Abstract
Background and objectives
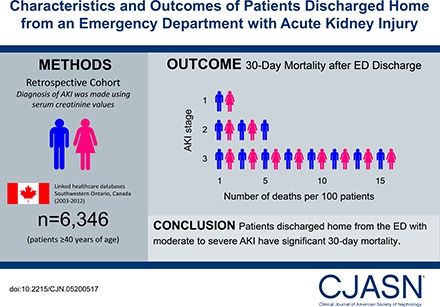
Patients discharged home from an emergency department with AKI are not well described. This study describes their characteristics and outcomes and compares these outcomes to two referent groups.
Design, setting, participants, & measurements
We conducted a population-based retrospective cohort study in Ontario, Canada from 2003 to 2012 of 6346 patients aged ≥40 years who were discharged from the emergency department with AKI (defined using serum creatinine values). We analyzed the risk of all-cause mortality, receipt of acute dialysis, and hospitalization within 30 days after discharge. We used propensity score methods to compare all-cause mortality to two referent groups. We matched 4379 discharged patients to 4379 patients who were hospitalized from the emergency department with similar AKI stage. We also matched 6188 discharged patients to 6188 patients who were discharged home from the emergency department with no AKI.
Results
There were 6346 emergency department discharges with AKI. The mean age was 69 years and 6012 (95%) had stage 1, 290 (5%) had stage 2, and 44 (0.7%) had stage 3 AKI. Within 30 days, 149 (2%) (AKI stage 1: 127 [2%]; stage 2: 15 [5%]; stage 3: seven [16%]) died, 22 (0.3%) received acute dialysis, and 1032 (16%) were hospitalized. An emergency department discharge versus hospitalization with AKI was associated with lower mortality (3% versus 12%; relative risk, 0.3; 95% confidence interval, 0.2 to 0.3). An emergency department discharge with AKI versus no AKI was associated with higher mortality (2% versus 1%; relative risk, 1.6; 95% confidence interval, 1.2 to 2.0).
Conclusions
Patients discharged home from the emergency department with AKI are at risk of poor 30-day outcomes. A better understanding of care in this at-risk population is warranted, as are testing strategies to improve care.
Keywords: Acute Kidney Injury; Aged; Canada; Confidence Intervals; creatinine; Emergency Service, Hospital; hospitalization; Humans; Kidney Function Tests; Ontario; Patient Discharge; Propensity Score; renal dialysis; Retrospective Studies; Risk
Introduction
AKI, defined as a sudden deterioration in kidney function, affects approximately 10% of hospitalized patients worldwide (1–3). Among hospitalized patients, AKI is associated with increased morbidity, mortality, and health care costs exceeding $10 billion annually in the United States (4–7). Furthermore, survivors of AKI hospitalization are at increased risk of cardiovascular disease (8), CKD (9), and ESRD (10,11).
AKI epidemiology is largely informed by studies conducted in hospitalized and critically ill patients (12). Less is known about patients who develop AKI as outpatients or who present to the emergency department (ED) with AKI and are managed in the community (13). ED visits are brief, and health care staff must decide whether a patient should be admitted to hospital or discharged home. A discharge home may mean the ED health care staff felt the AKI was reversible and could be managed in the community or it may represent an underappreciated population at risk of poor outcomes. We performed a detailed search of PubMed and other bibliographic databases in March of 2016 and found no study describing this population in detail.
We conducted this study to describe the characteristics and outcomes of patients discharged home from the ED with AKI and to compare outcomes to two referent ED groups: patients hospitalized with AKI and patients discharged home with no AKI. We hypothesize that an ED discharge with AKI will have lower risk of adverse outcomes compared with hospitalization with AKI, and a higher risk compared with an ED discharge with no AKI.
Materials and Methods
Study Design and Setting
We conducted a population-based, retrospective cohort study of adults in Southwestern Ontario, Canada, at the Institute for Clinical Evaluative Sciences Western facility. Our study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre in Toronto, Ontario. Southwestern Ontario has approximately 1.6 million residents with universal access to hospital care and physician services (14). Prescription drug coverage is a universal benefit for those aged ≥65 years. Participant informed consent was not required for this study. This study followed the STrengthening the Reporting of OBservational studies in Epidemiology guidelines (Supplemental Table 1) (15). To comply with privacy regulations for minimizing the chance of patient reidentification, results were suppressed in cells with five or fewer patients.
Data Sources
We ascertained patient and hospital characteristics, drug prescriptions, and outcome data from 13 health administrative databases (Supplemental Table 2). These datasets were linked using unique, encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences Western facility. We obtained vital statistics on all Ontario residents who were issued a health card from the Ontario Registered Persons Database. The Registered Persons Database mortality flag has a sensitivity of 94% and a positive predictive value of 100% (16). We used the Ontario Drug Benefit database to identify prescription drugs for eligible patients or those aged ≥65 years with an error rate <1% (17). We identified diagnostic and procedural information from the National Ambulatory Care Reporting System (ED visits) and the Canadian Institute for Health Information Discharge Abstract Database (hospitalizations). We used the International Classification of Diseases, ninth (pre-2002) and tenth revision (post-2002) codes to assess baseline comorbidities in the 5 years before the index date (Supplemental Table 3). We used the Ontario Health Insurance Plan database for health claims for physician services (Supplemental Tables 4–6). We used the Institute for Clinical Evaluative Sciences Physician Database to ascertain information on physician specialty. We derived total health care costs from several Institute for Clinical Evaluative Sciences databases (Supplemental Table 6) (18).
We obtained outpatient, ED, and inpatient serum creatinine, sodium, and potassium measurements from 13 hospitals (Supplemental Table 7) in Southwestern Ontario sharing the same electronic health record (Cerner, MO). Data are only available for patients aged ≥40 years. We also obtained outpatient serum creatinine and urine protein (albumin, total protein, and dipstick) measurements from Dynacare Medical Laboratories, which represents approximately one third of outpatient laboratory testing for Ontario residents. Dynacare does not have test results from the ED or hospital.
