Abstract
Viruses are capable of inducing a wide spectrum of glomerular disorders that can be categorized on the basis of the duration of active viremia: acute, subacute, or chronic. The variable responses of the adaptive immune system to each time period of viral infection results mechanistically in different histologic forms of glomerular injury. The unique presence of a chronic viremic carrier state with either hepatitis C (HCV) or HIV has led to the opportunity to study in detail various pathogenic mechanisms of viral-induced glomerular injury, including direct viral infection of renal tissue and the development of circulating immune complexes composed of viral antigens that deposit along the glomerular basement membrane. Epidemiologic data show that approximately 25%–30% of all HIV patients are coinfected with HCV and 5%–10% of all HCV patients are coinfected with HIV. This situation can often lead to a challenging differential diagnosis when glomerular disease occurs in this dual-infected population and requires the clinician to be familiar with the clinical presentation, laboratory workup, and pathophysiology behind the development of renal disease for both HCV and HIV. Both of these viruses can be categorized under the new classification of infection-associated GN as opposed to being listed as causes of postinfectious GN as has previously been applied to them. Neither of these viruses lead to renal injury after a latent period of controlled and inactive viremia. The geneses of HCV- and HIV-associated glomerular diseases share a total dependence on the presence of active viral replication to sustain renal injury so the renal disease cannot be listed under “postinfectious” GN. With the new availability of direct-acting antivirals for HCV and more effective combined antiretroviral therapy for HIV, successful remission and even regression of glomerular lesions can be achieved if initiated at an early stage.
Keywords: Glomerulonephritis, hantavirus kidney, polyarteritis nodosa HCV, focal segmental glomerulosclerosis, APOL1, Antigen-Antibody Complex, Antigens, Viral, Antiviral Agents, Carrier State, Coinfection, Diagnosis, Differential, Glomerular Basement Membrane, HIV Infections, Hepacivirus, Hepatitis C, Humans, Immune System, Viremia
Introduction
Since their initial discovery in 1898, viruses have challenged their human hosts with a greater degree of foreign genomic diversity than any other infectious organisms (1). Over 5000 DNA and RNA viruses have been structurally defined, and either by their own direct intracellular virulence or through the stimulation of natural host adaptive immune defense mechanisms they often lead to significant tubule-interstitial and glomerular injury (2).
One proposed schema that can be used to classify viral-induced GN can be on the basis of the duration of active viremia. Patients with recent self-limited acute viremia (days to weeks) develop significantly different forms of glomerular pathology as compared with patients with subacute viremia (weeks to months) and those with chronic persistent viremia (years). An outline of the most well documented viruses that fit into each of these categories and their individual glomerular lesions that have been described in the literature is shown in Table 1.
Table 1.
Viruses associated with GN
| Virus Type | Predominant Glomerular Histology |
|---|---|
| Acute | |
| Dengue | ICGN, MsPGN |
| Hantavirus | HFRS-MsPGN |
| Varicella-zoster | DPGN |
| Parvovirus | ICGN, PAN, MPA, TMA, IgA |
| HAV | ICGN, MsPGN |
| HBV | DPGN |
| CMV | cFSGS, MN, IgA, HSP, ICGN, MPGN, TMA |
| EBV | ICGN, MN, MsPGN |
| Coxsackie B | RPGN |
| Subacute | |
| Parvovirus | cFSGS |
| EBV | cFSGS, MN |
| HBV | PAN |
| HCV | PAN |
| Chronic | |
| HBV | MN, Type I MPGN, MPGN+MC, PAN, IgA, FSGS |
| HIV | HIVAN, HIVICK, TMA |
| HCV | Type I MPGN+MC, Type I MPGN, PAN, IgA, MN |
| HEV | MN |
ICGN, immune complex glomerulonephritis; MsPGN, mesangial proliferative GN; HFRS, hemorrhagic fever and renal syndrome; DPGN, diffuse proliferative GN; PAN, polyarteritis nodosa; MPA, microscopic polyarteritis; TMA, thrombotic microangiopathy; HAV, hepatitis A virus; HBV, hepatitis B virus; CMV, cytomegalovirus; cFSGS, collapsing FSGS; MN, membranous glomerulopathy; HSP, Henoch Shoenlein purpura; MPGN, membranoproliferative GN; EBV, Epstein-Barr virus; RPGN, rapidly progressive glomerulonephritis; HCV, hepatitis C virus; MC, mixed cryoglobulinemia; HIVAN, HIV-associated nephropathy; HIVICK, HIV immune complex disease of the kidney; HEV, hepatitis E virus.
