Abstract
Type 2 diabetic kidney disease (DKD) is the most common cause of CKD and ESRD worldwide, and carries with it enormous human and societal costs. The goal of this review is to provide an update on the diagnosis and management of DKD based on a comprehensive review of the medical literature. Topics addressed include the evolving presentation of DKD, clinical differentiation of DKD from non-DKD, a state-of-the-art evaluation of current treatment strategies, and promising emerging treatments. It is expected that the review will help clinicians to diagnose and manage patients with DKD.
Keywords: Costs and Cost Analysis, Diabetic Nephropathies, Disease Management Disease Management, Goals, Humans, Kidney Failure, Chronic, Publications, Renal Insufficiency, Chronic
Introduction
Type 2 diabetes is the most common cause of CKD and ESRD worldwide (1). In the United States, >40% of the >29 million individuals with type 2 diabetes have diabetic kidney disease (DKD) (2). In 2011, Medicare alone spent $25 billion caring for patients with presumed DKD (3).
In light of its widespread prevalence and massive health and financial toll, the diagnosis and management of DKD are of great clinical and societal relevance. This review is therefore designed to update clinicians on the diagnosis and management of DKD through a comprehensive review of the medical literature. We will focus on the evolving clinical presentation of DKD, strategies for diagnosis, contemporary treatment options, and promising new therapies.
In the management section, we emphasize the existing randomized trial data on preventing the development and/or progression of type 2 DKD. Because of well founded concerns that microalbuminuria may not represent established kidney disease (4), we excluded studies that had microalbuminuria as their only kidney-related outcome.
The Evolving Presentation of DKD
The classic description of DKD involved progressive stages of glomerular hyperfiltration, microalbuminuria, overt proteinuria, and a decline in the GFR, eventually leading to dialysis (5). In recent years, this concept has been increasingly challenged as evidence suggests that DKD in the contemporary era presents in a more heterogeneous manner.
Large cross-sectional studies reveal that a large minority of patients with type 2 diabetes and reduced kidney function present with normal levels of albuminuria (6–9). In the Developing Education on Microalbuminuria for Awareness of Kidney and Cardiovascular Risk in Diabetes Study, 17% of this international cohort of 6072 individuals with type 2 diabetes of mean duration of 8 years and an eGFR<60 ml/min per 1.73 m2 had normoalbuminuria (6). In one United States population-based study this number was reported to be as high as 33% (7). These findings are consistent with a recent report in which the prevalence of albuminuria in patients with type 2 diabetes decreased from about 21% in 1988–1994 to 16% in 2009–2014, despite a rise in the prevalence of reduced eGFR (1).
Whether these observations are a result of misdiagnosing DKD in patients who have another kidney disease, the effects of routine use of renin-angiotensin-aldosterone system (RAAS) blockers or other therapies, or other factors remains uncertain. Regardless, a less uniform presentation poses challenges for the clinician in terms of diagnosing DKD, selecting the appropriate treatment regimen, and identifying treatment success.
Differentiating DKD from Non-DKD
Retrospective studies of kidney biopsy specimens in cohorts of patients with type 2 diabetes identify several clinical features (presented in Table 1) that can help differentiate DKD from other kidney diseases (10–12). In patients who have had type 2 diabetes for at least 10 years and who do not present with atypical findings (see Table 1), a tissue diagnosis is not usually necessary (10,13). Such a strategy is supported by findings from the largest such study of kidney biopsy specimens performed in patients with type 2 diabetes (14). In 620 middle-aged patients from the United States with a median time from diagnosis of diabetes of 10 years, 37% of biopsy specimens were consistent with DKD, 36% had non-DKD, and 27% showed coexisting DKD and non-DKD. The most common causes of non-DKD were FSGS (22%), hypertensive nephrosclerosis (18%), acute tubular necrosis (17%), IgA nephropathy (11%), membranous nephropathy (8%), and pauci-immune GN (7%). Duration of diabetes ≥12 years was the best predictor of DKD alone (sensitivity 58%, specificity 73%, positive predictive value 56%, negative predictive value 75%). These numbers are quite good when considering that the patients probably underwent a biopsy because of their atypical presentation.
