Abstract
Aim
To present our experience comparing cisplatin- and cetuximab-based radiotherapy for locally-advanced head and neck squamous cell carcinoma.
Background
The comparative effectiveness of cisplatin-based chemoradiotherapy (CRT) versus cetuximab-based bioradiotherapy (BRT) for locally-advanced head and neck squamous cell carcinoma (LAHNSCC) continues to be explored.
Materials and methods
Outcomes of LAHNSCC patients treated with CRT (125) or BRT (34) at two institutions were compared retrospectively, with attention to overall survival (OS), cancer-specific survival (CSS), locoregional control (LRC), and distant control (DC). Univariate analysis (UVA) using Cox regression was performed to explore the association of intervention with survival and disease control, and multivariate (MVA) Cox regression was then performed to assess the association of intervention with survival.
Results
There were significant baseline differences between the CRT and BRT groups with respect to age, race, performance status, N-classification, tobacco history, and human papillomavirus status. UVA demonstrated inferiority of BRT versus CRT with respect to both OS (hazard ratio [HR] 2.19, 95% confidence interval [95%CI] 1.03–4.63, p = 0.04) and CSS (HR 3.33, 95%CI 1.42–7.78, p < 0.01), but non-significantly different outcomes in LRC (HR 0.99, 95%CI 0.37–2.61, p = 0.98) and DC (HR 2.01, 95%CI 0.78–5.37, p = 0.14). On MVA, there was no significant OS difference between interventions (HR 1.19, 95%CI 0.42–3.35, p = 0.74); there were too few events for the other outcomes to draw meaningful conclusions with MVA.
Conclusions
In our retrospective analysis, patients undergoing CRT experienced improved OS and CSS over those receiving BRT; however, disease control did not significantly differ. These findings may inform management of LAHNSCC patients.
Keywords: Head and neck cancer, Chemoradiotherapy, Cisplatin, Cetuximab, Radiosensitization
1. Background
Among the most significant paradigm shifts in the management of locally-advanced head and neck squamous cell carcinoma (LAHNSCC) is the emergence of an organ-preservation strategy that utilizes radiotherapy (RT) as principal therapy and attempts to forgo surgery.1, 2, 3, 4 The improvements in disease control and survival with the addition of platinum-based cytotoxic chemotherapy to RT constitutes another.5 Despite meaningfully improving oncologic outcomes, this combination approach entails considerable toxicity6 and may complicate adherence to the intended treatment regimen, with potential adverse consequences for disease control and survival.7, 8, 9 This has prompted a search for regimens offering comparable outcomes to chemoradiotherapy while simultaneously reducing toxicity.
An alternative emerged with the 2006 publication of the “Bonner trial”, which demonstrated acceptable oncologic outcomes when cetuximab, an anti-epidermal growth factor receptor antibody, was combined with RT, without any reported increase in toxicity over RT alone.10, 11 While cisplatin-based chemoradiotherapy (CRT) and cetuximab-based bioradiotherapy (BRT) have subsequently proven to be the most popular regimens for LAHNSCC,12 their comparative effectiveness continues to be debated. In a recent randomized trial, the addition of cetuximab to CRT offered no survival or local control benefit but did exacerbate acute toxicity.13
To date, the available randomized evidence comparing CRT to BRT consists of a single phase II trial which demonstrated no difference between the two arms in survival or disease control but was discontinued early due to slow enrollment, limiting its statistical power.14 While the results of additional randomized trials are eagerly anticipated,15, 16, 17 the findings from retrospective series may be informative and guide management. We therefore present our bi-institutional experience of LAHNSCC treated with CRT versus BRT.