In order to link a viral illness with a specific form of GN, certain supporting criteria must be met: (1) demonstration of the presence of active quantifiable viremia primarily through serologic PCR testing, (2) the identification of viral proteins/nucleic acid residues within renal tissue and/or within immune complexes deposited in the glomerular basement membrane, and (3) resolution/regression of the glomerular lesion concomitant with the host immune clearance of viremia or eradication of viremia through antiviral therapy.
However, recent emerging data has proposed a new relationship of GN associated with occult viral disease which is defined as the presence of viral nucleic acid in renal tissue and in peripheral blood mononuclear cells but with complete absence of detectable systemic viremia by standard PCR amplification techniques (3). Occult hepatitis C (HCV) has been detected in 30%–50% of patients with idiopathic membranous nephropathy, IgA, FSGS, ANCA positive vasculitis, and membranoproliferative GN (MPGN) (4). Similarly, occult hepatitis B (HBV) infection has been described with documented HBV antigens found in renal tissue but with absent viremia in selected cases of idiopathic membranous nephropathy and IgA (5). In many of the cases, viremia could only be identified by intensive ultracentrifugation of plasma. Possibly fulfilling the cause and effect nature of this finding, there is anecdotal evidence that antiviral therapy in select cases of occult HCV or HBV has led to resolution of the previously diagnosed “idiopathic” glomerular disease (6). Future research is needed to define the role of occult viremia from undetectable intracellular reservoirs with clinical renal disease.
This review will detail the pathophysiology, histopathology, and clinical syndromes associated with clinically active viral diseases, which will be presented in two separate parts. This issue (part 1) will focus on the glomerular syndromes associated with long-term chronic HCV and HIV carrier states. In the second issue (part 2) the focus will concentrate on the glomerular diseases seen with chronic HBV infection and it will also cover glomerular diseases seen with a variety of acute and subacute viral infections including cytomegalovirus, parvovirus, Epstein–Barr, hantavirus, and dengue.
HCV
With the current availability of direct-acting antivirals (DAA) which can achieve a viral remission of >95% for most HCV genotypes, the prevalence of glomerular disease in this population should progressively decline in the coming decade (7). Chronic HCV viremia is present in 150–170 million people worldwide (3% of the global population) and in 3.2 million people in the United States. Approximately 2–3 million new cases of HCV occur each year with 75%–90% of these patients becoming chronic carriers (8).
