Abstract
Background and objectives
Soluble urokinase plasminogen activator receptor is a novel biomarker strongly predictive of cardiovascular outcomes implicated in the pathogenesis of kidney disease. Soluble urokinase plasminogen activator receptor levels, however, correlate with declining kidney function. It is unclear whether soluble urokinase plasminogen activator receptor levels remain associated with outcomes in patients with ESRD.
Design, setting, participants, & measurements
We measured plasma soluble urokinase plasminogen activator receptor levels in 1175 patients (mean age =66±8 years old, 54% men) with type 2 diabetes mellitus on hemodialysis participating in the German Diabetes and Dialysis Study followed for a median of 4 years for outcomes including all-cause death, cardiovascular events, and infection-related mortality. Survival analysis was performed using stepwise Cox proportional hazards models adjusted for potential confounders. Also, adjustments were made for inflammatory markers (C-reactive protein and leukocyte count) and the oxidative stress marker asymmetric dimethyl arginine to investigate potential mediators of the relationship between soluble urokinase plasminogen activator receptor and outcomes.
Results
Median soluble urokinase plasminogen activator receptor levels were 10,521 pg/ml (interquartile range, 9105–12,543 pg/ml). When stratified by tertiles, patients with soluble urokinase plasminogen activator receptor >11,633 pg/ml (third tertile) had adjusted 1.6-fold higher mortality (hazard ratio, 1.60; 95% confidence interval, 1.27 to 2.03) compared with those with low soluble urokinase plasminogen activator receptor <9599 pg/ml (first tertile). Risks of sudden death and stroke were higher (adjusted hazard ratio, 1.98; 95% confidence interval, 1.27 to 3.09 and adjusted hazard ratio, 1.74; 95% confidence interval, 1.05 to 2.90, respectively), together accounting for higher incidence of cardiovascular events (adjusted hazard ratio, 1.48; 95% confidence interval, 1.15 to 1.89). Associations with outcomes persisted after adjusting for C-reactive protein, leukocyte count, and asymmetric dimethyl arginine. Addition of soluble urokinase plasminogen activator receptor to a risk factor model modestly improved risk discrimination for all-cause death (ΔC statistic, 0.02; 95% confidence interval, 0.00 to 0.03) and cardiovascular events (ΔC statistic, 0.02; 95% confidence interval, 0.00 to 0.05).
Conclusions
The association of soluble urokinase plasminogen activator receptor levels with outcomes persists in patients on hemodialysis. Additional study is warranted to characterize the underlying pathways of that association, which may yield opportunities to develop new therapeutic strategies.
Keywords: ESRD; dialysis; urokinase; diabetes; Arginine; Cause of Death; Child, Preschool; Death, Sudden; Diabetes Mellitus, Type 2; Humans; Incidence; Kidney Failure, Chronic; Leukocyte Count; Male; oxidative stress; Proportional Hazards Models; Receptors, Urokinase Plasminogen Activator; renal dialysis; Renal Insufficiency, Chronic; risk factors; Stroke; Survival Analysis
Introduction
Over 10% of the world’s population is estimated to be affected by CKD, with at least 2 million currently receiving dialysis for ESRD (1,2). Outcomes of patients on dialysis remain poor, with the risk of death from cardiovascular disease being 5–30 times higher compared with in the general population (3,4). Moreover, the worldwide prevalence and incidence of both CKD and ESRD continue to rise and are a testament to the lack of novel therapeutic targets and progress in the early identification and prevention of CKD (1,2). Recently, soluble urokinase plasminogen activator receptor (suPAR), a marker of immune activation thought to be involved in the pathogenesis of FSGS (5–7), was shown to be strongly associated with incident CKD (8–10). suPAR levels have consistently been associated with incident cardiovascular disease and poor outcomes in various groups, including the general population, patients with sepsis, and those with cardiovascular disease, HIV, cancer, and early-stage CKD (8,11–20). However, suPAR plasma levels strongly correlate with eGFR, and some have surmised that elevation in suPAR merely represents impaired kidney function (21–23). suPAR levels are much higher in patients on hemodialysis compared with healthy subjects (24), but whether they remain associated with outcomes in patients with ESRD is, however, unknown.
Given its implication in the pathogenesis of kidney disease and its strong association with both eGFR and outcomes, examining suPAR in the setting of ESRD would provide insight as to whether levels measured in patients with poor to absent kidney function would still be associated with outcomes, thus suggesting that suPAR levels at least partially represent kidney-independent processes that lead to mortality. Thus, we sought to (1) report the range of plasma suPAR levels and their determinants in ESRD, (2) investigate whether they are independently associated with relevant outcomes, (3) improve risk discrimination in patients with diabetes on hemodialysis enrolled in the German Diabetes and Dialysis Study (4D Study), and lastly, (4) determine whether suPAR levels identify a subgroup of patients who may benefit from statin therapy (25).
