Introduction
A 55-year-old woman with ESRD attributed to hypertension presented with nephrotic syndrome 9 months post–kidney transplant. She was on dialysis for 6 years before pediatric dual kidney transplant from a previously healthy 5-year-old black boy weighing 23.5 kg. Kidney sizes were 6.5 and 7.5 cm and deemed too small to allocate individually. Each had a renal artery implanted separately. No peri- or postoperative surgical complications occurred. The recipient was sensitized with a class 1 panel reactive antibodies of 88% and class 2 PRA of 0%, and weighed 62 kg. The transplant was a 2A/2B/1DR mismatch with cytomegalovirus (CMV) serology negative in the donor and positive in the recipient (D−R+). She received thymoglobulin induction and was maintained on prednisone, mycophenolate mofetil, and tacrolimus (trough levels 7–10 ng/ml). The allografts functioned promptly with patent vasculature on postoperative day 1. Her baseline serum creatinine was 1.2 mg/dl and proteinuria was absent by repeat urinalysis. At 6 months post-transplant, she developed fevers, leukopenia, and CMV viremia (6178 copies/ml), and was treated with oral valganciclovir and mycophenolate mofetil reduction with prompt viremia clearance. At 9 months post-transplant, peripheral edema and a 3-kg weight gain developed with 3+ proteinuria and hypoalbuminemia (2.7 g/dl). Her creatinine was stable at 1.2 mg/dl and tacrolimus trough was 6.9 ng/ml. Her platelet count was 172 k/mm3 and hemoglobin was stable at 11.2 g/dl. An ultrasound showed kidney sizes of 9.8 and 10.2 cm, with normal resistive indices and no notable findings. A biopsy was performed.
Differential Diagnosis
Dr. Klein
Nephrotic syndrome occurring within the first year post–kidney transplant may represent de novo, recurrent, or donor-derived glomerular disease. Neither the recipient nor the donor had any history of glomerular disease pretransplant, which reduces the likelihood of recurrent or donor-derived disease. The differential for de novo nephrotic syndrome post-transplant includes minimal change disease, FSGS, membranous nephropathy, membranoproliferative GN, IgA nephropathy, C3 GN, and paraprotein-related disease. Transplant-related causes of proteinuria and allograft dysfunction include antibody mediated rejection, virally mediated disease, and immunosuppression effects. These can result in transplant glomerulopathy, immune complex–mediated GN, FSGS, and thrombotic microangiopathy. Lastly, post-transplant lymphoproliferative disease can also present with proteinuria.
Clinical Diagnosis
Dr. Klein
Our patient had no documented history of pretransplant GN, with stable allograft function and no proteinuria until 9 months post-transplant. Her nephrotic syndrome developed after CMV infection, making CMV-related causes including the collapsing variant of FSGS high in the differential. The recipient was also sensitized (2A/2B/1DR mismatch) and underwent mycophenolate mofetil reduction after CMV viremia, which heightened concern for antibody mediated rejection.