2. Material and methods
We identified 248 patients with AJCC Stage III-IVB squamous cell carcinoma of the oropharynx or larynx diagnosed between 2004 and 2015 that were treated with radiation to at least 60 Gray and either concurrent cisplatin or cetuximab at the University of Colorado and the University of New Mexico. After excluding 43 patients undergoing induction chemotherapy and 46 receiving chemoradiotherapy in the postoperative setting, we were left with 125 CRT patients and 34 BRT patients receiving definitive radiation with systemic therapy. Generally at both institutions, cisplatin was the preferred radiosensitizer, while cetuximab was reserved for those patients whose comorbidities rendered them cisplatin-ineligible. Among CRT patients, the regimen of choice was cisplatin 100 mg/m2 every three weeks for a total of three doses, while BRT patients typically received cetuximab according to the schedule used in the Bonner trial, with a loading dose of 400 mg/m2 followed by 250 mg/m2 weekly with RT.10
Relevant sociodemographic and tumor-related characteristics of interest were selected a priori, including age at diagnosis, gender, race, Karnofsky Performance Status,18 AJCC T- and N-classification, tobacco use (as previously categorized19), human papillomavirus (HPV) status (either in situ hybridization for HPV DNA or immunohistochemistry for p16 overexpression), and primary site (oropharynx vs. larynx).
The primary endpoints were overall survival (OS), defined as time from LAHNSCC diagnosis to death of any cause; cancer-specific survival (CSS), defined as time from diagnosis to cancer-related death; locoregional control (LRC), defined as time from completion of RT to locoregional recurrence; and distant control (DC), defined as time from completion of RT to development of metastatic disease.
Statistical analyses were performed using SPSS V24.0 (SPSS Inc., Chicago, IL). Pearson chi-square tests were used to assess associations between variables and outcomes. Endpoints were examined using the Kaplan–Meier method, and groups were compared with the log-rank test. Cox proportional hazards regression was used to determine hazard ratios (HR) for each endpoint, with HR > 1 corresponding to increased risk for the specified event. All tests were two-sided with a p ≤ 0.05 level of significance. The Hosmer–Lemeshow test was used to check for the goodness-of-fit of regression models.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008. This work was approved by the appropriate institutional review committees of both institutions.
3. Results
3.1. Population characteristics
Median follow-up for our cohort was 27.2 months. Significant baseline differences were noted between treatment groups, with BRT patients more likely to have older age, non-white race, lower KPS, less-advanced nodal disease, significant tobacco use history, and HPV-negative disease (all p ≤ 0.02) (Table 1). No significant differences in RT administration were noted with respect to total dose or unplanned interruptions (both p ≥ 0.43).
Table 1.
Demographics and clinical characteristics.
| Patients (N = 159) | p | ||||
|---|---|---|---|---|---|
| CRT (n = 125) |
BRT (n = 34) |
||||
| # | % | # | % | ||
| Age at diagnosis, years | 0.01 | ||||
| Median | 58 | 63 | |||
| Range | 35–80 | 44–81 | |||
| Gender | 0.78 | ||||
| Male | 108 | 86.4 | 30 | 88.2 | |
| Female | 17 | 13.6 | 4 | 11.8 | |
| Race | 0.01 | ||||
| White | 114 | 91.2 | 25 | 73.5 | |
| Non-white | 11 | 8.8 | 9 | 26.5 | |
| KPS | 0.02 | ||||
| ≥90 | 92 | 78.0 | 19 | 57.6 | |
| <90 | 26 | 22.0 | 14 | 42.4 | |
| T-classification | 0.22 | ||||
| T1–T2 | 77 | 61.6 | 17 | 50.0 | |
| T3–T4 | 48 | 38.4 | 17 | 50.0 | |
| N-classification | <0.01 | ||||
| N0-1 | 27 | 21.6 | 19 | 55.9 | |
| N2-3 | 98 | 78.4 | 15 | 44.1 | |
| Tobacco use | 0.02 | ||||
| ≤10 pack-years | 48 | 38.4 | 6 | 17.6 | |
| >10 pack-years | 77 | 61.6 | 28 | 82.4 | |
| HPV status | 0.01 | ||||
| Negative | 23 | 18.4 | 14 | 41.2 | |
| Positive | 71 | 56.8 | 12 | 35.3 | |
| Unknown | 31 | 24.8 | 8 | 23.5 | |
| Site | 0.08 | ||||
| Oropharynx | 93 | 74.4 | 20 | 58.8 | |
| Larynx | 32 | 25.6 | 14 | 41.2 | |
| Radiation dose | 0.43 | ||||
| <69.3 Gy | 32 | 25.6 | 11 | 32.4 | |
| ≥69.3 Gy | 93 | 74.4 | 23 | 67.6 | |
| Radiation interruption | 0.86 | ||||
| <10 days | 122 | 97.6 | 33 | 97.5 | |
| ≥10 days | 3 | 2.4 | 1 | 2.5 | |
| Overall survival, months | 0.04 | ||||
| Median | 97.9 | 58.1 | |||
| 95%CI | 62.9–117.1 | 18.1–97.9 | |||
Abbreviations: CRT, chemoradiotherapy; BRT, bioradiotherapy; KPS, Karnofsky performance status; HPV, human papillomavirus; Gy, gray; 95%CI, 95% confidence interval.
