thank the anonymous reviewers for their helpful comments and corrections.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijppaw.2017.06.004.
Contributor Information
Sarah Martin-Solano, Email: sarahmartinsolano@gmail.com, sarah.martin@doct.ulg.ac.be, ssmartin@espe.edu.ec.
Gabriel A. Carrillo-Bilbao, Email: gacarrillo@uce.edu.ec.
William Ramirez, Email: ramirezw@cwu.edu.
Maritza Celi-Erazo, Email: mceli@uce.edu.ec.
Marie-Claude Huynen, Email: marie-claude.huynen@ulg.ac.be.
Bruno Levecke, Email: bruno.levecke@ugent.be.
Washington Benitez-Ortiz, Email: wbenitez@uce.edu.ec.
Bertrand Losson, Email: blosson@ulg.ac.be.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Addis C.J.J. Experiments on the relations between sex hormones and the growth of tapeworms (Hymenolepis diminuta) in rats. J. Parasitol. 1946;32:574–580. [PubMed] [Google Scholar]
- Altizer S., Nunn C.L., Thrall P.H., Gittleman J.L., Antonovics J., Cunningham A.A., Dobson A.P., Ezenwa V., Jones K.E., Pedersen A.B., Poss M., Pulliam J.R.C. Social organization and disease risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Syst. 2003;34:517–547. [Google Scholar]
- Armon R., Cheruti U. IWA Publishing; 2012. Environmental Aspects of Zoonotic Diseases. [Google Scholar]
- Arrojo L. Parasites of wild animals in captivity from Lima. Peru. Rev. Peru. Biol. 2002;9:118–120. [Google Scholar]
- Behie A.M., Kutz S., Pavelka M.S. Cascading effects of climate change: do hurricane-damaged forests increase risk of exposure to parasites? Biotropica. 2014;46:25–31. [Google Scholar]
- Beltran-Bech S., Richard F.-J. Impact of infection on mate choice. Anim. Behav. G. 2014:159–170. [Google Scholar]
- Berrocal A., Naranjo C. 1999. Pulmonary (Filaroides sp.) and Intestinal (Prosthenorchis sp.) Parasites of New World Monkeys. (In, International Symposium of the World Association of Veterinary Laboratory. IX., College Station, TX US) [Google Scholar]
- Bezjian M., Gillespie T.R., Chapman C.A., Greiner E.C. Coprologic evidence of gastrointestinal helminths of forest baboons, Papio anubis, in kibale national Park, Uganda. J. Wildl. Dis. 2008;44:878–887. doi: 10.7589/0090-3558-44.4.878. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Callirgos J., Fernández-Conga D., Montes-Aliaga D., Sanchez-Perea N., Valencia-Límaco W., Valderrama-Bazán W., Mayor-Aparicio P. Presencia de Prosthenorchis elegans (DIESING, 1851) en primates neotropicales obtenidos por caza de subsistencia en la comunidad nueva esperanza, Loreto-Perú. In: Iannacone J., editor. II Congreso Internacional de Parasitología Neotropical “El rol de la Parasitología Neotropical en la Salud Global”. Peruvian Association of Helminthology and Associates Invertebrates (APHIA), Ricardo Palma University; Lima, Perú: 2010. [Google Scholar]
- Cervera L., Donati G. Cancun; Mexico: 2012. Population density and habitat preference of mantled howler monkeys (Alouatta palliata aequatorialis) and white-fronted capuchin monkeys (Cebus albifrons aequatorialis) ein the pacoche forest, south-west ecuador. In, XXIV Congress of the International Primatological Society. [Google Scholar]
- Chapman C.A., Gillespie T.R., Goldberg T.L. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host-parasite interactions? Evol. Anthropol. 2005;14:134–144. [Google Scholar]
