Abstract
Background and objectives
The objective of this meta-analysis is to compare the incidences of cytomegalovirus and BK polyoma virus infections in renal transplant recipients receiving a mammalian target of rapamycin inhibitor (mTOR)–based regimen compared with a calcineurin inhibitor–based regimen.
Design, setting, participants, & measurements
We conducted a comprehensive search for randomized, controlled trials up to January of 2016 addressing our objective. Other outcomes included acute rejection, graft loss, serious adverse events, proteinuria, wound-healing complications, and eGFR. Two review authors selected eligible studies, abstracted data, and assessed risk of bias. We assessed quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation methodology.
Results
We included 28 randomized, controlled trials with 6211 participants classified into comparison 1: mTOR inhibitor versus calcineurin inhibitor and comparison 2: mTOR inhibitor plus reduced dose of calcineurin inhibitor versus regular dose of calcineurin inhibitor. Results showed decreased incidence of cytomegalovirus infection in mTOR inhibitor–based group in both comparison 1 (risk ratio, 0.54; 95% confidence interval, 0.41 to 0.72), with high quality of evidence, and comparison 2 (risk ratio, 0.43; 95% confidence interval, 0.24 to 0.80), with moderate quality of evidence. The available evidence neither confirmed nor ruled out a reduction of BK polyoma virus infection in mTOR inhibitor–based group in both comparisons. Secondary outcomes revealed more serious adverse events and acute rejections in mTOR inhibitor–based group in comparison 1 and no difference in comparison 2. There was no difference in graft loss in both comparisons. eGFR was higher in the mTOR inhibitor–based group in comparison 1 (mean difference =4.07 ml/min per 1.73 m2; 95% confidence interval, 1.34 to 6.80) and similar to the calcineurin inhibitor–based group in comparison 2. More proteinuria and wound-healing complications occurred in the mTOR inhibitor–based groups.
Conclusions
We found moderate- to high-quality evidence of reduced risk of cytomegalovirus infection in renal transplant recipients in the mTOR inhibitor–based compared with the calcineurin inhibitor–based regimen. Our review also suggested that a combination of a mTOR inhibitor and a reduced dose of calcineurin inhibitor may be associated with similar eGFR and rates of acute rejections and serious adverse events compared with a standard calcineurin inhibitor–based regimen at the expense of higher incidence of proteinuria and wound-healing complications.
Keywords: immunosuppression, cytomegalovirus, Meta-analysis, Calcineurin inhibitor, BK virus, cyclosporine, tacrolimus, sirolimus, everolimus, Calcineurin, Calcineurin Inhibitors, Confidence Intervals, Cytomegalovirus Infections, glomerular filtration rate, Incidence, kidney transplantation, Odds Ratio, Polyomavirus, proteinuria, Randomized Controlled Trials as Topic, Risk, Assessment, Sirolimus, Wound Healing

Introduction
Cytomegalovirus (CMV) infection (defined by detectable CMV DNA by PCR in the blood or pp65 antigenemia) may occur in up to 92% of renal transplant recipients (RTRs), and CMV disease (defined by CMV infection with organ involvement) may occur in up to 37%. The occurrence of either CMV infection or disease is an independent risk factor for patient mortality and long-term allograft dysfunction (1).
BK polyoma virus (BKPyV) infection can lead to polyoma virus–associated nephropathy (PyVAN) in 1%–10% of RTRs and is an important cause of early graft failure (2). Viremia, as assessed by PCR, of >104 copies and viruria of >107 copies correlate with the presence of PyVAN (3).
In addition to their use as immunosuppressive medications, mammalian target of rapamycin inhibitors (mTORis; e.g., sirolimus or everolimus) may also exhibit direct antiviral action, because activation of mammalian target of rapamycin in host cells is essential for CMV replication (4,5). Furthermore, it has been shown that RTRs treated with everolimus had a more robust CMV-specific CD8+ T cell response compared with those treated with cyclosporin or mycophenolic acid (MPA) (6). Similarly, in a recent study, sirolimus inhibited BKPyV replication in renal tubular epithelial cells, whereas tacrolimus activated it (7).
In a meta-analysis in RTRs, conversion to an mTORi-based regimen from a calcineurin inhibitor (CNI)–based regimen (e.g., cyclosporin or tacrolimus) resulted in a 37% decreased risk of CMV infection. A limitation of this systematic review was that most of the trials included compared mTORi with cyclosporin; therefore, no conclusion was possible regarding their comparison with tacrolimus. Also, the incidence of BKPyV infection was not assessed (8).
In a large patient-cohort study examining 48,292 RTRs, the incidence of PyVAN within 24 months of transplantation was 1.74% with use of an mTORi versus 3.67% if an mTORi was not used (9). However, in a recent retrospective analysis involving 348 RTRs, the incidence of BKPyV infection was independent of the type of induction or maintenance immunosuppressive regimen used (10).
On the basis of these emerging data, there is a need to summarize and update current evidence from randomized, controlled trials (RCTs) that assessed these infections in RTRs.
The objective of this systematic review is to compare the incidences of CMV and BKPyV infections in RTRs receiving an mTORi-based maintenance immunosuppression compared with a CNI-based regimen.
Materials and Methods
Protocol and Registration
Our review protocol is registered in PROSPERO (PROSPERO 2016:CRD42016033665; available at http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016033665).
Eligibility Criteria
We sought to include RCTs, blinded or nonblinded, comparing an mTORi-based regimen with a CNI-based regimen and reporting the incidence of at least one of the primary outcomes (CMV or BKPyV infection) with a follow-up time of at least 6 months. Inclusion criteria were RTRs irrespective of age, race, or sex.
We selected trials with an intervention arm consisting of an mTORi-based regimen (e.g., mTORi + MPA ± steroids) and a control arm consisting of a CNI-based regimen (e.g., CNI + MPA ± steroids; comparison 1). We also selected trials using an mTORi-based maintenance regimen consisting of an mTORi combined with a reduced-dose CNI compared with a standard CNI-based regimen (comparison 2). For both comparisons, we selected trials where cointerventions, including induction immunosuppressive therapy and additional immunosuppressive drugs, were similar for the two groups at study entry.
Outcomes
Table 1 details the primary and secondary outcomes assessed in our review.
Table 1.
Primary and secondary outcomes assessed
| Primary outcomes |
| Incidence of CMV infection (on the basis of a positive blood CMV PCR or antigenemia pp65 or as defined in included trials) |
| Incidence of BKPyV infection (on the basis of blood PCR >104 copies or urine PCR >107 copies or as defined in included trials) or a transplanted kidney biopsy typical of PyVAN |
| Secondary outcomes |
| Composite of the incidence of acute cellular rejection, AMR, or development of DSAs |
| Graft loss |
| Incidence of serious adverse events |
| Incidence of other viral, bacterial, or fungal infections |
| Incidence of PyVAN as defined by kidney biopsy |
| Incidence of CMV disease (retinitis, pneumonitis, hepatitis, or colitis) |
| Incidence of proteinuria |
| Incidence of wound-healing complications |
| eGFR or creatinine clearance at study termination |
CMV, cytomegalovirus; BKPyV, BK polyoma virus; PyVAN, polyoma virus–associated nephropathy; AMR, antibody-mediated rejection; DSA, donor-specific antibody.
Search Strategy
We searched the following electronic sources from inception to January of 2016: the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE. We did not use any language restrictions. We also searched the reference lists of included trials and related systematic reviews (records identified through other sources). Supplemental Table 1 details the electronic search strategy.