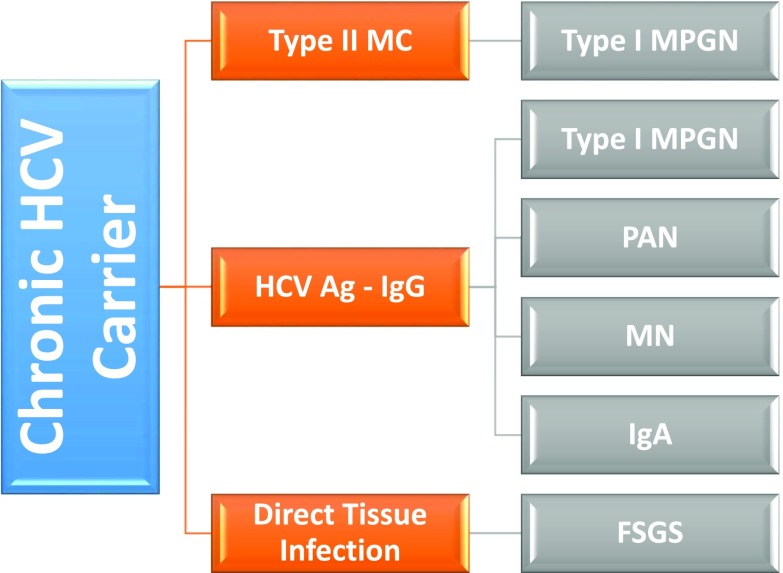
Similar to HBV, HCV-associated glomerular disease is primarily a consequence of viral antigen – immune complex formation with glomerular basement membrane deposition. The classic pathologic hallmark of HCV renal disease is type 1 MPGN as a consequence of type 2 mixed cryoglobulinemia (MC), although the same renal lesion may also develop from noncryoglobulinemic immune complexes (9). In addition, HCV is also an important cause of polyarteritis nodosa (PAN) from immune complex deposition in medium size blood vessels leading to renal ischemia and infarction similar to HBV PAN and idiopathic PAN (10). Other forms of glomerular immune complex deposition have been described in HCV patients, including mesangial proliferative and focal proliferative GN and IgA nephropathy (Figure 1).
Figure 1.
Glomerular disease in patients with chronic HCV infection. Ag, antigen; HCV, hepatitis C virus; MC, mixed cryoglobulinemia; MN, membranous glomerulopathy; MPGN, membranoproliferative GN; PAN, polyarteritis nodosa.
It is important to note that renal dysfunction in a patient with HCV is rarely a result of GN (<10%) and hospital admissions for MPGN from HCV have been decreasing for the past decade (11). The majority of renal diseases in HCV patients with liver disease are a consequence of acute tubular necrosis, hepatorenal syndrome, prerenal azotemia, and CKD and therefore it is essential to be familiar with the typical presentation and characteristics of acute GN in HCV patients and how to differentiate them from the more common diagnoses causing AKI in this population.
Type 1 MPGN with MC
HCV provides a unique opportunity to study the effects of a hepatotropic virus that also has unique lymphotropic potential (12). In the absence of this lymphotropism, HCV would be indistinguishable from a pathogenic standpoint from HBV and HIV in the development of typical IgG–viral particle immune complexes of varying size and virulence. In approximately 20% of type 1 MPGN cases caused by HCV, this standard sequence results in the production of noncryoglobulin immune complexes (13). However, in the majority of type 1 MPGN lesions, HCV is capable of specifically binding to CD5+ CD81+ B cells which subsequently leads to polyclonal type 3 and eventually monoclonal type 2 cryoglobulin synthesis of IgMκ. This IgMκ monoclonal antibody is a unique effect of HCV-driven B cell clonal proliferation and carries rheumatoid factor activity. Additional receptors that mediate HCV B cell stimulation involve the scavenger receptor SR-B3 and toll receptor TRL4 (14).
The entire cryoglobulin complex consists of monoclonal IgMκ binding to the Fc portion of non-neutralizing HCV-specific IgG1 and IgG3 that have ineffectively bound the structural E2 or nonstructural NS3 proteins of HCV or the actual HCV RNA itself. It is not surprising, given the sheer size of this complex, that it lodges along the subendothelial space creating large crystalline intraluminal thrombi completely distinct from a typical subendothelial immune complex as in SLE. Cryoglobulinemia is unusual (<5%) in all other forms of viral hepatitis (HBV, hepatitis A virus), HIV carriers, cytomegalovirus, parvovirus B19, and Epstein-Barr virus which reinforces the unique role of the HCV/B cell interaction (15).