3.2. Survival
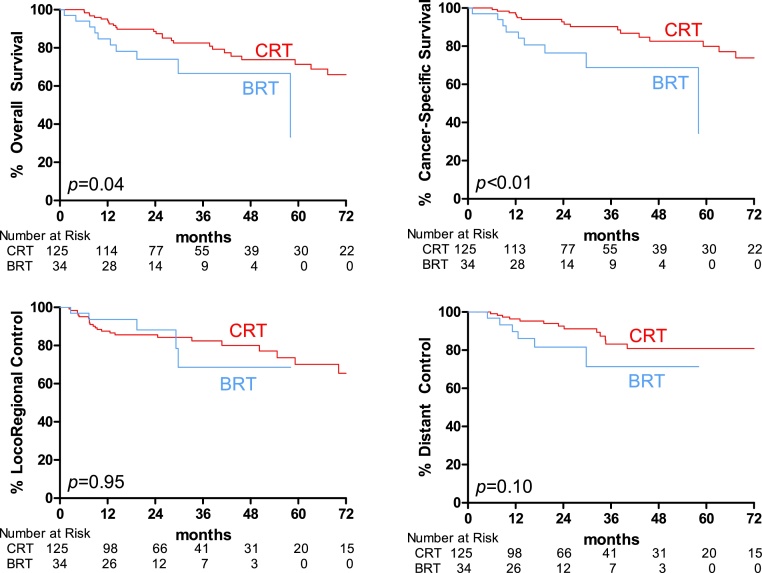
Among our 159 patients, 41 (25.8%) experienced death from any cause. The BRT group compared unfavorably to the CRT group when OS was examined via the Kaplan–Meier method (median 58.1 vs 97.9 months, p = 0.04) (Table 1, Fig. 1A), a finding that persisted on univariate Cox regression (HR 2.19, 95%CI 1.03–4.63, p = 0.04) (Table 2). Other factors associated with worse OS on UVA included KPS <90 (HR 2.88, 95%CI 1.46–5.66, p < 0.01), T3–4> disease (HR 2.64, 95%CI 1.41–4.97, p < 0.01), tobacco use >10 pack-years (HR 3.01, 95%CI 1.38–6.54, p < 0.01), while HPV-positive status was associated with improved OS (HR 0.38, 95%CI 0.18–0.81, p = 0.01). On MVA, lower KPS (HR 3.95, 95%CI 1.79–8.74, p < 0.01), heavy tobacco use (HR 3.18, 95%CI 1.10–9.15, p = 0.03), and HPV-negativity (HR 0.34, 95%CI 0.12–0.96, p = 0.04) remained independent predictors for survival; whereas BRT lost its association (HR 1.19, 95%CI 0.42–3.35, p = 0.74).
Fig. 1.