Selection Process
Two review authors (B.Y.T. and H.S.I.) screened in duplicate and independently the abstract and title of every record retrieved by the searches for eligibility. We retrieved the full text for all articles judged as potentially eligible by at least one of the two reviewers. The two reviewers then assessed in duplicate and independently the full texts for eligibility using a standardized and pilot tested screening form. They then compared their results and resolved any disagreements by consensus and when unsuccessful, with the help of a third reviewer (S.G.M.). We will present a preferred reporting items for systematic reviews and meta-analyses flow chart to summarize the study selection process (11).
Data Extraction
For studies that fulfilled our eligibility criteria, two review authors (B.Y.T. and H.S.I.) independently and in duplicate abstracted relevant information using standardized data extraction forms. We extracted information about the study design and the characteristics of the population, intervention, comparator, and outcomes (Table 2).
Table 2.
Characteristics of included studies
| Study Name | Study Design | Participants | Intervention | Control | Outcomes Assessed |
|---|---|---|---|---|---|
| Comparison 1: mTORi versus CNI | |||||
| Rostaing et al., 2015 (17) | Multicenter, open label, prospective, randomized trial | n=194; Country: France; mean age: 48 yr; men: 65%; immunologic risk: low/moderate; donor type: donor with brain stem death: 90%; living donor: 10% | EVR target trough level of 6–10 ng/ml | CsA target trough level of 100–150 ng/ml | Primary outcome: progression of IF/TA between 3 and 12 mo post-transplant; secondary outcomes included BPAR, eGFR (MDRD formula), adverse events, including CMV and BKPyV infections |
| Induction therapy: basiliximab in both groups | Cointerventions | Cointerventions | |||
| CMV prophylaxis: data not available | EC-MPS, steroids | EC-MPS, steroids | |||
| All patients were maintained on CsA + EC-MPS + steroids at baseline | Timing of conversion: 3 mo post-transplant | ||||
| Budde et al., 2015 (18) | Multicenter, open label, prospective, randomized, parallel-group study | n=93; Country: Germany; mean age: 51 yr; men: 63%; race: all white; donor type: deceased, noncardiac death: 54.3%; deceased, cardiac death: 13%; living related: 21%; living unrelated: 11%; immunologic risk: low | EVR target trough of 6–10 ng/ml | CsA trough target range 80–150 ng/ml or TAC target range of 5–10 ng/ml; cointerventions: EC-MPS, steroids | Primary efficacy outcome: eGFR (Nankivell formula) 12 mo after randomization, safety outcomes, including CMV infection |
| Induction therapy: data not available | Cointerventions: EC-MPS, steroids | ||||
| CMV prophylaxis: data not available | Timing of conversion: mean of 7 yr after kidney transplantation | ||||
| All patients maintained on CNI + EC-MPS ± steroids at baseline | |||||
| Budde et al., 2015 (5-yr follow-up) (19) | Multicenter, open label, prospective, randomized, parallel-group study | n=78; Country: Germany; mean age: 48 yr; men: 62%; race: all white; donor type: deceased donor: 70%; immunologic risk: low | EVR target trough of 6–10 ng/ml | CsA trough target of 80–150 ng/ml or TAC target of 5–10 ng/ml; cointerventions: EC-MPS, steroids | Primary efficacy outcome: eGFR (Nankivell formula) 5 yr after randomization, safety outcomes, including CMV and BKPyV infection |
| Induction therapy: data not available | Cointerventions: EC-MPS, steroids | ||||
| CMV prophylaxis: data not available | Timing of conversion: mean of 7 yr after kidney transplantation | ||||
| All patients maintained on CNI + EC-MPS ± steroids at baseline | |||||
| Budde et al., 2015 (20) | Multicenter, randomized, prospective, open label, parallel-group trial | n=232; Country: Germany, Switzerland; mean age: 47.3 yr; men: 62%; race: 97% white; immunologic risk: low | EVR target trough of 6–10 ng/ml; cointerventions: EC-MPS ± steroids | CsA trough target of 100–150 ng/ml; cointerventions: EC-MPS ± steroids | Primary efficacy outcome: eGFR (Nankivell formula) 5 yr after randomization, safety outcomes, including CMV infection |
| Induction therapy: basiliximab in both arms | Proportion of patients on steroids was balanced between the two groups | ||||
| CMV prophylaxis: data not available | Timing of conversion: 4.5 mo post-transplantation | ||||
| All patients maintained on CsA + EC-MPS + steroids at baseline | |||||
| Silva et al., 2013 (21) | Prospective, multicenter, randomized, controlled, parallel-group trial | n=297; Country: Brazil; mean age: 44.6 yr; men: 69%; race: white, 57%; black, 11%; mixed, 26%; other, 6%; immunologic risk: low; donor type: living related: 16%; living unrelated: 9%; deceased: 75% | SIR target trough level of 8–12 ng/ml | TAC trough target trough level of 5–10 ng/ml; cointerventions: EC-MPS + steroids | Primary outcome: eGFR at 24 mo (MDRD formula); secondary outcomes: acute rejection, adverse events, including CMV infection |
| Induction therapy: basiliximab in both arms | Cointerventions: EC-MPS + steroids | ||||
| CMV prophylaxis: data not available | Timing of conversion: 3 mo post-transplantation | ||||
| All patients maintained on TAC + EC-MPS + steroids at baseline | |||||
| Chhabra et al., 2013 (22) | Prospective, open label, single-center, randomized study | n=200; Country: United States; mean age: 49 yr; men: 52.8%; race: 53% white, 23% black, 20% Hispanic; donor type: deceased: 31%; living related: 41%; living unrelated: 27% | SIR target trough level 5–8 ng/ml | TAC target trough 6–8 ng/ml; cointerventions: MMF | Primary outcome: acute rejection at 24 mo; secondary outcomes: patient and graft survival, eGFR (MDRD formula), DSAs, and safety outcomes, including CMV and BKPyV infection |
| Induction therapy: alemtuzumab and methylprednisolone with rapid steroid elimination in both groups | |||||
| CMV prophylaxis: all patients receiving kidneys from CMV-positive donors were given valganciclovir 450 mg orally once daily for 6 mo; seronegative recipients receiving a kidney from a CMV-negative donor did not receive CMV prophylaxis | Cointerventions: MMF | ||||
| Timing of conversion: 12 mo post-transplantation | |||||
| All patients were maintained on a steroid-free regimen with TAC and MMF at baseline | |||||
| Bansal et al., 2013 (23) | Prospective, open label, randomized trial | n=60; Country: India; mean age: 30 yr; men: 86%; donor type: living related: 75%; living unrelated: 25%; immunologic risk: low | SIR target trough level 8–15 ng/ml; cointerventions: MMF + steroids; timing of conversion: 2 mo post-transplantation | TAC target trough level 6–8 ng/ml or CsA target trough level 150–250 ng/ml; cointerventions: MMF + steroids | Primary outcome: eGFR by MDRD at 6 mo; secondary outcomes: BPAR, side effects, including CMV infection |
| Induction therapy: 18% of population received induction therapy, balanced between the two groups | |||||
| CMV prophylaxis: data not available | |||||
| All patients maintained on CNI + MMF + steroids at baseline | |||||