Table 1.
Clinical features distinguishing type 2 diabetic kidney disease (DKD) from Other Causes of Kidney Disease
| Clinical Feature | Non-DKD | DKD |
|---|---|---|
| Onset of proteinuria | Rapid | Gradual |
| Progression of CKD | Rapid | Gradual |
| Duration of diabetes | <5 yr | >10 yr |
| Urinalysis | Active sediment (hematuria, pyuria, casts) | Bland sediment |
| Retinopathy | Absent | Present |
Until such time when noninvasive biomarkers for DKD are available, the decision to biopsy a patient will remain a clinical judgment that should take into account, for each individual, the risk of the procedure and the likelihood that if another diagnosis should be uncovered then appropriate treatment will not only be offered but will influence the prognosis.
Current Treatment Strategies
BP Control
The Kidney Disease Improving Global Outcomes clinical practice guidelines recommend that patients with DKD achieve systolic and diastolic targets of ≤130 and ≤80 mmHg, respectively, for patients with urine albumin excretion >30 mg/24 h (grade 2D). This guideline was made purely on the basis of observational data that demonstrate urine albumin levels are predictive of adverse cardiovascular and kidney outcomes and a lower BP is inversely associated with albuminuria (15,16).
However, data from randomized controlled trials are much less supportive of these goals. In the landmark UK Prospective Diabetes Study Group (UKPDS) trial of 1148 patients with type 2 diabetes and newly diagnosed hypertension (17), randomization to intensive versus less tight BP control (mean levels achieved, 144/82 versus 154/87 mmHg) significantly reduced the risk of developing microalbuminuria at 6 years of follow-up. However, by year 9 there was no difference between groups in prevalence of micro- or macroalbuminuria or kidney failure.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study (18) randomized 4733 patients with type 2 diabetes for a mean duration of 11 years and hypertension to tight versus standard systolic BP (mean achieved, 119 versus 134 mmHg) and followed them for an average of 5 years. Aggressive BP control significantly reduced the risk of developing microalbuminuria by 16% but not macroalbuminuria or kidney failure, defined as a serum creatinine >3.3 mg/dl, dialysis, or kidney transplantation.
In summary, there are no rigorous clinical trial data to support strict BP control for kidney protection in patients with type 2 diabetes, nor is information available on the ideal BP goal for patients with existing DKD. Individual clinical judgment is therefore indicated with the understanding that although there may be other important benefits of controlling BP in such patients, overly strict BP control may also carry risks. A post hoc analysis of the Irbesartan Diabetic Nephropathy Trial (IDNT) (discussed below) in 1590 adults with overt DKD found a systolic BP ≤120 (versus >120) mmHg to be associated with an increased risk of cardiovascular and all-cause mortality and congestive heart failure events (19).
Preferred Antihypertensive Agents
The use of RAAS blockers as first-line BP-lowering agents in patients with DKD is based on high quality randomized controlled trials throughout the range of type 2 diabetes and DKD (20–23).
In a study of patients with early DKD (21), the angiotensin receptor blocker (ARB) irbesartan was compared with placebo in 590 patients with type 2 diabetes, hypertension, normal mean creatinine clearance, and microalbuminuria. Over a median of 2 years, irbesartan reduced the likelihood of the development of macroalbuminuria or a rise in microalbuminuria by 30% (primary composite outcome) in a statistically significant and dose-dependent manner (44% reduction with 150 mg/d, 68% reduction with 300 mg/d, after adjustment for baseline albuminuria level and BP).