Kaplan–Meier curves for outcomes of interest. A, overall survival. B, cancer-specific survival. C, locoregional control. D, distant control. Abbreviations: CRT, cisplatin with radiotherapy; BRT cetuximab with radiotherapy.
Table 2.
Predictors of mortality (Cox regression).
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Age at diagnosis | ||||||
| continuous | 1.02 | 0.99–1.06 | 0.19 | 1.01 | 0.97–1.05 | 0.71 |
| Gender (v male) | ||||||
| female | 0.89 | 0.35–2.26 | 0.80 | 1.48 | 0.52–4.17 | 0.46 |
| Race (v white) | ||||||
| on-whiten | 1.49 | 0.62–3.54 | 0.37 | 1.54 | 0.47–5.01 | 0.48 |
| KPS (v ≥90) | ||||||
| <90 | 2.88 | 1.46–5.66 | <0.01 | 3.95 | 1.79–8.74 | <0.01 |
| T-classification (v T1–T2) | ||||||
| T3–T4 | 2.64 | 1.41–4.97 | <0.01 | 1.85 | 0.87–3.95 | 0.11 |
| N-classification (v N0–1) | ||||||
| N2–N3 | 1.75 | 0.84–3.68 | 0.14 | 2.40 | 0.93–6.22 | 0.07 |
| Tobacco use (v ≤10 pack-years) | ||||||
| >10 pack-years | 3.01 | 1.38–6.54 | <0.01 | 3.18 | 1.10–9.15 | 0.03 |
| HPV status (v negative) | ||||||
| positive | 0.38 | 0.18–0.81 | 0.01 | 0.34 | 0.12–0.96 | 0.04 |
| unknown | 0.65 | 0.30–1.41 | 0.27 | 0.33 | 0.12–0.87 | 0.03 |
| Site (v oropharynx) | ||||||
| larynx | 0.97 | 0.47–1.98 | 0.93 | 0.45 | 0.17–1.20 | 0.11 |
| Chemotherapy (v CRT) | ||||||
| BRT | 2.19 | 1.03–4.63 | 0.04 | 1.19 | 0.42–3.35 | 0.74 |
Abbreviations: CRT, chemoradiotherapy; BRT, bioradiotherapy; KPS, Karnofsky performance status; HPV, human papillomavirus; HR, hazard ratio; 95%CI, 95% confidence interval.
As found with OS, CSS was improved for CRT over BRT by both Kaplan–Meier (Fig. 1B) and Cox UVA (HR 3.33, 95%CI 1.42–7.78, p < 0.01) (Table 3). Those patients with lower performance status (HR 2.83, 95%CI 1.26–6.35, p = 0.01) and advanced primary tumors (HR 3.41, 95%CI 1.58–7.37, p < 0.01) were also more likely to die of cancer.
Table 3.
Predictors of cancer-specific mortality, locoregional failure, distant failure (all univariate analyses).