Materials and Methods
Study Design and Participants
The methodology of the 4D Study has previously been reported in detail (24). Briefly, the 4D Study was a prospective, randomized, controlled trial of 1255 patients with type 2 diabetes mellitus ages 18–80 years old who started hemodialysis within the last 2 years before enrollment. Between March of 1998 and October of 2002, patients were recruited from 178 dialysis centers in Germany. After a run-in period of 4 weeks, patients were randomly assigned to double-blinded treatment with either 20 mg atorvastatin (n=619) or placebo (n=636) once daily. Study visits took place three times before randomization (visits 1–3), at randomization (visit 4), at 4 weeks (visit 5), and then, every 6 months (visit 6, etc.) after randomization until the date of death, censoring, or the end of the study in March of 2004. At each follow-up, blood samples were taken, and clinical information, including any adverse events, and an electrocardiogram were recorded. For this post hoc analysis, we measured suPAR in a subpopulation of 1175 patients with available blood samples. The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the appropriate medical ethics committee. All patients gave their written informed consent before inclusion.
Data Collection
Information on age, sex, and smoking status was obtained through patient interviews. Smoking status was classified as never, former, or current. Comorbidities, including the presence of coronary artery disease and congestive heart failure, as well as the duration of diabetes mellitus and dialysis treatment were reported by the patients’ nephrologists. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting surgery, percutaneous coronary intervention, or the presence of coronary heart disease as documented by coronary angiography.
suPAR, C-Reactive Protein, and Asymmetric Dimethyl Arginine Measurements
Plasma suPAR was measured in blood samples taken at baseline during study visit 3 (1 week before randomization) by ELISA (suPARnostic kit; ViroGates, Copenhagen, Denmark), with a lower detection limit of 100 pg/ml and intra- and interassay variations of 2.75% and 9.17%, respectively. suPAR levels have been shown to be quite stable in long-term storage as well as at room temperature and are minimally affected by repeated freezing and thawing cycles (15,26). C-reactive protein (CRP) was measured by turbidimetry on a Modular PP analyzer (Roche Diagnostics, Mannheim, Germany). The interassay coefficient of variance for CRP was <5%. Asymmetric dimethyl arginine (ADMA) was measured by HPLC with solid-phase extraction and precolumn derivatization. Within-day coefficients of variation for ADMA were 3.1% (0.62 μmol/L) and 1.0% (2.0 μmol/L), and between-day coefficients of variation were 9% (0.62 μmol/L), and 2.2% (2.0 μmol/L). All blood samples were taken before the start of dialysis sessions and administration of drugs. Technicians measuring suPAR, CRP, and ADMA were blinded to the clinical and outcomes data.
Outcome Assessment
The primary end point of the 4D Study was defined as a composite of death from cardiac causes, fatal or nonfatal stroke, and nonfatal myocardial infarction, whichever occurred first (composite cardiovascular end point). Death from cardiac causes comprised sudden cardiac death, fatal myocardial infarction, death due to congestive heart failure, death due to coronary artery disease during or within 28 days after an intervention, and all other deaths attributable to coronary artery disease. Sudden cardiac death was considered as the presence of any of the following: death as verified by terminal rhythm disorders in an electrocardiogram, death as verified by witnesses observed death within 1 hour after the onset of cardiac symptoms, death confirmed by autopsy, or unexpected death, presumably or possibly of cardiac origin and in the absence of a potassium level ≥7.5 mmol/L before the start of the three most recent sessions of hemodialysis. Death due to heart failure was determined by the end point committee after detailed documents had been received. These included original reports from the general practitioners and hospitals, laboratory results, and all procedures performed as well as an autopsy report.
Myocardial infarction was diagnosed when two of the following three criteria were met: typical symptoms, elevated levels of cardiac enzymes (i.e., creatinine kinase MB above 5% of the total level of creatinine kinase, lactic dehydrogenase 1.5 times the upper limit of normal, or a troponin T level >2 ng/ml), or diagnostic changes on the electrocardiogram. When death occurred within 28 days after a myocardial infarction as diagnosed above, it was specified as death due to myocardial infarction. The classifications were made exclusively, with fatal myocardial infarction being classified as death and not being classified as sudden cardiac death. Stroke was defined as a neurologic deficit lasting longer than 24 hours. Computed tomographic or magnetic resonance imaging was available in all but 16 patients. All-cause mortality and the specific causes of death were secondary end points.
The 4D Study end points were centrally adjudicated by three members of the end point committee blinded to study treatment per predefined criteria (24).
For this analysis, sudden cardiac death, myocardial infarction (fatal and nonfatal), stroke (fatal and nonfatal), death due to congestive heart failure, combined cardiovascular events, all-cause mortality, and infectious mortality were all chosen as separate outcome measures. The categorization of these events was on the basis of the primary judgement of the end point committee during the 4D Study.
Statistical Analyses
Continuous variables are expressed as mean with SD or median with interquartile range (as appropriate), and categorical variables were expressed as percentages. The study population was divided into three groups stratified by suPAR tertiles at enrollment: ≤9599, >9599 to ≤11,633, and >11,633 pg/ml. We compared the distribution of baseline characteristics between tertiles of suPAR by chi-squared test (categorical variables) or ANOVA (continuous variables). We then used linear regression with log2-transformed suPAR as a dependent variable to identify and report the characteristics independently associated with suPAR levels.
