Abstract
The diagnosis of Meniere’s disease (MD) and vestibular migraine (VM) is primarily based on clinical criteria and their differentiation is often difficult. Currently, there are no known definitive diagnostic tests that can reliably distinguish the two conditions. Patients with MD and patients with VM are treated differently, therefore improving the diagnosis of these two pathologies should avoid errors in management. A systematic review was conducted according to PRISMA guidelines. Medline-Ovid and Embase databases were used to conduct a thorough search of English-language publications dating from 1948 to March 2016. The primary search objective was to identify all papers explicitly comparing MD and VM in order to clarify and validate the diagnosis of these two diseases. A total of 13 articles out of 831 were reviewed. Among other differences, MD showed later age of onset, more hearing loss, tinnitus, aural fullness, abnormal nystagmus, abnormal caloric testing results, abnormal vestibular evoked myogenic potential and endolymphatic hydrops. VM showed more headaches, photophobia, vomiting and aura. Even though differences were noted between the two diseases, only one study focused on assessing the differences between VM, MD and patients fulfilling both diagnostic criteria (MDVM). This study showed no difference between the three groups. Since the introduction of the new International Headache Society and Barany Society criteria for VM, no studies have focused on comparing these three groups. We strongly encourage authors to focus on comparing MD and VM from MDVM in future studies to help adequately distinguish the diagnosis of both diseases.
Keywords: Endolymphatic hydrops, Meniere’s disease, Vestibular migraine, Migrainous vertigo, Migraine-associated vertigo, Migraine-associated vestibulopathy, Migraine-related vertigo, Migraine-related vestibulopathy
Introduction
Decades go, Kuritzky et al [1] was the first to report significantly more vestibular symptoms in classical migraine patients compared to controls. Vestibular migraine (VM) then became an emerging diagnosis for vestibular symptoms in patients with current or previous headaches with migraine characteristics.
In 2001, Neuhauser et al [2] introduced the first criteria to define the disease (Table 1). However it was only in 2012 did the International Headache Society (IHS) and the Barany Society [3] validate these criteria into a well-established clinical entity (Table 2). Both these sets of criteria distinguish definite VM from probable VM (pVM). A study analyzed both these sets of criteria and determined that a significant difference exists between them, and the 2012 diagnostic criteria for VM limited the diagnosis of the disease to fewer patients, mainly because of the type, intensity, and duration of dizziness [4].
Table 1. Diagnostic Criteria for Vestibular Migraine Proposed by Neuhauser, 2001.
| Vestibular migraine, defined |
| 1) Vestibular symptoms at least of moderate intensity |
| 2) Current or past history of migraine, according to International Headache Society criteria |
| 3) One of the following migraine symptoms during at least two attacks of vertigo: migraine, photophobia, phonophobia, visual or other auras |
| 4) Other causes ruled out by an appropriate research |
| Probable vestibular migraine |
| 1) Vestibular symptoms of at least moderate intensity |
| 2) One of the following: |
| a) Current or past history of migraine according to 2004 criteria |
| b) Migraine symptoms during vestibular symptoms |
| c) Migraine precipitants of vertigo in more than 50% of attacks: food triggers, sleep problems, hormonal changes |
| d) Response to anti-migraine drugs in more than 50% of attacks |
| 3) Other causes ruled out by appropriate research |
Table 2. Diagnostic Criteria for Vestibular Migraine Proposed by Barany Society and the Third International Classification of Headache Disorders (ICHD-3), 2012.
| Vestibular migraine |
| 1) At least five episodes with vestibular symptoms of moderatea or severeb intensity, lasting 5 min to 72 h |
| 2) Current or previous history of migraine with or without aura according to the International Classification of Headache Disorders (ICHD) |
| 3) One or more migraine features with at least 50% of the vestibular episodes: a) headache with at least two of the following characteristics: one sided location, pulsating quality, moderatea or severeb pain intensity, aggravation of routine physical activity; b) photophobia and phonophobia; c) visual aura |
| 4) Not better accounted for by another vestibular or ICHD diagnosis |
| Probable vestibular migraine |
| 1) At least five episodes with vestibular symptoms of moderate or severe intensity, lasting 5 min to 72 h |
| 2) Only one of the criteria B and C for vestibular migraine is fulfilled (migraine history or migraine features during the episode) |
| 3) Not better accounted for by another vestibular or ICHD diagnosis |
aUsually interfere with daily activities. bUsually prohibit daily activities.
Many studies have demonstrated a significant overlap between symptoms of Meniere’s disease (MD) and VM [5]. Both diseases have distinct proposed pathophysiologic mechanisms. In the case of VM, vasospasm of the internal auditory artery was one of the first proposed explanations [6], followed by the implication of the trigemino-vascular system [7]. Also, based on a review of several imaging studies done on VM patients, Espinosa-Sanchez et al [8] hypothesize that VM may be due to a defect in sensory functioning at the levels of the vestibular system, thalamus and cortex. Some of these studies are mentioned subsequently in this article.
On the other hand, MD is a consequence of overaccumulation of endolymph in the inner ear, which occurs at the expense of the perilymphatic space. Inadequate absorption of endolymph by the endolymphatic sac seems to be the mechanism underlying this [9]; however, it remains controversial. Studies have shown that the endolymphatic duct may act as a valve to regulate endolymph equilibrium [10] and that any lesion can produce a faulty duct function, such as chemical exposure, viral infection, inflammation and ischemia [11]. Ischemia has also been proposed as an underlying mechanism for MD [12]. Such a common vascular mechanism could be a link between VM and MD.
MD is diagnosed by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) criteria [13] (Table 3). Diagnosis of the two pathologies is primarily based on clinical criteria. Differentiation of these two diseases is often difficult, so sometimes patients with VM are misdiagnosed as MD patients [14]. Currently, there are no known definitive diagnostic tests that can reliably distinguish the two conditions. Patients with MD and patients with VM are treated differently [15], therefore improving the diagnosis of these two pathologies should avoid errors in management.
Table 3. The 1995 American Academy of Otolaryngology-Head and Neck Surgery Diagnostic Criteria for Meniere’s Disease.
| Certain Meniere’s disease | Definite Meniere’s disease plus histopathologic confirmation |
| Definite Meniere’s disease | Two or more definitive spontaneous episodes of vertigo 20 minutes or longer |
| Audiometrically documented hearing loss on at least one occasion | |
| Tinnitus or aural fullness in the treated ear | |
| Other causes excluded | |
| Probable Meniere’s disease | One definitive episode of vertigo |
| Audiometrically documented hearing loss on at least one occasion | |
| Tinnitus or aural fullness in the treated ear | |
| Other causes excluded | |
| Possible Meniere’s disease | Episodic vertigo of the Meniere’s type without documented hearing loss or sensorineural hearing loss, fluctuating or fixed, with disequilibrium but without definitive episodes |
| Other causes excluded |
The purpose of this study is to review all articles explicitly demonstrating comparisons between VM and MD at the level of genetics, history, physical exam, audiometry, vestibular tests including caloric testing and vestibular evoked myogenic potential (VEMP) as well as endolymphatic sac size in order to clarify and validate the diagnosis of these two diseases. Moreover, we will be comparing articles using the recent VM IHS and Barany Society criteria with the ones using the older criteria to see if these changes have influenced the comparison of these two entities.