| Variable | CSM |
LRF |
DF |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Age at diagnosis | |||||||||
| continuous | 1.02 | 0.98–1.06 | 0.32 | 1.00 | 0.97–1.04 | 0.82 | 1.00 | 0.96–1.04 | 0.95 |
| Gender (v male) | |||||||||
| female | 0.77 | 0.23–2.53 | 0.66 | 0.93 | 0.32–2.70 | 0.89 | 0.31 | 0.04–2.30 | 0.25 |
| Race (v white) | |||||||||
| on-whiten | 1.83 | 0.70–4.81 | 0.22 | 1.67 | 0.64–4.37 | 0.30 | 1.50 | 0.44–5.14 | 0.52 |
| KPS (v ≥90) | |||||||||
| <90 | 2.83 | 1.26–6.35 | 0.01 | 1.52 | 0.69–3.35 | 0.30 | 1.01 | 0.36–2.85 | 0.99 |
| T-classification (v T1–T2) | |||||||||
| T3–T4 | 3.41 | 1.58–7.37 | <0.01 | 2.94 | 1.40–6.18 | <0.01 | 3.87 | 1.53–9.78 | <0.01 |
| N-classification (v N0–1) | |||||||||
| N2–N3 | 1.85 | 0.75–4.54 | 0.18 | 1.32 | 0.59–2.98 | 0.50 | 4.79 | 1.11–20.7 | 0.04 |
| Tobacco Use (v ≤10 pack-years) | |||||||||
| >10 pack-years | 2.26 | 0.96–5.30 | 0.06 | 2.80 | 1.14–6.91 | 0.03 | 1.62 | 0.62–4.23 | 0.32 |
| HPV status (v negative) | |||||||||
| positive | 0.42 | 0.17–1.03 | 0.06 | 0.52 | 0.22–1.21 | 0.13 | 0.45 | 0.15–1.35 | 0.16 |
| unknown | 0.57 | 0.22–1.48 | 0.25 | 0.63 | 0.24–1.67 | 0.35 | 0.90 | 0.30–2.68 | 0.85 |
| Site (v oropharynx) | |||||||||
| larynx | 0.96 | 0.41–2.26 | 0.92 | 1.40 | 0.65–3.01 | 0.39 | 0.63 | 0.21–1.89 | 0.41 |
| Chemotherapy (v CRT) | |||||||||
| BRT | 3.33 | 1.42–7.78 | <0.01 | 0.99 | 0.37–2.61 | 0.98 | 2.01 | 0.78–5.37 | 0.14 |
Abbreviations: CSM, cancer-spcific mortality; LRF, locoregional failure; DF, distant failure; CRT, chemoradiotherapy; BRT, bioradiotherapy; KPS, Karnofsky performance status; HPV, human papillomavirus; HR, hazard ratio; 95%CI, 95% confidence interval.
The 29 cancer-related deaths in our population are too few to analyze with MVA.
3.3. Disease control
In contrast to survival, disease control outcomes were not significantly different between BRT and CRT by Kaplan–Meier (Fig. 1C and D). Likewise, by UVA there were no significant differences by intervention for either LRC (HR 0.99, 95%CI 0.37–2.61, p = 0.98) or DC (HR 2.01, 95%CI 0.78–5.37, p = 0.14) (Table 3). However, advanced T-classification portended worse control for both endpoints (HR for LRC 2.94, 95%CI 1.40–6.18, p < 0.01; HR for DC 3.87, 95%CI 1.53–9.78, p < 0.01). UVA also demonstrated worse LRC among heavy smokers (HR 2.80, 95%CI 1.14–6.91, p = 0.03) and worse DC with advanced nodal disease (HR 4.79, 95%CI 1.11–20.7, p = 0.04).
With only 30 LRC events and 20 DC events, MVA was again limited by the low event rate observed in our study.
4. Conclusion
Our study adds another piece to the puzzle confronting oncologists who treat LAHNSCC. Among 125 patients undergoing CRT and 34 receiving BRT, OS and CSS were significantly improved with CRT; however, no differences were observed in LRC or DC. On MVA adjusting for covariates, no significant OS difference was noted between interventions. Our findings also validate the published literature in demonstrating comparatively poor outcomes in those patients with HPV-negative disease and those with extensive smoking history.
Guidelines from the National Comprehensive Cancer Network specify cisplatin-based CRT as the preferred intervention for LAHNSCC, with BRT an acceptable alternative for those medically unfit to receive cisplatin.20 However, a definitive answer regarding the comparative effectiveness of CRT versus BRT remains elusive, owing in no small part to the scant randomized evidence and conflicting findings reported in single-institutional retrospective series such as ours.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 At present, the bulk of published data derives from these retrospective, non-randomized series.
