Abstract
Background and objectives
We showed that mineralocorticoid receptor blockade (MRB) prevented acute and chronic cyclosporine nephropathy (CsA-Nx) in the rat. The aim of this translational study was to investigate the effect of long-term eplerenone administration on renal allograft function in children with biopsy-proven chronic allograft nephropathy (CAN).
Design, setting, participants, & measurements
Renal transplant children <18 years, biopsy-proven CAN, and a GFR>40 ml/min per 1.73 m2 were included. Patients with BK virus active nephritis, recurrence of renal disease, GFR decline in previous 3 months, or treated with calcium antagonists or antifungal drugs were excluded. They were randomized to receive placebo (n=10) or eplerenone 25 mg/d for 24 months (n=13). Visits were scheduled at baseline, 6, 12, and 24 months. At each period, a complete clinical examination was performed and blood and urine samples were taken. Urine creatinine, 8-hydroxylated-guanosine, heat shock protein 72 (HSP72), and kidney injury molecule (KIM-1) levels were also assessed. In kidney biopsy samples, the tubulo-interstitial area affected by fibrosis (TIF) and glomerulosclerosis were measured at baseline and after 24 months.
Results
The baseline eGFR was 80±6 in the placebo and 86±6 ml/min per 1.73 m2 in the eplerenone group; at 24 months it was 66±8 and 81±7 ml/min per 1.73 m2, respectively (P=0.33; 95% confidence intervals, −18 to 33 at baseline, and −11 to 40 after 24 months). The albumin-to-creatinine ratio was 110±74 in the placebo, and 265±140 mg/g in the eplerenone group; and after 24 months it was 276±140 and 228±88 mg/g, respectively (P=0.15; 95% confidence intervals, −283 to 593, and −485 to 391, respectively). In addition, the placebo exhibited a greater TIF, glomerulosclerosis, and urinary HSP72 compared with the eplerenone group.
Conclusions
Although this study was underpowered to provide definitive evidence that long-term eplerenone administration attenuates the progression of CAN in pediatric transplant patients, it encourages testing the potential benefit of MRB in this pediatric population.
Introduction
Chronic allograft nephropathy (CAN) is the main cause of renal graft dysfunction and loss (1). In renal biopsy samples, it is characterized by tubulo-interstitial fibrosis and tubular atrophy (IF/TA), and it can be due to immunologic or nonimmunologic factors, including previous history of acute cellular rejection, chronic humoral rejection, ischemia/reperfusion injury, infectious tubulo-interstitial nephritis, hypertension, dyslipidemia, and nephrotoxicity due to calcineurin inhibitors (CIs) (2). IF/TA is present in 53%–90% of protocol biopsy samples at 12 months post-transplant, and its severity correlates with renal dysfunction and proteinuria (3,4). Even though the use of CIs has improved graft survival in kidney transplant patients, their clinical use is often restricted due to their nephrotoxic side effects, which can manifest as acute or chronic nephrotoxicity (5). The acute form is reversible due to renal vasoconstriction. The chronic toxicity is not reversible and is characterized by renal vasoconstriction together with tubulo-interstitial fibrosis (TIF), arteriolopathy, and a progressive decrease in GFR that could lead to renal graft loss (6).
Over the past two decades, it has been recognized that aldosterone exerts deleterious effects in the vasculature, heart, and kidney (7). It promotes fibrosis and vascular remodeling through the induction of fibroblast growth and proliferation and by altering the expression of profibrotic factors (8–12). Furthermore, it has been shown that aldosterone/mineralocorticoid receptor (MR) activation increases reactive oxygen species generation, which in turn triggers an inflammatory response that further amplifies the initial TIF (13,14). In this sense, several observations suggest that aldosterone plays an essential role in the development of CI nephrotoxicity.
In a rat model of chronic cyclosporine A (CsA) nephrotoxicity, we showed that MR antagonism with spironolactone completely prevented renal dysfunction, renal fibrosis, and the upregulation of profibrotic molecules (15). Spironolactone administration improved renal function through the normalization of the imbalance of vasoactive factors produced by CsA in acute and chronic nephrotoxicity models (16). Indeed, specific MR deletion in smooth muscle cells in mice fully prevented acute CsA nephrotoxicity by preventing the increase in renal vascular resistance (17). We have also shown that mineralocorticoid receptor blockade (MRB) is effective enough to prevent renal vasoconstriction induced by ischemia/reperfusion when it is administered before and even after the ischemic process in the rat (18–20). In addition, in a model of pre-established chronic CsA nephrotoxicity, we showed that MRB prevented further deterioration of renal function and partially reversed the structural alterations together with a reduction in TGF-β levels (21). Finally, in a clinical pilot-study, we showed that MRB reduced oxidative stress in kidney transplant patients (22). This evidence strongly supports that MRB may be a useful strategy to fight against renal dysfunction and TIF induced by the chronic use of CIs.
Thus, we hypothesized that long-term administration of eplerenone could reduce CAN progression. Therefore, the aim of this translational study was to investigate whether MRB with eplerenone reduces the progression of CAN already present in pediatric patients.
Materials and Methods
Study Design and Patients
A prospective, randomized single blind study was conducted according to the recommendations of the World Medical Association’s Declaration of Helsinki. It was approved by the Hospital Internal Review Board and the Ethics Committee (HIM Protocol HIM/2009/015) and was registered in Current Controlled Trials (http://www.controlled-trials.com/ISRCTN19419571, 09/06/2010). Parental written consent and assent of the patient was obtained in all cases.