- Chapman C.A., Speirs M.L., Gillespie T.R., Holland T., Austad K.M. Life on the edge: gastrointestinal parasites from the forest edge and interior primate groups. Am. J. Primatol. 2006;68:397–409. doi: 10.1002/ajp.20233. [DOI] [PubMed] [Google Scholar]
- Chapman C.A., Bowman D.D., Ghai R.R., Gogarten J.F., Goldberg T.L., Rothman J.M., Twinomugisha D., Walsh C. Protozoan parasites in group-living primates: testing the biological island hypothesis. Am. J. Primatol. 2012;74:510–517. doi: 10.1002/ajp.20992. [DOI] [PubMed] [Google Scholar]
- Chinchilla M., Guerrero O.M., Gutierrez-Espeleta G.A., Sánchez R., Valerio Campos I. Parásitos en monos carablanca Cebus capucinus (Primates: cebidae) de Costa Rica. Parasitol. Latinoam. 2007;62:170–175. [Google Scholar]
- Côté I.M., Poulin R. Parasitism and group size in social animals: a meta-analysis. Behav. Ecol. 1995;6:159–165. [Google Scholar]
- Cristóbal-Azkarate J., Hervier B., Vegas-Carrillo S., Osorio-Sarabia D., Rodríguez-Luna E., Veà J.J. Parasitic infections of three Mexican howler monkey groups (Alouatta palliata mexicana) living in forest fragments in Mexico. Primates. 2010;51:231–239. doi: 10.1007/s10329-010-0193-7. [DOI] [PubMed] [Google Scholar]
- Darling S.T. Strongyloides infections in man and animals in the isthmian canal zone. J. Exp. Med. 1911;14:1–24. doi: 10.1084/jem.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- de la Torre S., Morales A.L., Link A., Cornejo F. 2008. Cebus albifrons.http://www.iucnredlist.org/ The IUCN Red List of Threatened Species. Version 2015.2. Downloaded on 27 August 2015. [Google Scholar]
- Defler T.R. On the ecology and behavior of Cebus albifrons in eastern Colombia. Primates. 1979;20:491–502. [Google Scholar]
- Derby A.M. 2008. Investigating How Ecology and Demography Influence Folivorous Primate Biomass in the Western Amazon Stony Brook University. [Google Scholar]
- Dunn F.L. Acanthocephalans and cestodes of South American monkeys and marmosets. J. Parasitol. 1963;49:717–722. [PubMed] [Google Scholar]
- Dunn F.L., Lambrecht F.L. On some filarial parasites of South American primates, with a description of Tetrapetalonema tamarinae n.sp. from the Peruvian tamarin marmoset, Tamarinus nigricollis (Spix, 1823) J. Helminthol. 1963;37:261–286. [Google Scholar]
- Eckert K., Hahn N., Genz A., Kitchen D., Stuart M., Averbeck G., Stromberg B., Markowitz H. Coprological surveys of Alouatta pigra at two sites in Belize. Int. J. Primatol. 2006;27:227–238. [Google Scholar]
- Elsheikha H.M., Khan N.A. Caister Academic Press; 2011. Essentials of Veterinary Parasitology. [Google Scholar]
- Evans W.S., Wong A., Hardy M., Currie R.W., Vanderwel D. Evidence that the factor used by the tapeworm, Hymenolepis diminuta, to direct the foraging of its intermediate host, Tribolium confusum, is a volatile attractant. J. Parasitol. 1998;84:1098–1101. [PubMed] [Google Scholar]
- Ewing S.A., Helland D.R., Anthony H.D., Leipold H.W. Occurrence of Athesmia sp. in the cinnamon ringtail monkey, Cebus albifrons. Lab. Anim. Care. 1968;18:488–492. [PubMed] [Google Scholar]
- Flynn R. The Iowa State University Press; Ames, Iowa: 1973. Parasites of Laboratory Animals. [Google Scholar]
- Foster A.O., Johnson C.M. An explanation for the occurrence of Capillaria hepatica ova in human faeces suggested by the finding of three new hosts used as food. Trans. R. Soc. Trop. Med. Hyg. 1939;32:639–644. [Google Scholar]