| Mjörnstedt et al., 2012 (24) | Prospective, multicenter, randomized, controlled, parallel-group trial | n=202; Country: Sweden, Norway, and Denmark; mean age: 55.5 yr; women: 31.4%; race: 97% white; immunologic risk: low; donor type: deceased donor: 73% | EVR target trough level of 6–10 ng/ml; cointerventions EC-MPS + steroids; timing of conversion: 7 wk post-transplantation | CsA target trough level 50–150 ng/ml; cointerventions EC-MPS + steroids | Primary outcome: change in renal function from week 7 to month 12 (iohexol or 51Cr-EDTA clearance); secondary outcomes: safety outcomes, including CMV and BKPyV infection |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: prophylactic treatment for CMV was given according to local practice | |||||
| All patients maintained on CsA + EC-MPS + steroids at baseline | |||||
| Guba et al., 2012 (25) (follow-up of Guba et al., 2010 [28]) | Prospective, multicenter, randomized, controlled, parallel-group trial | n=132; country: Germany; immunologic risk: low to moderate | SIR trough levels of 5–10 ng/ml; cointerventions: MMF + steroids; timing of conversion: 10–24 d post-transplantation | CsA trough levels 100–150 ng/ml; cointerventions: MMF + steroids | Primary outcome: eGFR at 36 mo (Nankivell formula); secondary end points: acute rejection, recipient and allograft survival, adverse events, including CMV infection |
| Induction therapy: ATG in both groups | |||||
| CMV prophylaxis: CMV-negative patients receiving kidneys from CMV-positive donors received prophylaxis according to local center practice | |||||
| All patients maintained on CsA + MMF + steroids at baseline | |||||
| Weir et al., 2011 (26) | Multicenter, randomized, prospective, open label trial | n=299; Country: United States; mean age: 48.7 yr; men: 62.8%; race: 50% white; 32% black; immunologic risk: low/moderate; donor type: deceased: 60%; living related: 28%; living unrelated: 12% | SIR to trough levels of 5–10 ng/ml | CNI (dosed according to center protocol); cointerventions: MMF ± steroids | Primary outcome: mean percentage change in renal function after 12 mo (clearance of cold iothalamate); secondary outcomes: acute rejection, graft loss, adverse events, including CMV and BKPyV infection |
| Induction therapy: induction therapy was used in 70% of patients in each group; use of ATG, basiliximab, daclizumab, and muromonab-CD3 was balanced between the two groups | Cointerventions: MMF ± steroids | ||||
| CMV prophylaxis: antiviral prophylaxis given to around 40% of patients in both groups | Timing of conversion: | ||||
| All patients maintained on CNI + MMF ± steroids at baseline | 30–180 d post-transplantation | ||||
| Heilman et al., 2011 (27) | Prospective, randomized, nonblinded trial | n=122; Country: United States; mean age: 54 yr; men: 60%; race: 75% white, 15% Hispanic, 10% black; immunologic risk: low; donor type: deceased: 50%; living: 50%; induction therapy: ATG in both groups | SIR to target trough level of at least 8 ng/ml; cointerventions: MMF; timing of conversion: 1 mo post-transplantation | TAC to trough of 5–8 ng/ml; cointerventions: MMF | Primary outcome: measured GFR by iothalamate at 1 yr; secondary outcomes: GFR at 2 yr, BPAR, adverse events, including CMV and BKPyV infection |
| CMV prophylaxis: valgancyclovir 450 mg daily for 3 mo if the recipient was CMV positive or the donor was positive and the recipient was negative | |||||
| All patients maintained at baseline on CNI + MMF with rapid corticosteroid withdrawal | |||||
| Guba et al., 2010 (28) | Prospective, multicenter, randomized, controlled, parallel-group trial | n=141; Country: Germany; mean age: 47 yr; men: 65%; race: 98% white; immunologic risk: low to moderate; donor type: after brain death: 88.4%; living related: 11.6% | SIR trough levels of 5–10 ng/ml; cointerventions: MMF + steroids; timing of conversion: 10–24 d after transplantation | CsA trough levels 100–150 ng/ml; cointerventions: MMF + steroids | Primary outcome: eGFR at 12 mo (Nankivell formula); secondary outcomes: acute rejection, recipient and allograft survival, adverse events, including CMV infection |
| Induction therapy: ATG in both groups | |||||
| CMV prophylaxis: CMV-negative patients receiving kidneys from CMV-positive donors received prophylaxis according to local center practice | |||||
| All patients were maintained on CsA + MMF + steroids at baseline | |||||
| Franz et al., 2010 (29) | Prospective, single-center, randomized, open label trial | n=127; Country: Switzerland; mean age: 48 yr; men: 70%; immunologic risk: low; donor type: cadaveric: 44%; living related: 31%; living unrelated: 25% | SIR to trough of 8–15 ng/ml; cointerventions: MMF + steroids | CsA to trough level of 200–250 ng/ml; cointerventions: MMF + steroids | Primary outcome: kidney function measured using serum creatinine level at 6 mo; secondary outcomes: patient and graft survival, no. of rejections, proteinuria, adverse events, including CMV infection |
| Induction therapy: not given | |||||
| CMV prophylaxis: data not available | |||||
| Lebranchu et al., 2009 (30) | Prospective, multicenter, randomized, open label trial | n=192; Country: France; mean age: 46.5 yr; men: 70%; immunologic risk: low; donor type: cadaveric, noncardiac death | SIR to target trough level of 5–10 ng/ml; cointerventions: MMF + steroids; timing of conversion: 3 mo post-transplantation | CsA to C2 levels 500–800 ng/ml; cointerventions: MMF + steroids | Primary outcome: creatinine clearance by Cockcroft–Gault formula at 52 wk; secondary outcomes: graft and patient survival, BPAR, adverse events, including CMV and BKPyV infections |
| Induction therapy: daclizumab in both groups | |||||
| CMV prophylaxis: all CMV-negative recipients who received a kidney from a CMV-positive donor received prophylaxis for CMV infection according to the center practice for a minimum of 12 wk | |||||
| All patients were maintained on CsA + MMF + steroids at baseline | |||||
| Durrbach et al., 2008 (31) | Prospective, multicenter, randomized, open label trial | n=69; Country: France; mean age: 52 yr; immunologic risk: low/moderate; donor type: cadaveric, expanded criteria donor | SIR to trough levels of 10–20 ng/ml; cointerventions: MMF + steroids | CsA to trough level of 75–200 ng/ml; cointerventions: MMF + steroids | Primary outcome: creatinine clearance (Cockcroft–Gault formula) at 6 mo; secondary outcomes: patient and graft survival, BPAR, adverse events, including CMV infection |
| Induction therapy: ATG in both groups | |||||
| CMV prophylaxis: valacyclovir or valganciclovir for 16 wk in CMV-negative recipients of a CMV-positive kidney | |||||
| Ekberg et al., 2007 (32) | Prospective, multicenter, randomized, controlled, parallel-group trial | n=1645; Country: international; mean age: 46 yr; men: 65%; race: 92% white; immunologic risk: low/moderate; donor type: deceased: 64%; living related: 29%; living unrelated: 6%; four arms: standard-dose CsA (excluded because different induction regimen), low-dose CsA, low-dose tacrolimus (control), low-dose SIR (intervention) | SIR trough level of 4–8 ng/ml; cointerventions: MMF + steroids | CsA trough level of 50–100 ng/ml or TAC trough level of 3–7 ng/ml; cointerventions: MMF + steroids | Primary outcome: eGFR (Cockcroft–Gault formula) at 12 mo; secondary end points: acute rejection, patient survival, graft survival, adverse events, including CMV infection |
| Induction therapy: daclizumab for 2 mo, except in standard-dose CsA group (this group was excluded from analysis) | |||||
| CMV prophylaxis: data not available; however, proportion of CMV-negative patients receiving kidneys from CMV- positive donors was balanced between groups (around 14%) | |||||