The development of MC and type 1 MPGN requires a considerable duration of viremia and a select host response. After a minimum of 5–10 years, 35%–50% of HCV carriers, regardless of HCV genotype, develop type 3 MC which carries a minimal risk of GN. After 10–15 years of viremia approximately 10% of patients within this subgroup transition and develop type 2 MC and systemic small vessel vasculitis (16). These patients demonstrate the characteristic skin rash (palpable purpura), polyneuropathy, gastrointestinal/pulmonary/cardiac/central nervous system vasculitis, and in 30% of patients a type 1 MPGN. From a nephrologic standpoint, these patients may present with hypertension and the nephritic syndrome but importantly 20% of type 1 MPGN patients present only with the nephrotic syndrome, making a renal biopsy essential for diagnosis.
The mechanisms behind the transition from type 3 to type 2 MC in a small fraction of HCV patients are not well defined. Host factors may play an important role with >65% of HCV-positive patients worldwide being men but there is a reversed female predominance in patients that develop type 2 MC. The response by sex to chronic antigenemia is different, with women showing a TH2 response and a greater likelihood of autoimmunity compared with a more typical TH1 response in men. In HCV patients, autoantibody production is especially prevalent with 66% showing anti-smooth muscle antibodies and 40% with a positive anti-nuclear antibody. Between 40% and 75% of HCV patients have some form of extrahepatic autoimmune complication (17).
Hypocomplementemia of the classic pathway is a distinguishing feature of type 2 MC with type 1 MPGN with C4 being low in 93% of patients, whereas C3 is depressed in only 53% due to both its role as an acute phase reactant and the possible activation of the alternate C pathway in some patients. At this stage of their HCV cycle, most patients will have serologic evidence of chronic active hepatitis with <15% showing advanced cirrhosis.
Left untreated, type 2 MC from HCV is a precursor sign for the emergence of B cell lymphoma in 6% of patients and an additional risk factor for cardiovascular death from coronary vasculitis (18). Therefore, treatment of type 2 MC and renal disease in HCV patients has a two-fold purpose: reduce the risk of malignancy and decrease patient cardiovascular mortality.
Treatment
Ongoing HCV viremia is at the epicenter for the generation of nephritogenic immune complexes. The development of DAAs against HCV has provided a revolutionary opportunity for long-term successful eradication of HCV and elimination of cryoglobulinemia (19). These oral agents have virtually replaced the need for IFN. HCV carries an innate ability to suppress natural IFNγ production in the host with a concomitant decrease in Treg cells that allow for persistence of viremia and the development of autoimmune sequela. Exogenous IFN used to be the backbone of therapy but was hampered by the need for prolonged therapy (48 weeks), poor patient tolerance, and an unsatisfactory sustained viral response (<65% on the basis of genotype). More limiting was the inability to use IFN in solid organ transplant recipients due to the risk of humoral rejection (20).
DAAs target three groups of nonstructural proteins called NS5A, NS5B, and NS3/4A. A variety of drugs in this class have been developed that are used in combination over a 12-week period without IFN and result in sustained viral remissions of >95% for all genotypes. In the setting of acute cryoglobulinemic vasculitis and GN, DAAs have resulted in clinical remission and disappearance of cryogobulinemia (21). Often, control of viremia alone will not suffice due to the relative delay in viral clearance over 4–8 weeks, and in some circumstances plasmapheresis, steroids, and rituximab are still needed to control the acute inflammatory vasculitic symptoms. DAAs are now the recommended drugs of choice in an IFN-free regimen for patients with HCV-related MPGN or PAN.
HIV
The HIV pandemic has continued, with 37 million active carriers in the world (1.1 million in the United States), 2 million newly infected cases per year, and another 15%–20% of patients still unaware of their HIV diagnosis. The HIV population is significantly less than the 240 million carriers of HBV and the 170 million carriers of HCV. On the basis of the 2013 World Health Organization treatment guidelines only 35%–40% of eligible patients globally are receiving combined antiretroviral therapy (cART), indicating that the majority of HIV patients are actively viremic (22).