- Friant S.C. Oxford Brookes University; Oxford: 2007. An Investigation of Gastro-intestinal Parasites in Captive and Semi-captive Cercopithecine Primates of Southern Nigeria. [Google Scholar]
- García-Solorza E., Levi-Castillo R. Filariasis in monkey Cebus albifrons aequatorialis in the coastal region of Ecuador (First note) Rev. Ecu. Entom. Paras. 1954;2:101–104. [Google Scholar]
- Garner E., Hemrick F., Rudiger H. Multiple helminth infections in cinnamon-ringtail monkeys (Cebus albifrons) Lab. Anim. Care. 1967;17:310–315. [PubMed] [Google Scholar]
- Ghandour A.M., Zahid N.Z., Banaja A.A., Kamal K.B., Bouq A.I. Zoonotic intestinal parasites of hamadryas baboons Papio hamadryas in the western and northern regions of Saudi Arabia. J. Trop. Med. Hyg. 1995;98:431–439. [PubMed] [Google Scholar]
- Gillespie T.R. Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. Int. J. Primatol. 2006;27:1129–1143. [Google Scholar]
- Gillespie T.R., Chapman C.A. Prediction of parasite infection dynamics in primate metapopulations based on attributes of forest fragmentation. Conserv. Biol. 2006;20:441–448. doi: 10.1111/j.1523-1739.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Gillespie T.R., Greiner E.C., Chapman C. Gastrointestinal parasites of the colobus mokeys of Uganda. J. Parasitol. 2005;91:569–573. doi: 10.1645/GE-434R. [DOI] [PubMed] [Google Scholar]
- Gillespie T.R., Nunn C.L., Leendertz F.H. Integrative approaches to the study of primate infectious disease: implications for biodiversity conservation and global health. Yrbk. Phys. Anth. 2008;51:53–69. doi: 10.1002/ajpa.20949. [DOI] [PubMed] [Google Scholar]
- Goldberg T.L., Gillespie T.R., Rwego I.B., Estoff E.E., Chapman C. Forest fragmentation as cause of bacterial transmission among primates, humans, and livestock, Uganda. Emerg. Infect. Dis. 2008;14:1375–1382. doi: 10.3201/eid1409.071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J.M., Nunn C.L., Verdú M. Centrality in primate–parasite networks reveals the potential for the transmission of emerging infectious diseases to humans. Proc. Natl. Acad. Sci. 2013;110:7738–7741. doi: 10.1073/pnas.1220716110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M.F., Serrano-Martínez E., Tantaleán V.M., Quispe H.M., Casas V.G. Identificación de parásitos gastrointestinales en primates no humanos del zoológico parque natural de Pucallpa. Perú. Rev. Investig. Vet. Peru. 2012;23:469–478. [Google Scholar]
- Hahn N.E., Proulx D., Muruthi P.M., Alberts S., Altmann J. Gastrointestinal parasites in free-ranging kenyan baboons (Papio cynocephalus and P. anubis) Int. J. Primatol. 2003;24:271–279. [Google Scholar]
- Hasegawa H., Huffman M.A., Chapman C. Useful diagnostic references and images of protozoans, helminths, and nematodes commonly found in wild primates. In: Huffman M.A., Chapman C., editors. Primate Parasite Ecology. Cambridge University Press; Cambridge: 2009. pp. 507–513. [Google Scholar]
- Helenbrook W.D., Wade S.E., Shields W.M., Stehman S.V., Whipps C.M. Gastrointestinal parasites of ecuadorian mantled howler monkeys (Alouatta palliata aequatorialis) based on fecal analysis. J. Parasitol. 2015;101:341–350. doi: 10.1645/13-356.1. [DOI] [PubMed] [Google Scholar]
- Homsy J. The International Gorilla Conservation Programme; Kigali: 1999. Ape Tourism and Human Diseases: How Close Should We Get? p. 86. In, Report of a Consultancy for the International Gorilla Conservation Programme. [Google Scholar]