We assessed the association of baseline suPAR with all-cause mortality as both a continuous variable (log2 transformed; interpreted as per doubling of suPAR) and a categorical variable. For the latter, the lowest suPAR tertile was used as the reference group. Survival analysis was performed using Cox proportional hazards models in a stepwise fashion adjusting for the following confounders. Model 1 included demographics and known traditional risk factors: age, sex, atorvastatin treatment, body mass index, hypertension, HDL and LDL cholesterol, and antiplatelet and angiotensin-converting enzyme inhibitor therapy. In model 2, we added the following potential confounders: diuretics use, heart failure, coronary artery disease, peripheral vascular disease, vascular access, levels of hemoglobin, albumin, and phosphate. Lastly, model 3 additionally incorporated markers of inflammation (leukocyte count, CRP, and the oxidative stress marker ADMA) as potential intermediate parameters. Changes in the hazard ratio (HR) and effect size of suPAR would suggest potential mediation by the aforementioned variables.
Similarly, we investigated suPAR and the risk of specific adverse cardiac and vascular outcomes, including sudden cardiac death, myocardial infarction, stroke, death due to heart failure, combined cardiovascular events, and death due to infection. Additionally, we have examined a competing risks model for each end point considering death by infection as the competing event (27).
We examined the incremental value of adding suPAR to clinical models predicting cardiovascular events (combined outcome) and all-cause death by calculating Harrel C concordance statistics (28).
Lastly, in sensitivity analyses, we determined whether treatment with atorvastatin modulated the association between suPAR and outcomes by examining the interaction term (suPAR × atorvastatin treatment) in our outcome analyses. Furthermore, we assessed the efficacy estimates of atorvastatin in subgroups defined by baseline suPAR tertiles.
We tested for proportional hazard by a hypothesis test on the basis of the Schoenfeld residuals and graphical methods (multivariate adjusted log-log plots), and it showed no evidence of violation (29,30). We accounted for multiple testing by taking the global Wald test statistic as the criterion to decide on significance of multilevel categorical predictor variables (e.g., tertiles of suPAR) as well as the global test of the proportional hazard condition.
All P values are reported two sided, with P<0.05 considered as statistically significant. Analyses were performed using the statistical software package STATA, version 13.0 (StataCorp 2013, Stata Statistical Software: Release 13; StataCorp LPCollege Station, TX).
Results
Patient Characteristics
Of the 1255 patients included in the 4D Study, 1175 (94%) had suPAR measured at baseline. Patients were 54% men, with a mean age of 66±8 years old and median suPAR of 10,521 pg/ml (interquartile range, 9105–12,543 pg/ml) (Table 1). Patients with high suPAR concentrations were more likely to be women, be smokers, and have congestive heart failure as well as a central venous catheter compared with patients with low suPAR concentrations (Table 1). High suPAR concentrations were, furthermore, associated with lower concentrations of albumin and hemoglobin; higher CRP, ADMA, and phosphate concentrations; and a higher leukocyte count. In multivariable regression analysis, suPAR levels were independently associated with the following continuous markers (β indicates change in suPAR in picograms per milliliter per population SD increase of marker): serum phosphate (β=231.7; P<0.001), ADMA (β=222.6; P<0.001), and albumin (β=−461.4; P<0.001). Furthermore, for categorical covariates (β indicates contrast compared with reference category), the multivariate model showed significantly increased suPAR concentrations in individuals with peripheral vascular disease (β=323.2; P=0.01) and women (β=578.1; P<0.001).
Table 1.
Patient characteristics stratified by tertiles of soluble urokinase plasminogen activator receptor concentration (picograms per milliliter) at baseline (study population n=1175)
| Characteristics | Tertile 1 ≤9599 pg/ml, n=391 | Tertile 2 >9599 to ≤11,633 pg/ml, n=392 | Tertile 3 >11633 pg/ml, n=392 | P Valuea |
|---|---|---|---|---|
| Age, yr | 66±8 | 66±8 | 67±8 | 0.01 |
| Men, % | 67 | 49 | 46 | <0.001 |
| Atorvastatin treatment, % | 48 | 53 | 48 | 0.34 |
| Systolic BP, mmHg | 145±21 | 147±21 | 145±23 | 0.28 |
| Diastolic BP, mmHg | 76±11 | 77±10 | 75±12 | 0.12 |
| Body mass index, kg/m2 | 27.5±4.1 | 27.2±4.6 | 27.8±5.6 | 0.17 |
| Duration of diabetes, yr | 18±9 | 19±8 | 18±8 | 0.52 |
| Time on hemodialysis, mo | 8±7 | 8±7 | 8±7 | 0.76 |
| Smoker, % | 8 | 8 | 10 | 0.46 |
| Vascular access, % | <0.001 | |||
| AV fistula | 90 | 84 | 78 | |
| AV graft | 5 | 10 | 12 | |
| Central venous catheter | 5 | 6 | 10 | |
| Coronary artery disease, % | 28 | 33 | 28 | 0.19 |
| Congestive heart failure, % | 30 | 37 | 41 | 0.01 |
| Peripheral vascular disease, % | 41 | 46 | 47 | 0.20 |
| LDL cholesterol, mg/dl | 125±28 | 128±32 | 125±29 | 0.27 |
| HDL cholesterol, mg/dl | 37±13 | 37±13 | 36±14 | 0.46 |
| Hemoglobin, g/dl | 11.1±1.3 | 10.9±1.3 | 10.7±1.4 | 0.003 |
| Albumin, g/dl | 3.90±0.3 | 3.82±0.3 | 3.74±0.3 | <0.001 |
| CRP, μg/ml | 5.4 (0.2–109) | 5.8 (0.2–117) | 7.7 (0.2–237) | <0.001 |
| Phosphate, mg/dl | 5.9±1.5 | 6.0±1.5 | 6.2±1.8 | 0.04 |
| Leukocyte count, ×109/L | 7.6±2.2 | 8.1±2.4 | 8.6±2.6 | <0.001 |
| HbA1c, % | 6.7±1.3 | 6.8±1.2 | 6.6±1.2 | 0.22 |
| ADMA, μmol/L | 0.8±0.1 | 0.9±0.2 | 0.9±0.2 | <0.001 |
| Use of diuretics, % | 80 | 79 | 81 | 0.60 |
| Use of angiotensin-converting enzyme inhibitors, % | 51 | 49 | 40 | 0.01 |
| Use of antiplatelet treatment, % | 52 | 51 | 54 | 0.72 |
AV, arteriovenous; CRP, C-reactive protein; HbA1c, hemoglobin A1c; ADMA, asymmetric dimethyl arginine.