RAAS inhibition has also been studied in patients with more advanced DKD. The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan Study (22) randomized 1513 patients with type 2 diabetes and proteinuric DKD (serum creatinine, 1.3–3.0 mg/dl; urine albumin-to-creatinine ratio [UACR] >300 mg/g creatinine or proteinuria >500 mg/g creatinine) to losartan or placebo for a mean of 3.4 years. Losartan therapy resulted in a 16% reduced risk in the primary composite end point of death, ESRD, or doubling of serum creatinine, with the individual risk of ESRD or doubling of serum creatinine dropping by 25% and 28%, respectively. Losartan reduced the median rate of decline in eGFR by 0.8 ml/min per 1.73 m2 per year.
Similarly, the IDNT (23) Study randomized 1715 patients with DKD and hypertension to irbesartan, amlodipine, or placebo and followed them over a mean of 2.6 years. Compared with placebo or amlodipine, use of irbesartan was associated with a 20% and 23% lower risk of developing the primary composite end point, respectively, which included doubling of baseline serum creatinine, ESRD, or death. With regard to individual end points, irbesartan lowered the risk of doubling of serum creatinine by 33% and 37%, respectively, and reduced the risk of ESRD by 23%, although this was of borderline statistical significance. The rate of decline in the eGFR was 5.5, 6.8, and 6.5 ml/min per 1.73 m2 per year in the irbesartan, amlodipine, and placebo arms, respectively.
Of note, although the data supporting the use of angiotensin-converting enzyme inhibitors (ACEIs) does not rise to the level of evidence for that of ARBs, these medication classes have been used interchangeably in general clinical practice and published guidelines.
Combination ACEI/ARB therapy was evaluated as a prespecified secondary end point in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (24), which randomized 25,620 patients with atherosclerotic vascular disease or diabetes and end-organ damage and a mean baseline creatinine of 1.06 mg/dl to ramipril, telmisartan, or both and followed them over a median of 56 months. Combination therapy was associated with significantly worse outcomes (greater number of events in the primary and secondary renal composite outcomes, which included doubling of serum creatinine, dialysis, or death). The Veterans Affairs Nephropathy in Diabetes Trial similarly assigned 1448 patients with type 2 diabetes, a baseline eGFR between 30 and 89.9 ml/min per 1.73 m2, and UACR≥300 mg/g to losartan alone or in combination with lisinopril (25). No cardiovascular or mortality benefit was observed with combination therapy, although trends suggesting benefits were observed with respect to the secondary end point (first occurrence of decline in eGFR) (hazard ratio [HR], 0.78; 95% confidence interval [95% CI], 0.58 to 1.05; P=0.10) and development of ESRD (HR, 0.66; 95% CI, 0.41 to 1.07; P=0.07). However, safety concerns over a higher incidence of hyperkalemia (6.3 versus 2.6 events per 100 person-years with monotherapy; P<0.001) and AKI (12.2 versus 6.7 events per 100 person-years; P<0.001) in the combination therapy arm led to early termination of the trial. Interestingly, a secondary analysis of this trial showed that participants who developed AKI in the dual therapy arm actually had a lower 30-day mortality (4.7% versus 15.0%; P<0.01), lower risk for first occurrence of reduction in eGFR (HR, 0.60; 95% CI, 0.37 to 0.98), and higher rate of kidney function recovery (75.9% versus 66.3%; P=0.04) during follow-up than individuals in the monotherapy arm. (26)
Direct renin inhibition as add-on therapy to ACEI/ARB treatment has been examined in two trials. The Aliskiren in the Evaluation of Proteinuria in Diabetes Trial (27) randomized 599 patients with type 2 diabetes, hypertension, and DKD (defined as UACR>300 mg/g, or >200 mg/g if on RAAS blockers) to aliskiren or placebo. Over a 6-month study period, aliskiren reduced the primary outcome of UACR by 20%, although a small effect on BP was also noted. In contrast, the much larger Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) (28) compared aliskiren to placebo in 8561 patients with type 2 diabetes and nephropathy (UACR>200 mg/g, eGFR≥30 ml/min per 1.73 m2, or eGFR≤60 ml/min per 1.73 m2 with 20 mg/g ≥UACR <200 mg/g or cardiovascular disease). ALTITUDE was terminated prematurely because of lack of effect on kidney-related outcomes, and a higher rate of hyperkalemia in the add-on arm.