- Hooge P.N., Eichenlaub B. Alaska Science Center—Biological Science Office, U.S. Geological Survey; Anchorage, AK: 2000. Animal Movement Extension to ArcView, Version 2.0. [Google Scholar]
- Hopkins M.E., Nunn C.L. Gap analysis and the geographical distribution of parasites. In: Morand S., Krasnov B., editors. The Biogeography of Host-parasite Interactions. Oxford University Press; Oxford: 2010. pp. 129–142. [Google Scholar]
- Howells M.E., Pruetz J., Gillespie T.R. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: the case of sympatric western chimpanzees (Pan troglodytes verus) and Guinea baboons (Papio hamadryas papio) at fongoli. Senegal. Am. J. Primatol. 2011;73:173–179. doi: 10.1002/ajp.20884. [DOI] [PubMed] [Google Scholar]
- INAMHI . INAMHI. INAMHI, Ecuador; Quito: 2014. Datos meteorológicos para las Provincias de Pastaza y de Napo del Instituto Nacional de Meteorología e Hidrología. [Google Scholar]
- Johnson-Delaney C.A. Parasites of captive nonhuman primates. Vet. Clin. North Am. Exot. Anim. Pract. 2009;12:563–581. doi: 10.1016/j.cvex.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Kowalewski M.M., Gillespie T.R. Ecological and anthropogenic influences on patterns of parasitism in free -ranging primates: a meta - analysis of the Genus Alouatta. In: Garber P.A., Estrada A., Strier K.B., Bicca -Marques J., Heymann E.W., editors. South American Primates, Developments in Primatology: Progress and Prospects. Springer Press; 2009. pp. 433–461. [Google Scholar]
- Kowalewski M.M., Salzer J.S., Deutsch J.C., Raño M., Kuhlenschmidt M.S., Gillespie T.R. Black and gold howler monkeys (Alouatta caraya) as Sentinels of ecosystem health: patterns of zoonotic Protozoa infection relative to degree of human–primate contact. Am. J. Primatol. 2011;73:75–83. doi: 10.1002/ajp.20803. [DOI] [PubMed] [Google Scholar]
- Kumar Rai S., Uga S., Ono K., Rai G., Matsumura T. Contamination of soil with helminth parasite eggs in Nepal. Southeast Asian J. Trop. Med. Public Health. 2000;31:388–393. [PubMed] [Google Scholar]
- Legesse M., Erko B. Zoonotic intestinal parasites in Papio anubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Trop. 2004;90:231–236. doi: 10.1016/j.actatropica.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Levecke B. Ghent University; Ghent: 2010. The Importance of Gastrointestinal protozoa in Captive Non-human Primates. [Google Scholar]
- Levecke B., Dreesen L., Dorny P., Verweij J.J., Vercammen F., Casaert S., Vercruysse J., Geldhof P. Molecular identification of Entamoeba spp. In captive nonhuman primates. J. Clin. Microbiol. 2010;48:2988–2990. doi: 10.1128/JCM.00013-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levecke B., Dorny P., Vercammen F., Visser L.G., Van Esbroeck M., Vercruysse J., Verweij J.J. Transmission of Entamoeba nuttalli and Trichuris trichiura from nonhuman primates to humans. Emerg. Infect. Dis. 2015;21:1871–1872. doi: 10.3201/eid2110.141456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly A.A., Mehlman P.T., Doran D. Intestinal parasites in gorillas, chimpanzees, and humans at mondika research site, dzanga-ndoki national Park, Central African Republic. Int. J. Primatol. 2002;23:555–573. [Google Scholar]