P value of ANOVA F statistic (for continuous outcomes) or Pearson chi square statistic (for categorical outcomes).
suPAR and Outcomes
The median follow-up period was 4 years. During follow-up, 577 patients died, including 150 patients who died of sudden cardiac death and 39 patients who died due to congestive heart failure. A total of 434 patients reached the primary combined end point, with myocardial infarction (fatal or nonfatal) and stroke (fatal or nonfatal) occurring in 185 and 96 patients, respectively.
All-Cause Mortality.
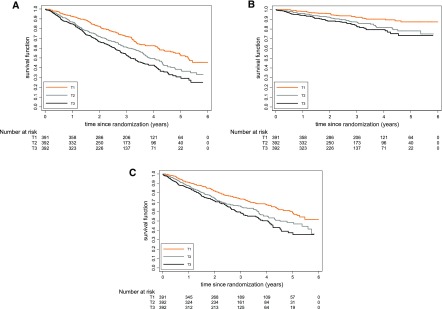
High plasma suPAR levels at baseline were associated with a higher mortality risk. When patients were divided into tertiles according to their suPAR levels, the unadjusted risk was incrementally higher: patients in the second tertile had a 51% higher risk (HR, 1.51; 95% confidence interval [95% CI], 1.23 to 1.85) and patients in the highest suPAR tertile exhibited an almost twofold higher mortality (HR, 1.90; 95% CI, 1.53 to 2.36) compared with patients with low suPAR levels in the first tertile (Figure 1A). The association remained significant after adjustment for confounders, which however, reduced the effect size (adjusted HR, 1.40; 95% CI, 1.12 to 1.76 for the second tertile and adjusted HR, 1.60; 95% CI, 1.27 to 2.03 for the third tertile compared with the first tertile) (Table 2). When we analyzed suPAR as a continuous variable, we consistently found a 14% higher mortality risk per doubling in suPAR concentrations (adjusted HR, 1.14; 95% CI, 1.03 to 1.27).
Figure 1.
Event-Free survival stratified by suPAR tertiles. Kaplan–Meier curves for the time to (A) all-cause mortality, (B) sudden cardiac death, and (C) combined cardiovascular events in subgroups of patients stratified by soluble urokinase plasminogen activator receptor concentrations at baseline (tertiles).
Table 2.
Risk (hazard ratio and 95% confidence interval) of sudden cardiac death, myocardial infarction, stroke, death due to heart failure, combined cardiovascular events, all-cause mortality, and death due to infection by tertiles of soluble urokinase plasminogen activator receptor at baseline (study population n=1175)
| Outcome | HRs Stratified by suPAR Tertiles at Baseline | ||
|---|---|---|---|
| Tertile 1 ≤9599 pg/ml, n=391 | Tertile 2 >9599 to ≤11,633 pg/ml, n=392 | Tertile 3 >11,633 pg/ml, n=392 | |
| All-cause mortality | |||
| Crude HR (95% CI) | 1 | 1.51 (1.23 to 1.85) | 1.90 (1.53 to 2.36) |
| Adjusteda HR (95% CI) | 1 | 1.54 (1.24 to 1.92) | 1.91 (1.51 to 2.40) |
| Adjustedb HR (95% CI) | 1 | 1.40 (1.12 to 1.76) | 1.60 (1.27 to 2.03) |
| Adjustedc HR (95% CI) | 1 | 1.37 (1.09 to 1.73) | 1.51 (1.19 to 1.92) |
| Cardiovascular eventsd | |||
| Crude HR (95% CI) | 1 | 1.49 (1.17 to 1.90) | 1.75 (1.38 to 2.20) |
| Adjusteda HR (95% CI) | 1 | 1.41 (1.09 to 1.81) | 1.73 (1.36 to 2.19) |
| Adjustedb HR (95% CI) | 1 | 1.30 (1.01 to 1.68) | 1.48 (1.15 to 1.89) |
| Adjustedc HR (95% CI) | 1 | 1.29 (1.00 to 1.67) | 1.45 (1.13 to 1.88) |
| Sudden cardiac death | |||
| Crude HR (95% CI) | 1 | 1.90 (1.21 to 3.00) | 2.31 (1.51 to 3.53) |
| Adjusteda HR (95% CI) | 1 | 1.81 (1.13 to 2.91) | 2.27 (1.45 to 3.55) |
| Adjustedb HR (95% CI) | 1 | 1.62 (1.01 to 2.61) | 1.98 (1.27 to 3.09) |
| Adjustedc HR (95% CI) | 1 | 1.62 (1.01 to 2.61) | 1.95 (1.22 to 3.10) |
| Myocardial infarction | |||
| Crude HR (95% CI) | 1 | 1.76 (0.94 to 3.32) | 0.84 (0.36 to 1.96) |
| Adjusteda HR (95% CI) | 1 | 2.03 (1.06 to 3.87) | 0.89 (0.39 to 2.04) |
| Adjustedb HR (95% CI) | 1 | 1.91 (0.98 to 3.72) | 0.80 (0.34 to 1.90) |
| Adjustedc HR (95% CI) | 1 | 1.84 (0.93 to 3.63) | 0.76 (0.31 to 1.88) |
| Stroke | |||
| Crude HR (95% CI) | 1 | 1.55 (0.90 to 2.65) | 2.41 (1.46 to 3.98) |