While these include patients with diverse clinicopathologic features and report a variety of endpoints, a rough dividing line can be made between those series that generally suggest comparable outcomes between CRT and BRT (Alabama-Birmingham,21 Taiwan,24 Moffitt,28 University of Oklahoma,33 MD Anderson30) and those that tend to demonstrate superiority of CRT over BRT (Washington University,23 British Columbia,34 William Beaumont,25 Stanford,29 LSU-Shreveport,35 Gustave Roussy,26, 32 New Delhi,36 Memorial Sloan-Kettering22, 27, 31). Intriguingly, those series that stratify by HPV status tend to report identical findings for their HPV-positive cohorts as for their overall populations,28, 29, 31, 32 and one series was composed entirely of patients with HPV-positive disease.30
This combined experience from the University of Colorado and the University of New Mexico offers some additional insight. Our unadjusted results suggest superior survival outcomes with CRT over BRT but comparable disease control outcomes between the interventions. However, the OS advantage of CRT is attenuated on MVA, and with only 34 BRT patients and a small number of both cancer-related deaths and failures, our population may be too small to comment on the superiority of one radiosensitizing agent over another.
A variety of explanations has been offered in attempting to account for the conflicting findings from these series. Most hinge on interseries differences between study populations and covariates, which limit comparison and generalizability between series; on intraseries imbalances in the size of the CRT and BRT groups, which suggest a lack of statistical power; and on intraseries asymmetry in the composition of the groups, which introduces the potential for selection bias. These critiques could appropriately be leveled at our analysis: our study is similarly unable to overcome the possibility of selection bias and also exhibits baseline differences between its comparison groups.
In comparison to retrospective studies, the design of prospective randomized controlled trials allows them to mitigate both selection bias and intergroup imbalance. The available randomized evidence on this subject consists of a phase II trial from Italy,14 which assigned patients with LAHNSCC to RT with cetuximab or cisplatin once weekly. While well-designed and well-balanced, the trial regrettably accrued more slowly than anticipated, enrolling 70 participants out of its intended 130. Possibly stemming from the resulting inadequate statistical power, no differences were observed in survival or disease control. Notably, adherence to planned therapy in both arms was inferior to that reported in historical comparisons, with only 28% of BRT and 20% of CRT subjects receiving all doses of concurrent systemic therapy, 66% of BRT and 47% of CRT patients receiving no systemic therapy dose reduction, and 12% of BRT participants experiencing RT interruption of >10 days. Alarmingly, 13% of BRT and 3% of CRT patients experienced a fatal treatment-related adverse event. Perhaps underlying these findings, the trial was enriched with locally-advanced disease, with T4 patients comprising 43% of the total population. These results, combined with the absence of HPV data, may limit the applicability of this trial to many patients with LAHNSCC.
Three additional randomized trials comparing CRT to BRT are currently or have recently finished enrolling subjects.15, 16, 17 Each allocates HPV-positive oropharyngeal cancer patients to either CRT or BRT; however, there are important distinctions. RTOG1016 is unique in utilizing an accelerated fractionation regimen of RT, resulting in a 6 week course, while the others use conventional fractionation over 7 weeks. Likewise, TROG12.01 stands out for using weekly cisplatin, while the other two specify cisplatin administration on a triweekly schedule. While results from these trials are eagerly anticipated, it should be noted that their conclusions may have limited generalizability to patients with HPV-negative oropharyngeal or non-oropharyngeal cancers.
As our study is retrospective, non-randomized, and drawn from two institutions, it has several limitations, including the aforementioned potential for selection bias. Baseline differences between our CRT and BRT cohorts, notably in age, performance status, nodal involvement, tobacco history, and HPV status, may have biased our results. Despite attempting to control for these differences using MVA where able, it is possible that unmeasured differences may have influenced our findings. For example, we were unable to determine the HPV status for nearly one in four patients in our analysis, raising the possibility that complete data may alter our findings; however, these patients comprised a minority of our population, and HPV status that was ascertained was similarly apportioned between our two cohorts. Finally, our BRT group was comparatively small with 34 subjects, potentially rendering our analysis underpowered and susceptible to type II error.