Patients
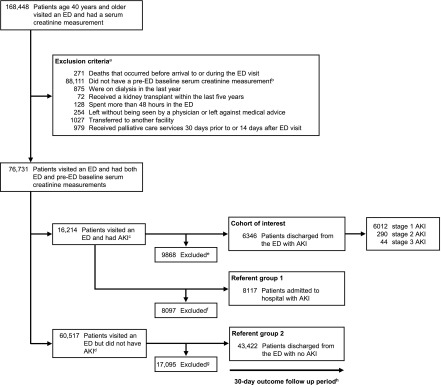
We established a cohort of Ontario residents aged ≥40 years who visited an ED and had at least one serum creatinine measurement at the ED visit and 7–365 days before the ED visit (pre-ED baseline) (Figure 1). We excluded patients who: (1) died on arrival or during the ED visit; (2) received dialysis 1 year before the ED visit (because AKI would not be relevant on dialysis and to ensure stable kidney function after the discontinuation of dialysis); (3) received a kidney transplant in the 5 years before the ED visit (to ensure AKI was not related to transplant rejection); (4) spent >48 hours in the ED (to exclude those without a disposition plan); (5) left against medical advice or without being seen by an ED physician; (6) were transferred to another facility; or (7) received palliative care 30 days before or 14 days after the ED visit (to exclude those who did not receive active medical management). The ED visit date served as the index or cohort entry date. If an individual had multiple ED serum creatinine measurements, we selected the highest value. If multiple pre-ED baseline serum creatinine values were available, we selected the most recent one.
Figure 1.
Study flow diagram. aPatients were excluded in order as listed. bWe selected the most recent pre-ED baseline serum creatinine measurement. cIf an individual had more than one ED visit with AKI, as defined by ED and pre-ED serum creatinine measurements, we selected the first ED visit with AKI. dIf an individual had more than one ED visit with a serum creatinine measurement, we selected the first ED visit. This group does not include patients with a subsequent ED visit with AKI (n=9549); these patients were preferentially classified as having AKI. eExcluded: improvement in AKI severity (discharged n=447, admitted n=1248); admitted to hospital (n=8117); assigned an ED diagnosis of AKI (International Classification of Diseases, Tenth Revision, code N17) (n=56). fExcluded: patients with an improvement in AKI severity (n=1695); discharged home from ED (n=6402). gExcluded: patients admitted to hospital from the ED with no AKI. hFollow-up was complete during the 30-day period. ED, emergency department.
Patients were assigned to one of three groups on the basis of AKI and ED disposition: (1) discharge home with AKI, (2) hospitalization with AKI, or (3) discharge home with no AKI (Figure 1). There was no crossover of patients between groups. Preference was given to the group discharged home from the ED with AKI if patients were also eligible for one of the other two groups. For all ED visits with AKI, we excluded patients with an improvement in AKI severity during the ED visit and those assigned AKI as the main ED diagnosis, in order to concentrate on patients less likely to be treated and resolved before discharge. For ED visits with no AKI, we selected the first ED visit in which the individual had both ED and pre-ED baseline serum creatinine measurements.
Kidney Function
AKI was defined as a relative increase in serum creatinine by ≥50%, or an absolute increase of ≥0.3 mg/dl from the pre-ED baseline. Patients were identified and staged according to AKI severity as outlined in the 2012 Kidney Disease: Improving Global Outcomes guidelines (Supplemental Table 8) (19). Urine output was not available in our data sources. Pre-ED baseline serum creatinine measurements were chosen no earlier than 7 days before the ED visit to avoid potentially unstable baseline values. Baseline kidney function was reported as the eGFR (in ml/min per 1.73 m2), derived from the CKD Epidemiology Collaboration equation (20). We stratified baseline kidney function into five CKD groups (21): CKD stage 1, 2, or normal kidney function (eGFR≥60 ml/min per 1.73 m2); stage 3a (eGFR of 45 to <60 ml/min per 1.73 m2); stage 3b (eGFR of 30 to <45 ml/min per 1.73 m2); stage 4 (eGFR of 15 to <30 ml/min per 1.73 m2); and stage 5 (eGFR<15 ml/min per 1.73 m2 when not on dialysis).
Outcomes
In the descriptive analysis, the outcomes assessed were all-cause mortality, receipt of hospital-based acute dialysis, hospitalization, at least one outpatient physician visit (family physician, internist, nephrologist, or urologist), at least one outpatient serum creatinine or urine protein test, and total health care costs, all within 30 days of ED discharge (outcome codes defined in Supplemental Table 6). Health care costs were reported in Canadian dollars and adjusted for inflation to 2013. In the two subpopulation analyses, the primary outcome was all-cause mortality and the secondary outcome was hospital-based acute dialysis, all within 30 days of ED discharge.
Statistical Analyses
We compared patients discharged home from the ED with AKI to two separate propensity score-matched referent ED groups: (1) patients hospitalized with AKI (AKI subpopulation) and (2) patients discharged home with no AKI (discharge subpopulation) (Supplemental Figure 1).
In the AKI subpopulation, we matched each patient from our discharged home cohort to one patient admitted to hospital from the ED with AKI on the logit of the propensity score and AKI stage. In the discharged subpopulation, we matched each patient to one patient discharged from the ED with no AKI on the logit of the propensity score and baseline CKD stage. We derived the propensity scores from logistic regression models and included 92 and 91 baseline variables for the AKI and discharged subpopulations, respectively (Supplemental Table 7). We included Ontario Drug Benefit eligibility in the propensity score to account for individuals who did not have provincial drug coverage. We used greedy, nearest neighbor matching (without replacement) with a specified caliper width ±0.2 times the SD of the logit of the propensity score (22).
Serum sodium and potassium values were missing in <10% of patients. We performed single imputation using the median value (23). Income quintile and rural residency status were missing in <2% of patients. We imputed the middle quintile for income and “no” for rural residency status.