Materials and Methods
Literature review
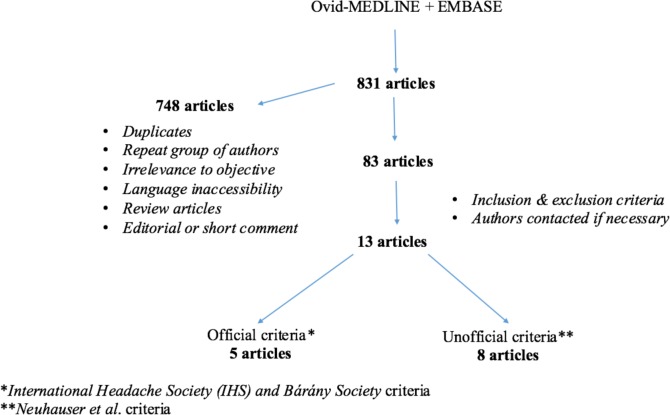
Medline-Ovid and Embase were used to perform a thorough literature review for English-language publications, dating from 1948 to March 2016. Endolymphatic hydrops, Meniere’s disease, vestibular migraine, migrainous vertigo, migraine-associated vertigo, migraine-associated vestibulopathy, migraine-related vertigo, and migraine-related vestibulopathy were the keywords used alone and in various combinations to perform this search. An outline of our review methodology can be seen in Figure 1.
Figure 1.
Study methodology - flow chart.
Inclusion and exclusion criteria
To be included in this paper, reviewed studies must have reported explicit comparisons between the patients diagnosed with MD who met the 1995 AAO-HNS criteria and patients diagnosed with VM based on either Neuhauser’s criteria or the IHS and Barany Society criteria. Articles were then subdivided into subgroups according to which criteria were used for VM diagnosis. All pediatric patients were also excluded.
Data collection
Each study was thoroughly analyzed to extract all available data and assure eligibility for inclusion of every patient. Genetics, MD and VM criteria overlap, age, age of onset, female to male ratio, tinnitus, aural fullness, hearing loss (HL), vomiting, photophobia, phonophobia, visual aura, migraine headaches, headache-related variables, audiometry test scores, caloric tests scores, VEMP scores, endolymphatic sac hydrops and size by electrocochleography (ECOG) or imaging respectively and other vestibular tests were considered in our review.
Results
A total of 13 articles were included in our review. Five of these used the official IHS and Barany Society criteria and eight used the older Neuhauser criteria.
No articles in our review explicitly compared VM and MD on the level of genetics. However, we did find articles describing both entities separately. These will be discussed later in this article.
With regards to findings on history, comparisons between VM and MD were found in five articles [16-20] (Table 4).
Table 4. Comparisons in Historical Findings Between Meniere’s Disease (MD) and Vestibular Migraine (VM).
| Findings | Article | Criteria | MD | VM | P-value |
|---|---|---|---|---|---|
| Sex (female %) | Neff et al [16] | Unofficial | 35 | 83 | < 0.0001 |
| Martin-Sanz et al [20] | Official | Nil | Nil | ND | |
| Age (years) | Hong et al [19] | Unofficial | 49 | 43 | ND |
| Martin-Sanz et al [20] | Official | 46 | 37 | ND | |
| Age of onset (years) | Neff et al [16] | Unofficial | 51 | 41 | 0.0007 |
| Lopez-Escamez et al [18] | Official | 48 | 43 | 0.007 | |
| Evolution | Martin-Sanz et al [20] | Official | Nil | Nil | ND |
| Vertigo Duration (hours) | Neff et al [16] | Unofficial | 47 | 19 | < 0.0001 |
| Illness duration (months) | 12 | 6 | < 0.0001 | ||
| Non-vertiginous dizziness (%) | 50 | 78 | < 0.0001 | ||
| Fluctuating HL (%) | 78 | 14 | < 0.0001 | ||
| Progressive HL (%) | 93 | 22 | < 0.0001 | ||
| HL related to vertigo (%) | 43 | 44 | 0.91 | ||
| Tinnitus (%) | Neff et al [16] | Unofficial | 96 | 55 | < 0.0001 |
| Lopez-Escamez et al [18] | Official | 83 | 46 | < 0.001 | |
| Tinnitus related to vertigo (%) | Neff et al [16] | Unofficial | 59 | 50 | 0.47 |
| Aural Fullness (%) | Neff et al [16] | Unofficial | 78 | 51 | 0.0026 |
| Lopez-Escamez et al [18] | Official | 80 | 34 | < 0.001 | |
| Aural Fullness related to vertigo (%) | Neff et al [16] | Unofficial | 65 | 70 | 0.71 |
| Otalgia (%) | 17 | 27 | 0.09 | ||
| Palpitations (%) | Lopez-Escamez et al [18] | Official | 34 | 50 | 0.008 |
| Anxiety (%) | 78 | 91 | 0.024 | ||
| Headache (%) | Neff et al [16] | Unofficial | 81 | 99 | 0.0026 |
| Lopez-Escamez et al [18] | Official | 41 | 95 | < 0.001 | |
| Frequent headache (daily or weekly) | Neff et al [16] | Unofficial | 19 | 67 | < 0.0001 |
| Headache duration (> day) | 8 | 43 | 0.0012 | ||
| Headache severity (mod/severe) (%) | 26 | 96 | < 0.0001 | ||
| HA age of onset (years) | 23 | 28 | 0.44 | ||
| Migraine-type headache (%) | Lopez-Escamez et al [18] | Official | 8 | 69 | < 0.001 |
| Phonophobia (%) | Neff et al [16] | Unofficial | 63 | 82 | 0.1 |
| Lopez-Escamez et al [18] | Official | 62 | 80 | < 0.001 | |
| Photophobia (%) | Neff et al [16] | Unofficial | 40 | 86 | < 0.0001 |
| Lopez-Escamez et al [18] | Official | 41 | 80 | < 0.001 | |
| Nausea or vomiting with HA (%) | Neff et al [16] | Unofficial | 20 | 72 | < 0.0001 |
| Vomiting (%) | Lopez-Escamez et al [18] | Official | 84 | 69 | 0.002 |
| Headache triggers (%) | Neff et al [16] | Unofficial | 11 | 69 | < 0.0001 |
| Balance symptoms with HA | 31 | 81 | 0.07 | ||
| Frequency of balance symptoms with HA | 29 | 80 | < 0.0001 | ||
| Aura (%) | Neff et al [16] | Unofficial | 22 | 62 | < 0.0001 |
| Lopez-Escamez et al [18] | Official | 11 | 32 | < 0.001 | |
| Family Hx of vertigo or dizziness (%) | Neff et al [16] | Unofficial | 17 | 30 | 0.16 |
| Family Hx of HL (%) | 33 | 25 | 0.74 | ||
| Family Hx of Migraine (%) | 26 | 61 | 0.0017 | ||
| History of motion sickness (%) | Neff et al [16] | Unofficial | 20 | 51 | 0.0023 |
| MSQ score to riding in a car | Sharon et al [17] | Unofficial | 0.5 | 1.07 | 0.048 |
Nil: data not mentioned; ND: no difference noted but no P-value given; MSQ: motion sensitivity questionnaire; HL: hearing loss; HA: headache; Mod: moderate; Hx: history. Official: International Headache Society and Barany Society criteria (2012); Unofficial: Neuhauser criteria (2001).