| Flechner et al., 2007 (33) (5-yr follow-up of Flechner et al., 2002 [36]) | Randomized, prospective trial | n=61; Country: United States; mean age: 48 yr; men: 68%; race: 20% white, 8% black, 3% Asian; immunologic risk: low; donor type: deceased: 66%; living related: 23%; living unrelated: 11% | CsA to trough levels of 200–250 ng/ml.; cointerventions: MMF + steroids | Primary outcomes: renal function (eGFR, MDRD formula), acute rejection at 5 yr; secondary end points: patient and graft survival, medical and surgical complications, adverse event, including CMV infections | |
| SIR trough level 5–10 ng/ml; cointerventions: MMF + steroids | |||||
| Büchler et al., 2007 (34) | Prospective, multicenter, randomized study | n=150; Country: France; mean age: 38.7 yr; men: 62%; race: white, 95%; immunologic risk: low to moderate; donor type: all deceased donors | SIR to trough level of 10–15 ng/ml; cointerventions: MMF ± steroids | CsA trough of 75–150 ng/ml; cointerventions: MMF ± steroids | Primary outcome: graft function–assessed eGFR at 12 mo (Nankivell formula); secondary end points: patient and graft survival at 12 mo, acute rejection, incidence of CMV infection, adverse events |
| Induction therapy: ATG in both groups | |||||
| CMV prophylaxis: all CMV-negative patients who received a kidney from a CMV-positive donor received valacyclovir for 16 wk | |||||
| Larson et al., 2006 (35) | Prospective, open label, randomized study | n=165; Country: United States; mean age: 49 yr; men: 54%; race: 96% white; immunologic risk: low; donor type: living: 83%; deceased: 17% | SIR to trough of 10–15 ng/ml; cointerventions: MMF + steroids | Tacrolimus to trough levels of 6–8 ng/ml; cointerventions: MMF + steroids | Outcomes: graft survival, patient survival, acute rejection at 12 mo, renal functions via iothalamate clearance, and adverse events, including CMV infections |
| Induction therapy: ATG in both groups | |||||
| CMV prophylaxis: data not available | |||||
| Flechner et al., 2002 (36) | Randomized, prospective trial | n=61; Country: United States; mean age: 48 yr; men: 68%; race: 20% white, 8% black, 3% Asian; immunologic risk: low; donor type: deceased: 66%; living related: 23%; living unrelated: 11% | SIR trough to 5–10 ng/ml; cointerventions: MMF + steroids | CsA to trough levels of 200–250 ng/ml; cointerventions: MMF + steroids | Primary outcomes: renal function (eGFR; MDRD formula), acute rejection at 12 mo; secondary end points: patient and graft survival, medical and surgical complications, adverse events, including CMV infections |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: 90 d of either oral acyclovir or ganciclovir was given according to donor-recipient serology | |||||
| Kreis et al., 2000 (37) | Randomized, open label, parallel group, multicenter trial | n=78; Country: France, Germany, Spain, Belgium; mean age: 43 yr; men: 70%; race: 95% white; immunologic risk: low to moderate; donor type: all cadaveric | SIR to trough 15 ng/ml; cointerventions: MMF + steroids | CsA to trough of 100–200 ng/ml; cointerventions: MMF + steroids | Primary outcome: BPAR at 12 mo; secondary outcomes: patient and graft survival and graft function (eGFR with Nankivell formula); safety outcomes, including CMV infection |
| Induction therapy: none given | |||||
| CMV prophylaxis: standard prophylactic therapies for CMV were required | |||||
| Groth et al., 1999 (38) | Randomized, open label, parallel-group, multicenter study | n=83; Country: Sweden, United Kingdom, France, Spain; mean age: 47 yr; men: 71%; race: 90% white; immunologic risk: low to moderate; donor type: all cadaveric | SIR to trough level 15 ng/ml; cointerventions: azathioprine + steroids | CsA to trough of 100–200 ng/ml; cointerventions: azathioprine + steroids | Primary outcome: acute rejection, graft loss, and death at 12 mo; secondary outcomes: time to first rejection episode and graft function; safety outcomes, including CMV infection |
| Induction therapy: none given | |||||
| CMV prophylaxis: standard prophylactic therapies per local practice were required and recommended for CMV for 6 mo | |||||
| Comparison 2: mTORi + low-dose CNI versus regular-dose CNI + MPA | |||||
| Tedesco-Silva et al., 2015 (39) | Single-center, prospective, randomized, open label, controlled trial | n=300; Country: Brazil; mean age: 45 yr; men: 67%; race: 50% white; immunologic risk: low/moderate; donor type: deceased: 79%; living: 21% | EVR to trough between 4 and 8 ng/ml + TAC to trough 3–5 ng/ml (low-dose TAC); cointerventions: prednisone; timing of interventions: day 1 post-transplantation | TAC to trough 6–8 ng/ml (regular-dose TAC) + MMF; cointerventions: prednisone | Primary outcome: cumulative incidence of CMV infection after 12 mo; secondary outcomes: acute rejection, eGFR (MDRD formula), adverse events |
| Induction therapy: three arms: group 1: ATG (excluded from this analysis because of different induction regimen), basiliximab for group 2 (intervention), and group 3 (control) | |||||
| CMV prophylaxis: not given in both groups, a preemptive strategy of weekly monitoring of CMV replication for 6 mo was used | |||||
| Suszynski et al., 2013 (40) | Prospective, randomized, controlled trial | n=440; Country: United States; mean age: 48 yr; men: 83%; race: 93% white; immunologic risk: low/moderate; donor type: deceased: 28.5%; living related: 43.7; living unrelated: 27.8 | SIR for a trough level 8–12 μg/L + TAC to trough of 3–7 μg/L | CSA for a trough of 150–200 μg/L + MMF | Primary outcome: composite of a return to dialysis, death with function, chronic rejection at 10 yr; secondary outcomes: adverse events, including CMV and BKPyV infections |
| Induction therapy: ATG in all groups | |||||
| CMV prophylaxis: CMV prophylaxis consisted of iv ganciclovir during hospitalization followed by oral ganciclovir or oral valganciclovir for 3 mo | |||||
| Three arms: arm 1: CsA/MMF (control group); arm 2: high-dose TAC + low-dose sirolimus (excluded from analysis); arm 3: low-dose TAC/high-dose sirolimus (intervention group) | |||||
| Takahashi et al., 2013 (41) | Prospective, multicenter, randomized, controlled trial | n=122; Country: Japan; mean age: 42 yr; men: 75.4%; immunologic risk: low/moderate; donor type: deceased: 1.6%; living related: 59%; living unrelated: 39.3% | EVR trough level 3–8 ng/ml + CsA to trough of 25–50 ng/ml (low-dose CNI); cointerventions: steroids; intervention started within 24–36 h post-transplantation | CsA to trough 100–250 ng/ml + MMF; cointerventions: steroids | Primary outcome: composite of BPAR, graft loss, death, or loss to follow-up at month 12; secondary end points: eGFR at 12 mo (MDRD formula), adverse events, including CMV infection |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: was mandatory for CMV-negative patients receiving kidneys from CMV-positive donors; the duration of prophylaxis was not defined in the protocol; all other patients were treated according to local practice | |||||