Baseline characteristics included demographic information, comorbidities, prior health care utilization, medications dispensed, laboratory results, and ED characteristics. We compared characteristics using standardized differences to assess balance after propensity score matching because this metric is less sensitive to large sample sizes (24,25). A standardized difference of ≥10% was considered clinically meaningful (26). After matching, we used modified Poisson regression to express risk in relative terms, account for matched data, and test for interaction (27,28). We defined statistical significance as a two-sided P value of <0.05.
Results
Characteristics of Patients Discharged Home from the ED with AKI
Cohort selection is presented in Figure 1. There were 6346 patients discharged home from the ED with AKI included in the cohort, of which 6012 (95%) had stage 1, 290 (5%) had stage 2, and 44 (0.7%) had stage 3 AKI. Selected characteristics of this cohort are shown in Table 1 (additional characteristics in Supplemental Table 9). The mean age was 69 years and 47% were women. The most common preexisting comorbidities were hypertension (75%), diabetes (38%), and coronary artery disease (34%). Baseline medication information was available for 73% of patients. Commonly prescribed medications in the 120 days before the ED visit were angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (60%), antibiotics (44%), nonpotassium-sparing diuretics (57%), and nonsteroidal anti-inflammatory drugs (19%). CKD, defined by a baseline eGFR of <60 ml/min per 1.73 m2, was present in 38% of patients. Compared with patients with stage 1 AKI, patients with stage 2 or 3 AKI were more likely to be women, reside in long-term care, or a have lower baseline serum creatinine value; they were less likely to have coronary artery disease, heart failure, CKD, or be prescribed β-blockers, calcium channel blockers, lithium, oral hypoglycemic agents, or insulin. The three most frequent main diagnoses assigned by ED physicians were throat or chest pain (9%), abdominal or pelvic pain (7%), and renal colic (5%) (Supplemental Table 10).
Table 1.
Baseline characteristics of patients discharged home from the emergency department (ED) with AKI
| Characteristic | Patients Discharged Home from the ED with AKI, n (%)a | |||
|---|---|---|---|---|
| All Patients | AKI Stageb | |||
| 1 | 2 | 3 | ||
| Cohort size | 6346 | 6012 | 290 | 44 |
| Age, yr, mean (SD) | 69 (13) | 69 (13) | 68 (14) | 65 (13)ठ|
| 40 to <65 | 2326 (37) | 2189 (36) | 112 (39) | 25 (57)ठ|
| 65 to <80 | 2475 (39) | 2351 (39) | 114 (39) | 10 (23)ठ|
| ≥80 | 1545 (24) | 1472 (25) | 64 (22) | 9 (21) |
| Women | 2948 (47) | 2745 (46) | 177 (61)† | 26 (59)‡ |
| Year of cohort entry (index date) | ||||
| 2003–2005 | 1593 (25) | 1497 (25) | 87 (30)† | 9 (21)‡§ |
| 2006–2008 | 2903 (46) | 2769 (46) | 116 (40)† | 18 (41)‡ |
| 2009–2011 | 1850 (29) | 1746 (29) | 87 (30) | 17 (39)‡§ |
| Rural residence | 969 (15) | 912 (15) | 51 (18) | 6 (14)§ |
| Comorbid conditionsc | ||||
| Hypertension | 4783 (75) | 4525 (75) | 222 (77) | 36 (82)ठ|
| Diabetes | 2405 (38) | 2292 (38) | 100 (35) | 13 (30)ठ|
| Heart failure | Not reported | 1325 (22) | 48 (17)† | ≤5 (≤11)‡§ |
| Coronary artery diseased | 2160 (34) | 2069 (34) | 78 (27)† | 13 (30)‡ |
| Chronic liver disease | Not reported | 399 (7) | 24 (8) | ≤5 (≤11)§ |
| Nephrolithiasis | Not reported | 162 (3) | 11(4) | ≤5 (≤11)‡§ |
| Aggregated diagnosis group point scoree | ||||
| 0–5 | 1756 (28) | 1669 (28) | 79 (27) | 9 (21)‡§ |
| ≥6 | 4589 (72) | 4343 (72) | 211 (73) | 35 (80)‡§ |
| Medication utilizationf | ||||
| Ontario Drug Benefit eligible patients | 4605 (73) | 4367 (73) | 213 (73) | 25 (57)ठ|
| Medication classg | ||||
| Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers | 2750/4605 (60) | 2614/4367 (60) | 122/213 (57) | 14/25 (56) |
| Antibiotics | 2041/4605 (44) | 1931/4367 (44) | 97/213 (46) | 13/25 (52)ठ|
| Antipsychotic medications | Not reported | 284/4367 (7) | 18/213 (9) | ≤5/25 (≤20) |
| Nonpotassium-sparing diuretics | 2609/4605 (57) | 2465/4367 (56) | 128/213 (60) | 16/25 (64)‡ |
| Potassium-sparing diuretics | Not reported | 511/4367 (12) | 34/213 (16)† | ≤5/25 (≤20)‡§ |
| Nonsteroidal anti-inflammatory drugsh | 892/4605 (19) | 849/4367 (19) | 34/213 (16) | 9/25 (36)ठ|
| No. of unique drug names, median (interquartile range) | 5 (0–10) | 5 (0–10) | 5 (0–10) | 0 (0–7) |
| Pre-ED baseline kidney functioni | ||||
| Baseline serum creatinine in mg/dl, mean (SD) | 1.1 (0.6) | 1.2 (0.6) | 0.9 (0.3)† | 1.1 (1.0)§ |
| No. of d taken before ED visit, mean (SD) | 133 (102) | 132 (102) | 140 (105) | 152 (116)ठ|