Comparisons between MD and VM in physical exam findings were found in two articles [16, 21]. Two articles [16, 20] showed comparisons in auditory tests results. Endolymphatic hydrops was mentioned in two other articles [20, 22]. The details of these results are shown in detail in Table 5 [16, 20-22].
Table 5. Comparisons in Physical Exam Findings, Auditory Tests Results and Endolymphatic Hydrops Between MD and VM.
| Findings (unit) | Article | Criteria | MD | VM | P-value |
|---|---|---|---|---|---|
| Abnormal head-shaking nystagmus (%) | Neff et al [16] | Unofficial | 62 | 15 | < 0.0001 |
| Shin et al [21] | 71 | 50 | < 0.05 | ||
| Abnormal head-thrust (%) | Neff et al [16] | 37 | 3 | < 0.0001 | |
| Abnormal vibration induced nystagmus (%) | Neff et al [16] | 60 | 12 | < 0.0001 | |
| Shin et al [21] | 42 | 32 | < 0.05 | ||
| Abnormal smooth pursuit (%) | Neff et al [16] | 5 | 8 | 0.09 | |
| Abnormal saccades (%) | 5 | 0 | 0.46 | ||
| Initial PTA ≥ 25 dB (%) | Neff et al [16] | Unofficial | 83 | 7 | 0.0011 |
| Worst PTA ≥ 25 dB (%) | 100 | 9 | < 0.0001 | ||
| Initial discrimination ≥ 25 dB (%) | 68 | 2 | < 0.0001 | ||
| Change in discrimination (%/month) | 86 | 2 | < 0.0001 | ||
| Initial hearing class B-D (%) | 71 | 5 | < 0.0001 | ||
| Worst hearing class B-D (%) | 95 | 6 | < 0.0001 | ||
| Low tone hearing loss pattern (%) | 40 | 0 | < 0.0001 | ||
| PTA for dMD vs. dVM (dB) | Martin-Sanz et al [20] | Official | Nil | Nil | > 0.05 |
| PTA for pMD vs. pVM (dB) | 35 | 16 | D | ||
| Significant vestibular ELH (%) | Nakada et al [22] | Official | 79 | 14 | < 0.01 |
| Significant Cochlear ELH (%) | 30 | 0 | < 0.05 | ||
| ELH on ECOG (%) (SP/AP amplitude ratio) | Martin-Sanz et al [20] | 82 | 24 | < 0.05 |
MD: Meniere’s disease; VM: vestibular Migraine; dMD: definite Meniere’s disease; dVM: definite vestibular migraine; pMD: probable Meniere’s disease; pVM: probable vestibular migraine; PTA: pure tone average; ELH: endolymphatic hydrops; ECOG: electrocochleography; dB: decibels; Nil: data not mentioned; D: difference noted but no P-value given; official: International Headache Society and Barany Society criteria (2012); unofficial: Neuhauser criteria (2001). Hearing class according to AAO-HNS hearing preservation reporting guidelines (view Table 7). SP: summating potential; AP: action potential.
When analyzing vestibular testing, seven articles mentioned comparisons between MD and VM with regards to caloric testing [16, 17, 19-21, 23, 24]. Three articles described comparisons in VEMP [16, 24-27] and two of these also described rotary chair comparisons [16, 24]. Finally, two other articles [23, 28] mentioned variations in video head impulse testing (vHIT) between MD and VM. Data of these results are shown in detail in Table 6 [16, 17, 19, 20, 23-28].
Table 6. Comparisons in Vestibular Tests Results Between MD and VM.
| Findings | Article | Criteria | MD | VM | P-value |
|---|---|---|---|---|---|
| Mean caloric asymmetry (%) | Neff et al [16] | Unofficial | 33 | 13 | < 0.0001 |
| Blodow et al [23] | Official | 38 | 16 | 0.005 | |
| Sharon et al [17] | Unofficial | 40 | 24 | 0.0007 | |
| Abnormal caloric asymmetry (%) | Neff et al [16] | Unofficial | 63 | 17 | < 0.0001 |
| Blodow et al [23] | Official | 67 | 22 | 0.002 | |
| Hong et al [19] | Unofficial | 48 | 23 | < 0.05 | |
| Shin et al [21] | Unofficial | 47 | 25 | < 0.05 | |
| Taylor et al [24] | Unofficial | OR = 26.36 for MD vs. VM | < 0.001 | ||
| Sensitivity = 74.5% | |||||
| Martin-Sanz et al [20] | Official | Nil | Nil | > 0.05 | |
| Mean directional preponderance (%) | Neff et al [16] | Unofficial | 19 | 13 | 0.09 |
| Abnormal directional preponderance (%) | 29 | 15 | 0.15 | ||
| Abnormal rotary chair phase (%) | Neff et al [16] | Unofficial | 68 | 18 | < 0.0001 |
| Rotary chair gain towards affected ear (Hz) | Neff et al [16] | Unofficial | 25 | 36 | < 0.0001 |
| Taylor et al [24] | Unofficial | 16 | 25 | 0.014 | |
| Rotary chair gain towards unaffected ear (Hz) | Taylor et al [24] | Unofficial | 18 | 23 | 0.165 |
| Abnormal rotary chair TC towards affected ear (Hz) | 16 | 25 | 0.009 | ||
| Abnormal rotary chair TC towards un affected ear (Hz) | 17 | 24 | 0.033 | ||
| Abnormal rotary chair symmetry (%) | Neff et al [16] | Unofficial | 35 | 29 | 0.02 |
| Abnormal vHIT hVOR gain (%) | Blodow et al [23] | Official | 37 | 9 | 0.025 |
| Presence of vHIT CAQEM (%) | Heuberger et al [28] | Official | Nil | Nil | 0.01 |
| Abnormal VEMP (%) | Neff et al [16] | Unofficial | 45 | 16 | 0.0068 |
| cVEMP 250 Hz TB amplitude asymmetry ratios (%) | Taylor et al [24] | Unofficial | 40 | 5 | < 0.001 - 0.024 |
| cVEMP 500 Hz TB amplitude asymmetry ratios (%) | 60 | 10 | < 0.001 - 0.024 | ||
| cVEMP 1 kHz TB amplitude asymmetry ratios (%) | 40 | 15 | < 0.001 - 0.024 | ||
| cVEMP 2 kHz TB amplitude asymmetry ratios (%) | 30 | 15 | < 0.001 - 0.024 | ||
| cVEMP 500 Hz/1kHz TB amplitude ratio | 0.89 | 1.11 | 0.007 | ||
| cVEMP 500 Hz TB detection rate (%) | Murofushi et al [25] | Unofficial | 63 | 100 | 0.0003 |
| cVEMP mean corrected amplitudes | 0.59 | 1.49 | 0.001 | ||
| cVEMP click amplitudes (µV) | Zuniga et al [26] | Unofficial | Nil | Nil | ND |
| cVEMP click peak-to-peak amplitudes (µV) | 29 | 38 | 0.625 | ||
| cVEMP click latencies (ms) | Nil | Nil | ND | ||
| cVEMP click amplitudes for right side | Baier et al [27] | Unofficial | Nil | Nil | 0.22 |
| cVEMP click amplitudes for left side (ms) | Nil | Nil | 0.744 | ||
| cVEMP click latencies p13 right side (ms) | 16 | 16 | > 0.01 | ||
| cVEMP click latencies p13 left side (ms) | 16 | 16 | > 0.01 | ||
| cVEMP click latencies n23 right side (ms) | 25 | 25 | > 0.01 | ||
| cVEMP click latencies n23 left side (ms) | 26 | 25 | > 0.01 | ||
| oVEMP click reflex latencies (ms) | Zuniga et al [26] | Unofficial | 11.1 | 9.8 | 0.028 |
| oVEMP 500 Hz TB reflex latencies (ms) | 11.1 | 10.4 | 0.041 | ||
| oVEMP 500 Hz TB reflex amplitudes | 0.98 | 3.4 | 0.007 | ||
| oVEMP reflex hammer midline tap amplitudes (mV) | 4.6 | 5.45 | 0.21 | ||
| oVEMP mini-shaker tap amplitudes (mV) | 3.9 | 5 | 0.217 | ||
| oVEMP reflex hammer midline tap latencies (ms) | 8 | 7.5 | 0.879 | ||
| oVEMP reflex mini-shaker tap latencies (ms) | 9.8 | 9.8 | 0.597 | ||