| Adjusteda HR (95% CI) | 1 | 1.41 (0.81 to 2.44) | 2.30 (1.39 to 3.78) |
| Adjustedb HR (95% CI) | 1 | 1.27 (0.74 to 2.17) | 1.74 (1.05 to 2.90) |
| Adjustedc HR (95% CI) | 1 | 1.26 (0.73 to 2.18) | 1.74 (1.02 to 2.98) |
| Death due to heart failure | |||
| Crude HR (95% CI) | 1 | 0.98 (0.42 to 2.28) | 2.40 (1.11 to 5.20) |
| Adjusteda HR (95% CI) | 1 | 1.12 (0.45 to 2.82) | 2.64 (1.15 to 6.03) |
| Adjustedb HR (95% CI) | 1 | 1.09 (0.43 to 2.77) | 2.42 (1.02 to 5.71) |
| Adjustedc HR (95% CI) | 1 | 1.05 (0.41 to 2.69) | 2.08 (0.88 to 4.91) |
| Death due to infection | |||
| Crude HR (95% CI) | 1 | 1.21 (0.77 to 1.85) | 1.40 (1.02 to 2.18) |
| Adjusteda HR (95% CI) | 1 | 1.24 (0.76 to 2.04) | 1.44 (0.93 to 2.22) |
| Adjustedb HR (95% CI) | 1 | 1.09 (0.67 to 1.78) | 1.03 (0.66 to 1.59) |
| Adjustedc HR (95% CI) | 1 | 1.07 (0.65 to 1.76) | 0.96 (0.62 to 1.50) |
HR, hazard ratio; suPAR, soluble urokinase plasminogen activator receptor; 95% CI, 95% confidence interval.
Adjusted HR: adjustments were made for age, sex, body mass index, hypertension, LDL, HDL cholesterol, and antiplatelet and angiotensin-converting enzyme inhibitor therapy.
Adjusted HR: adjustments were made for age, sex, body mass index, hypertension, LDL, HDL cholesterol, antiplatelet and angiotensin-converting enzyme inhibitor therapy, heart failure, coronary artery disease, peripheral vascular disease, diuretics, vascular access, hemoglobin, albumin, and phosphate.
Adjusted HR: adjustments were made for age, sex, body mass index, hypertension, LDL, HDL cholesterol, antiplatelet and angiotensin-converting enzyme inhibitor therapy, heart failure, coronary artery disease, peripheral vascular disease, diuretics, vascular access, hemoglobin, albumin, phosphate, C-reactive protein, leukocyte count, and asymmetric dimethyl arginine.
Combined cardiovascular events were defined as a composite of death from cardiac causes, fatal or nonfatal stroke, and nonfatal myocardial infarction, whichever occurred first. Death from cardiac causes comprised sudden cardiac death, fatal myocardial infarction, death due to congestive heart failure, death due to coronary heart disease during or within 28 days after an intervention, and all other deaths attributable to coronary heart disease.
We investigated potential intermediate pathways and additionally adjusted our analyses for CRP, leukocyte count, and ADMA as parameters of inflammation and endothelial dysfunction. With these additional adjustments, the effect estimates slightly decreased further (third versus first tertile adjusted HR, 1.51; 95% CI, 1.19 to 1.92), suggesting little contribution of the surmised mechanisms to the association between suPAR levels and mortality.
The results of our competing risk analyses were similar: patients of the second and third suPAR tertiles had 47% and 64% higher risks of death, respectively, compared with patients of the first suPAR tertile.
Cardiovascular Outcomes.
A higher risk of sudden death was also observed at high levels of suPAR (third versus first tertile HR, 2.31; 95% CI, 1.51 to 3.53) (Figure 1B). This association largely persisted after adjustment for confounders, with patients in the third tertile having an almost twofold higher risk compared with those in the first tertile (adjusted HR, 1.98; 95% CI, 1.27 to 3.09). Similarly, the analyses using suPAR as a continuous variable also revealed an increase in the risk of sudden death per doubling in suPAR levels (adjusted HR, 1.18; 95% CI, 1.00 to 1.38). suPAR was also associated with a higher risk of death attributed to congestive heart failure (third versus first tertile adjusted HR, 2.42; 95% CI, 1.02 to 5.71) and a higher risk to experience a stroke (third versus first tertile adjusted HR, 1.74; 95% CI, 1.05 to 2.90) (Table 2).