Pathology
Dr. Hennigar
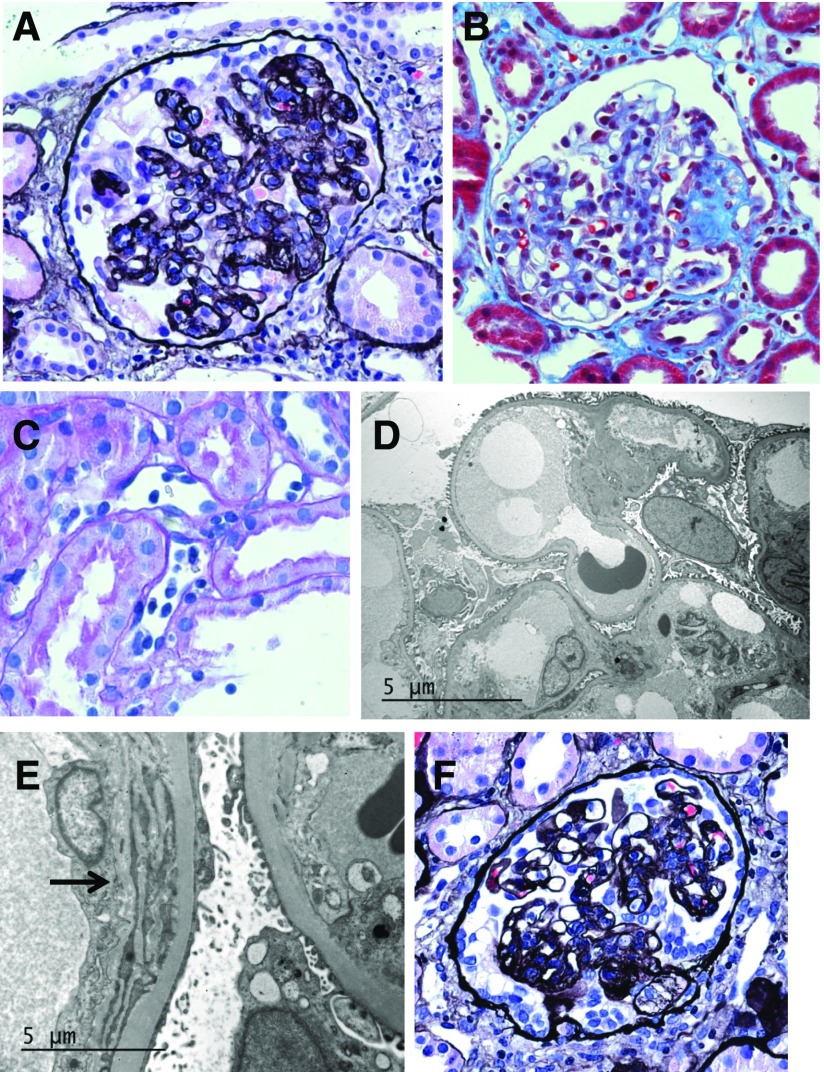
Tissue submitted for light microscopy consisted of a single core of cortex that contained 21 glomeruli, none of which were globally sclerotic. About 20% of glomeruli exhibited retraction of the tuft, with collapse and corrugation of the capillary loops (Figure 1A). A few glomeruli also showed segmental sclerosis (Figure 1B). Glomerular epithelium overlying areas of tuft collapse and sclerosis displayed cellular hypertrophy with reactive nuclear atypia, prominent protein resorption droplets, and cytoplasmic vacuolization. Hypertrophy was accompanied by hyperplastic changes in several glomeruli. Affected glomeruli also showed mild mesangial proliferation. Mild endocapillary proliferation was present in <25% of glomeruli and was interpreted as mild glomerulitis. Silver stains revealed rare double contours along glomerular capillary loops. There was patchy mild chronic interstitial inflammation and minimal tubulointerstitial scarring. No tubulitis was identified. A few tubules were ectatic, lined by attenuated epithelial cells, and contained cast material positive for periodic acid–Schiff, i.e., microcystic tubular dilation. Proximal tubules were lined by reactive epithelial cells. Arteries and arterioles were unremarkable. There was moderate peritubular capillaritis (Figure 1C). Immunoperoxidase staining for polyoma SV40, CMV, and parvovirus was negative. Immunofluorescence for IgG, IgA, IgM, C3, C4, C1q, albumin, fibrinogen, and κ and λ light chains was negative within glomeruli. Immunofluorescence for C4d was negative along peritubular capillaries.
Figure 1.
Morphologic findings from serial biopsies. (A–E) The initial biopsy was performed 10 months post-transplant and the specimen showed (A) focal collapsing lesions characterized by retraction of the tuft and epithelial cell hypertrophy and hyperplasia (Jones methenamine silver stain; original magnification, ×400); (B) FSGS (Masson trichrome stain; original magnification, ×400); (C) peritubular capillaritis (periodic acid–Schiff stain; original magnification, ×400); (D) minimal foot process effacement (Unstained; original magnification ×4000); and (E) multilamination of glomerular basement membranes (arrow, unstained; original magnification ×12,000). (F) The second biopsy was performed at 12 months post-transplant and the specimen continued to show collapsing lesions (Jones methenamine silver stain; original magnification, ×400).