Glycemic Control
The putative benefit of glycemic control on DKD has been the focus of several major studies. The previously mentioned UKPDS Study (29) randomized 3867 patients with newly diagnosed type 2 diabetes to intensive versus conventional glycemic control (median achieved hemoglobin A1c [HbA1c], 7.0% versus 7.9%) using oral hypoglycemic agents or insulin, followed them for up to 15 years, and studied a number of clinical end points. The most consistent finding from a kidney standpoint was a reduction in the development of microalbuminuria in the intensive glycemic control group, but macroalbuminuria and doubling of serum creatinine were also significantly lower in that group at 9 and 12 (but not 15) years of follow-up, with events in these two categories being infrequent.
Another trial measuring the effect of tight glycemic control on kidney outcomes was the Veterans Affairs Diabetes Trial (30) which randomized 1791 veterans with mean duration of type 2 diabetes of 11.5 years, mean HbA1c of 9.4%, and serum creatinine ≤1.6 mg/dl to intensive (achieved mean HbA1c of 6.9%) versus standard (achieved mean HbA1c of 8.4%) glucose control. Over a 5–7 year follow-up period the secondary outcome of nephropathy was assessed. The intensive control group had a significantly lower rate of progression to micro- and macroalbuminuria and any increase in albuminuria, but no difference was noted between the groups in terms of decline in GFR.
Two very large studies examined the effect of glycemic control in populations that included patients with mild to moderate DKD. The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation Trial (31) followed 11,140 patients with type 2 diabetes randomized to intensive versus standard glycemic control (mean achieved HbA1c, 6.5% versus 7.3%) for a median of 5 years. A total of 19% of participants at baseline had an eGFR<60 ml/min per 1.73 m2 and 31% had microalbuminuria or greater. Intensive glycemic control was associated with improvements in a variety of kidney outcomes, including a 9% reduction in new microalbuminuria, 30% reduction in new macroalbuminuria, and 65% reduction in ESRD (with relatively few of these events).
Contrasting findings were reported in the ACCORD Study, which randomized 10,251 patients with type 2 diabetes and high cardiovascular risk to intensive versus standard glycemic control (median HbA1c, 6.4% versus 7.5%) and followed them for an average of 3.5 years. At baseline, median eGFR was 90 ml/min per 1.73 m2 and 33% of participants had microalbuminuria or greater. Although the trial was terminated early because of higher mortality rates in the intensive glucose control arm, kidney-related outcomes were measured as prespecified secondary end points (32). Intensive glycemic control was significantly associated with a 21% and 31% lower incidence of micro- and macroalbuminuria, respectively, a 7% higher rate of doubling of serum creatinine, or a >20 ml/min per 1.73 m2 decrease in eGFR, with no effect found on development of ESRD.
In summary, intensive glycemic control appears to have a beneficial effect on micro- or macroalbuminuria, with conflicting data on whether it protects kidney health in the long term. The higher mortality risk associated with stricter glycemic control reported in the ACCORD Study is also of obvious concern.
Preferred Diabetes Regimen
Growing evidence suggests that certain medication classes offer kidney protection independent of diabetes control. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are the most exciting such class. SGLT-2 is almost exclusively expressed in the proximal kidney tubule and is believed to be the main mechanism for tubular reclamation of glucose. SGLT-2 inhibition not only lowers HbA1c by inhibiting tubular glucose uptake but also leads to weight loss and lower BP. Because the actions of SGLT-2 inhibitors require filtration through the glomerulus, its beneficial effects may be blunted at lower levels of GFR.