- Little M.D. Comparative morphology of six species of Strongyloides (Nematoda) and redefinition of the genus. J. Parasitol. 1966;52 [PubMed] [Google Scholar]
- Little M.D. 1981. Differentiation of Nematode Larvae in Coprocultures: Guidelines for Routine Practice in Medical Laboratories; pp. 144–150. WHO Technical Reports Series No 666. [Google Scholar]
- MacIntosh A.J.J., Jacobs A., Garcia C., Shimizu K., Mouri K., Huffman M.A., Hernandez A.D. Monkeys in the middle: parasite transmission through the social network of a wild primate. PLoS One. 2012;7:e51144. doi: 10.1371/journal.pone.0051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-López S., Maldonado-López Y., Gómez-Tagle Ch A., Cuevas-Reyes P., Stoner K.E. Patterns of infection by intestinal parasites in sympatric howler monkey (Alouatta palliata) and spider monkey (Ateles geoffroyi) populations in a tropical dry forest in Costa Rica. Primates. 2014;55:383–392. doi: 10.1007/s10329-014-0413-7. [DOI] [PubMed] [Google Scholar]
- McGrew W.C., Tutin C.E.G., Collins D.A., File S.K. Intestinal parasites of sympatric Pan troglodytes and Papio Spp. at two sites: gombe (Tanzania) and Mt. Assirik (Senegal) Am. J. Primatol. 1989;17:147–155. doi: 10.1002/ajp.1350170204. [DOI] [PubMed] [Google Scholar]
- Michaud C., Tantalean M., Ique C., Montoya E., Gozalo A. A survey for helminth parasites in feral New World non-human primate populations and its comparison with parasitological data from man in the region. J. Med. Primatol. 2003;32:341–345. doi: 10.1046/j.1600-0684.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Milozzi C., Bruno G., Cundom E., Mudry M.D., Navone G.T. Intestinal parasites of Alouatta caraya (Primates, Ceboidea): preliminary study in semi-captivity and in the wild in Argentina. Mastozool. neotrop. 2012;19:163–178. [Google Scholar]
- Monteiro R.V., Dietz J.M., Jansen A.M. The impact of concomitant infections by Trypanosoma cruzi and intestinal helminths on the health of wild golden and golden-headed lion tamarins. Res. Vet. Sci. 2010;89:27–35. doi: 10.1016/j.rvsc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Montenegro M.M.V. 2011. Ecologia de Cebus flavius (Schreber, 1774) em remanescentes de Mata Atlântica no estado da Paraíba. [Google Scholar]
- Muehlenbein M.P. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at ngogo, kibale national Park, Uganda. Am. J. Primatol. 2005;65:167–179. doi: 10.1002/ajp.20106. [DOI] [PubMed] [Google Scholar]
- Mul I., Paembonan W., Singleton I., Wich S., van Bolhuis H. Intestinal parasites of free-ranging, Semicaptive,and captive Pongo abelii in Sumatra, Indonesia. Int. J. Primatol. 2007;28:407–420. [Google Scholar]
- Müller B. Tierärztliche Hochschule Hannover; Hannover: 2007. Determinants of the Diversity of Intestinal Parasite Communities in Sympatric New World Primates (Saguinus mystax, Saguinus fuscicollis, Callicebus cupreus) [Google Scholar]
- Müller B., Mätz-Rensing K., Pérez Yamacita J.G., Heymann D.E.W. Pathological and parasitological findings in a wild red titi monkey, Callicebus cupreus (Pitheciidae, Platyrrhini) Eur. J. Wildl. Res. 2010;56:601–604. [Google Scholar]
- Müller-Graf C.D.M., Collins D.A., Woolhouse M.E.J. Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology. 1996;112:489–497. doi: 10.1017/s0031182000076952. [DOI] [PubMed] [Google Scholar]