Hz: Hertz; TB: tone burst; vHIT: video head impulse test; hVOR: horizontal vestibulo-ocular reflex; CAQEM: covert anti-compensatory quick eye movements; nil: data not mentioned; official: International Headache Society and Barany Society criteria (2012); unofficial: Neuhauser criteria (2001). oVEMP: ocular vestibular evoked myogenic potential; cVEMP: cervical vestibular evoked myogenic potential; µV: microvolt; ms: millisecond; mV: millivolt; ND: no difference noted but no P-value given.
Discussion
Genetics
Three families experiencing migraine and vertigo were first reported by Baloh et al [29]. These families also experienced loss of peripheral vestibular function a year later. In VM, studies have shown a familial occurrence with an autosomal dominant pattern with moderate to high penetrance [30, 31]. However, Sanger sequencing [31] and other methods [32] have been used to try and identify a genomic region responsible for this inheritance pattern. These measures were unsuccessful. This indicates the possibility of a polygenic inheritance in VM patients. Nevertheless, a susceptibility allele with female penetrance may exist since an 11q region was identified in most affected females. Bahmad et al [33] also demonstrated an autosomal dominant inheritance pattern when studying 10 family members suffering from VM. A location in chromosome 5q35 between rs244895 and D5S2073 markers was discovered when using genome-wide linkage analysis and subsequent fine mapping. In another study, a genome-wide scan identified, on chromosome 6q at marker D6S1556, a suggestive linkage in four families suffering from migraine and vestibular pathology [34].
As for MD, studies have shown familial cases going to as high as 20% [35, 36], with an autosomal dominant inheritance pattern with incomplete penetrance [35-39]. Many families with earlier onset disease and severe symptom tendencies have been described in successive generations [35, 37, 39-42]. This suggests anticipation in familial MD. Numerous families with simultaneous migraine and MD have also been described [43]. A study by Morrison et al [44] showed that HLA-C and HLA-A on chromosome 6 were likely loci in sporadic and familial cases of MD. Furthermore, linkage analysis identified a candidate region on chromosome 12p12.3 in three Swedish families [39]. Another study also identified an allelic association on chromosome 12p12.3 between D12S373 and GT27 markers [42]. Another study on 19 families suffering from MD with a 39% prevalence of migraine demonstrated linkage on chromosome 5, and 13 of these families had linkage to the D5S644 region [35]. The third and fourth generations of patients suffering from migraines actually presented with earlier onset of MD.
In summary, both diseases seem to have a predominantly autosomal dominant type of hereditary pattern. However, VM would be of moderate to high penetrance whereas MD would be of incomplete penetrance. Also, chromosomes 5 and 6 seem to be implicated in both diseases. Given its high prevalence, VM seems to be more of a polygenic disease, similarly to MD [30, 31].
History
Two studies [16, 18] in our review had substantial data on comparative symptomatology between MD and VM. Of note, the article by Neff et al [16] was based on an updated version of the 2001 Neuhauser criteria and the article by Lopez-Escamez et al [18] was based on the recent IHS and Barany society criteria. In general, these two studies showed similar results. When compared with MD, VM showed statistically significant lower age of onset, less tinnitus, aural fullness and HL, more vomiting, headaches, aura and photophobia. Few contradicting conclusions were noted when comparing both articles.
Neff et al [16] and Ghavami et al [45] were the only two authors to mention an overlap between the criteria of both diagnoses. In Ghavami et al, 15 of 37 patients with definite MD fulfilled the new criteria for VM (41% of all patients and 79% of those with migraine according to the IHS criteria). Neff et al [16] presented 55 patients with MD and 71 patients with VM with an overlap of 21 patients meeting both criteria (38% of MD patients fulfilled VM criteria).
However, Neff et al [16] compared both population with a third group composed of patients qualifying for both VD and MD criteria (MDVM). No single clinical feature was able to adequately separate MD, VM and MDVM. In the paper by Lopez-Escamez et al [18], patients fulfilling the criteria for both MD and VM were excluded from the study. Also, Neff et al made no distinction between definite VM and probable VM, unlike the article by Lopez-Escamez et al.
Also, Hong et al [19] found no difference in the age of the patients between VM and MD but does not mention age of onset. Martin-Sanz et al [20] mention no difference in sex when comparing VM and MD, which comes into conflict with the difference noted by Neff et al [16]. Firstly, both these studies use different diagnostic criteria to diagnose VM. Secondly, in the study by Martin-Sanz et al [20], they mention a change in diagnosis of 18.8% of patients. Sex data previous to the diagnosis change are the only ones mentioned and no mention is made about the data after the diagnosis change. However, it is important to note that no sex predominated when MD and VM patients in Neff et al [16] were compared with the MDVM group.
Sharon et al [17] were the only article found in the literature comparing motion sensitivity between MD and VM which showed a significantly higher prevalence of motion sensitivity to riding in a car in the VM population. A possible explanation for the susceptibility to motion sickness described in migraine and VM patients could be a result of an overly sensitized thalamus. Espinosa-Sanchez et al [8] have hypothesized that thalamic ventral posterolateral nucleus and ventral posteromedial nucleus sensitization may be caused by migraines. These would therefore lead to an enhancement of vestibular and other sensory perceptions. This conclusion is based on animal model studies with electrophysiological and neuroanatomical analysis as well as functional neuroimaging techniques in humans [17, 46].
Reversible vasospasm of the internal auditory artery was the first proposed explanation for migraine-associated vestibular symptoms [6]. This would consequently explain the sudden onset of auditory and vestibular symptoms in such patients. This mechanism establishes a possible pathophysiological link between VM and MD.