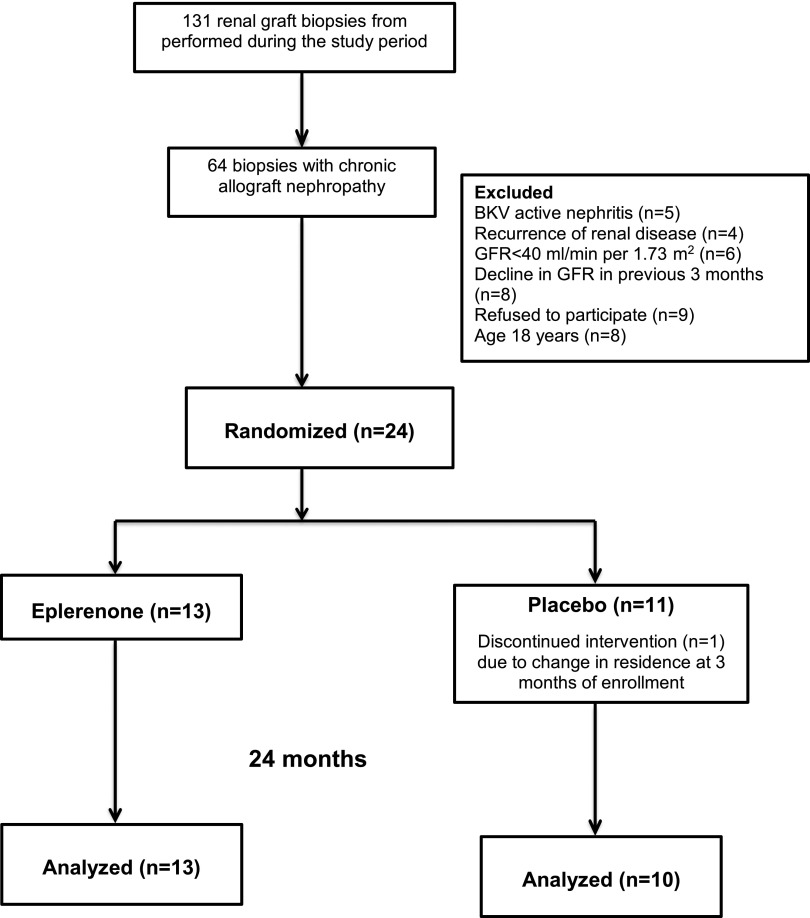
A total of 131 renal graft biopsies were performed from March of 2009 to September of 2011 in our institution, and only 64 biopsy samples were diagnosed with CAN (Figure 1). From these biopsy samples, 40 were excluded due to BK virus active nephritis in five patients; recurrence of renal disease in four patients; GFR<40 ml/min per 1.73 m2 in six patients; a decline in GFR in the previous 3 months (n=8); refusal to participate in nine patients; and age≥18 years in eight patients. Therefore, all included patients (n=24) had biopsy-proven CAN and a GFR>40 ml/min per 1.73 m2 body surface area by both the Schwartz and Zappitelli cystatin C– and serum creatinine–based prediction equation. Patients receiving calcium antagonists, clarithromycin, erythromycin, itraconazole, or fluconazole were excluded.
Figure 1.
Study flow diagram. Twenty-four renal transplant children fulfilling the inclusion criteria from 131 studied renal graft biopsy samples. The children were randomized to receive placebo (n=11) or eplerenone (n=13) for 24 months. Only one patient from the placebo group discontinued the study treatment.
Once the CAN diagnosis was confirmed, each patient was invited to participate in the study, and when the consent form was signed, the patient was randomized to placebo or eplerenone treatment. Thus, the study was a parallel arm study, and the allocation was a 1:1 ratio using a computer-generated table of random numbers using permuted blocks of four. Group assignment was in sequentially numbered sealed and opaque envelopes prepared by an independent technician. The allocation sequence was generated by an independent statistician, and the clinical researcher invited and enrolled the participants. The study was blinded to the patients and care providers but not to the clinical researchers.
The placebo group (P) received 180 mg/d of calcium carbonate, and the eplerenone group (Ep) received Inspra (Pfizer) at an initial dose of 12.5 mg/d for 2 weeks, which was increased to 25 mg/d for 24 months.
The CI dose was reduced by 20% in all participants. Visits were scheduled at baseline, 6, 12, 18, and 24 months. Clinical examinations were performed at each visit. A second biopsy was performed after 24 months of treatment.
The primary outcome was change in GFR after 24 months of treatment, and the secondary outcomes included acute rejection episodes, graft loss, patient deaths, adverse effects, and changes in: proteinuria, TGF-β, aldosterone levels, interstitial fibrosis, and urinary heat shock protein 72 (HSP72).
Considering a median difference of 10 ml/min per 1.73 m2 between the groups after 24 months of treatment, a standard deviation of 14 ml/min per 1.73 m2, a power of 80%, and 95% confidence intervals, 31 patients were required in each arm, although we were not able to recruit 62 patients within the study period.
Laboratory Tests
Serum creatinine, electrolytes, transaminases, complete blood cell count, and urinalysis were performed at each visit. Serum aldosterone and TGF-β, as well as urinary kidney injury molecule–1 (KIM-1), were assessed at 0, 6, 12, and 24 months. Whereas urinary HSP72 levels were tested at 0 and 24 months.