The incidence of cardiovascular events as a combined outcome was markedly higher at higher concentrations of suPAR (Figure 1C). Patients in the highest suPAR tertile had a 48% higher risk of developing a cardiovascular event after adjustment for confounders. Again, the competing risk analyses showed similar results, with 29% and 45% higher risks for patients of the second and third suPAR tertiles, respectively, compared with patients of the first suPAR tertile.
In contrast to the results seen for sudden death, stroke, and death due to heart failure, suPAR did not show an association with myocardial infarction. In both continuous (HR, 0.77; 95% CI, 0.49 to 1.20) and categorical analyses, the incidence of myocardial infarction did not increase over varying concentrations of suPAR (third versus first tertile adjusted HR, 0.80; 95% CI, 0.34 to 1.90) (Table 2).
suPAR and Death Due to Infection.
High suPAR concentrations were not associated with a higher risk of death due to infections (HR, 1.11; 95% CI, 0.87 to 1.38 per doubling of suPAR). Accordingly, patients with the highest suPAR concentrations in the third tertile did not have a higher risk of death due to infection compared with patients with low suPAR concentrations in the first tertile (third versus first tertile adjusted HR, 1.03; 95% CI, 0.66 to 1.59).
Risk Discrimination.
Lastly, we explored whether addition of suPAR to models incorporating traditional risk factors (model 1), models incorporating expanded clinical characteristics (model 2), and a full model that included markers of immune activation improved the C statistic (Table 3). In summary, addition of suPAR to a traditional risk factor model predicting all-cause death and cardiovascular outcomes improved the C statistic significantly (ΔC statistic, 0.02; 95% CI, 0.00 to 0.03 and ΔC statistic, 0.02; 95% CI, 0.00 to 0.05, respectively) but did not improve it when added to the expanded models.
Table 3.
Risk discrimination metrics for all-cause death and cardiovascular events
| Model | All-Cause Death | Cardiovascular Events | ||
|---|---|---|---|---|
| C Statistic (95% CI) | ∆C Statistic (95% CI) | C Statistic (95% CI) | ∆C Statistic (95% CI) | |
| Model 1: RFs | 0.61 (0.58 to 0.63) | — | 0.58 (0.55 to 0.61) | — |
| Model 2: RFs and suPAR | 0.63 (0.60 to 0.65)a | 0.02 (0.00 to 0.03)a | 0.60 (0.57 to 0.63)a | 0.03 (0.00 to 0.05)a |
| Model 3: RFs and clinical | 0.67 (0.65 to 0.70) | — | 0.64 (0.61 to 0.67) | — |
| Model 4: RFs, clinical, and suPAR | 0.68 (0.65 to 0.70) | 0.01 (−0.00 to 0.01) | 0.65 (0.62 to 0.67) | 0.01 (−0.01 to 0.01) |
| Model 5: RFs, clinical, and inflammation | 0.68 (0.66 to 0.70) | — | 0.65 (0.62 to 0.67) | — |
| Model 6: RFs, clinical, inflammation, and suPAR | 0.68 (0.66 to 0.71) | 0.00 (−0.00 to 0.01) | 0.65 (0.62 to 0.67) | 0.00 (−0.01 to 0.01) |
All models 1–6 include age, sex, body mass index, hypertension, LDL cholesterol, HDL cholesterol, use of angiotensin-converting enzyme inhibitors, and antiplatelet therapy. Models 3–6 have, in addition, congestive heart failure, coronary artery disease, peripheral vascular disease, use of diuretics, vascular access, hemoglobin levels, and albumin levels. Lastly, C-reactive protein, leukocyte count, and asymmetric dimethyl arginine levels are incorporated in models 5 and 6. The change in C statistic reported is relative to the previous model not including suPAR. 95% CI, 95% confidence interval; RF, risk factor; —, baseline model; suPAR, soluble urokinase plasminogen activator receptor.
Values reflect statistically significant change in C statistic.
suPAR, Statin Therapy, and Outcomes.
In addition, we sought to determine whether atorvastatin therapy influenced survival in subjects who were stratified according to their baseline suPAR values in sensitivity analyses by including an interaction term in the outcome analyses. The term was nonsignificant (P>0.20), suggesting the absence of a multiplicative interaction (Table 4).
Table 4.