Another possible explanation may be local extravasation of basilar and anterior inferior cerebellar artery plasma which would consequently lead to excitation of the trigeminal nerve [7]. Supporting this theory, studies have shown that vasoactive neuropeptides are present in the inner ear and vestibular sensory fibers of the trigeminal nerve [47, 48].
Physical examination
Two articles [16, 21] in our review, based on the same VM diagnostic criteria, mention an explicit comparison between MD and VM with regards to physical examination. They agree that abnormal headshake nystagmus (HSN) and abnormal vibration-induced nystagmus (VIN) are more frequent in the MD population. It is important to note that in Neff et al [16], no single physical exam was able to adequately separate MD and VM from MDVM. In Shin et al [21] overlap between the two diseases was not mentioned.
It has been shown that peripheral spontaneous nystagmus may be caused or enhanced by painful stimulation of the trigeminal nerve [49]. However, HSN and VIN were not evaluated in this study. Regardless, this provides a possible link between migraine and peripheral nystagmus.
Other possible etiologies of peripheral-type nystagmus are vasospasm of the internal auditory artery [6] and aberrant canal-otolith integration [50]. The latter would be the source of a heightened sensitivity to head motions and would be due to a processing error in the caudal cerebellar vermis [51]. Peripheral nystagmus in patients with VM may in fact be caused by this precise dysfunction.
Unfortunately, this does not explain the presence of vertical nystagmus in some cases of VM [52]. It is however known that infraction of the labyrinth is often associated with brainstem or cerebellar infaction [53], which would explain the presence of central nystagmus in VM patients.
Furthermore, a meta-analysis has indicated that migraine is probably an independent risk factor of stroke [54].
Audiometry
Only two articles [16, 20] in our review compared audiometric results between VM and MD. HL in general is not viewed as a hallmark of the presentation of VM patients, unlike MD patients whose diagnosis depends on it. It is not surprising to see that in Neff et al [16] all audiometry results identified worst results in MD patients. The American Academy of Otolaryngology-Head and Neck Surgery reported in 1995 the hearing preservation guidelines (Table 7).
Table 7. 1995 American Academy of Otolaryngology-Head and Neck Surgery Hearing Preservation Reporting Guidelines.
| Class | Pure-tone thresholds | Speech discrimination (%) |
|---|---|---|
| A | ≤ 30 dB | ≥ 70 |
| B | > 30 dB, ≤ 50 dB | ≥ 50 |
| C | > 50 dB | ≥ 50 |
| D | Any level | < 50 |
Martin-Sanz et al [20] showed that results were only significantly different when comparing probable MD group with probable VM. Reasons for this once again might be caused by the use of different diagnostic criteria used for VM or secondary to the 18.8% changes in diagnosis between VM and MD. Also, Neff et al did not show results of VM and MD patients separated in their different categories, e.g. (definite vs. probable). It is important to also note that in Neff et al no audiometric results were able to adequately separate MD and VM from MDVM.
Elevation of auditory nerve action potential and reduction of cochlear otoacoustic emissions have been shown to be caused by capsaicin [55]. This provides a link between trigeminal nerve stimulation and cochlear dysfunction in patients suffering from VM. In addition, a reduction of labelled nerve cell bodies in the anteromedial portion of the trigeminal ganglion of hydropic guinea pigs has also been demonstrated [56]. These two studies indicate that the trigeminal innervation to the cochlea could be involved in inner ear homeostatic disturbances, linking migraine with hearing problems and MD.
Endolymphatic hydrops
Two studies [20, 22] compared endolymphatic hydrops (ELH) with different methods (MRI or electrocochleography) between VM and MD patients, and both agreed that ELH was more frequent in MD patients. However, in Martin-Sanz et al [20], sub-analysis was not made to distinguish vestibular vs. cochlear ELH. Also Gurkov et al [57] showed ELH in four of 19 patients with VM; however, three of those also fulfilled criteria for definite MD and one patient for probable MD. These patients could be a part of the MDVM group.
Other imaging modalities have been used to study the brains of patients suffering from migraines. Dorsal brainstem activation during migraine attacks and vestibulo-thalamo-cortical pathway activation in VM patients have been demonstrated on position-emission tomography [58-60].
Moreover, an increase in mediodorsal thalamic activation following ipsilateral caloric stimulation was shown in VM patients using blood-oxygen-level dependent functional magnetic resonance imaging (BOLD-fMRI). Also, the degree of activation correlated with the frequency of migraine attacks in VM patients [61].
Finally, using MRI-based voxel-based morphometry, VM patients showed a decrease in gray matter volume in the temporal, supramarginal and inferior occipital gyri, the cingulate and dorsolateral prefrontal cortices, posterior insula and superior parietal lobules [62]. This corroborates the theory of aberrant cortical processing of nociceptive and vestibular processing.
Therefore, enhanced vestibular organ perception and its interactions with the brainstem, thalamus and cortex can explain the link between migraine and some vestibular disorders, including VM [63].
None of the aforementioned imaging modalities have been used to analyze the brain of MD patients. It would be interesting to see if these are potential investigating tools to help in differentiating MD from VM.
Caloric testing
Six of the seven articles [16, 17, 19, 21, 23, 24] in our review describing caloric testing found more abnormal test results among the MD patients. Five of these [16, 17, 19, 21, 24] based their VM diagnosis on old criteria and two of these based it on the recent criteria.
However, in Neff et al [16], no single caloric test was able to adequately separate MD and VM from MDVM. All other studies did not consider a group composed of patients fulfilling both MD and VM criteria.
However, Martin-Sanz et al [20] did not observe any significant differences (P > 0.05) in the proportion of normal caloric test between the VM and MD populations. Here, only a change in initial diagnosis could explain this discordance since an article with the same VM criteria [23] found significant differences between MD and VM.
VEMP
Five articles [16, 24-27] in our review compared MD and VM on the basis of VEMP results. All reviewed articles in this review were based on the old criteria.
Regarding cervical vestibular evoked myogenic potential (cVEMP), two studies [26, 27] found no difference in latencies or amplitudes between MD and VM patients. However, Taylor et al [24] showed that cVEMP asymmetry ratios for 500 Hz tone bursts were significantly higher for MD than VM. Also they show that the ratio of cVEMP amplitude generated by tone bursts at a frequency of 500 Hz to that generated by 1 kHz was significantly lower for MD affected ears than for VM or controls ears. In concordance, Murofushi et al [25] showed significantly smaller cVEMP amplitudes to 500 Hz tone busts on the affected side of MD.
Taylor et al [24] mentioned these differences in observations between their study and Baier et al [27], and their explanation was a narrower normal range resulting in a higher proportion of abnormal results in Baier et al [27] as well as the use of different stimuli (clicks vs. tone busts). The different types of stimuli could also explain the different results found in Murofushi et al [25] and Zuniga et al [26]. Interestingly, Taylor et al [24] who showed that the 500 Hz/1 kHz frequency ratio, 500 Hz asymmetry ratio and caloric test combined, separated MD from VM with a sensitivity of 90.0% and specificity of 70.0%.