| Cibrik et al., 2013 (42) (24-mo follow-up of Tedesco Silva et al., 2010 [44]) | Prospective, multicenter, randomized, controlled trial | n=833; Country: international; mean age: 45 yr; men: 68%; immunologic risk: low/moderate; donor type: deceased heart beating: 46.2%; deceased after cardiac death: 0.4%; living related: 35.7%; living unrelated: 17.3% | EVR to trough 3–8 or 6–12 ng/ml (two arms) + reduced-dose CsA (trough target 25–50 ng/ml); cointerventions: ± steroids; intervention initiated immediately after transplantation | Standard-dose CsA (trough target 100–250 ng/ml) + MPA; cointerventions: ± steroids | Primary outcome: treated acute rejection, death, graft loss, or loss to follow-up at 24 mo; secondary outcomes: eGFR (Nankivell and MDRD formulas); safety outcomes, adverse events, including CMV and BKPyV infection |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: a minimum of 30 d of CMV prophylaxis was mandatory for CMV donor-positive/recipient-negative transplants; treatment with ganciclovir, CMV hyper-Ig, acyclovir, or valacyclovir was permitted and administered according to local practice | |||||
| Bertoni et al., 2011 (43) | Prospective, single-center, randomized, open label trial | n=106; Country: Italy; mean age: 49 yr; immunologic risk: low/moderate; donor type: deceased | EVR target trough level of 8–12 ng/ml + CsA C2: 250–300 ng/ml; cointerventions: steroids | CsA target C2 of 500–700 ng/ml + EC-MPS; cointerventions steroids | Outcomes: eGFR (Cockcroft–Gault) at 12 mo, BPAR, adverse events, including CMV infection |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: not given; a preemptive strategy was used | |||||
| Tedesco Silva et al., 2010 (44) | Prospective, multicenter, randomized, controlled trial | n=833; Country: international; mean age: 45 yr; men: 68%; immunologic risk: low/moderate; donor type: deceased heart beating: 46.2%; deceased after cardiac death: 0.4%; living related: 35.7%; living unrelated: 17.3% | EVR to trough 3–8 or 6–12 ng/ml (two arms) + reduced-dose CsA (trough target 25–50 ng/ml); cointerventions: ± steroids; intervention initiated immediately after transplantation | Standard-dose CsA (trough target 100–250 ng/ml) + MPA; cointerventions: ± steroids | Primary outcome: treated acute rejection, death, graft loss, or loss to follow-up at 24 mo; secondary outcomes: eGFR (Nankivell and MDRD formulas); safety outcomes, adverse events, including CMV and BKPyV infection |
| Induction therapy: basiliximab in both groups | |||||
| CMV prophylaxis: a minimum of 30 d of CMV prophylaxis was mandatory for CMV donor-positive/recipient-negative transplants; treatment with ganciclovir, CMV hyper-Ig, acyclovir, or valacyclovir was permitted and administered according to local practice |
mTORi, mammalian target of rapamycin inhibitor; CNI, calcineurin inhibitor; EVR, everolimus; CsA, cyclosporin A; IF/TA, interstitial fibrosis/tubular atrophy; BPAR, biopsy-proven acute rejection; MDRD, Modification of Diet in Renal Disease; CMV, cytomegalovirus; BKPyV, BK polyoma virus; EC-MPS, enteric-coated mycophenolate sodium; TAC, tacrolimus; SIR, sirolimus; MMF, mycophenolate mofetil; DSA, donor-specific antibody; ATG, antithymocyte globulin; MPA, mycophenolic acid.
Assessment of Risk of Bias
Two review authors (B.Y.T. and H.S.I.) assessed the risk of bias of each included study independently and in duplicate. We resolved disagreements by consensus and when unsuccessful, with the help of a third reviewer. We assessed risk of bias using The Cochrane Collaboration’s Risk of Bias tool (12,13). We used the following criteria: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants, providers, data collectors, outcome adjudicators, and data analysts (performance bias and detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other bias (including early stopping for benefit). We judged risk of bias criteria as low risk, high risk, or unclear risk and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (12). We will present a risk of bias summary figure.
Data Analyses
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (95% CIs). For continuous outcomes data, we calculated the mean differences (MDs; difference in means) with 95% CIs. In terms of evidence synthesis, we used a random effects model for the primary meta-analysis (14). We assessed heterogeneity (inconsistency) between study results by visual inspection of the forest plots and use of the I2 statistic. An I2 statistic of 50% or more indicated a considerable level of heterogeneity (12). To explain any heterogeneity, we conducted subgroup analyses on the basis of the following categories: early introduction of mTORi (<3 months) versus late introduction (≥3 months), type of CNI (cyclosporin or tacrolimus), and type of mTORi (sirolimus or everolimus).
Grading of the Certainty of Evidence
We graded the quality of the evidence for each outcome using the grading of recommendations assessment, development and evaluation approach as detailed in Tables 3 and 4 (15). We also developed summary of findings (SOF) tables using the GRADEpro/GDT tool (16).
Table 3.
Summary of findings, comparison 1: Mammalian target of rapamycin inhibitor versus calcineurin inhibitor (all other cointerventions are identical)
| Outcomes | No. of Participants (Studies) Follow-Up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Risk with CNI | Risk Difference with mTORi | ||||
| CMV infection | 3914 (19 RCTs) | ⊕⊕⊕⊕ High | RR, 0.54 (0.41 to 0.72) | 110 per 1000 | 50 Fewer per 1000 (65–31 fewer) |
| BK virus infection | 1989 (12 RCTs) | ⊕⊕⊕◯ Moderatea | RR, 0.71 (0.40 to 1.25) | 35 per 1000 | 10 Fewer per 1000 (21 fewer to 9 more) |
| Incidence of other viral, bacterial, or fungal infections | 2682 (12 RCTs) | ⊕⊕⊕⊕ High | RR, 1.08 (1.02 to 1.15) | 524 per 1000 | 42 More per 1000 (10–79 more) |
| Composite of incidence of acute cellular or antibody-mediated rejection or DSAs | 3921 (19 RCTs) | ⊕⊕⊕◯ Moderateb | RR, 1.39 (1.09 to 1.77) | 194 per 1000 | 76 More per 1000 (17–150 more) |
| Graft loss | 3589 (17 RCTs) | ⊕⊕⊕◯ Moderatea | RR, 1.10 (0.74 to 1.65) | 41 per 1000 | 4 More per 1000 (11 fewer to 27 more) |
| Serious adverse events | 2173 (7 RCTs) | ⊕⊕⊕◯ Moderatec | RR, 1.26 (1.02 to 1.56) | 387 per 1000 | 101 More per 1000 (8–217 more) |
| Proteinuria | 2844 (12 RCTs) | ⊕⊕⊕⊕ High | RR, 2.35 (1.52 to 3.64) | 41 per 1000 | 55 More per 1000 (21–107 more) |
| BK nephropathy defined by kidney biopsy | 561 (3 RCTs) | ⊕⊕◯◯ Lowa,d | RR, 0.54 (0.20 to 1.49) | 35 per 1000 | 16 Fewer per 1000 (28 fewer to 17 more) |
| Wound-healing complications | 1826 (7 RCTs) | ⊕⊕⊕⊕ High | RR, 1.62 (1.22 to 2.15) | 82 per 1000 | 51 More per 1000 (18–94 more) |
| eGFR or creatinine clearance, ml/min per 1.73 m2 | 3768 (18 RCTs) | ⊕⊕⊕◯ Moderatee | — | MD 4.07 higher (1.34–6.8 higher) | |
The risk in the intervention group (and its 95% CI) is on the basis of the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence were used. The approach classifies the quality of evidence into four categories: high, moderate, low, and very low. It takes into account the following factors: risk of bias, imprecision, inconsistency, indirectness, and publication bias. High quality: we are very confident that the true effect is close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. GRADE, grading of recommendations assessment, development and evaluation; 95% CI, 95% confidence interval; CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor; CMV, cytomegalovirus; RCT, randomized, controlled trial; RR, risk ratio; DSA, donor-specific antibody; —, risk ratio not applicable for continuous variables; MD, mean difference.