Effects of atorvastatin therapy on 4-year risk of death from all causes in patients stratified according to baseline soluble urokinase plasminogen activator receptor levels
| suPAR Tertile | HR | 95% CI | P Value |
|---|---|---|---|
| Bottom | 0.89 | 0.67 to 1.18 | 0.40 |
| Middle | 0.87 | 0.65 to 1.17 | 0.35 |
| Top | 1.07 | 0.80 to 1.43 | 0.65 |
suPAR, soluble urokinase plasminogen activator receptor; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In this post hoc analysis of patients with diabetes on hemodialysis enrolled in the 4D Study, we found that plasma suPAR levels (1) are elevated in ESRD and have a wide range; (2) remain independently associated with sex, cardiovascular risk factors (such as smoking), and known cardiovascular disease (such as congestive heart failure); and most importantly, (3) were strongly associated with incident adverse outcomes, including all-cause death and cardiovascular outcomes. The association with outcomes was independent of traditional cardiovascular risk factors, coronary artery disease, or congestive heart failure as well as measures of inflammation (CRP) or oxidative stress (ADMA). Moreover, addition of suPAR to a traditional risk factor model modestly improved risk discrimination indices. Lastly, statin therapy did not affect the association between suPAR and outcomes in this population. These findings have two important implications: first, they highlight suPAR as a potentially useful biomarker of risk in patients with ESRD, and second, they provide evidence that elevated suPAR levels are not solely reflective of decreased GFR and still are informative in the setting of kidney dysfunction and ESRD.
The importance of suPAR in kidney disease has taken front stage in recent years, with accumulating evidence of its role in the pathogenesis of FSGS (5–7) and more recently, its strong association with incident kidney function decline (8,9). The mechanisms underlying this association have been on the basis of FSGS studies and are thought to involve the activation of αvβ3-integrins on podocytes, leading to their effacement and glomerular dysfunction (31,32). suPAR levels in plasma, however, strongly correlate with eGFR, and some have hypothesized that chronically elevated suPAR may be the result of decreased clearance due to subclinical impairment in kidney function (21–23). Although a significant component of suPAR levels may be attributed to decreased GFR, here we show that patients with ESRD on dialysis still exhibit a wide range of suPAR levels (≤599 to >11,633 pg/ml), despite the protein not being dialyzed, and its levels remained associated with clinical characteristics and outcomes described in previous studies of subjects without kidney disease and with lower ranges of suPAR (8,11–20,33).
suPAR levels have been associated with poor outcomes in various populations, including the general population, critically ill patients, and those with cardiovascular disease, HIV, cancer, or CKD (8,11–20). suPAR is strongly associated with traditional cardiovascular risk factors as well as inflammatory markers, such as CRP, and it is thought to be involved in the pathogenesis of atherosclerosis (33). Consistent with other studies, we found suPAR to be associated with outcomes independent of cardiovascular risk factors and coronary artery disease as well as CRP and ADMA, markers of inflammation and oxidative stress, respectively. The decrease in HR estimates when adjusting for these factors, although not evidence of causation, suggests possible mediation via inflammation, oxidative stress, and cardiovascular disease. Similarly, although suPAR has been found to be predictive of incident kidney disease, the fact that elevated levels in ESRD remain associated with outcomes suggests that kidney dysfunction is, at most, only partially contributing to this association (8). Although elevated suPAR levels have been described in sepsis, HIV, and various disease states, we did not find an association between suPAR and death from infectious causes. This is likely because the cohort did not include critically ill patients or those with an active infection. Given the nonspecific association with outcomes, suPAR levels likely represent upstream pathophysiologic processes common to multiple disease states, which are currently unaccounted for when using conventional measures and biomarkers of risk. Addition of suPAR to a traditional risk factor model led to statistically significant but marginal improvement in risk discrimination indices, which is likely due to the high-risk nature of the cohort. Additional studies are needed to determine whether measuring suPAR would be clinically useful in the setting of ESRD.
Lastly, this study is the first to explore a potential interaction between suPAR levels and the effect of statin therapy on outcomes. We did not find an interaction; however, definite conclusions cannot be derived. First, the study was not powered to detect differences in outcomes on the basis of both therapy and suPAR levels, and second, the 4D Study did not find a significant main effect of statin therapy on outcomes in patients with diabetes on dialysis. These findings cannot be applied to lower-risk patients without known kidney disease. Additional studies are needed to determine whether suPAR levels would be useful to identify other groups of patients who would benefit from statin therapy.
Potential limitations of our study need to be acknowledged. It was a post hoc analysis within a selected cohort of German patients with type 2 diabetes mellitus undergoing hemodialysis. Therefore, the relationship between high suPAR and adverse outcomes may not be generalizable to other patient populations. Despite careful adjustments for possible confounders, we cannot rule out residual confounding. However, because known important confounders were considered, the effect of potential residual confounding is likely to be small. Furthermore, we cannot draw conclusions regarding causality from our data. Our data generate new hypotheses that high suPAR levels may reflect a novel pathophysiologic process related to a poor outcome in patients on dialysis. The main strengths of this study are that we could analyze specific outcomes, including sudden cardiac death, stroke, and heart failure death. Additional strengths include the long-term follow-up, adequate sample size, and high incidence of prespecified and centrally adjudicated end points.
In conclusion, suPAR levels are significantly elevated in patients with ESRD and remain associated with adverse outcomes. Thus, although suPAR levels strongly correlate with eGFR, the persistent association with outcomes in ESRD suggests that mechanisms beyond impaired kidney function determine suPAR levels and underlie that association. Additional study is needed to elucidate these mechanisms and determine whether suPAR represents a potential therapeutic target in patients with or at risk for CKD.
Disclosures
C. Wei and J.R. have pending and/or issued patents on novel strategies for kidney therapeutics and stand to gain royalties from their commercialization. J.R. and S.S. are co-founders of TRISAQ, a biotechnology company in which they have financial interest, including stock.