Electron microscopic analysis of a single glomerulus revealed mild increase in mesangial matrix with sparse mesangial electron densities. Several capillary loops were occluded by swollen endothelial cells. Subendothelial electron-lucent widening was nominal. There was only mild foot process effacement (Figure 1D). A few capillary loops showed multilamination or early duplication of glomerular basement membranes (Figure 1E). No immune-type deposits were found along capillary walls. A single peritubular capillary displayed multilamination of surrounding basement membrane (about five layers).
Pathologic Diagnosis
Dr. Hennigar
The primary diagnosis was collapsing glomerulopathy (CGP) superimposed upon changes suspicious for C4d-negative chronic active antibody mediated rejection. The latter diagnosis was on the basis of changes interpreted as early chronic transplant glomerulopathy with glomerulitis, moderate peritubular capillaritis, and acute tubular injury in the absence of cellular rejection (per 2013 Banff Classification of Renal Allograft Pathology) (1).
Clinicopathologic Follow-Up
Dr. Klein and Dr. Hennigar
Testing was negative for (2) donor-specific antibodies (LABScreen Single Antigen Assay, One Lambda; Thermo Fisher Scientific); (3) CMV, HIV, Epstein–Barr, and parvovirus viremia; and (4) BK viruria. Despite the negative C4d and donor-specific antibodies, features suggesting chronic antibody mediated rejection prompted treatment with a short prednisone pulse of 20 mg daily for 1 week, 10 mg daily for 1 week, then continuation of 5 mg daily along with increase of mycophenolate mofetil to 1000 mg twice daily. Lisinopril was initiated for proteinuria. The patient's nephrotic syndrome progressed, with urinary protein-to-creatinine ratio of 7.2, worsening hypoalbuminemia (2.2 g/dl), hyperlipidemia (total cholesterol, 314; LDL, 215), and increased creatinine (1.7 mg/dl). Her tacrolimus trough level was 8.5 ng/ml and lactate dehydrogenase, total bilirubin, and platelet counts were normal. A rebiopsy was performed at 12 months post-transplant and her specimen showed advancing CGP lesions involving 40% of glomeruli (Figure 1F). Interstitial fibrosis was increased to 25%. Her sample continued to show changes suspicious for chronic antibody mediated rejection (double contours along capillary loops, glomerulitis, peritubular capillaritis), but no signs of cellular rejection. Immunostaining for Ig, complement (including C4d), light chains, fibrinogen, CMV, and polyoma SV40 was negative.
To treat nephrotic range proteinuria, corticotropin injection gel (H.P. Acthar) was administered at 80 U subcutaneously, twice weekly. After completion of 22 weeks of therapy, her urinary protein-to-creatinine ratio had stabilized at 4.0, creatinine remained at 1.6–1.9 mg/dl, and hypoalbuminemia had resolved (3.5 g/dl). At 6 months after H.P. Acthar gel completion, she represented with worsening edema and increased creatinine (2.2 mg/dl). Her urinary protein-to-creatinine ratio and serum albumin were stable (2.3 and 3.5 mg/dl, respectively) and tacrolimus trough was 6.0 ng/ml. A third allograft biopsy was performed at 2.2 years post-transplant. Her specimen showed CGP with 50% glomerular obsolescence and progression to 70% interstitial fibrosis. Silver stains continued to show occasional double contours along glomerular capillary loops. Glomerulitis had subsided but peritubular capillaritis persisted. There were no signs of cellular rejection. A single artery exhibited mild intimal fibrosis and her arterioles were unremarkable. Immunostaining for Ig, complement (including C4d), light chains, fibrinogen, and polyoma SV40 was negative.