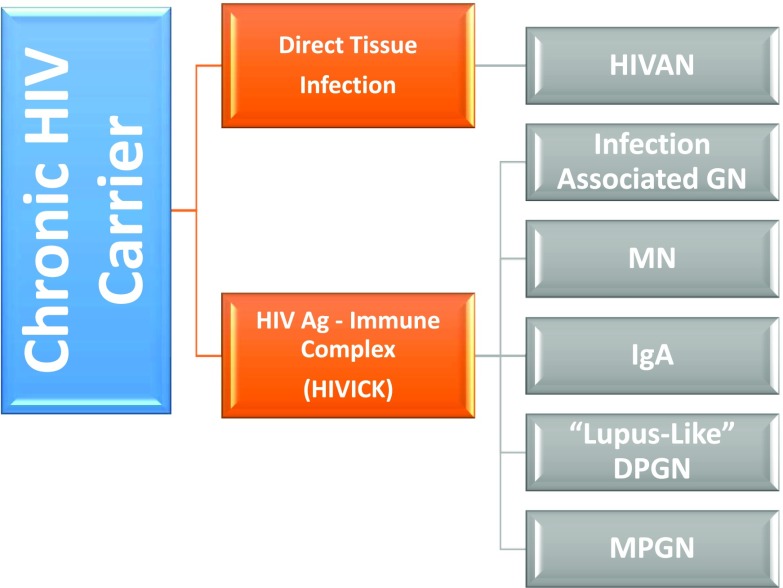
The differential diagnosis of glomerular disease in the setting of HIV infection will be significantly influenced by (1) the treatment status of the patient: cART-naïve or actively on cART, and (2) by their coinfection status with other viruses such as with HBV (5%–10%) or with HCV (25%–30%). As with all of the viral diseases previously discussed the prerequisite need for ongoing active viremia is essential for the development of the classic HIV-related glomerular syndromes: HIV-associated nephropathy (HIVAN) and HIV immune complex disease of the kidney (HIVICK) (Figure 2).
Figure 2.
Glomerular disease associated with chronic HIV infection. Ag, antigen; DPGN, diffuse proliferative GN; HIVAN, HIV-associated nephropathy; HIVICK, HIV immune complex disease of the kidney; MC, mixed cryoglobulinemia; MN, membranous glomerulopathy; MPGN, membranoproliferative GN.
HIVAN
HIVAN represents a unique manifestation of glomerular injury that arises from direct viral infection of renal tissue, particularly the visceral and parietal epithelial cells, without the presence of immune complexes. HIVAN comprises a constellation of four individual pathologic findings in the renal biopsy that may not all be present simultaneously: collapsing FSGS (cFSGS), microcystic dilation of the tubules, interstitial nephritis, and the presence of intracytoplasmic tubulo-reticular inclusions (“TRI-IFN footprints”). By definition, the only specific requirement to fulfill the diagnosis of HIVAN is the presence of cFSGS in the setting of HIV infection with the remaining lesions found in variable frequencies (23).
HIVAN develops almost exclusively in patients of black race origin who are homozygous or heterozygous for APOL1 G1 or G2 variants. The lifetime risk of HIVAN in an untreated HIV-infected black patient is 2%–10%, however, this risk increases to 50% if that person is homozygous for the APOL1 variants. APOLI variants, initially an evolutionary advantage for protection against trypanosomiasis in Africa, now represent the single most important risk factor for the development of HIVAN worldwide (24).
The histopathologic lesions of HIVAN in a susceptible host require translation of the nine-gene HIV genome which then leads to altered phenotype changes in the podocytes with loss of maturation markers (synaptopodin. podocalyxin) and upregulation of proliferation markers (Ki-67). The main HIV genes responsible for these changes are TAT (transactivator of transcription), NEF (negative factor), and Vpr (viral protein r) (25).