Supplementary Material
Acknowledgments
We thank all patients who participated in the German Diabetes and Dialysis Study. We also thank all investigators, study nurses, and collaborators involved in patient recruitment, sample, and data handling and the laboratory staffs at the University of Freiburg, the University of Würzburg, and the University of Chicago.
This study is supported by departmental funds from Rush University Medical Center and in part, National Institutes of Health grant R01DK101350 (to J.R.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10881016/-/DCSupplemental.
References
- 1.Woo KT, Choong HL, Wong KS, Tan HB, Chan CM: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 81: 1044–1045, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LYC, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA: US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67[Suppl 1]: S1–S305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd , Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members, American Heart Association Statistics Committee, Stroke Statistics Subcommittee : Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 133: e38–e360, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Thunø M, Macho B, Eugen-Olsen J: suPAR: The molecular crystal ball. Dis Markers 27: 157–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiser J: Circulating permeability factor suPAR: From concept to discovery to clinic. Trans Am Clin Climatol Assoc 124: 133–138, 2013 [PMC free article] [PubMed] [Google Scholar]
- 7.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison SJ: Chronic kidney disease: suPAR in CKD. Nat Rev Nephrol 12: 3, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Schulz CA, Persson M, Christensson A, Hindy G, Almgren P, Nilsson PM, Melander O, Engström G, Orho-Melander M: Soluble urokinase-type plasminogen activator receptor (suPAR) and impaired kidney function in the population-based Malmö diet and cancer study. Kidney Int Rep 2: 239–247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbel Y, Strauss BH: suPAR: A cardiac biomarker with a future? Can J Cardiol 31: 1223–1224, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Botha S, Fourie CMT, Schutte R, Eugen-Olsen J, Pretorius R, Schutte AE: Soluble urokinase plasminogen activator receptor as a prognostic marker of all-cause and cardiovascular mortality in a black population. Int J Cardiol 184: 631–636, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Cyrille NB, Villablanca PA, Ramakrishna H: Soluble urokinase plasminogen activation receptor--an emerging new biomarker of cardiovascular disease and critical illness. Ann Card Anaesth 19: 214–216, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, Thorball CW, Velegraki A, Kremastinos DT, Lerakis S, Sperling L, Quyyumi AA: Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc 3: e001118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, Petersen J, Pielak T, Møller LN, Jeppesen J, Lyngbaek S, Fenger M, Olsen MH, Hildebrandt PR, Borch-Johnsen K, Jørgensen T, Haugaard SB: Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med 268: 296–308, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Haupt TH, Petersen J, Ellekilde G, Klausen HH, Thorball CW, Eugen-Olsen J, Andersen O: Plasma suPAR levels are associated with mortality, admission time, and charlson comorbidity index in the acutely admitted medical patient: A prospective observational study. Crit Care 16: R130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyngbæk S, Marott JL, Sehestedt T, Hansen TW, Olsen MH, Andersen O, Linneberg A, Haugaard SB, Eugen-Olsen J, Hansen PR, Jeppesen J: Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham risk score. Int J Cardiol 167: 2904–2911, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Meijers B, Poesen R, Claes K, Dietrich R, Bammens B, Sprangers B, Naesens M, Storr M, Kuypers D, Evenepoel P: Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int 87: 210–216, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Persson M, Engström G, Björkbacka H, Hedblad B: Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the Malmo Diet and Cancer study. Atherosclerosis 220: 502–505, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Donadello K, Scolletta S, Taccone FS, Covajes C, Santonocito C, Cortes DO, Grazulyte D, Gottin L, Vincent JL: Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J Crit Care 29: 144–149, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Musetti C, Quaglia M, Cena T, Chiocchetti A, Monti S, Clemente N, Magnani C, Dianzani U, Stratta P: Circulating suPAR levels are affected by glomerular filtration rate and proteinuria in primary and secondary glomerulonephritis. J Nephrol 28: 299–305, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Harita Y, Ishizuka K, Tanego A, Sugawara N, Chikamoto H, Akioka Y, Tsurumi H, Miura K, Gotoh Y, Tsujita M, Yamamoto T, Horike K, Takeda A, Oka A, Igarashi T, Hattori M: Decreased glomerular filtration as the primary factor of elevated circulating suPAR levels in focal segmental glomerulosclerosis. Pediatr Nephrol 29: 1553–1560, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, Wong HN, Troost JP, Gadegbeku CA, Gipson DS, Kretzler M, Nihalani D, Holzman LB; Nephrotic Syndrome Study Network : A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int 87: 564–574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlak K, Pawlak D, Mysliwiec M: Excess soluble urokinase-type plasminogen activator receptor in the plasma of dialysis patients correlates with increased fibrinolytic activity. Thromb Res 119: 475–480, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J: Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem 52: 1284–1293, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 28.Harrell FE Jr., Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Grambsch PM, Therneau TM: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526, 1994 [Google Scholar]
- 30.Hess KR: Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 14: 1707–1723, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Maile LA, Busby WH, Gollahon KA, Flowers W, Garbacik N, Garbacik S, Stewart K, Nichols T, Bellinger D, Patel A, Dunbar P, Medlin M, Clemmons D: Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology 155: 4665–4675, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL: suPAR: A new biomarker for cardiovascular disease? Can J Cardiol 31: 1293–1302, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.