Discussion
CGP is classified as a variant of FSGS according to the Columbia schema, and preempts all other variants with its aggressiveness (2). Non-HIV CGP arising post-transplant tends to exhibit the same clinical aggression as its native counterpart. The vast majority of patients present with nephrotic range proteinuria and kidney failure (3–7). CGP can be an early or late complication of transplantation with a mean time to onset post-transplant of 24.9 months (range, 0.23–129.5 months) (4–7). Previous studies estimated the incidence of recurrent CGP at 20%–25%, with higher rates assigned to de novo disease (3, 6). Graft loss occurs quickly, irrespective of time to onset. Almost all patients progress to ESRD within 3 years compared with 40% of post-transplant patients having noncollapsing forms of FSGS (3). Interestingly, only 5% of patients are black, which contrasts with the much higher prevalence of CGP among blacks outside of the transplant population.
FSGS with collapsing features was first recognized as a component of AIDS- and HIV-related nephropathy. The collapsing phenotype was therefore tightly associated with HIV infection and considered an acquired condition. CGP is now recognized as associated with multiple conditions other than HIV (Table 1), indicative of a final common pathway arising from genetic and acquired disease. CGP is therefore viewed as a pattern of histologic injury rather than a disease.
Table 1.
Conditions associated with collapsing glomerulopathy
| Conditions |
|---|
| Genetic diseases |
| ApoL1-associated nephropathy |
| CoQ2 nephropathy |
| Action myoclonus renal failure |
| Mandibuloacral dysplasia |
| Galloway–Mowat syndrome |
| Infectious diseases |
| HIV |
| Cytomegalovirus |
| Parvovirus B19 |
| Epstein–Barr virus |
| Human T-lymphotropic virus type 1 |
| Campylobacter |
| Tuberculosis |
| Parasitic diseases |
| Autoimmune diseases |
| SLE |
| Mixed connective tissue disease |
| Still disease |
| Hematopoietic diseases |
| Leukemias |
| Multiple myeloma |
| Hemophagocytic lymphohistiocytosis |
| Drugs |
| Bisphosphonates |
| Valproic acid |
| Calcineurin inhibitors |
| Interferons |
| Anabolic steroids |
| Vascular diseases |
| Thrombotic microangiopathy |
| Atheroembolic disease |
| Diabetes mellitus |
| Sickle cell disease |
We attempted to attach a disease cause to our patient by ruling out the most relevant conditions listed in Table 1. The clinical history strongly suggested the possibility of CMV-related glomerular disease. However, immunoperoxidase staining for CMV was negative in the first and second biopsy specimens, and genetic testing of both specimens failed to detect insertion of CMV genomic material. Furthermore, testing for other viral causes (HIV, parvovirus, polyomavirus, Epstein–Barr virus, and hepatitis C) was negative.
Because the donor was black, we also explored the possibility of transmitted APOL1 risk alleles by the donor kidneys. Allelic variations within the gene encoding ApoL1 confer markedly increased susceptibility to kidney disease among global populations derived from sub-Saharan Africa (8). Paired expression of two APOL1 variant alleles (termed G1 and G2) is linked to nondiabetic kidney diseases, many of which exhibit a collapsing phenotype (9). There is growing evidence that secondary modifiers (i.e., second hit) or specific epistatic interactions may be required for the clinical manifestation of ApoL1-related diseases (9). Moreover, APOL1 risk alleles may be transmitted via donor kidneys to allograft recipients and render the recipient susceptible to CGP or other forms of ApoL1-associated nephropathy (10). We speculated that APOL1 G1/G2 alleles were transmitted from the black donor to our patient, who then experienced a second hit in the form of CMV infection. Indeed, a similar scenario was reported by Shah et al. (10). Accordingly, APOL1 risk variant genotyping was performed using real-time polymerase chain reaction applied to frozen tissue from the second biopsy specimen. The assay revealed only a single risk allele (G2), meaning that ApoL1 was unlikely to confer susceptibility to CGP in our recipient.