| No. with serum creatinine ≤0.6 mg/dl | Not reported | 215 (4) | 44 (15)† | ≤5 (≤11)‡§ |
| Baseline eGFR, ml/min per 1.73 m2 | ||||
| ≥60 | 3919 (62) | 3654 (61) | 230 (80)† | 35 (80)‡ |
| 45 to <60 | Not reported | 1029 (17) | 34 (12)† | ≤5 (≤11)‡§ |
| 30 to <45 | Not reported | 833 (14) | 19 (7)† | ≤5 (≤11)‡ |
| <30 | Not reported | 496 (8) | 7 (2)† | ≤5 (≤11)§ |
| Albumin-to-creatinine ratio, mg/g | 1689 (27) | 1602 (27) | 77 (27) | 10 (23) |
| <30 | Not reported | 959 (16) | 49 (17) | ≤5 (≤11)‡§ |
| 30 to <300 | Not reported | 262 (4) | 13 (5) | ≤5 (≤11) |
| ≥300 | Not reported | 381 (6) | 15 (5) | ≤5 (≤11) |
| ED visit laboratory values | ||||
| Serum potassium in mEq/L, mean (SD) | 4.2 (0.6) | 4.2 (0.6) | 4.1 (0.8)† | 4.2 (0.8)§ |
| Serum sodium in mEq/L, mean (SD) | 137 (5) | 137 (5) | 136 (5)† | 134 (5)‡§ |
| Serum creatinine in mg/dl | ||||
| Mean (SD) | 1.7 (0.8) | 1.6 (0.7) | 2.0 (0.8)† | 3.8 (2.1)‡§ |
| Median (interquartile range) | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 1.8 (1.4–2.3) | 3.4 (2.3–4.8) |
| Absolute change in serum creatinine in mg/dl | ||||
| Mean (SD) | 0.5 (0.4) | 0.5 (0.2) | 1.1 (0.5)† | 2.7 (1.4)‡§ |
| Median (interquartile range) | 0.4 (0.4–0.6) | 0.4 (0.4–0.5) | 1.0 (0.8–1.3) | 2.5 (1.6–3.7) |
| Percent change in serum creatinine, % | ||||
| Mean (SD) | 52 (34) | 48 (18) | 129 (26)† | 294 (128)‡§ |
| Median (interquartile range) | 45 (35–60) | 44 (34–57) | 121 (108–144) | 265 (224–359) |
| Previous health care utilizationj | ||||
| ED visit in the previous 30 d | 1114 (18) | 1062 (18) | 45 (16) | 7 (16) |
| Hospitalization in the previous 30 d | 481 (8) | 451 (8) | 24 (8) | 6 (14)ठ|
| Family physician visit | 6251 (99) | 5924 (99) | 283 (98) | 44 (71)ठ|
| General internist visit | 1470 (23) | 1385 (23) | 76 (26) | 9 (21)§ |
| Nephrology visit | Not reported | 270 (5) | 14 (5) | ≤5 (≤11) |
| Urology visit | 1132 (18) | 1084 (18) | 40 (14)† | 8 (18)§ |
| Imaging and investigations | ||||
| Computed tomography with contrast | Not reported | 175 (3) | 7 (2) | ≤5 (≤11) |
| Coronary angiogram or revascularization | Not reported | 138 (2) | 6 (2) | ≤5 (≤11)‡§ |
| ED patient acuity and wait times | ||||
| Canadian triage acuity scalek | ||||
| 1 and 2 | 1321 (21) | 1271 (21) | 43 (15)† | 7 (16)‡ |
| 3 | 3797 (60) | 3579 (60) | 193 (67)† | 25 (57)§ |
| 4 and 5 | 1228 (19) | 1162 (19) | 54 (19) | 12 (27)ठ|
| Time (in hr) waiting for physician assessment, mean (SD) | ||||
| Canadian triage acuity scale 1 and 2 | 0.6 (0.8) | 0.6 (0.8) | 0.5 (0.6)† | 0.3 (0.5)‡§ |
| Canadian triage acuity scale 3 | 1.3 (1.3) | 1.3 (1.3) | 1.5 (1.3) | 1.6 (1.1)ठ|
| Canadian triage acuity scale 4 and 5 | 1.6 (1.4) | 1.6 (1.4) | 1.7 (1.4) | 1.4 (1.2)ठ|
A standardized difference of ≥10% was found between the following two AKI subgroup comparisons: †stage 1 versus stage 2; ‡stage 1 versus stage 3; and §stage 2 versus stage 3. To convert serum creatinine from traditional units (milligram per deciliter) to SI units (micromole), multiply by 88.42.
Reported as n (%) unless otherwise noted. To comply with privacy regulations for minimizing the chance of patient reidentification, numbers of patients were suppressed in five or fewer patients. The total number of patients was not reported if there were other calculations that could result in the reidentification of five or fewer patients.
Stage 1, evidence of a relative increase in serum creatinine value of ≥50% to <100% or ≥0.3 mg/dl from baseline; stage 2, evidence of a relative increase in serum creatinine value of ≥100% to <200% from baseline; and stage 3, evidence of a relative increase in serum creatinine value of >200% from baseline or an absolute increase in serum creatinine value to ≥4 mg/dl or the initiation of dialysis.
Look-back window for comorbidities was 5 years unless otherwise noted.
Does not include angina.
The Aggregated Diagnosis Groups point score, derived from the John Hopkins Adjusted Clinical Groups system, is a weighted measure of health care utilization as a proxy measure for comorbidity and accounts for the duration of condition, severity of condition, diagnostic certainty, etiology of the condition, and specialty care involvement. The higher Aggregated Diagnosis Groups score, the greater the comorbidity. Individuals with an Aggregated Diagnosis Groups score of 0–2 reflect low health care costs with no prior hospitalizations; 3–5 reflects high health care costs but no prior hospitalizations; and a score of ≥6 reflects high health care costs and at least one prior hospitalization.
Look-back window for medication utilization was 120 days.
Percentages reported are on the basis of the number of Ontario Drug Benefit eligible patients (those aged ≥65 years).