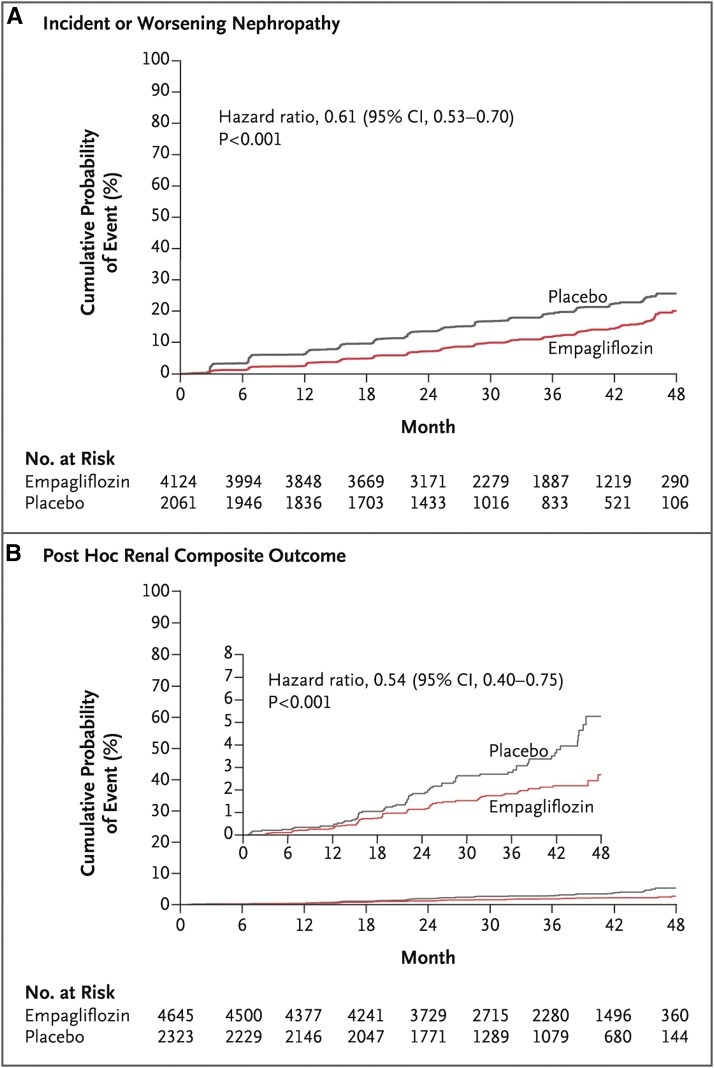
SGLT-2 inhibitors have been shown to influence kidney function and health. For example, they transiently lower GFR (33) presumably by stimulating the tubulo-glomerular feedback mechanism, and also reduce albuminuria (34). The most impressive evidence for renoprotection comes from the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) Trial, which randomized 7020 patients with type 2 diabetes and cardiovascular disease to the SGLT-2 receptor empagliflozin at two doses versus placebo (35). At baseline, 26% of patients had an eGFR<60 ml/min per 1.73 m2 and 40% had micro- or macroalbuminuria. After a median of 3.1 years of follow-up, not only was empagliflozin use linked to fewer cardiovascular and death events but it also improved kidney-related outcomes. Specifically, it was associated with a statistically significant lower rate of incident macroalbuminuria (38%), doubling of serum creatinine and eGFR≤45 ml/min per 1.73 m2 (44%), and initiation of RRT (55%), although the latter end points were few in number (Figure 1). These findings were consistent throughout the range of baseline eGFR and dose of empagliflozin.
Figure 1.
Empagliflozin was associated with improved renal outcomes compared to placebo. (A) Estimates of the probability of the first occurrence of the prespecified composite renal outcomes defined as incident or worsening nephropathy. (B) Estimates of the probability of the post hoc renal composite outcomes defined as doubling of serum creatinine level, death from renal disease, or requirement of RRT. Hazard ratios shown are from Cox regression analysis (35). 95% CI, 95% confidence interval.