Regarding ocular vestibular evoked myogenic potentials (oVEMPs), Zuniga et al [26] showed longer latencies and lower amplitudes in MD patients with clicks. Taylor corroborated these findings by demonstrating significantly higher air conduction click-oVEMP abnormalities in MD patients. Stimuli in both these studies were comparable.
Whereas Neff et al [16] described a significantly smaller amount of abnormal VEMP (VM 16%; MD 45%; P = 0.0068); however, it was not specified if these VEMPs were oVEMPs or cVEMP. In Neff et al [16], no VEMP results were able to adequately separate MD and VM from MDVM.
No other study in our review tried to differentiate MD and VM from MDVM group.
Other vestibular tests
Two articles [16, 24] mentioned rotary chair results. Both agreed that rotary chair gain was significantly smaller in MD than VM. However, Neff et al [16] did not mention in the article if rotation was toward the affected or unaffected ear and no rotary chair results were able to adequately separate MD and VM from MDVM.
Blodow et al [23] were the only study that showed significantly more abnormal horizontal vestibulo-ocular reflex gain in vHIT in MD patients compared to VM. Heuberger et al [28] were the only study to show that covert anti-compensatory quick eye movements (CAQEMs) during vHIT could be used to differentiate VM from MD with a sensitivity of 46%, a specificity of 81% and an accuracy of 65%. He also showed that with a combination of vestibulo-ocular reflex gain and CAQEM analysis, VM could be differentiated from MD with a specificity of 100% and an accuracy of 60% (P = 0.02).
Old vs. new VM criteria
A total of five articles in our review used the IHS criteria and eight articles used the older criteria based on the 2001 Neuhauser criteria. Differences between criteria were noted at the level of history, audiometry and caloric testing. All differences noted between articles with different criteria implicated the article by Martin-Sanz et al [20]. As we mentioned above, reasons for the differences could very well be due to the difference in criteria; however, since this article is the only aberrant one, its methodology could also explain these differences. For example, a change in diagnosis of 18.8% patients could be the explanation behind all of these different results.
In summary, even though different criteria seem to tend towards similar results when comparing MD and VM, we suggest that the new IHS criteria and the new amended MD criteria announced by the 2015 Equilibrium Committee [64] be used in future studies (Table 8) and to create a third category which is MDVM.
Table 8. Amended 2015 Criteria for Diagnosis of MD by the European Academy of Otology and Neurotology.
| Definite | Two or more spontaneous episodes of vertigo, each lasting 20 min to 12h |
| Audiometrically documented low to midfrequency sensorineural hearing loss in one ear, defining the ear on one occasion before, during or after one episode of vertigo | |
| Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the affected ear | |
| Not better accounted for by another vestibular diagnosis | |
| Probable | Two or more episodes of vertigo or dizziness, each lasting 20 min to 24 h |
| Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the affected ear | |
| Not better accounted for by another vestibular diagnosis |
Limitations and Literature Critique
When this study was initially designed, the goal was to create a meta-analysis of all data on MD and VM to then compare the results of all patients suffering from either or both diseases. Unfortunately, because of insufficient data currently available on VM compared to the extensive data on MD, comparisons between both entities in this way would not have been possible. Also, given the different criteria for VM, subgroups for each criteria used would have had to be created, making the VM populations even smaller. A review of studies explicitly comparing both groups seemed to be the best second option and also limited the bias of pooling different populations from different centers into the same group.
Very few articles distinguished and compared patients with overlapping criteria for MD and VM with those not overlapping. In fact, some articles excluded the overlap group while others did not consider them. In Neff et al [16], it is well demonstrated that none of the signs, symptoms or investigations were able to distinguish MD and VM from MDVM. This pushes us to encourage future articles using the most recent criteria by the IHS and Barany Society to study and compare the overlapping group with the non-overlapping groups and consequently help to further the validation of the diagnostic criteria for both diseases.
We also noted that not all articles made the distinction between the “definite” and “probable” subgroups for MD and VM, as well as the “possible” subgroup for MD in their analysis of differences between patients. Also, none of the articles included in this review apply the new amended criteria for MD [64].
Conclusions
In this review, many differences were identified between MD and VM, some statistically significant. Before the introduction of the new IHS and Barany Society criteria, overlaps in all of these fields were identified in Neff et al [16] between MD and VM and MDVM patients; however, none could confidently differentiate MD and VM from MDVM. Since the introduction of the most recent criteria, no studies have focused on assessing the differences between these three groups. Consequently, it is difficult to say if the current diagnostic criteria reduce ambiguity between these two clinical entities. In order to answer these numerous questions, we strongly encourage authors to focus on comparing MD and VM from MDVM in future studies using the recent IHS criteria for VM.
Financial Support
None.
Funding
No funding was received for this work from any of the following organizations: NIH, Welcome Trust, HHMI or other.
Conflicts of Interest
None.
Abbreviations
- VM
vestibular migraine
- HIS
International Headache Society
- MD
Meniere’s disease
- AAO-HNS
American Academy of Otolaryngology-Head and Neck Surgery
- VEMP
vestibular evoked myogenic potential
- HL
hearing loss
- vHIT
video head impulse testing
- MDVM
Meniere’s disease and vestibular migraine
- HSN
headshake nystagmus
- VIN
vibration-induced nystagmus
- ELH
endolymphatic hydrops
- CAQEM
covert anti-compensatory quick eye movement
References
- 1.Kuritzky A, Toglia UJ, Thomas D. Vestibular function in migraine. Headache. 1981;21(3):110–112. doi: 10.1111/j.1526-4610.1981.hed2103110.x. [DOI] [PubMed] [Google Scholar]
- 2.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56(4):436–441. doi: 10.1212/WNL.56.4.436. [DOI] [PubMed] [Google Scholar]