Kidney Allograft Biopsies
Baseline biopsies were performed by the Nephrology Department and “protocol” biopsies were routinely suggested to all patients at 6 and 12 months postrenal transplant. Additional kidney biopsy samples were obtained when graft dysfunction was documented. Biopsies were performed with ultrasonogram guidance and an automatic Bard Magnum biopsy instrument. Renal tissue was processed for light microscopy and was evaluated by the same nephropathologist who was blinded to the treatment group. Lesions were diagnosed and graded according to the Banff 2007 criteria (23). C4d staining was performed by immunohistochemistry in paraffin sections.
The extent of TIF was quantified by computer-assisted morphometry in trichrome-stained slides. Morphometric analysis was performed using a Nikon microscope equipped with a full color DS-Ri1 camera and NIS-Elements AR 4.50.00 image analysis software. The biopsy samples taken at the beginning and at the end of the study were blinded to the treatment group and analyzed. Several pictures were taken of each biopsy sample to reach an area of at least 245,000 μ2 to be assessed. In addition, the percentages of segmental and global glomerulosclerosis (GS), as well as affected glomeruli percentage, were determined.
Renal Function Evaluation
Serum creatinine was measured using a SYNCHRON CX3 (Beckman) validated by isotope dilution mass spectrometry-traceable assay. Cystatin C was assessed using the BN ProSpec System analyzer (Siemens Healthcare Diagnostics, NY) with the N Latex Cystatin C kit. GFR was estimated by the Zappitelli formula for transplant recipients using serum creatinine and cystatin C concentrations (24). Proteinuria was measured by urinary albumin-to-creatinine ratio. Serum TGF-β1 was measured at baseline and 12 months by ELISA (R&D Systems catalog SB100B). Serum aldosterone was measured at baseline, 6, 12, and 24 months by commercial ELISA following the procedures described by the manufacturer (EIA-4128; DRG International Inc., NJ).
Urinary Biomarker Levels
Urinary KIM-1 levels were analyzed using a commercially available ELISA kit (CSB-E08807 h; Cusabio Biotech). All procedures were performed according to the manufacturer’s instructions. Urinary HSP72 levels were evaluated by western blot analysis. Briefly, 10 μl of urine was loaded in 8.5% SDS-PAGE gels and transferred into polyvinylidene difluoride membranes. The membranes were blocked with 5% blotting-grade reagent, incubated overnight at 4°C with the antibody against HSP72 (C92F3A-5; Enzo Life Sciences), washed with tris-buffered saline-Tween, and incubated with the secondary antibody IgG goat–anti-mouse horseradish peroxidase. The proteins were detected using a chemiluminescence kit (Millipore), and the bands were scanned for densitometric analysis.
Urinary Oxidative Stress
The urinary levels of 8-hydroxy 2 deoxyguanosine as an oxidative DNA damage marker were assessed by using a commercially available kit (ab201734; Abcam) following the manufacturer’s instructions.
Statistical Analyses
Descriptive statistics for continuous measures are reported as the mean and SD when normally distributed or median (25th–75th percentiles) for skewed variables. Categoric/binary measures are presented as percentages. Variables were analyzed for a normal distribution with the Kolmogorov–Smirnov test. For normally distributed data, two-way repeated measures ANOVA (RM ANOVA) was performed followed by a Bonferroni post hoc test, otherwise we used Kruskal–Wallis test with Dunn’s Multiple Comparison post hoc analysis. Primary outcome analysis was made by both intention-to-treat and per protocol. Histopathologic findings and urinary 8-hydroxylated-guanosine levels were analyzed by one-way ANOVA followed by a Bonferroni multiple comparisons post hoc test. The HSP72 percentage of change was analyzed by unpaired t test. Statistical significance was defined when P<0.05. GraphPad Prism 6 was used for graphical representation and statistical analyses. Post hoc power calculation was performed with the App Sample Size Calculator for Clinical Research version 2.0 for iOs.
Results
Twenty-three children were analyzed: ten in the placebo group and 13 in the eplerenone group; only one patient from the placebo group discontinued the study treatment (Figure 1). Patient demographics are depicted in Table 1. All patients received induction with anti-CD25. There were no differences in age, sex, type of immunosuppression, or post-transplant time, and 70% of patients had a history of an acute cellular rejection episode. The adherence to the study visits was 90% in the placebo group and 100% in the eplerenone group. In our institution, once a patient is diagnosed with CAN, CI doses are reduced by 20%. The CI levels were monitored at each visit to maintain tacrolimus trough levels between 4 and 6 ng/ml and cyclosporine trough levels between 75 and 100 ng/ml. The degree of immunosuppression between groups was similar; there were no statistically significant differences in the leukocyte count between the groups throughout the study (RM ANOVA P=0.61).
Table 1.