95% CI does not rule out or confirm difference between the intervention and control groups.
Heterogeneity detected I2=67%.
Heterogeneity detected I2=63%.
Unclear risk of bias in some of the included trials.
Heterogeneity detected I2=74%.
Table 4.
Summary of findings, comparison 2: Mammalian target of rapamycin inhibitor plus reduced-dose calcineurin inhibitor versus regular-dose calcineurin inhibitor plus mycophenolic acid/azathioprine (all other cointerventions are identical)
| Outcomes | No. of Participants (Studies) Follow-Up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Risk with Regular-Dose CNI + MMF/AZA | Risk Difference with mTORi + Reduced-Dose CNI | ||||
| CMV infection | 1547 (5 RCTs) | ⊕⊕⊕◯ Moderatea | RR, 0.43 (0.24 to 0.80) | 231 per 1000 | 132 Fewer per 1000 (176–46 fewer) |
| BK virus infection | 1320 (3 RCTs) | ⊕⊕◯◯ Lowb,c | RR, 0.57 (0.12 to 2.72) | 32 per 1000 | 14 Fewer per 1000 (28 fewer to 56 more) |
| Incidence of other viral, bacterial, or fungal infections | 1028 (2 RCTs) | ⊕⊕◯◯ Lowc,d | RR, 1.08 (0.87 to 1.34) | 505 per 1000 | 40 More per 1000 66 fewer to 172 more) |
| Acute cellular or antibody-mediated rejection | 1553 (5 RCTs) | ⊕⊕⊕◯ Moderatec | RR, 0.88 (0.70 to 1.09) | 192 per 1000 | 23 Fewer per 1000 (57 fewer to 17 more) |
| Graft loss | 1547 (5 RCTs) | ⊕⊕⊕◯ Moderate | RR, 1.03 (0.64 to 1.65) | 105 per 1000 | 3 More per 1000 (38 fewer to 68 more) |
| Serious adverse events | 1150 (3 RCTs) | ⊕⊕◯◯ Lowc,e | RR, 0.87 (0.62 to 1.20) | 632 per 1000 | 82 Fewer per 1000 (240 fewer to 126 more) |
| CMV disease | 1441 (4 RCTs) | ⊕⊕⊕◯ Moderatef | RR, 0.42 (0.21 to 0.82) | 119 per 1000 | 69 Fewer per 1000 (94–22 fewer) |
| Proteinuria | 1150 (3 RCTs) | ⊕⊕⊕⊕ High | RR, 1.47 (1.04 to 2.06) | 99 per 1000 | 46 More per 1000 (4–105 more) |
| Wound-healing complications | 1150 (3 RCTs) | ⊕⊕⊕◯ Moderateg | RR, 1.71 (1.16 to 2.50) | 230 per 1000 | 163 More per 1000 (37–345 more) |
| eGFR or creatinine clearance, ml/min per 1.73 m2 | 1543 (5 RCTs) | ⊕⊕◯◯ Lowc,h | — | MD 3.36 higher (4.31 lower to 11.02 higher) | |
The risk in the intervention group (and its 95% CI) is on the basis of the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence were used. The approach classifies the quality of evidence into four categories: high, moderate, low, and very low. It takes into account the following factors: risk of bias, imprecision, inconsistency, indirectness, and publication bias. High quality: we are very confident that the true effect is close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. GRADE, grading of recommendations assessment, development and evaluation; mTORi, mammalian target of rapamycin inhibitor; CNI, calcineurin inhibitor; MPA, mycophenolic acid; AZA, azathioprine; CMV, cytomegalovirus; RCT, randomized, controlled trial; RR, risk ratio; —, risk ratio not applicable for continuous variables; MD, mean difference.
Heterogeneity detected I2=81%.
Heterogeneity detected I2=71%.
95% CI does not rule out or confirm difference between the intervention and control groups.
Heterogeneity detected I2=53%.
Heterogeneity detected I2=85%.
Heterogeneity detected I2=58%.
Heterogeneity detected I2=56%.
Heterogeneity detected I2=84%.
Results
Search Results
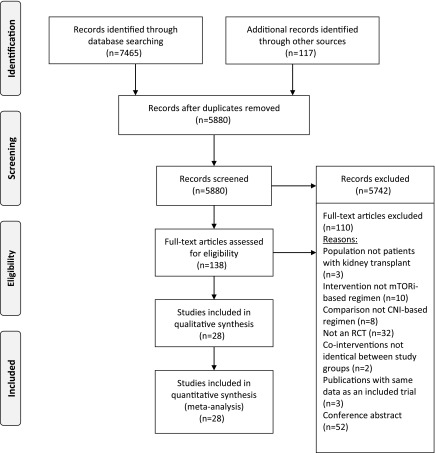
Figure 1 shows the study flow chart. Of 5880 screened citations, we identified 24 eligible original studies and four follow-up reports with a total of 6211 participants.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses study flow chart. Of 5880 screened citations, we included 28 studies with a total of 6211 participants. CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor; RCT, randomized, controlled trial.
Included Studies
Table 2 summarizes the characteristics of the studies included in our two comparisons. All trials were in the English language. All of the included studies were randomized, controlled, parallel-group trials. (17–44). Most of the trials included RTRs with low/moderate immunologic risk. Most of the included trials used induction therapy at the time of transplantation. Induction therapy consisted of basiliximab in 11 trials (17,20,21,24,33,36,39,41–44), antithymocyte globulin in seven trials (25,27,28,31,34,35,40), daclizumab in two trials (30,32), and alemtuzumab in one trial (22). One trial used different induction therapies consisting of antithymocyte globulin, basiliximab, daclizumab, and muromonab-CD3 in a balanced way between the two arms (26). Three trials did not give any induction therapy (29,37,38), whereas one trial did not provide any details concerning the induction therapy (18,19). One trial did not specify the type of induction therapy; however, it used it in 18% of the population in a way balanced between the two arms (23). In most of the included trials, as highlighted in Table 2, CMV prophylaxis was administered according to local center practice.
Risk of Bias in Included Studies
Supplemental Figure 1 and Supplemental Tables 2 and 3 summarize the assessment of risk of bias in included studies. For most of the assessed parameters, most of the included trials were judged to have a low risk of bias. An unclear risk of bias was judged for some trials, especially when methods of random sequence generation, allocation concealment, and blinding were not specified.
Effects of Intervention: Comparison 1, Primary Outcomes
Incidence of CMV Infection.