- Munene E., Otsyula M., Mbaabu D.A.N., Mutahi W.T., Muriuki S.M.K., Muchemi G.M. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet. Parasitol. 1998;78:195–201. doi: 10.1016/s0304-4017(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Muriuki S.M.K., Murugu R.K., Munene E., Karere G.M., Chai D.C. Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Trop. 1998;71:73–82. doi: 10.1016/s0001-706x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Nizeyi J.B., Sebunya D., Dasilva A.J., Cranfield M.R., Pieniazek N.J., Graczyk T.K. Cryptosporidiosis in people sharing habitats with free-ranging Mountain gorillas (Gorilla gorilla beringei), Uganda. Am. J. Trop. Med. Hyg. 2002;66:442–444. doi: 10.4269/ajtmh.2002.66.442. [DOI] [PubMed] [Google Scholar]
- Nuñez-Iturri G., Howe H.F. Bushmeat and the fate of trees with Seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica. 2007;39:348–354. [Google Scholar]
- Nunn C.L., Altizer S. Oxford University Press; 2006. Infectious Disease and Primate Social Systems, Infectious Diseases in Primates: Behavior, Ecology and Evolution; p. 400. [Google Scholar]
- Nunn C.L., Tae-Won Dokey A. Ranging patterns and parasitism in primates. Biol. Lett. 2006;2:351–354. doi: 10.1098/rsbl.2006.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn C.L., Altizer S., Jones K.E., Sechrest W. Comparative tests of parasite species richness in primates. Am. Nat. 2003;162:597–614. doi: 10.1086/378721. [DOI] [PubMed] [Google Scholar]
- Ocaido M., Dranzoa C., Cheli P. Gastrointestinal parasites of baboons (Papio anubis) interacting with humans in West bugwe forest reserve, Uganda. Afr. J. Ecol. 2003;41:356–359. [Google Scholar]
- Pacheco L.R., Neri F.M., Frahia V.T., De Melo A.L. Parasitismo natural em Sauás, Callicebus nigrifrons (Spix, 1823): variacao na eliminacao de ovos de nematoda e cestoda. Neotrop. Primates. 2003;11:29–32. [Google Scholar]
- PAHO . WHO; Washington D.C: 2003. Zoonoses and Communicable Diseases Common to Man and Animals. [Google Scholar]
- Parr N., Fedigan L., Kutz S. Predictors of parasitism in wild white-faced capuchins (Cebus capucinus) Int. J. Primatol. 2013;34:1137–1152. doi: 10.1159/000348287. [DOI] [PubMed] [Google Scholar]
- Parr N.A., Fedigan L.M., Kutz S.J., Babik W. A cropological survey of parasites in white-faces capuchins (Cebus capucinus) from Sector Santa rosa, ACG, Costa Rica. Folia Primatol. 2013;84:102–114. doi: 10.1159/000348287. [DOI] [PubMed] [Google Scholar]
- Pérez J., Ramírez M., Hernández C.A. Surgical intervention for removing intestinal Prosthenorchis sp. in a captive white-footed tamarin (Saguinus leucopus) Rev. Colom. Cienc. Pecua. 2008;21:2008. [Google Scholar]
- Perez J., Peña J., Soler-Tovar D. Spiny-headed worms (Prosthenorchis elegans) in Silvery-brown bare-face tamarins (Saguinus leucopus): a case report. In: Defler T.R., Stevenson P.R., Bueno M., Guzman-Caro D.C., editors. Colombian Primates Endangered, Asociación Primatológica Colombiana. 2013. pp. 139–150. [Google Scholar]
- Pérez García J., Ramirez D.M., Hernández C.A. Prosthenorchis sp. en titíes grises (Saguinus leucopus). Revisión de tema. Rev. CES Med. Zootec. 2007;2 [Google Scholar]
- Phillips K.A., Haas M.E., Grafton B.W., Yrivarren M. Survey of the gastrointestinal parasites of the primate community at Tambopata National Reserve. Peru. J. Zool. 2004;264:149–151. [Google Scholar]