Does not include acetylsalicylic acid.
Pre-ED visit look-back window was 7–365 days.
Look-back window for health care utilization was 365 days unless otherwise noted.
Patients with a Canadian Triage Acuity Scale score of 1 or 2 need to be seen immediately 98% of the time or within 15 minutes 95% of the time, respectively. Patients with a Canadian Triage Acuity Scale score of 3 need to be seen within 30 minutes 90% of the time or 60 minutes 85% of the time, respectively. Patients with a Canadian Triage Acuity Scale score of 4 or 5 need to be seen within 120 minutes 80% of the time.
Outcomes
During the 30 days after an ED discharge with AKI, 149 (2%) patients died, 22 (0.3%) received hospital-based acute dialysis, and 1032 (16%) required hospitalization (Table 2). There were 4287 (68%) patients who were seen at least once by a physician as an outpatient. Outpatient serum creatinine testing occurred at least once in 1446 (23%) patients. Outpatient urine protein testing occurred in at least 11% of patients. When assessing patients by AKI severity, 127 (2%) with stage 1, 15 (5%) with stage 2, and seven (16%) with stage 3 AKI died within 30 days of ED discharge. Compared with patients with stage 1 AKI, the likelihood of hospitalization and total health care costs were higher in patients with stage 2 or 3 AKI.
Table 2.
Thirty-day outcomes of patients discharged home from the emergency department (ED) with AKI
| Outcome | Patients Discharged Home from the ED with AKI, n (%)a | |||
|---|---|---|---|---|
| All Patients (n=6346) | AKI Stageb | |||
| 1 (n=6012) | 2 (n=290) | 3 (n=44) | ||
| All-cause mortality | 149 (2) | 127 (2) | 15 (5) | 7 (16) |
| Receipt of hospital-based acute dialysis | 22 (0.3) | Not reported | ≤5 (≤2) | ≤5 (≤11) |
| At least one hospitalization | 1032 (16) | 956 (16) | 62 (21) | 14 (32) |
| At least one outpatient | ||||
| Physician clinic visitc | 4287 (68) | 4062 (68) | 197 (68) | 28 (64) |
| Serum creatinine test | 1446 (23) | 1339 (22) | 89 (31) | 18 (41) |
| Urine test for proteind | Not reported | 713 (12) | 41 (14) | ≤5 (≤11) |
| Total health care costs, $e | ||||
| Mean (SD) | 3522 (7079) | 3499 (7135) | 3856 (6065) | 4429 (5454) |
| Median (interquartile range) | 1172 (661–3020) | 1164 (657–2955) | 1342 (749–4372) | 1748 (699–6478) |
Reported as n (%) unless otherwise noted. To comply with privacy regulations for minimizing the chance of patient reidentification, numbers of patients were suppressed in five or fewer patients. The total number of patients was not reported if there were other calculations that could result in the reidentification of five or fewer patients.
AKI stage 1, evidence of a relative increase in serum creatinine value of ≥50% to <100% or ≥0.3 mg/dl from baseline; stage 2, evidence of a relative increase in serum creatinine value of ≥100% to <200% from baseline; and stage 3, evidence of a relative increase in serum creatinine value of >200% from baseline or an absolute increase in serum creatinine value to ≥4 mg/dl or the initiation of dialysis.
Seen by any one of the following physicians as outpatient: family medicine, internal medicine, nephrology, or urology.
Urine protein tests included any one of dipstick, protein, or albumin-to-creatinine ratio.
Reported in Canadian dollars, adjusted for inflation to 2013.
AKI Subpopulation
Among patients who had AKI upon presentation to the ED, we matched 4379 patients discharged home to 4379 patients whose ED visit resulted in hospitalization (Table 3). There were 4091 (93%) patients with stage 1, 244 (6%) with stage 2, and 44 (1%) with stage 3 AKI. Groups were balanced for 91 out of 92 characteristics (Supplemental Table 11). The primary outcome of 30-day all-cause mortality is presented in Table 3. Compared with patients admitted to hospital with AKI, fewer patients discharged home with AKI died (130 [3%] versus 522 [12%]; relative risk, 0.3; 95% confidence interval, 0.2 to 0.3; P<0.001). The difference was attenuated in a subgroup of patients with increased AKI severity (P value for interaction <0.001). Fewer patients discharged home with AKI received hospital-based acute dialysis within 30 days of an ED visit, although this difference was not statistically significant (19 [0.4%] versus 33 [0.8%]; relative risk, 0.6; 95% confidence interval, 0.3 to 1.0; P=0.06).
Table 3.
Thirty-day risk of all-cause mortality and other adverse outcomes in the AKI subpopulation
| Outcome | Events, n/N (%) | Relative Riska (95% Confidence Interval) | P Value | |
|---|---|---|---|---|
| Patients in the ED with AKI | ||||
| Discharged Home | Admitted to Hospital | |||
| All-cause mortality | 130/4379 (3) | 522/4379 (12) | 0.3 (0.2 to 0.3) | <0.001 |
| AKI severity | ||||
| Stage 1 | 108/4091 (3) | 477/4091 (12) | 0.2 (0.2 to 0.3) | <0.001b |
| Stage 2 | 15/244 (6) | 38/244 (16) | 0.4 (0.2 to 0.7) | |
| Stage 3 | 7/44 (16) | 7/44 (16) | 1.0 (0.4 to 2.6) | |
| Receipt of hospital-based acute dialysis | 19/4379 (0.4) | 33/4379 (0.8) | 0.6 (0.3 to 1.0) | 0.06 |
Patients admitted to hospital from the ED with AKI served as the referent group.
P value for interaction.