- 3.Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, Bisdorff A. et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 4.Salmito MC, Morganti LO, Nakao BH, Simoes JC, Duarte JA, Gananca FF. Vestibular migraine: comparative analysis between diagnostic criteria. Braz J Otorhinolaryngol. 2015;81(5):485–490. doi: 10.1016/j.bjorl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepard NT. Differentiation of Meniere's disease and migraine-associated dizziness: a review. J Am Acad Audiol. 2006;17(1):69–80. doi: 10.3766/jaaa.17.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Baloh RW. Neurotology of migraine. Headache. 1997;37(10):615–621. doi: 10.1046/j.1526-4610.1997.3710615.x. [DOI] [PubMed] [Google Scholar]
- 7.Vass Z, Steyger PS, Hordichok AJ, Trune DR, Jancso G, Nuttall AL. Capsaicin stimulation of the cochlea and electric stimulation of the trigeminal ganglion mediate vascular permeability in cochlear and vertebro-basilar arteries: a potential cause of inner ear dysfunction in headache. Neuroscience. 2001;103(1):189–201. doi: 10.1016/S0306-4522(00)00521-2. [DOI] [PubMed] [Google Scholar]
- 8.Espinosa-Sanchez JM, Lopez-Escamez JA. New insights into pathophysiology of vestibular migraine. Front Neurol. 2015;6:12. doi: 10.3389/fneur.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paparella MM, Djalilian HR. Etiology, pathophysiology of symptoms, and pathogenesis of Meniere's disease. Otolaryngol Clin North Am. 2002;35(3):529–545, vi. doi: 10.1016/S0030-6665(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 10.Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191(1-2):90–100. doi: 10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Kimura RS. Animal models of inner ear vascular disturbances. Am J Otolaryngol. 1986;7(2):130–139. doi: 10.1016/S0196-0709(86)80042-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS, Kimura RS. Ischemia of the endolymphatic sac. Acta Otolaryngol. 1992;112(4):658–666. doi: 10.3109/00016489209137456. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere’s Disease. Otolaryngol Head Neck Surg. 1995;113(3):181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 14.Cha YH, Brodsky J, Ishiyama G, Sabatti C, Baloh RW. The relevance of migraine in patients with Meniere's disease. Acta Otolaryngol. 2007;127(12):1241–1245. doi: 10.1080/00016480701242469. [DOI] [PubMed] [Google Scholar]
- 15.Huppert D, Strupp M, Muckter H, Brandt T. Which medication do I need to manage dizzy patients? Acta Otolaryngol. 2011;131(3):228–241. doi: 10.3109/00016489.2010.531052. [DOI] [PubMed] [Google Scholar]
- 16.Neff BA, Staab JP, Eggers SD, Carlson ML, Schmitt WR, Van Abel KM, Worthington DK. et al. Auditory and vestibular symptoms and chronic subjective dizziness in patients with Meniere's disease, vestibular migraine, and Meniere's disease with concomitant vestibular migraine. Otol Neurotol. 2012;33(7):1235–1244. doi: 10.1097/MAO.0b013e31825d644a. [DOI] [PubMed] [Google Scholar]
- 17.Sharon JD, Hullar TE. Motion sensitivity and caloric responsiveness in vestibular migraine and Meniere's disease. Laryngoscope. 2014;124(4):969–973. doi: 10.1002/lary.24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, Lempert T, Teggi R, von Brevern M, Bisdorff A. Accompanying Symptoms Overlap during Attacks in Meniere's Disease and Vestibular Migraine. Front Neurol. 2014;5:265. doi: 10.3389/fneur.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong HR, Shim DB, Kim TS, Shim BS, Ahn JH, Chung JW, Yoon TH. et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere's disease and vestibular neuritis. Acta Otolaryngol. 2013;133(12):1236–1241. doi: 10.3109/00016489.2013.820343. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Sanz E, Vargas Salamanca E, Marques Cabrero A, Esteban J, Muerte I, Sanz-Fernandez R. Value of clinical data and vestibular testing in a population of 101 patients with recurrent vestibulopathy. Clin Otolaryngol. 2014;39(5):311–315. doi: 10.1111/coa.12287. [DOI] [PubMed] [Google Scholar]
- 21.Shin JE, Kim CH, Park HJ. Vestibular abnormality in patients with Meniere's disease and migrainous vertigo. Acta Otolaryngol. 2013;133(2):154–158. doi: 10.3109/00016489.2012.727469. [DOI] [PubMed] [Google Scholar]
- 22.Nakada T, Yoshida T, Suga K, Kato M, Otake H, Kato K, Teranishi M. et al. Endolymphatic space size in patients with vestibular migraine and Meniere's disease. J Neurol. 2014;261(11):2079–2084. doi: 10.1007/s00415-014-7458-9. [DOI] [PubMed] [Google Scholar]
- 23.Blodow A, Heinze M, Bloching MB, von Brevern M, Radtke A, Lempert T. Caloric stimulation and video-head impulse testing in Meniere's disease and vestibular migraine. Acta Otolaryngol. 2014;134(12):1239–1244. doi: 10.3109/00016489.2014.939300. [DOI] [PubMed] [Google Scholar]
- 24.Taylor RL, Zagami AS, Gibson WP, Black DA, Watson SR, Halmagyi MG, Welgampola MS. Vestibular evoked myogenic potentials to sound and vibration: characteristics in vestibular migraine that enable separation from Meniere's disease. Cephalalgia. 2012;32(3):213–225. doi: 10.1177/0333102411434166. [DOI] [PubMed] [Google Scholar]
- 25.Murofushi T, Ozeki H, Inoue A, Sakata A. Does migraine-associated vertigo share a common pathophysiology with Meniere's disease? Study with vestibular-evoked myogenic potential. Cephalalgia. 2009;29(12):1259–1266. doi: 10.1111/j.1468-2982.2009.01860.x. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga MG, Janky KL, Schubert MC, Carey JP. Can vestibular-evoked myogenic potentials help differentiate Meniere disease from vestibular migraine? Otolaryngol Head Neck Surg. 2012;146(5):788–796. doi: 10.1177/0194599811434073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baier B, Dieterich M. Vestibular-evoked myogenic potentials in "vestibular migraine" and Meniere's disease: a sign of an electrophysiological link? Ann N Y Acad Sci. 2009;1164:324–327. doi: 10.1111/j.1749-6632.2009.03868.x. [DOI] [PubMed] [Google Scholar]
- 28.Heuberger M, Saglam M, Todd NS, Jahn K, Schneider E, Lehnen N. Covert anti-compensatory quick eye movements during head impulses. PLoS One. 2014;9(4):e93086. doi: 10.1371/journal.pone.0093086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baloh RW, Jacobson K, Fife T. Familial vestibulopathy: a new dominantly inherited syndrome. Neurology. 1994;44(1):20–25. doi: 10.1212/WNL.44.1.20. [DOI] [PubMed] [Google Scholar]
- 30.Kim JS, Yue Q, Jen JC, Nelson SF, Baloh RW. Familial migraine with vertigo: no mutations found in CACNA1A. Am J Med Genet. 1998;79(2):148–151. doi: 10.1002/(SICI)1096-8628(19980901)79:2<148::AID-AJMG11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.von Brevern M, Ta N, Shankar A, Wiste A, Siegel A, Radtke A, Sander T. et al. Migrainous vertigo: mutation analysis of the candidate genes CACNA1A, ATP1A2, SCN1A, and CACNB4. Headache. 2006;46(7):1136–1141. doi: 10.1111/j.1526-4610.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Jen JC, Cha YH, Nelson SF, Baloh RW. Phenotypic and genetic analysis of a large family with migraine-associated vertigo. Headache. 2008;48(10):1460–1467. doi: 10.1111/j.1526-4610.2007.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahmad F Jr, DePalma SR, Merchant SN, Bezerra RL, Oliveira CA, Seidman CE, Seidman JG. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol. 2009;118(9):670–676. doi: 10.1177/000348940911800912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jen JC, Wang H, Lee H, Sabatti C, Trent R, Hannigan I, Brantberg K. et al. Suggestive linkage to chromosome 6q in families with bilateral vestibulopathy. Neurology. 2004;63(12):2376–2379. doi: 10.1212/01.WNL.0000149498.79541.49. [DOI] [PubMed] [Google Scholar]