Patient demographics
| Characteristic | Placebo | Eplerenone |
|---|---|---|
| n=10 | n=13 | |
| Age (mean±SD), yr | 14.4±2.4 | 15.5±3.0 |
| Sex, n (%) | ||
| Male | 5 (50) | 10 (77) |
| Female | 5 (50) | 3 (23) |
| Graft source, n (%) | ||
| Living related donor | 8 (80) | 10 (77) |
| Deceased donor | 2 (20) | 3 (23) |
| Cause of ESRD, n (%) | ||
| Unknown | 6 (60) | 6 (46) |
| Structural | 3 (30) | 5 (38) |
| Glomerulopathies | 1 (10) | 2 (16) |
| Mo post-transplant, median (25th, 75th percentile) | 11 (8, 34) | 10 (9, 23) |
| Baseline eGFR, ml/min per 1.73 m2 | 80±6 | 86±6 |
| Immunosuppression, n (%) | ||
| Sirolimus | 2 (20) | 2 (15) |
| Calcineurin inhibitor | 8 (80) | 12 (92) |
| Cyclosporine | 1 (13) | 0 (0) |
| Tacrolimus | 7 (88) | 11 (85) |
| Mycophenolate mofetil | 10 (100) | 13 (100) |
| Prednisone | 10 (100) | 12 (92) |
| Tacrolimus trough levels, ng/ml (mean±SD) | ||
| Baseline | 7.0±1.6 | 6.5±1.5 |
| 24 mo | 5.1±1.3 | 5.2±1.7 |
| Mycophenolate mofetil, mg/m2 (mean±SD) | ||
| Baseline | 732±113 | 708±97 |
| 24 mo | 718±68 | 698±125 |
| Prednisone (mg/kg) mean±SD | ||
| Baseline | 0.12±0.01 | 0.12±0.03 |
| 24 mo | 0.11±0.01 | 0.11±0.01 |
| History of acute rejection, n (%) | 7 (70) | 9 (69) |
| CMV risk, n (%) | ||
| Medium (D+/R+) | 10 (100) | 12 (92) |
| High (D+/R−) | 0 (0) | 1 (8) |
| Hospitalization during the study, n (%) | ||
| Positive CMV antigenemia | 1 (10) | 2 (15) |
| Gastroenteritis | 2 (20) | 1 (8) |
| Urinary tract infection | 1 (10) | 2 (15) |
| All | 4 (40) | 5 (38) |
CMV, cytomegalovirus.
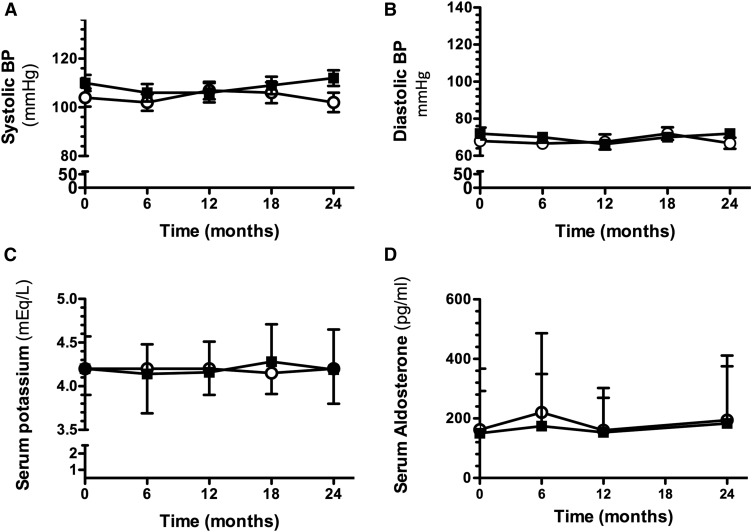
During the study follow-up, all of the children in both groups presented with normal systolic and diastolic BP, as illustrated in Figure 2, A and B. Serum potassium levels were monitored throughout the study; eplerenone did not alter them, and they were similar between groups (Figure 2C). Aldosterone levels also remained unaltered (Figure 2D). No adverse events attributed to eplerenone were observed throughout the study.
Figure 2.
Effect of long-term eplerenone administration on BP, serum potassium, and aldosterone levels in placebo- (○) or eplerenone-treated patients (■). (A) Systolic BP, (B) diastolic BP, (C) serum potassium levels, and (D) serum aldosterone levels. The data are expressed as mean±SD.
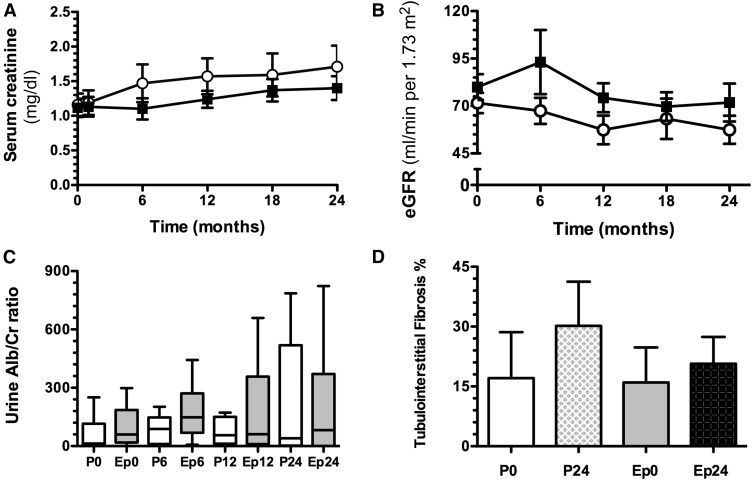
At the beginning of the study, both groups displayed similar serum creatinine levels, as shown in Figure 3A: 1.1±0.5 mg/dl in the placebo versus 1.1±0.5 mg/dl in the eplerenone group (P=0.84). Both groups exhibited greater levels of serum creatinine levels at 24 months, being more pronounced in the placebo group than in the eplerenone group, but the difference did not reach statistical significance (1.7±0.9 mg/dl versus 1.4±0.61 mg/dl [P=0.38; 95% confidence intervals at baseline, −0.9– to 0.05, and after 24 months, –0.7 to 1.2). The eGFR was 80±6 at baseline and progressively decreased to 74±7 at 6 months and to 66±8 ml/min per 1.73 m2 at 24 months in the placebo group. In contrast, eplerenone-treated children exhibited stable eGFR throughout the study: 86±6, 87±6, and 81±7 ml/min per 1.73 m2, however, the difference between groups was NS (RM ANOVA, P=0.33; 95% confidence intervals at baseline, −18 to 33, and after 24-months, −11 to 40). In the intention-to-treat analysis, we included the baseline values of the patient in the placebo group that was lost during the follow-up, and for missing observations the last value was carried forward; there were no statistically significant differences in the eGFR at 24 months (P=0.64). The albuminuria-to-creatinine