Although the early evidence for SGLT-2 inhibition appears promising, it remains to be seen whether these results will be duplicated in several ongoing clinical trials that have primary kidney-related outcomes (Clinicaltrials.gov identifiers: NCT02065791, NCT02065791, and NCT01989754). In addition, the US Food and Drug Administration recently released a warning about the risk of AKI with SGLT-2 inhibitor use (36). Although the EMPA-REG OUTCOME Trial did not report higher rates of AKI, postmarketing surveillance will be important in determining its frequency.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are another class of diabetes drugs with possible kidney protective effects. DPP-4 inhibitors block the degradation of molecules like glucagon-like peptide-1, leading to augmented pancreatic secretion of insulin. A post hoc analysis of 217 patients with type 2 diabetes and micro- or macroalbuminuria on RAAS blockers collected from several phase 3 randomized, placebo-controlled clinical trials found that use of the DPP-4 inhibitor linagliptin for 24 weeks led to a 32% drop in albuminuria, independent of BP or HbA1c values (37). More recently, a post hoc analysis of the Trial Evaluating Cardiovascular Outcomes with Sitagliptin, which included 14,735 patients with type 2 diabetes and cardiovascular disease randomized to the DPP-4 inhibitor sitagliptin versus placebo as add-on therapy, reported that over the 4-year follow-up period, the sitagliptin group had a marginally lower median UACR (−0.18 mg/g) and lower mean eGFR (−1.34 ml/min per 1.73 m2) (38).
Weight Reduction and Diet
Because the development of type 2 diabetes is strongly linked to dietary habits and excess adiposity, it is reasonable to consider strategies targeting these factors in the management of DKD. Although there are no dietary recommendations tailored specifically for patients with DKD, the Action for Health in Diabetes Trial (39) compared the effect of intensive lifestyle intervention (i.e., decreased caloric intake and increased physical activity) versus standard diabetes support and education on the prespecified secondary end point of DKD. Participants included 5145 overweight or obese patients with type 2 diabetes and baseline mean albuminuria and serum creatinine levels within the normal range. Over 10 years of follow-up, the intensive lifestyle group had a 4 kg reduction in weight compared with the group on standard treatment. They also had a statistically significant 31% lower cumulative incidence of developing very high-risk CKD (40). Although preliminary, these findings suggest that diet and weight can modify the development and progression of DKD.
Whether bariatric surgery, the most effective and sustained of the weight reduction strategies, is effective in treating DKD has recently been comprehensively reviewed (41). An even more recent case-control study of 985 patients who underwent bariatric surgery and matched controls also addressed this question (42). Over a median follow-up of 4 years, bariatric surgery was associated with a 58% lower risk of ≥30% decline in eGFR and a 57% lower risk of doubling of serum creatinine or ESRD. Similar findings were seen in the approximately 40% of individuals with type 2 diabetes who were included in the study. Results should be interpreted cautiously in light of the influential effect large weight reduction has on serum creatinine (and eGFR) through the loss of muscle mass and the relatively small number of individuals who achieved the clinical end points. Nevertheless, in light of the positive results and ongoing advances in surgical weight reduction strategies, bariatric surgery may turn out to be an important addition to the clinical armamentarium for DKD.
Emerging Therapies for DKD
This section reviews several novel therapies for DKD that are being studied in phase 3+ clinical trials.
Endothelin-1 Receptor A Antagonists
Endothelin-1 receptor A activation within the kidney results in increased oxidative stress, podocyte injury, vasoconstriction, fibrosis, and inflammation (43).
A phase 2 clinical trial, the Reducing Residual Albuminuria in Subjects with Diabetes and Nephropathy with Atrasentan Trial, showed that the selective endothelin-1 receptor A antagonist atrasentan lowered albuminuria and BP in patients with DKD (44). A post hoc analysis showed decreased renal risk with use of atrasentan and served as the basis for the Study of Diabetic Nephropathy with Atrasentan (SONAR) (Clinicaltrials.gov identifier: NCT01858532) (45). SONAR is expected to enroll 4148 patients with DKD and has a composite end point of ESRD, doubling of serum creatinine, or death. Its expected completion date is late 2018.