The one major question as yet unanswered is the method for direct HIV entry into the podocytes, mesangium, and tubular cells because they lack CD4 receptors and chemokine receptors used by HIV for entry into nonrenal tissue. The use of lipid rafts for freely circulating Tat and Vpr proteins or binding of the whole HIV virion to DEC-205 (dendritic cell receptor) or dendritic cell specific ICAM-3-grabbing nonintegrin may allow for direct tissue infection (26).
Although nephrotic range proteinuria is the expected consequence of cFSGS in HIVAN, early collapsing lesions can be seen in untreated HIV patients with only microalbuminuria (27). Therefore, HIVAN needs to be considered in any cART-naïve HIV patient of black race ancestry with any degree of abnormal albuminuria or proteinuria.
Because HIVAN is a direct consequence of viral replication, cART has been demonstrated to attenuate the rate of decline of GFR and actually cause histologic regression with a return of the normal podocyte phenotype within the glomerular lesions (28). This favorable response is predicated on achieving viral remission. Deterioration of renal function in a patient successfully treated for HIVAN on cART may be a reflection of the intrinsic nephrotoxicity of either the protease inhibitors or the non-nucleotide(s) reverse transcription inhibitors rather than due to HIV nephropathy (29). Alternatively, a condition entitled immune reconstitution syndrome with AKI from acute interstitial nephritis or immune complex GN may occur shortly after starting cART. Finally, because coinfection with HBV or HCV is common, renal impairment after cART may also reflect the glomerular injury from these ongoing untreated and possibly unrecognized viral infections.
New emerging therapy for HIVAN includes renin-angiotensin-aldosterone system inhibition, interruption of the mTOR pathway, and retinoic acid derivatives (30,31). Upregulation of angiotensin 2 with possible costimulation of the mTOR pathway and involving the AT2 receptor lends support for the benefit of direct blockade of these pathways. Experimentally, HIVAN lesions fail to develop with the use of renin-angiotensin-aldosterone system and mTOR inhibitors. The application of retinoids in HIVAN and other glomerular diseases is currently being investigated. HIV downregulates the retinoic acid receptor supporting a potential clinical role for this therapy as an adjunct to cART.
HIVICK
HIVICK represents a constellation of histopathologic diseases rather than a specific glomerular syndrome of its own such as HIVAN. This is why it is impossible to describe an actual typical presentation or outcome for HIVICK because it represents a pathophysiologic grouping of a wide variety of glomerular diseases each with a different presentation and prognosis. All glomerular diseases comprising HIVICK result from the deposition of immune complexes in the glomerulus, consisting of either HIV antigens or as a natural result of typical postinfectious immune complexes that occur due to the increased risk of infections from a compromised immune system found in HIV patients not on cART (32). Like HIVAN, HIVICK is a complication of active HIV viremia in a cART-naïve patient or a patient with cART resistance. Glomerular lesions from coinfected HIV patients with HBV or HCV are not considered HIVICK and are classified separately as secondary lesions due to their individual viruses.
Approximately 50% of glomerular histology within the HIVICK category can be described as postinfectious GN, with the remaining half resulting from immune complex deposition consisting of HIV antigens in the form of membranous, IgA, MPGN, and a “lupus-like” diffuse proliferative GN (33). Some studies have excluded patients with IgA from having HIVICK assuming it may be an idiopathic lesion, however, the colocalization of the HIVp24 and gp120 antigens with IgA in the presence of active HIV disease and typical IgA histology on biopsy clearly suggests that IgA can be and likely should be considered a manifestation of HIVICK (34).
Each of these lesions has their own distinct prognosis, with membranous and the “lupus- like” proliferative lesions progressing the fastest toward ESRD. In general, HIVICK progresses at a slower pace toward ESRD than untreated HIVAN, with 70% of HIVAN patients requiring dialysis within 2 years of diagnosis compared with only 34% of HIVICK patients (35).
The majority of studies do not show a major effect of cART on the progression of HIVICK. cART has been able to induce histologic regression in HIVAN but this has only been infrequently seen in HIVICK. This is likely due to the permanent destructive injury to the glomerular basement membrane from immune complex deposition in HIVICK compared with the reversible intracellular phenotypic changes of the podocyte characteristic of HIVAN.