ratio progressively increased in the placebo group (from 110±74 to 276±140 mg/g), as seen in Figure 3C, which contrasted with the effect observed in the children receiving eplerenone, in whom the ratio remained constant throughout the follow-up period (from 265±140 to 228±88 mg/g), despite higher levels at the beginning of the study. No differences were found between the groups along the study (RM ANOVA, P=0.15; 95% confidence intervals at baseline, −283 to 593, and after 24 months, −485 to 391). At the end of the study, the placebo group exhibited a trend toward higher percentage of TIF-affected area compared with the eplerenone group (P=0.06; 95% confidence intervals at baseline, −13.5 to 15.7, and after 24 months, 5.1 to 24.1, Figure 3D). The IF/TA and inflammation scores were similar between groups (Table 2).
Figure 3.
Long-term eplerenone administration effect on renal function and tubulo-interstitial fibrosis. (A) Serum creatinine, (B) eGFR by the Zappitelli formula, (C) urinary albumin-to-creatinine ratio, and (D) percentage of tubulo-interstitial fibrosis assessed by morphometry analysis in placebo- (○) or eplerenone-treated patients (■). The data are expressed as mean±SD, except for urinary albumin-to-creatinine ratio expressed as median and 25th–75th percentiles. Alb/Cr, albumine/creatinine ratio; Ep0, eplerenone month 0; Ep6, eplerenone month 6; Ep12, eplerenone month 12; Ep24, eplerenone month 24; P0, placebo month 0; P6, placebo month 6; P12, placebo month 12; P24, placebo month 24.
Table 2.
Classification of renal biopsy samples at baseline and 24 months of treatment according to Banff 2007 classification (23)
| Variable | Baseline, n (%) | 24 mo, n (%) |
|---|---|---|
| Placebo (n=10) | ||
| IF/TA | ||
| Grade I | 9 (90) | 6 (60) |
| Grade II | 0 | 3 (30) |
| Grade III | 1 (10) | 1 (10) |
| C4d staining | ||
| C4d0 | 10 (100) | 9 (90) |
| C4d1 | 0 | 0 |
| C4d2 | 0 | 1 (10) |
| C4d3 | 0 | 0 |
| Total inflammation | ||
| ti1 | 3 (30) | 3 (30) |
| ti2 | 0 | 0 |
| Eplerenone (n=13) | ||
| IF/TA | ||
| Grade I | 12 (92) | 10 (77) |
| Grade II | 1 (8) | 2 (15) |
| Grade III | 0 | 1 (8) |
| C4d staining | ||
| C4d0 | 13 (100) | 9 (69) |
| C4d1 | 0 | 0 |
| C4d2 | 0 | 3 (23) |
| C4d3 | 0 | 1 (8) |
| Total inflammation | ||
| ti1 | 5 (38.4) | 2 (15.3) |
| ti2 | 1 (8) | 1 (8) |
IFTA Grade I, <25% cortical area; II, 26%–50%; III, >50%. C4d staining, C4d0: negative, 0% biopsy sample; C4d1: minimal detection 1 <10%; C4d2: positive focal 10%–50%; C4d3 positive diffuse >50%. Scoring of total inflammation (ti) of renal allograft, ti1: 10%–25% of parenchyma inflamed, and ti2: 26%–50%. IF/TA, interstitial fibrosis/tubular atrophy.
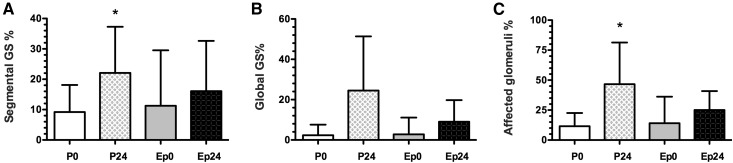
As shown in Figure 4, the placebo group exhibited a higher percentage of segmental GS (P=0.04; 95% confidence intervals at baseline, −38 to 33, and after 24 months, −12 to 55) and affected glomeruli (P=0.04; 95% confidence intervals at baseline, −23.4 to 22.6, and after 24 months, −6.3 to 37.0).
Figure 4.
Effect of long-term eplerenone treatment on glomerular injury. Glomerulosclerosis percentage was quantified in renal biopsy samples from placebo- or eplerenone-treated groups at 0 and 24 months. (A) FSGS, (B) global glomerulosclerosis, and (C) total glomerulosclerosis in placebo 0 (white bars), placebo 24 (squared bars), eplerenone 0 (gray bars), and eplerenone 24 (black bars). The data are expressed as mean±SD, *P<0.05 versus placebo 0 group. Ep0, eplerenone month 0; Ep24, eplerenone month 24; GS, glomerulosclerosis; P0, placebo month 0; P24, placebo month 24.