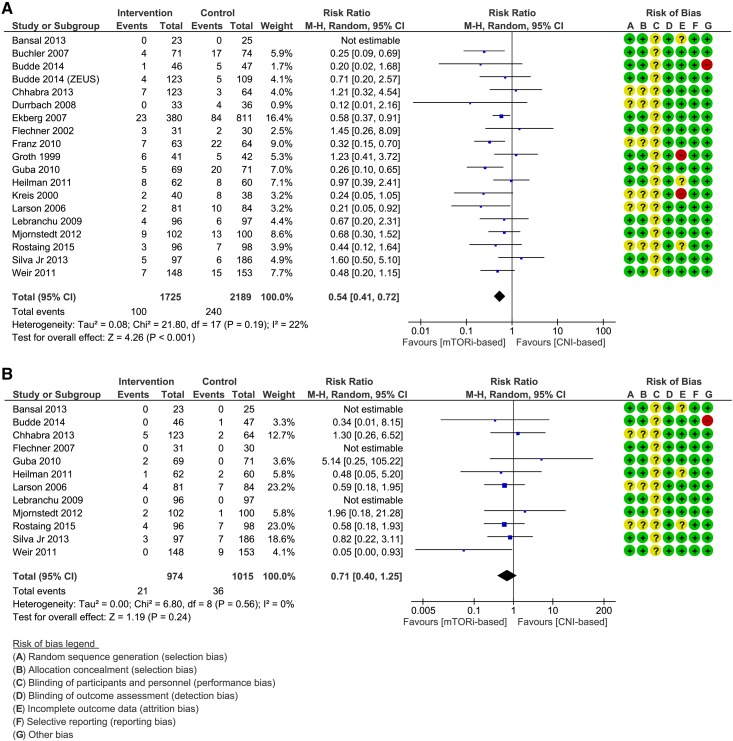
The analysis included 19 trials with follow-up time ranging from 6 months to 5 years. The results showed a significant 46% decreased risk of CMV infections favoring the mTORi-based group (RR, 0.54; 95% CI, 0.41 to 0.72) (Figure 2A). Quality of evidence was judged to be high (SOF in Table 3).
Figure 2.
Forest plots, comparison 1. Results showed a significant 46% decreased risk of Cytomegalovirus infections in mTORi-based group and no significant difference between the two groups with regards to BK polyoma virus infection. (A) Cytomegalovirus infection. (B) BK polyoma virus infection. 95% CI, 95% confidence interval; CNI, calcineurin inhibitor; M-H, Mantel-Haenszel method; mTORi, mammalian target of rapamycin inhibitor.
Incidence of BKPyV Infection.
The analysis included 12 trials and showed no significant difference between the two groups (RR, 0.71; 95% CI, 0.40 to 1.25) (Figure 2B). Quality of evidence was judged to be moderate on the basis of imprecision (SOF in Table 3).
Effects of Intervention: Comparison 1, Secondary Outcomes
Incidence of Other Infections and Serious Adverse Events.
The results show an 8% increase in the incidence of other infections (RR, 1.08; 95% CI, 1.02 to 1.15) and a 26% increase in the incidence of serious adverse events (SAEs; RR, 1.26; 95% CI, 1.02 to 1.56) in the mTORi-based group (Supplemental Figures 2 and 3).
Composite Incidence of Acute Rejection or Donor-Specific Antibodies and Incidence of Graft Loss.
The results showed an increased risk of acute rejection or donor-specific antibodies (DSAs) in the mTORi-based group (RR, 1.39; 95% CI, 1.09 to 1.77); however, no significant difference was shown in the incidence of graft loss at last follow-up (Supplemental Figures 4 and 5).
Incidence of PyVAN by Kidney Biopsy and CMV Disease.
Only three trials reported the incidence of PyVAN as defined by a kidney biopsy, and one trial reported the incidence of CMV disease. The result did not show a significant difference between the two groups (Supplemental Figure 6).
Incidence of Proteinuria and Wound-Healing Complications.
There was a significant increased risk of proteinuria (RR, 2.35; 95% CI, 1.52 to 3.64) and wound-healing complications (RR, 1.62; 95% CI, 1.22 to 2.15) in the mTORi-based group (Supplemental Figures 7 and 8).
eGFR.
The analysis included 18 trials with follow-up ranging from 6 months to 5 years. The results showed a significant MD of 4.07 ml/min per 1.73 m2 favoring the mTORi-based group (MD =4.07 ml/min per 1.73 m2; 95% CI, 1.34 to 6.80) (Supplemental Figure 9).
Effects of Intervention: Comparison 2, Primary Outcomes
Incidence of CMV Infection.
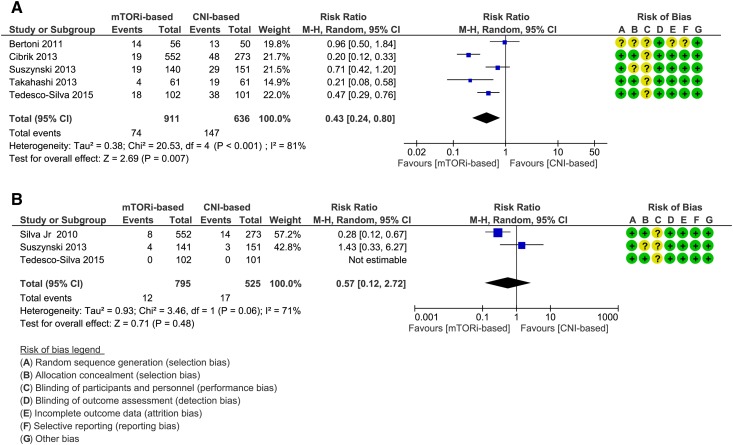
The analysis included five trials, and the results showed a significant 57% decreased risk of CMV infection favoring the mTORi-based group (RR, 0.43; 95% CI, 0.24 to 0.80) (Figure 3A). Quality of evidence was judged to be moderate on the basis of heterogeneity (SOF in Table 4).
Figure 3.
Forest plots, comparison 2. Results showed a significant 57% decreased risk of Cytomegalovirus infections in mTORi-based group and no significant difference between the two groups with regards to BK polyoma virus infection. (A) Cytomegalovirus infection. (B) BK polyoma virus infection. 95% CI, 95% confidence interval; CNI, calcineurin inhibitor; M-H, Mantel-Haenszel method; mTORi, mammalian target of rapamycin inhibitor.
Incidence of BKPyV Infection.
The analysis included three trials, and the results showed no significant difference between the two groups (RR, 0.57; 95% CI, 0.12 to 2.72) (Figure 3B). Quality of evidence was judged to be moderate on the basis of imprecision (SOF in Table 4).
Effects of Intervention: Comparison 2, Secondary Outcomes
Incidence of Other Infections and SAEs.
The analysis included three trials and showed a nonsignificant difference between the two groups with regards to these outcomes (Supplemental Figures 10 and 11).
Incidence of Acute Rejection, Graft Loss, and DSAs.
The analysis included five trials, and the results showed a nonsignificant trend toward decreased incidence of acute cellular rejection or antibody-mediated rejection favoring the mTORi-based group (RR, 0.88; 95% CI, 0.70 to 1.09), with no difference with regards to graft loss (Supplemental Figures 12 and 13). None of the included trials assessed the incidence of DSAs.
Incidence of PyVAN by Kidney Biopsy and CMV Disease.
Analysis of four included trials showed a significant 58% decreased risk of CMV disease favoring the mTORi-based group (RR, 0.42; 95% CI, 0.21 to 0.82) (Supplemental Figure 14). Only one trial assessed BK nephropathy as defined by kidney biopsy; therefore, no pooled analysis was possible.
Incidence of Proteinuria and Wound-Healing Complications.
The analysis included three trials and showed a significant increased risk of proteinuria (RR, 1.47; 95% CI, 1.04 to 2.06) and wound-healing complications (RR, 1.71; 95% CI, 1.16 to 2.50) in the mTORi-based group (Supplemental Figures 15 and 16).
eGFR.
The analysis included five trials, and the results showed no significant difference between the two groups at the end of follow-up (MD =3.36 ml/min per 1.73 m2; 95% CI, −4.32 to 11.02) (Supplemental Figure 17).