- Pinto J.M.S., Catenacci L.S., Colosio A.C., De Vleeschouwer M.K. 2008. Ocorrência de Prosthenorchis elegans (DIESING, 1861) TRAVASSOS, 1915 em Leontopithecus chrysomelas (Mico-Leão-De-Cara-Dourada) na reserva biológica (rebio), de Una - BA; p. 4. In, 35° Congresso Brasileiro de Medicina Veterinária, 2008 GramadoRS. Anais do 35° Conbravet. [Google Scholar]
- Pissinatti L., Pissinatti A., Burity C.H.F., Mattos D.G., Jr., Tortelly R. Ocorrência de Acanthocephala em Leontopithecus (Lesson, 1840), cativos: aspectos clínicopatológicos. Callitrichidae-Primates. Arq. Bras. Med. Vet. Zootec. 2007;59:1473–1477. [Google Scholar]
- Pozo R.W.E. Habitat preferences of six non-atelidae primates of Yasuni. Ecuad. Ecol. Apl. 2004;3:128–133. [Google Scholar]
- Regan C.S., Yon L., Hossain M., Elsheikha H.M. Prevalence of Entamoeba species in captive primates in zoological gardens in the UK. PeerJ. 2014;2 doi: 10.7717/peerj.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renquist D., Whitney R. Zoonoses acquired from pet primates. Vet. Clin. North Am. Small Anim. Pract. 1987;17:219–240. doi: 10.1016/s0195-5616(87)50614-3. [DOI] [PubMed] [Google Scholar]
- Rose L. Sex differences in diet and foraging behavior in white-faced capuchins (Cebus capucinus) Int. J. Primatol. 1994;15:95–114. [Google Scholar]
- Rwego I.B., Isabirye-Basuta G., Gillespie T.R., Goldberg T.L. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in bwindi impenetrable national Park, Uganda. Conserv. Biol. 2008;22:1600–1607. doi: 10.1111/j.1523-1739.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Santa Cruz A.C.M., Borda J.T., Gomez L., de Rott M.I.O. Endoparasitosis in captive Cebus apella. Lab. Prim. Newsl. 2000;39:10. [Google Scholar]
- Stoner K.E. Prevalence and intensity of intestinal parasites in mantled howling monkeys (Alouatta palliata) in notheasthern Costa Rica: implications for conservation biology. Conserv. Biol. 1996;10:539–546. [Google Scholar]
- Stoner K.E., González-Di Pierro A.M. Intestinal parasitic infections in Alouatta pigra in tropical rainforest in lacandona, chiapas, Mexico: implications for behavioral ecology and conservation. In: Estrada A., Garber P.A., Pavelka M.S.M., Luecke L., editors. New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior, and Conservation. Springer; New York: 2005. [Google Scholar]
- Stuart M.D., Greenspan L.L., Glander K.E., Clarke M.R. A coprological survey of parasites of wild mantled howling monkeys, Alouatta palliata palliata. J. Wildl. Dis. 1990;26:547–549. doi: 10.7589/0090-3558-26.4.547. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Yanagi T., Pandey K., Cheng X.-J., Kobayashi S., Sherchand J.B., Kanbara H. An Entamoeba sp. strain isolated from rhesus monkey is virulent but genetically different from Entamoeba histolytica. Mol. Biochem. Parasitol. 2007;153:107–114. doi: 10.1016/j.molbiopara.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Yanagi T., Lama C., Pandey K., Feng M., Kobayashi S., Sherchand J.B. Prevalence of Entamoeba nuttalli infection in wild rhesus macaques in Nepal and characterization of the parasite isolates. Parasitol. Int. 2013;62:230–235. doi: 10.1016/j.parint.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Tavela A.d.O., Fuzessy L.F., Silva V.H.D.e., Silva F.d.F.R.d., Junior M.C., Silva I.d.O., Souza V.B. Helminths of wild hybrid marmosets (Callithrix sp.) living in an environment with high human activity. Rev. Bras. Parasitol. Vet. 2013;22:391–397. doi: 10.1590/S1984-29612013000300012. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Coop R.L., Wall R.L. John Wiley & Sons; 2013. Veterinary Parasitology. [Google Scholar]