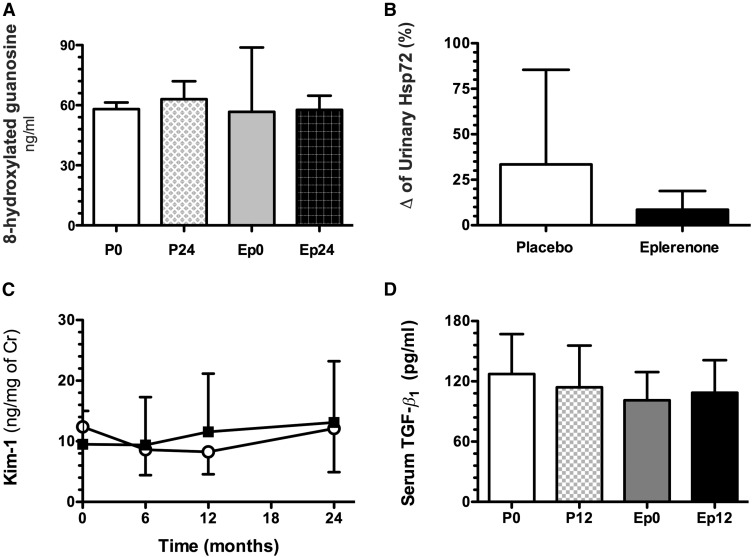
The placebo group showed higher 8-hydroxylated-guanosine levels after 24 months compared with the basal measurement (P=0.16). In contrast, in the patients treated with eplerenone, the urinary 8-hydroxylated-guanosine levels remained constant throughout the follow-up (Figure 5A), however, no difference between the groups was observed. In accordance with these findings, the percentage change from baseline levels of urinary HSP72 (25–27) show a trend to increase in the placebo compared with the eplerenone group (33.4±18.4% versus 8.5±3.0%, P=0.12, Figure 5B). No changes were observed in either urinary KIM-1 (Figure 5C) or serum TGF-β1 levels (Figure 5D).
Figure 5.
Long-term eplerenone administration on oxidative stress and urinary renal injury biomarkers. (A) 8-Hydroxylated guanosine (white bars) in placebo 24 (squared bars), eplerenone 0 (gray bars), and eplerenone 24 (black bars). (B) Percentage of change in urinary HSP72 compared with baseline in placebo (white bars) and eplerenone 24 (black bars). (C) Urinary KIM-1 in placebo- (○) or eplerenone-treated pediatric patients (■). (D) TGF-β1 at baseline and 12 months of treatment. The data are expressed as mean±SD. Cr, creatinine; Ep0, eplerenone month 0; Ep24, eplerenone month 24; P0, placebo month 0; P24, placebo month 24.
As a post hoc analysis, we calculated the power for the δ in eGFR (from baseline to 24 months) as primary outcome, and the δ for the following secondary outcomes: renal tissue fibrosis and urinary HSP72. We did not perform the analysis of the remaining secondary outcomes as there were no cases in any of the groups (as patient death or graft loss) or there were no differences among the groups. For the δ in eGFR, by the comparison of two group means method, the means for the placebo and eplerenone group were 15 and 5 ml/min per 1.73 m2, respectively, common SD 25, α 0.05. Considering a sample size of 13 patients, the calculated power was 26%; for the δ of renal fibrosis the means for the placebo and eplerenone group were 13% and 3.4%, respectively, common SD 11.5, α 0.05, and the calculated power 66%. For the δ of urinary HSP72, the means for the placebo and eplerenone group were 33.4% and 8.5%, respectively, common SD 52, α 0.05, and the calculated power 32%; therefore, the study was underpowered.
Discussion
In this small study, we found that the decline in eGFR and proteinuria was more pronounced in the placebo- than in the eplerenone-treated group; nevertheless, the differences were not statistically significant between the groups. However, the structural injury was significantly greater in the placebo-treated children.
In 1996, Hostetter and coworkers (28) showed for the first time that aldosterone plays a role in CKD in a model of subtotal nephrectomy in the rat. On the basis of this study, we and others have documented the effectiveness of MRB with spironolactone or eplerenone in reducing glomerular and tubulo-interstitial injury in several experimental models, including hypertensive rat (2,29–31), CsA nephrotoxicity (15,16,21), subtotal nephrectomy (32), CKD induced by ischemia/reperfusion injury (33,34), unilateral ureteral obstruction (35,36), and diabetic nephropathy (37,38).
Previous studies from our laboratory revealed the aldosterone involvement in kidney injury induced by ischemic processes such as CsA nephrotoxicity or by ischemia/reperfusion (15,16,18,21). In our first study, we showed that MRB reduced arteriolopathy and TIF in a chronic CsA nephrotoxicity model in the rat (15). Interestingly, the rats that received spironolactone were protected against renal dysfunction. Afterwards, we showed that MRB also prevented acute CsA nephrotoxicity, which is only characterized by renal vasoconstriction through the re-establishment of the balance of vasoactive pathways, such as nitric oxide and endothelin, as well as angiotensin and adenosine receptors (16). Another mechanism contributing to the vasoconstriction mediated by aldosterone is through reducing glucose-6-phosphate dehydrogenase (G6PD), a key enzyme that maintains the balance between nitric oxide and reactive oxygen species (39). Moreover, a recent report showed that the genetic deletion of MR in smooth muscle cells reduces the effect of CsA on increased vascular resistance in the kidney (17). Of note, the benefit of MRB was also observed in a model of preexisting chronic CsA nephrotoxicity in which spironolactone blunted the progression of kidney dysfunction and TIF induced by CsA (21). These findings pointed out the role of aldosterone in modulating vascular kidney tone, specifically in contributing to renal vasoconstriction, a hallmark of this type of nephropathy. Considering our experimental findings, we proposed this translational study, in which the effect of eplerenone treatment over 2 years in children with CAN was assessed.