Discharge Subpopulation
We matched 6188 patients discharged home from the ED with AKI to 6188 patients discharged home with no AKI (Table 4). The groups were balanced on 89 out of 91 characteristics (Supplemental Table 12). The primary outcome of 30-day all-cause mortality is presented in Table 4. Compared with patients discharged with no AKI, patients discharged with AKI had a higher risk of death (136 [2%] versus 87 [1%]; relative risk, 1.6; 95% confidence interval, 1.2 to 2.0; P=0.001). The association between AKI and mortality was not modified by CKD stage (P value for interaction <0.6). More patients discharged with AKI received hospital-based acute dialysis within 30 days of ED discharge (19 [0.3%] versus seven [0.1%]; relative risk, 2.7; 95% confidence interval, 1.2 to 6.0; P=0.01).
Table 4.
Thirty-day risk of all-cause mortality and other adverse outcomes in the discharge subpopulation
| Variable | Events, n/N (%) | Relative Riska (95% Confidence Interval) | P Value | |
|---|---|---|---|---|
| Patients Discharged from the ED | ||||
| AKI | No AKI | |||
| All-cause mortality | 136/6188 (2) | 87/6188 (1) | 1.6 (1.2 to 2.0) | 0.001 |
| Pre-ED CKD stage, eGFR in ml/min per 1.73 m2b | ||||
| ≥60 | 67/3904 (2) | 47/3904 (1) | 1.4 (1.0 to 2.1) | 0.57c |
| 45 to <60 | 34/1054 (3) | 16/1054 (2) | 2.1 (1.2 to 3.8) | |
| 30 to <45 | 16/803 (2) | 13/803 (2) | 1.2 (0.6 to 2.6) | |
| <30d | 19/427 (4) | 11/427 (3) | 1.7 (0.8 to 3.6) | |
| Receipt of hospital-based acute dialysis | 19/6188 (0.3) | 7/6188 (0.1) | 2.7 (1.2 to 6.0) | 0.01 |
ED, emergency department.
Patients discharged home from the ED with no AKI served as the referent group.
Derived from pre-ED serum creatinine measurements 7–365 days before the ED visit and is reported in ml/min per 1.73 m2.
P value for interaction.
Patients with an eGFR of 15 to <30 ml/min per 1.73 m2 and those with <15 ml/min per 1.73 m2 (not on dialysis) were combined because of a low number of events.
Discussion
In this population-based cohort study of adults discharged home from the ED with AKI, 149 (2%) died within 30 days and this proportion increased with AKI severity. Compared with patients who were hospitalized with AKI, patients discharged from the ED with AKI had lower risk of death and subsequent dialysis. Although these two groups had comparable characteristics, the divergence in outcomes highlights the accuracy of ED clinicians in discerning subtle clinical differences in patients with AKI. Sicker patients destined for worse outcomes were appropriately hospitalized. Nonetheless, the adverse outcomes of AKI after an ED discharge are clearly highlighted when such patients were compared with a similar cohort of ED patients without AKI. An ED discharge with AKI was associated with a 1.6-fold increase in mortality and an increased need for hospital-based acute dialysis within 30 days of discharge. Among the groups studied, an ED discharge with AKI represents an intermediate risk population.
The results of our study provide important insights into the characteristics and outcomes of patients discharged home from the ED with AKI. In our literature review, we found two observational studies of patients with community-acquired AKI managed by primary care or ED physicians (29,30). However, neither study performed a separate and comprehensive analysis of ED discharges with AKI.
We found comparable 30-day mortality in a matched subgroup of patients with stage 3 AKI in the AKI subpopulation. Patients discharged home with severe AKI appear to be at risk of poor outcomes. This may reflect progression of an underlying illness because 32% of patients were admitted to hospital within 30 days of the ED visit. However, the use of administrative data cannot account for important factors such as hemodynamic stability and the ED physician’s determination of safety and appropriateness for discharge home. Further details would require a detailed chart review, best collected in a prospective fashion.
The 30-day mortality risk in patients discharged home from the ED with AKI is not insignificant when compared with other ED patient groups. For example, fewer patients die within 30 and 90 days of an ED discharge with chest pain (0.2%) and a transient ischemic attack (2%), respectively (31,32). Without conducting a formal analysis and accounting for differences in study methodology, the higher risk is likely because our cohort has a greater burden of comorbidity. Conversely, other studies showed that more patients die within 30 days of ED discharge with heart failure (4%) or unstable angina (5%) (33,34). The lower risk is likely because a proportion of AKI in our cohort may be caused by mild, reversible hemodynamic changes and thus confer better short-term outcomes. We also found that most patients had mild AKI and that mortality was far more common than the need for dialysis. Mortality does not appear to be caused by kidney failure—it is more likely that AKI is a marker of severe illness.
The need for hospitalization within 30 days of an ED discharge with AKI occurred in 1032 (16%) patients, similar to the 30-day readmission rates (15%–20%) among AKI survivors discharged after hospitalization (35,36). We also found a discrepancy between the proportion of patients who received outpatient physician follow-up (68%) and those who had repeat serum creatinine measurements (23%). This raises questions of whether AKI was the main reason for the outpatient visit, physicians recognized the presence of AKI, and if appropriate measures were taken to avoid hospitalization.
Our results suggest there is an opportunity to explore health system strategies to improve the identification and management of patients discharged home from the ED with AKI. Several rapid access clinics for patients discharged from the ED with chest pain, heart failure, or a transient ischemic attack have been shown to improve patient outcomes (37–39). AKI survivors discharged after hospitalization appear to benefit from follow-up clinics (40). A similar model could be adapted for patients discharged home from the ED with AKI, supported by an automated surveillance system to aid in the identification of AKI.