In summary, our bi-institutional analysis demonstrates improved OS and CSS for CRT as compared to BRT, while LRC and DC were similar; however, this survival advantage was not maintained after adjusting for imbalanced covariates. While large randomized trials continue to accrue and mature, these findings add to the available literature on this topic and may be useful to guide clinical decision-making.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Kramer S., Gelber R.D., Snow J.B. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73-03 of the radiation therapy oncology group. Head Neck Surg. 1987;10:19–30. doi: 10.1002/hed.2890100105. [DOI] [PubMed] [Google Scholar]
- 2.Pfister D.G., Strong E., Harrison L. Larynx preservation with combined chemotherapy and radiation therapy in advanced but resectable head and neck cancer. J Clin Oncol. 1991;9:850–859. doi: 10.1200/JCO.1991.9.5.850. [DOI] [PubMed] [Google Scholar]
- 3.Narayan K., Crane C.H., Kleid S., Hughes P.G., Peters L.J. Planned neck dissection as an adjunct to the management of patients with advanced neck disease treated with definitive radiotherapy: for some or for all? Head Neck. 1999;21:606–613. doi: 10.1002/(sici)1097-0347(199910)21:7<606::aid-hed4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Garden A.S., Asper J.A., Morrison W.H. Is concurrent chemoradiation the treatment of choice for all patients with stage III or IV head and neck carcinoma? Cancer. 2004;100:1171–1178. doi: 10.1002/cncr.20069. [DOI] [PubMed] [Google Scholar]
- 5.Pignon J., Bourhis J., Domenge Co, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 6.Machtay M., Moughan J., Trotti A. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson A.G., Robertson C., Perone C. Effect of gap length and position on results of treatment of cancer of the larynx in Scotland by radiotherapy: alinear quadratic analysis. Radiother Oncol. 1998;48:165–173. doi: 10.1016/s0167-8140(98)00038-3. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal D.I., Liu L., Lee J.H. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 9.Suwinski R., Sowa A., Rutkowski T., Wydmanski J., Tarnawski R., Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/s0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 10.Bonner J.A., Harari P.M., Giralt J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Bonner J.A., Harari P.M., Giralt J. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 12.Wong S.J., Harari P.M., Garden A.S. Longitudinal oncology registry of head and neck carcinoma (LORHAN) Cancer. 2011;117:1679–1686. doi: 10.1002/cncr.25721. [DOI] [PubMed] [Google Scholar]
- 13.Ang K.K., Zhang Q., Rosenthal D.I. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magrini S.M., Buglione M., Corvò R. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase ii trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.63.1671. JCO631671. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Research United Kingdom . 2013. De-ESCALaTE HPV determination of epidermal growth factor receptor-inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus-positive oropharyngeal squamous cell carcinoma. [Google Scholar]
- 16.Radiation Therapy Oncology Group . 2011. RTOG 1016 Phase III trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV-associated oropharynx cancer. [Google Scholar]
- 17.Trans-Tasman Radiation Oncology Group . 2013. TROG 12.01 a randomised trial of weekly cetuximab and radiation versus weekly cisplatin and radiation in good prognosis locoregionally advanced HPV-associated oropharyngeal squamous cell carcinoma. [Google Scholar]