Additionally, another important finding of this study was the trend observed in untreated patients, in which albuminuria increased throughout the follow-up, whereas in the eplerenone group it remained constant. A beneficial effect of MRB on proteinuria has been reported in several studies in the population of patients with CKD (40,41). This finding was also supported by the lower levels of TIF and GS exhibited in the eplerenone than in the placebo group. Similarly, Piecha et al. demonstrated that MRB not only prevented but also reversed GS induced by subtotal nephrectomy in the rat (32).
Furthermore, in a previous pilot study, we found that in the acute phase after renal transplantation in patients receiving a kidney from a living donor, spironolactone treatment showed a benefit in reducing oxidative stress (22). In accordance with this study, we found that urinary 8-hydroxylated guanosine levels, a marker of DNA oxidative damage, tended to increase in the placebo group by the end of the study, and the values were higher than in the eplerenone group. This marginal effect might be explained by two factors: the chronic contribution of immune players to the development of kidney fibrosis independent of the MRB, and the low numbers of children included.
In the clinical setting, there is concern regarding the use of MR antagonists in patients with CKD due to the risk of hyperkalemia. Therefore, we decided to use a fixed dose of 25 mg/d in renal transplant recipients with an eGFR of at least 60 ml/min per 1.73 m2. By using this dose, we did not observe significant changes in serum potassium values throughout the study. Moreover, eplerenone has a lower affinity than spironolactone for progestere, androgen, and estrogen receptors, and therefore presents a lower risk of sexual adverse events such as menstrual disorders and gynecomastia (42,43). Despite being more expensive, these aspects must be considered when administering an MRB to children long-term. Supporting our findings regarding the safety of eplerenone administration in patients with reduced renal function, our previous study in kidney transplant patients and a recent report showed that no severe hyperkalemic events were found when spironolactone or eplerenone was administered at a dose of 25 mg/d for 5 days or 8 weeks, respectively (22,44). In addition, new nonsteroidal MR blockers, such as finerenone, have been demonstrated to reduce the risk of hyperkalemic events, although they have not yet been used in pediatric patients. Therefore, a similar study must be performed to assess their efficacy in improving renal function in children with CAN (45).
The limitations of our study include the small sample size, the fixed eplerenone dose that was not weight-adjusted, and the lack of HLA antibody measurement.
Although this study was underpowered to provide definitive evidence that eplerenone attenuates the progression of CAN, we consider these results encouraging to continue testing the potential benefit of MRB in these pediatric patients.
Disclosures
None.
Acknowledgments
We thank Dr. Rodolfo Rivas Ruiz for his valuable support in the statistical analysis.
This project was supported by grants from the Mexican Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología: 087381 to M.M. and 235855, 181267, and 272390 to N.A.B.) and by the National University of Mexico (Universidad Nacional Autónoma de México:IN223915 to N.A.B.).
The results presented in this paper have not been published previously in whole or in part, except as an abstract presented at the American Transplant Congress, May 1–5, 2010 (San Diego, CA) and the International Pediatric Transplant Association Congress, July 13–16, 2013 (Warsaw, Poland).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Haas M: Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: What is in a name? Curr Opin Nephrol Hypertens 23: 245–250, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto M, Amer H, Textor S, Schwab T, Cosio FG: The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 11: 698–707, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Viero RM, da Silva MG, dos Santos DC, de Carvalho MF, de Andrade LG: The role of renin-angiotensin system in the chronic allograft nephropathy: An immunohistochemical study. Ren Fail 37: 827–834, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Abramowicz D, Wissing KM, Broeders N: Nephrotoxicity of calcineurin inhibitors: New therapeutic approaches. Transplant Proc 32[1A Suppl]: 3S–5S, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bobadilla NA, Gamba G: New insights into the pathophysiology of cyclosporine nephrotoxicity: A role of aldosterone. Am J Physiol Renal Physiol 293: F2–F9, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Jaisser F, Farman N: Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacol Rev 68: 49–75, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Wolf G: Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int 70: 1914–1919, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y: Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol 294: F1287–F1295, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, López-Andrés N: Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 33: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Görlach A, Wulff P, Daut J, Dalton ND, Ross J Jr., Flögel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F: SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med (Berl) 84: 396–404, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Irita J, Okura T, Kurata M, Miyoshi K, Fukuoka T, Higaki J: Osteopontin in rat renal fibroblasts: Functional properties and transcriptional regulation by aldosterone. Hypertension 51: 507–513, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kadoya H, Satoh M, Sasaki T, Taniguchi S, Takahashi M, Kashihara N: Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J 29: 3899–3910, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Yamahara H, Kishimoto N, Nakata M, Okazaki A, Kimura T, Sonomura K, Matsuoka E, Shiotsu Y, Adachi T, Matsubara H, Iwasaka T, Mori Y: Direct aldosterone action as a profibrotic factor via ROS-mediated SGK1 in peritoneal fibroblasts. Kidney Blood Press Res 32: 