The heterogeneous nature of HIVICK and the limited number of published studies on outcomes makes it difficult to make a recommendation on the benefit of cART. Because 50% of the lesions are postinfectious these would not be expected to improve with cART, whereas the remaining immune complex glomerular diseases should theoretically show benefit depending on their degree of chronicity.
Idiopathic FSGS
The demographics of glomerular disease in HIV patients have been changing on the basis of the specific demographics of the population being reported (36). Currently, in Western Europe and in the United States, noncollapsing FSGS is emerging as the most common glomerular lesion seen. These results are self-fulfilling if the population is predominantly cART-treated. Because APOL1 polymorphisms, which are restricted to black race origin, are vitally important for the development of HIVAN, any study dealing with European or largely white HIV patients will clearly never show a significant percentage of HIVAN or FSGS lesions and may show more HIVICK (37).
Summary
The pathogenic mechanisms of glomerular disease in HCV and HIV patients exemplify the wide spectrum of immunologic and microbiologic pathways utilized by viruses in general to cause renal disease. Conclusive evidence exists both for HIV and HCV for direct viral infection of renal tissue as well as the development of immune complexes partially consisting of viral antigens. These same principles can be applied to other viral infections that will be discussed in part 2.
Although corticosteroids have been used in selected patients with HIVAN and lymphocytotoxic immunosuppression has been advocated for patients with HCV-associated cryoglobulinemia, invariably the ultimate control of active viremia remains the key objective in the management of both HIV- and HCV-associated GN.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Morgan GJ: What is a virus species? Radical pluralism in viral taxonomy. Stud Hist Philos Biol Biomed Sci 59:64–70, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Johnson RJ: The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int 86: 905–914, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Fowell AJ, Sheron N, Rosenberg WM: Renal hepatitis C in the absence of detectable serum or hepatic virus. Liver Int 28: 889–891, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Fabrizi F, Messa P, Martin P: Novel evidence on hepatitis C virus-associated glomerular disease. Kidney Int 86: 466–469, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Li D, Gao G, Jiang H, Tang Z, Yu Y, Zang G: Hepatitis B virus-associated glomerulonephritis in HBsAg serological-negative patients. Eur J Gastroenterol Hepatol 27: 65–69, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Kong D, Wu D, Wang T, Li T, Xu S, Chen F, Jin X, Lou G: Detection of viral antigens in renal tissue of glomerulonephritis patients without serological evidence of hepatitis B virus and hepatitis C virus infection. Int J Infect Dis 17: e535–e538, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Yau AH, Yoshida EM: Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review. Can J Gastroenterol Hepatol 28: 445–451, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shire NJ, Sherman KE: Epidemiology of Hepatitis C Virus: A Battle on New Frontiers. Gastroenterol Clin North Am 44: 699–716, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Gill K, Ghazinian H, Manch R, Gish R: Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int 10: 415–423, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadoun D, Terrier B, Semoun O, Sene D, Maisonobe T, Musset L, Amoura Z, Rigon MR, Cacoub P: Hepatitis C virus-associated polyarteritis nodosa. Arthritis Care Res (Hoboken) 63: 427–435, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Tong X, Spradling PR: Increase in nonhepatic diagnoses among persons with hepatitis C hospitalized for any cause, United States, 2004-2011. J Viral Hepat 22: 906–913, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Böckle BC, Sepp NT: Hepatitis C virus and autoimmunity. Auto Immun Highlights 1: 23–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozkok A, Yildiz A: Hepatitis C virus associated glomerulopathies. World J Gastroenterol 20: 7544–7554, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zignego AL, Gragnani L, Piluso A, Sebastiani M, Giuggioli D, Fallahi P, Antonelli A, Ferri C: Virus-driven autoimmunity and lymphoproliferation: the example of HCV infection. Expert Rev Clin Immunol 