Our study has limitations. First, we could not capture patients who had baseline pre-ED serum creatinine measurements in other outpatient laboratories or hospitals. Second, use of administrative data limited our ability to ascertain information regarding AKI awareness by ED physicians. For many patients, we suspect that AKI was in fact recognized, appropriately managed, and deemed safe for discharge home. Third, we excluded patients with an improvement in AKI severity in order to exclude those recognized and treated. These individuals may differ systematically from those who did not have an improvement in AKI severity. Fourth, hospitalization may not have been preventable because of underlying illness. Alternatively, these patients could have been appropriately hospitalized after a visit to a rural ED, where patients were strategically discharged and instructed to seek further care at a tertiary care center. Such patients would be considered an ED discharge. There were also limitations in our two subpopulation analyses. First, as with all observational studies, our results are subject to confounding because propensity score matching will only ensure balance on measured characteristics. The ED main diagnosis was not included in our propensity score models because the diagnosis is often preliminary and there is significant disagreement with main diagnoses assigned at later stages of patient care (41–43). Second, referent subpopulations are only generalizable to ED discharges with AKI included in the match. We could not match 1967 (31%) patients in the AKI subpopulation and 228 (4%) patients in the discharged subpopulation. Third, medication information was only available for a subset of patients eligible for provincial drug coverage. We minimized the effect of missing medication information by balancing the comorbidities for which these medications are indicated.
In summary, patients discharged home from the ED with AKI are at significant risk of 30-day mortality and hospitalization. Compared with hospital admission with AKI and an ED discharge with no AKI, an ED discharge with AKI is at intermediate risk for adverse outcomes. Research into risk factors for adverse outcomes, further characterization of ED and outpatient care, and testing health system strategies to identify and mitigate gaps in care is warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dynacare Medical Laboratories for their use of the outpatient laboratory database and the team at London Health Sciences Centre, St. Joseph’s Health Care London, and all of the Thames Valley Hospitals for providing access to the Cerner laboratory database. We thank IMS Brogan Inc. for use of their Drug Information Database. We thank Stephanie Dixon, Sonja Gandhi, and Racquel Jandoc for their administrative support and analytic advice (all from the Institute for Clinical Evaluative Sciences Western in London, Ontario, Canada). No one received compensation for their role in the study. This project was conducted by the Institute for Clinical Evaluative Sciences Western facility, with members of the Institute for Clinical Evaluative Sciences Kidney, Dialysis, and Transplantation Research Program.
R.R.A. is supported by the Clinical Investigator Program at Western University. S.A.S. is supported by a Kidney Research Scientist Core Education and National Training Program postdoctoral fellowship (cofunded by the Kidney Foundation of Canada, Canadian Society of Nephrology, and Canadian Institutes of Health Research). M.T.J. is supported by a Canadian Institutes of Health Research new investigator award. E.D.S. is supported by Veterans Affairs Health Services Research and Development grant IIR 13-073 and the Vanderbilt Center for Kidney Disease. M.E.M. is supported by Veterans Affairs Health Services Research and Development grants IIR 11-272 and 13-052, and the Center for Population Health Informatics at Vanderbilt University Medical Center. A.X.G. is supported by the Dr. Adam Linton Chair in Kidney Health Analytics.
R.R.A. drafted the manuscript and all other authors provided revisions. R.R.A., R.W., and A.X.G. contributed to the research idea and study design. All authors contributed to data analyses and interpretation of the data. E.M. conducted the statistical analyses. All authors gave final approval of the version to be published and agreed to act as guarantors of the work.
Research personnel who worked on this project were supported by the Lilibeth Caberto Kidney Clinical Research Unit and the Institute for Clinical Evaluative Sciences Western facility. This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
The sponsors had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Parts of this material are on the basis of data and information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of the Canadian Institute for Health Information.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Reconfiguring Health Care Delivery to Improve AKI Outcomes,” on pages 1203–1205.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10431016/-/DCSupplemental.
References
- 1.Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, Kolhe NV: Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 7: 533–540, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Porter CJ, Juurlink I, Bisset LH, Bavakunji R, Mehta RL, Devonald MA: A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant 29: 1888–1893, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, Parikh CR, Mizera R, Jones PM, Tiboni M, Font A, Cegarra V, Gomez MF, Meyhoff CS, VanHelder T, Chan MT, Torres D, Parlow J, Clanchet Mde N, Amir M, Bidgoli SJ, Pasin L, Martinsen K, Malaga G, Myles P, Acedillo R, Roshanov PS, Walsh M, Dresser G, Kumar P, Fleischmann E, Villar JC, Painter T, Biccard B, Bergese S, Srinathan S, Cata JP, Chan V, Mehra B, Wijeysundera DN, Leslie K, Forget P, Whitlock R, Yusuf S, Devereaux PJ: Perioperative aspirin and clonidine and risk of acute kidney injury: A randomized clinical trial. JAMA 312: 2254–2264, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ: Long-term risk of coronary events after AKI. J Am Soc Nephrol 25: 595–605, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M: Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet 376: 2096–2103, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG: Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Wald R, Quinn RR, Adhikari NK, Burns KE, Friedrich JO, Garg AX, Harel Z, Hladunewich MA, Luo J, Mamdani M, Perl J, Ray JG: Risk of chronic dialysis and death following acute kidney injury. Am J Med 125: 585–593, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Zhang JH, Peduzzi P, Star R, Young E, Fissel R, Fissel W, Patel U, Belanger K, Raine A, Ricci N, Lohr J, Arora P, Cloen D, Wassel D, Yohe L, Choudhury D, Amanzadeh J, Penfield J, Hussain M, Katneni R, Sajgure A, Swann A, Dolson G, Ramanathan