Mineralocorticoid Receptor Antagonists
There is growing interest in the protective role of agents that block the RAAS cascade downstream. Steroidal mineralocorticoid receptor (MR) antagonists like spironolactone and eplerenone have a limited role to play as adjunct therapy to ACEI/ARBs because of hyperkalemia and other adverse effects. It is thought that this is, in part, because of variations in cell-specific effects of steroidal MR antagonists and incomplete antagonism. Identification of nonsteroidal MR antagonists that have a predictable antagonistic response and more tolerable side effect profile is currently underway. Finerenone is one such agent under investigation. The Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy Study showed that addition of finerenone to ACEIs/ARBs resulted in improvement in UACR (46). The Efficacy and Safety of Finerenone in Subjects with Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease Study (Clinicaltrials.gov identifier: NCT02545049) has a planned enrollment of 6400 patients with type 2 diabetes, has kidney outcomes as secondary end points, and an estimated completion date in early 2019. The Efficacy and Safety of Finerenone in Subjects with Type 2 Diabetes Mellitus and Diabetic Kidney Disease Study has a planned enrollment of 4800 patients, a kidney end point as the primary end point, and an estimated completion date in mid-2019.
TGF-β Inhibitors
Fibrosis is a hallmark of DKD and TGF-β1 is a potent profibrotic molecule. Pirfenidone interferes with the secretion, expression, and action of TGF-β1 by an unknown mechanism. It is currently being evaluated in a phase 3 trial (Clinicaltrials.gov identifier: NCT02689778) with planned recruitment of 62 patients with type 2 diabetes and with changes in albuminuria and GFR as primary end points. It is expected to end in early 2018. Given its modest size, this trial should be expected to be more hypothesis generating than definitive in nature.
Phosphodiesterase Inhibitors
The nonselective phosphodiesterase inhibitor pentoxifylline has demonstrated anti-inflammatory, antifibrotic, and antiproteinuric effects in several small studies. It is currently being evaluated in a prospective clinical trial (Clinicaltrials.gov identifier: NCT01377285) of 350 patients with advanced DKD versus CKD stages 3 and 4 in nonpatients with diabetes. The study has a primary kidney end point and is expected to be completed in December 2018.
5-Hydroxytryptamine 2a Receptor Antagonists
The selective 5-hydroxytryptamine 2a receptor antagonist sarpogrelate hydrochloride has been shown to have renoprotective effects in a series of diabetic animal studies. The Sarpogrelate on the Nephropathy in Type 2 Diabetes Study (Clinicaltrials.gov identifier: NCT01869881) was designed to evaluate the safety and efficacy of sarpogrelate hydrochloride in 166 patients with type 2 diabetes and albuminuria or overt proteinuria. The study has a primary renal outcome. The status of the study remains unknown as the last Clinicaltrials.gov update was in July of 2014.
Discussion
The contemporary management of DKD, as seen in Table 2, offers some reasons for optimism, including the proven efficacy of RAAS blockers, the excitement over SGLT-2 inhibitors, and the possibility of novel therapies on the horizon. Yet critical gaps remain in our understanding of DKD. Diagnosis of DKD remains a matter of subjective assessment because a noninvasive biomarker is lacking. This limitation makes it more difficult to design clinical trials to identify effective treatments for DKD. Whether nonproteinuric DKD requires a different treatment strategy than proteinuric DKD is not known. The roles of BP and glycemic control in managing DKD still remain controversial despite decades of study. In addition, the influence of diet and obesity on the development and progression of DKD is also unclear. Insights into these issues will be necessary to further advance clinical management of DKD.
Table 2.