- 35.Arweiler-Harbeck D, Horsthemke B, Jahnke K, Hennies HC. Genetic aspects of familial Meniere's disease. Otol Neurotol. 2011;32(4):695–700. doi: 10.1097/MAO.0b013e318216074a. [DOI] [PubMed] [Google Scholar]
- 36.Birgerson L, Gustavson KH, Stahle J. Familial Meniere's disease: a genetic investigation. Am J Otol. 1987;8(4):323–326. [PubMed] [Google Scholar]
- 37.Morrison AW, Bailey ME, Morrison GA. Familial Meniere's disease: clinical and genetic aspects. J Laryngol Otol. 2009;123(1):29–37. doi: 10.1017/S0022215108002788. [DOI] [PubMed] [Google Scholar]
- 38.Hietikko E, Kotimaki J, Kentala E, Klockars T, Sorri M, Mannikko M. Finnish familial Meniere disease is not linked to chromosome 12p12.3, and anticipation and cosegregation with migraine are not common findings. Genet Med. 2011;13(5):415–420. doi: 10.1097/GIM.0b013e3182091a41. [DOI] [PubMed] [Google Scholar]
- 39.Klar J, Frykholm C, Friberg U, Dahl N. A Meniere's disease gene linked to chromosome 12p12.3. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(5):463–467. doi: 10.1002/ajmg.b.30347. [DOI] [PubMed] [Google Scholar]
- 40.Frykholm C, Larsen HC, Dahl N, Klar J, Rask-Andersen H, Friberg U. Familial Meniere's disease in five generations. Otol Neurotol. 2006;27(5):681–686. doi: 10.1097/01.mao.0000226315.27811.c8. [DOI] [PubMed] [Google Scholar]
- 41.Fung K, Xie Y, Hall SF, Lillicrap DP, Taylor SA. Genetic basis of familial Meniere's disease. J Otolaryngol. 2002;31(1):1–4. doi: 10.2310/7070.2002.19261. [DOI] [PubMed] [Google Scholar]
- 42.Gabrikova D, Frykholm C, Friberg U, Lahsaee S, Entesarian M, Dahl N, Klar J. Familiar Meniere's disease restricted to 1.48 Mb on chromosome 12p12.3 by allelic and haplotype association. J Hum Genet. 2010;55(12):834–837. doi: 10.1038/jhg.2010.122. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira CA, Ferrari I, Messias CI. Occurrence of familial Meniere's syndrome and migraine in Brasilia. Ann Otol Rhinol Laryngol. 2002;111(3 Pt 1):229–236. doi: 10.1177/000348940211100307. [DOI] [PubMed] [Google Scholar]
- 44.Morrison AW, Mowbray JF, Williamson R, Sheeka S, Sodha N, Koskinen N. On genetic and environmental factors in Meniere's disease. Am J Otol. 1994;15(1):35–39. [PubMed] [Google Scholar]
- 45.Ghavami Y, Mahboubi H, Yau AY, Maducdoc M, Djalilian HR. Migraine features in patients with Meniere's disease. Laryngoscope. 2016;126(1):163–168. doi: 10.1002/lary.25344. [DOI] [PubMed] [Google Scholar]
- 46.Murdin L, Chamberlain F, Cheema S, Arshad Q, Gresty MA, Golding JF, Bronstein A. Motion sickness in migraine and vestibular disorders. J Neurol Neurosurg Psychiatry. 2015;86(5):585–587. doi: 10.1136/jnnp-2014-308331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vass Z, Dai CF, Steyger PS, Jancso G, Trune DR, Nuttall AL. Co-localization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience. 2004;124(4):919–927. doi: 10.1016/j.neuroscience.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn SK, Khalmuratova R, Jeon SY, Kim JP, Park JJ, Hur DG, Balaban CD. Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci Lett. 2009;465(2):151–156. doi: 10.1016/j.neulet.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Marano E, Marcelli V, Di Stasio E, Bonuso S, Vacca G, Manganelli F, Marciano E. et al. Trigeminal stimulation elicits a peripheral vestibular imbalance in migraine patients. Headache. 2005;45(4):325–331. doi: 10.1111/j.1526-4610.2005.05069.x. [DOI] [PubMed] [Google Scholar]
- 50.Lewis RF, Priesol AJ, Nicoucar K, Lim K, Merfeld DM. Abnormal motion perception in vestibular migraine. Laryngoscope. 2011;121(5):1124–1125. doi: 10.1002/lary.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King S, Wang J, Priesol AJ, Lewis RF. Central Integration of Canal and Otolith Signals is Abnormal in Vestibular Migraine. Front Neurol. 2014;5:233. doi: 10.3389/fneur.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polensek SH, Tusa RJ. Nystagmus during attacks of vestibular migraine: an aid in diagnosis. Audiol Neurootol. 2010;15(4):241–246. doi: 10.1159/000255440. [DOI] [PubMed] [Google Scholar]
- 53.Lee H, Sohn SI, Jung DK, Cho YW, Lim JG, Yi SD, Lee SR. et al. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke. 2002;33(12):2807–2812. doi: 10.1161/01.STR.0000038692.17290.24. [DOI] [PubMed] [Google Scholar]
- 54.Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol. 2010;1:16. doi: 10.3389/fneur.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng J, Dai C, Steyger PS, Kim Y, Vass Z, Ren T, Nuttall AL. Vanilloid receptors in hearing: altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J Neurophysiol. 2003;90(1):444–455. doi: 10.1152/jn.00919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vass Z, Shore SE, Nuttall AL, Miller JM. Endolymphatic hydrops reduces retrograde labeling of trigeminal innervation to the cochlea. Exp Neurol. 1998;151(2):241–248. doi: 10.1006/exnr.1998.6813. [DOI] [PubMed] [Google Scholar]
- 57.Gurkov R, Kantner C, Strupp M, Flatz W, Krause E, Ertl-Wagner B. Endolymphatic hydrops in patients with vestibular migraine and auditory symptoms. Eur Arch Otorhinolaryngol. 2014;271(10):2661–2667. doi: 10.1007/s00405-013-2751-2. [DOI] [PubMed] [Google Scholar]
- 58.Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128(Pt 4):932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 59.Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998;121(Pt 9):1749–1758. doi: 10.1093/brain/121.9.1749. [DOI] [PubMed] [Google Scholar]
- 60.Shin JH, Kim YK, Kim HJ, Kim JS. Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia. 2014;34(1):58–67. doi: 10.1177/0333102413498940. [DOI] [PubMed] [Google Scholar]
- 61.Russo A, Marcelli V, Esposito F, Corvino V, Marcuccio L, Giannone A, Conforti R. et al. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014;82(23):2120–2126. doi: 10.1212/WNL.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 62.Obermann M, Wurthmann S, Steinberg BS, Theysohn N, Diener HC, Naegel S. Central vestibular system modulation in vestibular migraine. Cephalalgia. 2014;34(13):1053–1061. doi: 10.1177/0333102414527650. [DOI] [PubMed] [Google Scholar]
- 63.Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34(12):947–958. doi: 10.1177/0333102414526069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goebel JA. 2015 Equilibrium Committee Amendment to the 1995 AAO-HNS Guidelines for the Definition of Meniere's Disease. Otolaryngol Head Neck Surg. 2016;154(3):403–404. doi: 10.1177/0194599816628524. [DOI] [PubMed] [Google Scholar]