V, Tasby G, Bacallao R, Jaradat M, Graves K, Li Q, Krause M, Shaver M, Alam M, Morris K, Bland T, Satter E, Kraut J, Felsenfeld A, Levine B, Nagami G, Vaghaiwalla B, Duffney J, Moore J, Schein RM, Cely C, Jaimes E, Kett D, Quartin A, Arcia M, Barchi-Chung A, Batuman V, Alper A, Dreisbach A, Simon E, Kulivan C, Aslam N, Ramkumar M, Grum E, Rogers P, Weisbord S, Geffel C, Watnick S, Wahba I, Kelly D, Walczyk J, Feldman G, Mogyorosi A, Viol G, Halverson M, Schmid S, Totten H, Gabbai F, Mullaney S, Smith R, Dingsdale J, Woods S, Johansen K, Lovett D, O’Hare A, McCarthy J, Rosado-Rodriguez C, Galera A, Rodriguez-Vega G, Rodriguez W, Vilchez C, Young B, Andress D, Lindner A, Galvin G, Crowley S, Gourley N, Peixoto A, Perkal M, Joncas C, Paganini E, Demirjian S, Yared J, Brienza R, Garcia M, Seifert T, Sweeney L, Rabb H, Atta M, Brower R, Choi M, Eustace J, Scheel P, Heck E, Rahman H, Niles J, Bazari H, Smirnakis K, Steele D, Thadhani R, Laliberte K, Leeman B, McCarthy C, Pescatore M, Szerlip H, Fall P, Jagadeesan M, Mulloy L, Paulson W, White J, Sickafoose N, Chertow G, Cho K, Gropper M, Liu K, Matthay M, Borovitz K, Koenigsberg M, Rodriguez S, Contreras G, Schein RM, Cohn S, Diego J, Kett D, Quartin A, Carvalho C, Carvalho D, Castro M, de la Cuesta C, Espinal I, Hurtado A, Oyuela P, Aslam N, Unruh M, Kellum J, Burr R, Donahoe M, Marszalek J, Shields M, Venkataraman R, Weisbord S, Aubrecht J, Sterling H, Mandich L, Finkel K, Shaw A, Foringer J, Samuels J, Efron B, Rocco M, Deterding E, Moossavi S, Bethea C, McBride D, Warren S, Vijayan A, Kollef M, Sambandam K, Hammer E, Hoffman M, Stokes J, Connors AF Jr, Feldman H, Greer J, Koch GG, Stewart T, Wittes J, Palevsky PM, Overberger P, Michler S, Peduzzi P, Antonelli M, O’Connor TZ, Zhang JH, Dellert K, Durant L, Franchini R, Kossack A, McBride V, O’Neil S, Roy T, Russo J, Vitale J, Sather M, Fye C, Haakenson CM, Krueger DH, Swanson K, Thornton J, Dalzell C, Horney R, Smith MW, Siroka A, Su P, Brophy M, Humphries D, Govan D, O’Leary TJ, Huang GD, Feussner JR, Star RA, Eggers P: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A: Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 9: 1007–1014, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Canada: Estimates of population, Canada, provinces and territories. 2015. Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=510005. Accessed March 15, 2016
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 61: 344–349, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jha P, Deboer D, Sykora K, Naylor CD: Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: A population-based comparison. J Am Coll Cardiol 27: 1335–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D: Coding accuracy of administrative drug claims in the Ontario drug benefit database. Can J Clin Pharmacol 10: 67–71, 2003 [PubMed] [Google Scholar]
- 18.Wodchis W, Bushmeneva K, Nikitovic M, McKillop I: Guidelines on person level cost using administrative databases in Ontario. Health System Performance Research Network 1: 1–70, 2013 [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan YC: Multiple imputation for missing data: Concepts and new development (Version 9.0). SUGI Proc 1–13, 2000. Available at http://stats.idre.ucla.edu/wp-content/uploads/2016/02/multipleimputation.pdf. Accessed March 15, 2016 [Google Scholar]
- 24.Mamdani M, Sykora K, Li P, Normand S-LT, Streiner DL, Austin PC, Rochon PA, Anderson GM: Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330: 960–996, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 26.Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, Fremes SE: A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol 32: 259–265, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Zou GY, Donner A: Extension of the modified poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 22: 661–670, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Zou GY: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Talabani B, Zouwail S, Pyart RD, Meran S, Riley SG, Phillips AO: Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton) 19: 282–287, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Hobbs H, Bassett P, Wheeler T, Bedford M, Irving J, Stevens PE, Farmer CK: Do acute elevations of serum creatinine in primary care engender an increased mortality risk? BMC Nephrol 15: 206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasserman J, Perry J, Dowlatshahi D, Stotts G, Stiell I, Sutherland J, Symington C, Sharma M: Stratified, urgent care for transient ischemic attack results in low stroke rates. Stroke 41: 2601–2605, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Czarnecki A, Chong A, Lee DS, Schull MJ, Tu JV, Lau C, Farkouh ME, Ko DT: Association between physician follow-up and outcomes of care after chest pain assessment in high-risk patients. Circulation 127: 1386–1394, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Lee DS, Schull MJ, Alter DA, Austin PC, Laupacis A, Chong A, Tu JV, Stukel TA: Early deaths in patients with heart failure discharged from the emergency department a population-based analysis. Circ Heart Fail 3: 228–235, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP: Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 342: 1163–1170, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Koulouridis I, Price LL, Madias NE, Jaber BL: Hospital-acquired acute kidney injury and hospital readmission: A cohort study. Am J Kidney Dis 65: 275–282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekhri N, Feder GS, Junghans C, Hemingway H, Timmis AD: How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 93: 458–463, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu JV: Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation 122: 1806–1814, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Dutta D, Bowen E, Foy C: Four-year follow-up of transient ischemic attacks, strokes, and mimics: A retrospective transient ischemic attack clinic cohort study. Stroke 46: 1227–1232, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Campbell SG, Murray DD, Hawass A, Urquhart D, Ackroyd-Stolarz S, Maxwell D: Agreement between emergency physician diagnosis and radiologist reports in patients discharged from an emergency department with community-acquired pneumonia. Emerg Radiol 11: 242–246, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Farchi S, Camilloni L, Giorgi Rossi P, Chini F, Lori G, Tancioni V, Papini P, Borgia P, Guasticchi G: Agreement between emergency room and discharge diagnoses in a population of injured inpatients: Determinants and mortality. J Trauma 62: 1207–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Raven MC, Lowe RA, Maselli J, Hsia RY: Comparison of presenting complaint vs discharge diagnosis for identifying “nonemergency” emergency department visits. JAMA 309: 1145–1153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.