- 18.Mor V., Laliberte L., Morris J.N., Wiemann M. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfister D.G., Spencer S., Brizel D.M. Head and neck cancers, version 1.2015. J Natl Comp Cancer Network. 2015;13:847–855. doi: 10.6004/jnccn.2015.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caudell J.J., Sawrie S.M., Spencer S.A. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71:676–681. doi: 10.1016/j.ijrobp.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Koutcher L., Sherman E., Fury M. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915–922. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Ley J., Mehan P., Wildes T.M. Cisplatin versus cetuximab given concurrently with definitive radiation therapy for locally advanced head and neck squamous cell carcinoma. Oncology. 2013;85:290–296. doi: 10.1159/000355194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu M.H., Wang L.W., Lu H.J. Cisplatin-based chemotherapy versus cetuximab in concurrent chemoradiotherapy for locally advanced head and neck cancer treatment. BioMed Res Int. 2014;2014:904341. doi: 10.1155/2014/904341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J., Baschnagel A.M., Chen P. A matched-pair comparison of intensity-modulated radiation therapy with cetuximab versus intensity-modulated radiation therapy with platinum-based chemotherapy for locally advanced head neck cancer. Int J Clin Oncol. 2014;19:240–246. doi: 10.1007/s10147-013-0540-y. [DOI] [PubMed] [Google Scholar]
- 26.Levy A., Blanchard P., Bellefqih S. Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlenther Onkol. 2014;190:823–831. doi: 10.1007/s00066-014-0626-0. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro L.Q., Sherman E.J., Riaz N. Efficacy of concurrent cetuximab vs. 5-fluorouracil/carboplatin or high-dose cisplatin with intensity-modulated radiation therapy (IMRT) for locally-advanced head and neck cancer (LAHNSCC) Oral Oncol. 2014;50:947–955. doi: 10.1016/j.oraloncology.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strom T.J., Trotti A.M., Kish J. Comparison of every 3 week cisplatin or weekly cetuximab with concurrent radiotherapy for locally advanced head and neck cancer. Oral Oncol. 2015;51:704–708. doi: 10.1016/j.oraloncology.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Tang C., Chan C., Jiang W. Concurrent cetuximab versus platinum-based chemoradiation for the definitive treatment of locoregionally advanced head and neck cancer. Head Neck. 2015;37:386–392. doi: 10.1002/hed.23609. [DOI] [PubMed] [Google Scholar]
- 30.Nien H.H., Sturgis E.M., Kies M.S. Comparison of systemic therapies used concurrently with radiation for the treatment of human papillomavirus-associated oropharyngeal cancer. Head Neck. 2016;38(Suppl. 1):E1554–E1561. doi: 10.1002/hed.24278. [DOI] [PubMed] [Google Scholar]
- 31.Riaz N., Sherman E., Koutcher L. Concurrent chemoradiotherapy with cisplatin versus cetuximab for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39:27–31. doi: 10.1097/COC.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou D., Levy A., Blanchard P. Concurrent chemoradiotherapy with cisplatin or cetuximab for locally advanced head and neck squamous cell carcinomas: does human papilloma virus play a role? Oral Oncol. 2016;59:50–57. doi: 10.1016/j.oraloncology.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Smith M.L., Arain A.N., Herman T.S., Bogardus CR, Jr., Matthiesen C.L., Thompson J.S. Cisplatin versus cetuximab combined with radiation therapy for definitive management of locally advanced squamous cell carcinoma of the head and neck: a matched cohort retrospective analysis. Int J Radiat Oncol Biol Phys. 2015;93:E344. [Google Scholar]
- 34.Ye A.Y., Hay J.H., Laskin J.J., Wu J.S., Ho C.C. Toxicity and outcomes in combined modality treatment of head and neck squamous cell carcinoma: cisplatin versus cetuximab. J Cancer Res Therapeut. 2013;9:607–612. doi: 10.4103/0973-1482.126455. [DOI] [PubMed] [Google Scholar]
- 35.Peddi P., Shi R., Nair B., Ampil F., Mills G.M., Jafri S.H. Cisplatin, cetuximab, and radiation in locally advanced head and neck squamous cell cancer: a retrospective review. Clin Med Insights Oncol. 2015;9:1–7. doi: 10.4137/CMO.S18682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawat S., Ahlawat P., Kakria A. Comparison between weekly cisplatin-enhanced radiotherapy and cetuximab-enhanced radiotherapy in locally advanced head and neck cancer: first retrospective study in Asian population. Asia-Pacific J Clin Oncol. 2016 doi: 10.1111/ajco.12581. [DOI] [PubMed] [Google Scholar]