Contemporary management of diabetic kidney disease (DKD)
| Therapeutic Options | Comment |
|---|---|
| BP control | Guidelines’ goal of <130/<80 mmHg is not supported by data from randomized controlled trials. Ideal BP is not known |
| RAAS blockade | Strongest evidence supports ARB use. Combination ACEI/ARB or direct renin inhibitor use are not supported by data |
| Glycemic control | Intensive control (HbA1c≤7%) protects against micro- or macroalbuminuria but data are conflicting regarding effects on CKD progression. Also carries risk of serious complications |
| SGLT-2 inhibition | Promising evidence for renoprotection from secondary end point in EMPA-REG OUTCOME Trial. Awaiting results of additional confirmatory trials |
| Weight loss | Preliminary data suggest a benefit but require confirmation |
RAAS, renin-angiotensin-aldosterone system; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; HbA1c, hemoglobin A1c; SGLT-2, sodium-glucose cotransporter 2; EMPA-REG OUTCOME, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RA, Wang Y, Zhu V, Rupnow MF: Chronic kidney disease in US adults with type 2 diabetes: An updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 7: 415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: A report from an ADA Consensus Conference. Am J Kidney Dis 64: 510–533, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Bakris GL, Molitch M: Microalbuminuria as a risk predictor in diabetes: The continuing saga. Diabetes Care 37: 867–875, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB: Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: Results from the DEMAND study. Cardiorenal Med 2: 1–10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer HJ, Nguyen QD, Curhan G, Hsu CY: Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289: 3273–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G; Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29: 1802–1809, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, Atkins RC: Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 32: 1497–1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, Han SH, Yoo TH, Kang SW: Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract 92: 198–204, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA: Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 27: 322–328, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Bermejo S, Soler MJ, Gimeno J, Barrios C, Rodríguez E, Mojal S, Pascual J: Predictive factors for non-diabetic nephropathy in diabetic patients. The utility of renal biopsy. Nefrologia 36: 535–544, 2016. [DOI] [PubMed]
- 13.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, Wada J, Makino H: Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 69: 237–242, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, D’Agati VD: The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 8: 1718–1724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KDOQI: KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49[Suppl 2]: S12–S154, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care 39[Suppl 1]: S4–S5, 2016 [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail-Beigi F, Craven TE, O’Connor PJ, Karl D, Calles-Escandon J, Hramiak I, Genuth S, Cushman WC, Gerstein HC, Probstfield JL, Katz L, Schubart U; ACCORD Study Group: Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 81: 586–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ; Collaborative Study Group: Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 16: 2170–2179, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G; Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators: Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351: 1941–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators: Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Palevsky PM, Zhang JH, Seliger SL, Emanuele N, Fried LF; VA NEPHRON-D Study: Incidence, severity, and outcomes of AKI associated with dual renin-angiotensin system blockade. Clin J Am Soc Nephrol 11: 1944–1953, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK; AVOID Study Investigators: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Heerspink HJ, Persson F, Brenner BM, Chaturvedi N, Brunel P, McMurray JJ, Desai AS, Solomon SD, Pfeffer MA, Parving HH, de Zeeuw D: Renal outcomes with aliskiren in patients with type 2 diabetes: A prespecified secondary analysis of the ALTITUDE randomised controlled trial. Lancet Diabetes Endocrinol 4: 309–317, 2016 [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 30.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators: Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S; ADVANCE Collaborative Group: Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD trial group: Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD: Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia 59: 2036–2039, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR), 2016. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. Accessed February 15, 2017
- 37.Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M: Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 36: 3460–3468, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, Engel SS, Lopes RD, McGuire DK, Riefflin A, Rodbard HW, Sinay I, Tankova T, Wainstein J, Peterson ED, Holman RR; TECOS Study Group: Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: Outcomes from TECOS. Diabetes Care 39: 2304–2310, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Look AHEAD Research Group: Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: A secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2: 801–809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Supp 3: 1–150, 2013. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf. Accessed February 15, 2017
- 41.Friedman AN, Wolfe B: Is bariatric surgery an effective treatment for type II diabetic kidney disease? Clin J Am Soc Nephrol 11: 528–535, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, Kramer H, Hartle JE, Carey D, Appel LJ, Grams ME: Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 90: 164–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS: Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schievink B, de Zeeuw D, Smink PA, Andress D, Brennan JJ, Coll B, Correa-Rotter R, Hou FF, Kohan D, Kitzman DW, Makino H, Parving HH, Perkovic V, Remuzzi G, Tobe S, Toto R, Hoekman J, Lambers Heerspink HJ: Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur J Prev Cardiol 23: 758–768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group: Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]