Calcineurin inhibitors have been implicated in post-transplant CGP. It is unclear, however, if the collapsing phenotype is the direct result of drug-induced glomerular injury or the result of drug-induced vasculopathy and subsequent ischemia (11). A relationship between ischemia and CGP is supported by studies reporting collapsing lesions in the setting of cholesterol atheroembolism, thrombotic microangiopathy, vascular rejection, diabetic vasculopathy, and kidney infarction (12–16). However, our patient showed no signs of vascular injury until the third biopsy, which revealed only mild intimal fibrosis of a single artery.
Two biopsy specimens also showed changes suspicious for C4d-negative antibody mediated rejection, with duplication of glomerular basement membranes, glomerulitis, and peritubular capillaritis. No donor-specific antibodies were detected despite repeated testing. Other non-HLA antibodies such as those targeting major histocompatibility complex class 1-related chain A, angiotensin II type 1 receptor, and antiendothelial antibodies were unfortunately not tested. Therefore, the possibility of non-HLA antibody mediated rejection cannot be excluded.
Of note, the finding of only mild foot process effacement in our patient seems counterintuitive in an aggressive case of CGP. It should be emphasized, however, that Laurinavicius et al. (17) showed that extensive foot process effacement was present in only 57% and 38% of patients with and without HIV, respectively, with CGP.
Irrespective of intervention, reported outcomes in renal transplant recipients with CGP remain poor, with allograft failure and return to dialysis in over half of patients by 2 years postdiagnosis, particularly if moderate to advanced fibrosis is present on the biopsy specimen (4, 7, 18–20). If a specific cause is identified (Table 1) then targeted therapy may prevail, e.g., antiviral therapy or withdrawal of causative medications. There is limited data on the use of steroids, plasmapheresis, cyclophosphamide, inhibitors of the renin-aldosterone-angiotensin system, corticotropin injection gel, and rituximab to guide therapy in CGP post-transplant.
This case highlights the rapidly progressive course and high risk for early allograft loss in patients with post-transplant CGP. Superior allograft outcomes approaching those of living donor kidneys are normally observed after deceased donor transplants from pediatric donors if technical issues and thrombosis do not result in early graft loss. Despite excellent initial allograft function and lack of surgical complications, the recipient has an eGFR of 14 ml/min at 2.5 years post-transplant and is being prepared for a return to dialysis. Further investigation into etiologies resulting in CGP is warranted, as well as information regarding the risk of recurrent CGP if retransplant is pursued.
Disclosures
R.A.H is an ad hoc lecturer at Sanofi Pharmaceuticals and is coinvestigator in the Thrombotic Microangiopathy and Genetic Variants Involving the Alternative Complement Pathway in Patients with Hypertensive Emergency Study, which is sponsored by Alexion Pharmaceuticals. C.L.K is on the Speakers Bureau for Alexion Pharmaceuticals and is a contributor for UpToDate.com.