185–193, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Feria I, Pichardo I, Juárez P, Ramírez V, González MA, Uribe N, García-Torres R, López-Casillas F, Gamba G, Bobadilla NA: Therapeutic benefit of spironolactone in experimental chronic cyclosporine A nephrotoxicity. Kidney Int 63: 43–52, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Rojas JM, Derive S, Blanco JA, Cruz C, Martínez de la Maza L, Gamba G, Bobadilla NA: Renocortical mRNA expression of vasoactive factors during spironolactone protective effect in chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol 289: F1020–F1030, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, Berger S, Warnock DG, Chatziantoniou C, Jaffe IZ, Rieu P, Jaisser F: Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int 89:354–362, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mejía-Vilet JM, Ramírez V, Cruz C, Uribe N, Gamba G, Bobadilla NA: Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol 293: F78–F86, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Pozos K, Barrera-Chimal J, Garzón-Muvdi J, Pérez-Villalva R, Rodríguez-Romo R, Cruz C, Gamba G, Bobadilla NA: Recovery from ischemic acute kidney injury by spironolactone administration. Nephrol Dial Transplant 27: 3160–3169, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Barrera-Chimal J, Prince S, Fadel F, El Moghrabi S, Warnock DG, Kolkhof P, Jaisser F: Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol 27: 398–404, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA: Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol 292: F131–F139, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ojeda-Cervantes M, Barrera-Chimal J, Alberú J, Pérez-Villalva R, Morales-Buenrostro LE, Bobadilla NA: Mineralocorticoid receptor blockade reduced oxidative stress in renal transplant recipients: A double-blind, randomized pilot study. Am J Nephrol 37: 481–490, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L: Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis 48: 221–230, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Barrera-Chimal J, Pérez-Villalva R, Cortés-González C, Ojeda-Cervantes M, Gamba G, Morales-Buenrostro LE, Bobadilla NA: Hsp72 is an early and sensitive biomarker to detect acute kidney injury. EMBO Mol Med 3: 5–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales-Buenrostro LE, Salas-Nolasco OI, Barrera-Chimal J, Casas-Aparicio G, Irizar-Santana S, Pérez-Villalva R, Bobadilla NA: Hsp72 is a novel biomarker to predict acute kidney injury in critically ill patients. PLoS One 9: e109407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega-Trejo JA, Pérez-Villalva R, Barrera-Chimal J, Carrillo-Pérez DL, Morales-Buenrostro LE, Gamba G, Flores ME, Bobadilla NA: Heat shock protein 72 (Hsp72) specific induction and temporal stability in urine samples as a reliable biomarker of acute kidney injury (AKI). Biomarkers 20: 453–459, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr.: Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 31: 451–458, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Rocha R, Chander PN, Zuckerman A, Stier CT Jr.: Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33: 232–237, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L: Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63: 797–803, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak M, Ritz E: Regression of glomerulosclerosis in subtotally nephrectomized rats: Effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol Renal Physiol 295: F137–F144, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Barrera-Chimal J, Pérez-Villalva R, Ortega JA, Sánchez A, Rodríguez-Romo R, Durand M, Jaisser F, Bobadilla NA: Mild ischemic injury leads to long-term alterations in the kidney: Amelioration by spironolactone administration. Int J Biol Sci 11: 892–900, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, Reyna J, Uribe N, Gamba G, Bobadilla NA: Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int 83: 93–103, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Trachtman H, Weiser AC, Valderrama E, Morgado M, Palmer LS: Prevention of renal fibrosis by spironolactone in mice with complete unilateral ureteral obstruction. J Urol 172: 1590–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Sun F, Zhong X, Shao Y, Yoshimura A, Liu Y: Eplerenone-mediated aldosterone blockade prevents renal fibrosis by reducing renal inflammation, interstitial cell proliferation and oxidative stress. Kidney Blood Press Res 37: 557–566, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR: Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol 17: 1362–1372, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK: Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 147: 5363–5373, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J: Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med 13: 189–197, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuboi N, Kawamura T, Okonogi H, Ishii T, Hosoya T: The long-term antiproteinuric effect of eplerenone, a selective aldosterone blocker, in patients with non-diabetic chronic kidney disease. J Renin Angiotensin Aldosterone Syst 13: 113–117, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Sengul E, Sahin T, Sevin E, Yilmaz A: Effect of spironolactone on urinary protein excretion in patients with chronic kidney disease. Ren Fail 31: 928–932, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Mulatero P, Milan A, Williams TA, Veglio F: Mineralocorticoid receptor blockade in the protection of target organ damage. Cardiovasc Hematol Agents Med Chem 4: 75–91, 2006 [DOI] [PubMed] [Google Scholar]
- 43.McManus F, McInnes GT, Connell JM: Drug insight: Eplerenone, a mineralocorticoid-receptor antagonist. Nat Clin Pract Endocrinol Metab 4: 44–52, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Bertocchio JP, Barbe C, Lavaud S, Toupance O, Nazeyrollas P, Jaisser F, Rieu P: Safety of eplerenone for kidney-transplant recipients with impaired renal function and receiving cyclosporine A. PLoS One 11: e0153635, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE: Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail 18: 28–37, 2016 [DOI] [PubMed] [Google Scholar]