- Tello J.G. Frugivores at a fruiting Ficus in south-eastern Per. J. Trop. Ecol. 2003;19:717–721. [Google Scholar]
- Tenorio Mati V.L., Ferreira Junior F.C., Alves Pinto H., de Melo A.L. Strongyloides cebus (Nematoda: Strongyloididae) in Lagothrix cana (Primates: atelidae) from the Brazilian Amazon: aspects of clinical presentation, anatomopathology, treatment, and parasitic biology. J. Parasitol. 2013;99:1009–1018. doi: 10.1645/13-288.1. [DOI] [PubMed] [Google Scholar]
- Tenorio Mati V.L., Raso P., de Melo A.L. Strongyloides stercoralis infection in marmosets: replication of complicated and uncomplicated human disease and parasite biology. Parasit. Vectors. 2014;7:579. doi: 10.1186/s13071-014-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborgh J. Princeton University Press; Princeton, New Yersey: 1983. Five New World Primates: a Study in Comparative Ecology (Monographs in Behavior and Ecology) [Google Scholar]
- Thienpont D., Rochette F., Vanparijs O.F.J. Janssen Research Foundation; 1986. Diagnóstico de las helmintiasis por medio del examen coprológico. [Google Scholar]
- VanderWaal K.L., Atwill E.R., Isbell L.A., McCowan B. Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis) J. Anim. Ecol. 2014;83:406–414. doi: 10.1111/1365-2656.12137. [DOI] [PubMed] [Google Scholar]
- Verweij J., Vermeer J., Brienen E.T., Blotkamp C., Laeijendecker D., van Lieshout L., Polderman A. Entamoeba histolytica infections in captive primates. Parasitol. Res. 2003;90:100–103. doi: 10.1007/s00436-002-0808-z. [DOI] [PubMed] [Google Scholar]
- Vitazkova S.K., Wade S.E. Parasites of free-ranging black howler monkeys (Alouatta pigra) from Belize and Mexico. Am. J. Primatol. 2006;68:1089–1097. doi: 10.1002/ajp.20309. [DOI] [PubMed] [Google Scholar]
- Vitazkova S.K., Wade S.E. Effects of ecology on the gastronintestinal parasites of Alouatta pigra. Int. J. Primatol. 2007;28:1327–1343. [Google Scholar]
- Wenz A., Heymann E.W., Petney T.N., Taraschewski H.F. The influence of human settlements on the parasite community in two species of Peruvian tamarin. Parasitology. 2010;137:675–684. doi: 10.1017/S0031182009991570. [DOI] [PubMed] [Google Scholar]
- Wolfe N.D., Daszak P., Marm Kilpatrick A., Burke D.S. Bushmeat hunting, deforestation, and prediction of zoonotic disease emergence. Emerg. Infect. Dis. 2005;11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P., Pond J., Meehan T. Surgical removal of Prosthenorchis elegans from six species of Callitrichidae. Am. Assoc. Zoo Veterinarian Annu. Proc. 1990;95 [Google Scholar]
- Wren B.T., Remis M.J., Gillespie T.R. vol. 291. XXIII International Primatological Society; KYOTO: 2010. (Social Grooming in Primates: Hygienic or Health Risk?). [Google Scholar]
- Wren B.T., Gillespie T.R., Camp J.W., Remis M.J. Helminths of vervet monkeys, Chlorocebus aethiops, from loskop dam nature reserve, South Africa. Comp. Parasitol. 2015;82:101–108. [Google Scholar]
- Yamashita J. Ecological relationships between parasites and primates. I. Helminth parasites and primates. Primates. 1963;4:1–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.