Acknowledgments
The authors would like to acknowledge Helen Liapis, Senior Consultant at Arkana Laboratories, who organized the ASN Clinicopathological Correlation Conference and offered sound advice. We would also like to thank Gregory A. Storch, Professor of Pediatrics, Chief of the Division of Pediatric Laboratory Medicine, and Medical Director of the Clinical Laboratories at St. Louis Children’s Hospital, whose laboratory performed the cytomegalovirus testing. Testing for APOL1 genotyping was performed by Marjorie Beggs, Supervisor of the Molecular Laboratory at Arkana Laboratories in Little Rock, Arkansas.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee: Banff 2013 meeting report: Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 2.D'Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Lager DJ, Qian X, Stegall MD, Larson TS, Griffin MD: Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant 21: 2607–2614, 2006 [DOI] [PubMed] [Google Scholar]
- 4.IJpelaar DH, Farris AB, Goemaere N, Amann K, Goldschmeding R, Nguyen TQ, Farkash E, van den Heuvel MC, de Heer E, Bruijn JA, Colvin RB, Bajema IM: Fidelity and evolution of recurrent FSGS in renal allografts. J Am Soc Nephrol 19: 2219–2224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canaud G, Dion D, Zuber J, Gubler MC, Sberro R, Thervet E, Snanoudj R, Charbit M, Salomon R, Martinez F, Legendre C, Noel LH, Niaudet P: Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: Course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 25: 1321–1328, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Schachter ME, Monahan M, Radhakrishnan J, Crew J, Pollak M, Ratner L, Valeri AM, Stokes MB, Appel GB: Recurrent focal segmental glomerulosclerosis in the renal allograft: Single center experience in the era of modern immunosuppression. Clin Nephrol 74: 173–181, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Unagami K, Kawanishi K, Shimizu T, Kanzawa T, Toki D, Okumi M, Omoto K, Horita S, Koike J, Honda K, Nagashima Y, Ishida H, Tanabe K, Nitta K: A case of recurrent focal segmental glomerulosclerosis after kidney transplantation associated with variant conversion in the Columbia classification. Nephrology (Carlton) 20[Suppl 2]: 96–100, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, Winkler CA, Kopp JB: APOL1 kidney disease risk variants: An evolving landscape. Semin Nephrol 35: 222–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah PB, Cooper JE, Lucia MS, Boils C, Larsen CP, Wiseman AC: APOL1 polymorphisms in a deceased donor and early presentation of collapsing glomerulopathy and focal segmental glomerulosclerosis in two recipients. Am J Transplant 16: 1923–1927, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Goes NB, Colvin RB: Case records of the Massachusetts General Hospital. Case 12-2007. A 56-year-old woman with renal failure after heart-lung transplantation. N Engl J Med 356: 1657–1665, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Greenberg A, Bastacky SI, Iqbal A, Borochovitz D, Johnson JP: Focal segmental glomerulosclerosis associated with nephrotic syndrome in cholesterol atheroembolism: Clinicopathological correlations. Am J Kidney Dis 29: 334–344, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Nadasdy T, Allen C, Zand MS: Zonal distribution of glomerular collapse in renal allografts: Possible role of vascular changes. Hum Pathol 33: 437–441, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Canaud G, Bruneval P, Noël LH, Correas JM, Audard V, Zafrani L, Rabant M, Timsit MO, Martinez F, Anglicheau D, Thervet E, Patey N, Legendre C, Zuber J: Glomerular collapse associated with subtotal renal infarction in kidney transplant recipients with multiple renal arteries. Am J Kidney Dis 55: 558–565, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Salvatore SP, Reddi AS, Chandran CB, Chevalier JM, Okechukwu CN, Seshan SV: Collapsing glomerulopathy superimposed on diabetic nephropathy: Insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol Dial Transplant 29: 392–399, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Buob D, Decambron M, Gnemmi V, Frimat M, Hoffmann M, Azar R, Gheerbrant JD, Guincestre T, Noël C, Copin MC, Glowacki F: Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int 90: 1321–1331, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Laurinavicius A, Hurwitz S, Rennke HG: Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int 56: 2203–2213, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Grcevska L, Polenakovik M: Collapsing glomerulopathy: Clinical characteristics and follow-up. Am J Kidney Dis 33: 652–657, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Sureshkumar KK, Dosani I, Jasnosz KM, Arora S; De novo collapsing glomerulopathy: An unusual cause of early graft failure following kidney transplantation. Case Rep Transplant 2011: 263970, 2011. [DOI] [PMC free article] [PubMed]
- 20.Kanodia KV, Vanikar AV, Patel RD, Suthar KS, Nigam LK, Patel HV, Kute V, Trivedi HL: Collapsing glomerulopathy: A single centre clinicopathologic study of seven years. J Clin Diagn Res 10: EC15–EC17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]