11: 15–31, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Terrier B, Marie I, Lacraz A, Belenotti P, Bonnet F, Chiche L, Graffin B, Hot A, Kahn JE, Michel C, Quemeneur T, de Saint-Martin L, Hermine O, Léger JM, Mariette X, Senet P, Plaisier E, Cacoub P: Non HCV-related infectious cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey and systematic review of the literature. J Autoimmun 65: 74–81, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Ferri S, Muratori L, Lenzi M, Granito A, Bianchi FB, Vergani D: HCV and autoimmunity. Curr Pharm Des 14: 1678–1685, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Dammacco F, Racanelli V, Russi S, Sansonno D: The expanding spectrum of HCV-related cryoglobulinemic vasculitis: a narrative review. Clin Exp Med 16: 233–242, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Tasleem S, Sood GK: Hepatitis C Associated B-cell Non-Hodgkin Lymphoma: Clinical Features and the Role of Antiviral Therapy. J Clin Transl Hepatol 3: 134–139, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N, Kim AY, Thadhani R, Chung RT: Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 63: 408–417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrizi F, Martin P, Cacoub P, Messa P, Donato FM: Treatment of hepatitis C-related kidney disease. Expert Opin Pharmacother 16: 1815–1827, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N, Kim AY, Thadhani R, Chung RT: Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 63: 408–417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, Resch S, Ryckman T, Møgedal S, Dybul M, Goosby E, Watts C, Kilonzo N, McManus J, Sidibé M; UNAIDS–Lancet Commission: Defeating AIDS--advancing global health. Lancet 386: 171–218, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wyatt CM, Meliambro K, Klotman PE: Recent progress in HIV-associated nephropathy. Annu Rev Med 63: 147–159, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K: APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant 31: 349–358, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB: HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol 11: 150–160, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Ross MJ: Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int 86: 266–274, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Han TM, Naicker S, Ramdial PK, Assounga AG: A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int 69: 2243–2250, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Fabian J, Naicker S, Goetsch S, Venter WD: The clinical and histological response of HIV-associated kidney disease to antiretroviral therapy in South Africans. Nephrol Dial Transplant 28: 1543–1554, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Kalyesubula R, Perazella MA: Nephrotoxicity of HAART. AIDS Res Treat 2011: 562790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar D, Konkimalla S, Yadav A, Sataranatarajan K, Kasinath BS, Chander PN, Singhal PC: HIV-associated nephropathy: role of mammalian target of rapamycin pathway. Am J Pathol 177: 813–821, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallipattu SK, He JC: The beneficial role of retinoids in glomerular disease. Front Med (Lausanne) 2: 16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, Atta MG: Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol 8: 1524–1532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth J, Hamzah L, Jose S, Horsfield C, O’Donnell P, McAdoo S, Kumar EA, Turner-Stokes T, Khatib N, Das P, Naftalin C, Mackie N, Kingdon E, Williams D, Hendry BM, Sabin C, Jones R, Levy J, Hilton R, Connolly J, Post FA; HIV/CKD Study and the UK CHIC Study: Clinical characteristics and outcomes of HIV-associated immune complex kidney disease [published online ahead of print January 18, 2016] Nephrol Dial Transplant doi:10.1093/ndt/gfv436, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Rollino C, Vischini G, Coppo R: IgA nephropathy and infections. J Nephrol 29: 463–468, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Nobakht E, Cohen SD, Rosenberg AZ, Kimmel PL: HIV-associated immune complex kidney disease. Nat Rev Nephrol 12(5): 291–300, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Mallipattu SK, Salem F, Wyatt CM: The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney Int 86: 259–265, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, Ronco P, Pialoux G, Plaisier E: HIV-associated kidney glomerular diseases: changes with time and HAART. Nephrol Dial Transplant 27: 2349–2355, 2012 [DOI] [PubMed] [Google Scholar]