Subgroup Analyses, Investigation for Heterogeneity, and Sensitivity Analyses
Subgroup analysis was only possible for comparison 1 because of the limited number of trials in comparison 2. With regard to CMV infection, although the effect was more pronounced when mTORis were introduced early, subgroups analysis showed no significant difference between early and late introduction of mTORi on decreasing the incidence of CMV infection (Supplemental Figure 18). Likewise, there was no significant difference when results were stratified according to the type of CNI or mTORi used (Supplemental Figure 19). Subgroups analysis did not find any significant difference for BKPyV infection and secondary outcomes, including acute rejection, proteinuria, and GFR. However, the use of sirolimus was associated with a trend toward decreased eGFR compared with tacrolimus. Subgroup analysis for wound-healing complications was not possible, because most trials that assessed this outcome compared sirolimus with cyclosporin and most introduced mTORi early (Supplemental Figures 20–27).
Discussion
In summary, we found moderate- to high-quality evidence that confirms a significant reduction of CMV infection in RTRs receiving an mTORi-based regimen (both alone or combined with a reduced-dose CNI). Our analysis did not find a significant difference in the incidence of BKPyV infection between an mTORi-based regiment and a CNI-based regimen; however, this finding should be interpreted cautiously. In fact, the wide 95% CI for the association of mTORi use with reduction in BKPyV infection (RR, 0.71; 95% CI, 0.40 to 1.25) indicates that the available evidence neither confirms nor rules out a clinically significant differential effect of the compared regimens on BKPyV infection. In other words, our meta-analysis may have been underpowered to detect such an effect. Moreover, there is evidence that an mTORi-based regimen may reduce BKPyV infection. In a virologic study, sirolimus effectively inhibited BKPyV replication in renal tubular epithelial cells, and in a large patient-cohort study, incidence of BKPyV infection was lower in patients when an mTORi was used (7,9). Therefore, well powered RCTs are needed to confirm or rule out a substantive difference in the incidence of BKPyV infection between an mTORi-based regimen and a CNI-based regimen.
Our results also confirm previous data showing increased incidence of proteinuria and wound-healing complications in the mTORi-based group. SAEs, which led to drug discontinuation in some of the included studies, were more common in the mTORi-based group when mTORi was used alone (comparison 1) and similar between the two groups when mTORi was used with a reduced-dose CNI (comparison 2). In addition, our review showed an increased risk of acute rejection in the mTORi-based group when an mTORi was used alone and no difference when used in addition to a reduced-dose CNI. However, there was no difference in the incidence of graft loss at the end of follow-up in the two comparisons. Kidney graft function as assessed by eGFR or creatinine clearance favored the mTORi-based regimen when mTORi was used alone and was similar to the CNI-based group in comparison 2.
The balance between the benefit of reducing CMV infection and the harm of increasing rejection is best illustrated in our SOF tables. In comparison 1, SOF in Table 3 shows that, for 1000 participants taking mTORi, we would expect 76 more rejections (17–150 more) and 50 fewer CMV infections (65–31 fewer) compared with in the CNI-based group. However, for comparison 2, the balance is more clearly in favor of mTORi. Indeed, SOF in Table 4 shows that, for 1000 participants receiving mTORi and reduced-dose CNI, we would expect 132 fewer CMV infections (176–46 fewer) and 23 fewer rejections (57 fewer to 17 more) compared with in the CNI-based groups. Taken together, we found that the combination of an mTORi and a reduced-dose CNI might offer the benefit of decreased incidence of CMV infection with similar rates of acute rejection, SAEs, and preservation of eGFR compared with a CNI-based regimen; however, it is at the expense of increased risk of proteinuria and wound-healing complications.
Strengths and Limitations
One of the strengths of our review is the inclusion of well designed RCTs with an overall low risk of bias. In addition, we excluded trials that used different induction immunosuppressive therapy between groups, which permitted us to determine with confidence the difference between an mTORi-based regimen and a CNI-based regimen on the studied outcomes.
Limitations of our systematic review include the fact that our search strategy excluded trials that did not assess the incidence of CMV or BKPyV infections, which limited the power when assessing other specified secondary outcomes. Therefore, careful interpretation of our results is necessary with regards to secondary outcomes, including acute rejection, graft loss, and renal function. Another limitation is the heterogeneity of CMV preventive strategies between included trials. The possibility that current preventive strategies, such as extending CMV pharmacologic prophylaxis to 6 months in high-risk patients, might reduce the risk of CMV infection and secondarily attenuate the absolute reduction of that infection with an mTORi-based regimen needs to be investigated in future RCTs. Also, most of the included trials involved RTRs with low to moderate immunologic risk, and our results may not apply to RTRs with high immunologic risk.
Comparison with Other Systematic Reviews
Our results are in concert with a previously published systematic review that showed improvement in GFR and decreased incidence of CMV in an mTORi-based group at the expense of increased risk for acute rejection and increased incidence of drug discontinuation secondary to side effects compared with in patients who remained on their initial CNI-based regimen. Limitations of that review were the exclusion of trials that used mTORi as an initial immunosuppressive regimen; therefore, only six trials assessed incidence of CMV infection and were included in the analysis, and BKPyV infections were not assessed (8). Our review analyzed data from 24 trials that assessed incidence of CMV infection and 15 trials that assessed the incidence of BK infection, which makes the results of our review more robust with regards to these two outcomes.In another recently published systematic review, a CNI minimization strategy that included some of the trials included in comparison 2 of our review resulted in a 29% decreased risk of CMV infection (45). Our review showed a significant 57% decreased risk of CMV infection and a significant 58% decreased risk of CMV disease favoring a CNI minimization strategy incorporating an mTORi (comparison 2). In conclusion, our review confirmed a significant decreased risk of CMV infections in RTRs when an mTORi-based regimen was used compared with a CNI-based regimen across all studied comparisons. No definite conclusion was possible with regards to BKPyV infection. As for secondary outcomes, our review showed an increased risk of acute rejection and SAEs when an mTORi was used alone (comparison 1) and no significant difference with regards to these two outcomes when an mTORi was combined with a reduced-dose CNI (comparison 2). Therefore; our review suggested that a combination of an mTORi and a reduced-dose CNI may offer a reasonable risk-to-benefit ratio with regards to decreased risk of CMV, similar rates of acute rejections, SAEs, and comparable kidney function compared with a standard CNI-based regimen. There remained, however, a higher incidence of proteinuria and wound-healing complications when an mTORi was used. (SOF in Tables 3 and 4). Note, however, that comparison 2 included only five RCTs, which may have limited the power of our review when assessing secondary outcomes. Therefore, our findings need to be confirmed in future RCTs incorporating a prespecified CMV preventive strategy and should be adequately powered to assess CMV and BKPyV infections in addition to acute rejection, SAEs, and kidney function.
Disclosures
None.
Supplementary Material
Acknowledgments
Parts of this article were accepted for a poster presentation at the American Transplant Congress 2017 in Chicago, Illinois from April 29 to May 3, 2017.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13221216/-/DCSupplemental.
References
- 1.De Keyzer K, Van Laecke S, Peeters P, Vanholder R: Human cytomegalovirus and kidney transplantation: A clinician’s update. Am J Kidney Dis 58: 118–126, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, Shapiro R, Steiger J, Suthanthiran M, Trofe J: Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 79: 1277–1286, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Wiseman AC: Polyomavirus nephropathy: A current perspective and clinical considerations. Am J Kidney Dis 54: 131–142, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Nashan B, Gaston R, Emery V, Säemann MD, Mueller NJ, Couzi L, Dantal J, Shihab F, Mulgaonkar S, Seun Kim Y, Brennan DC: Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation 93: 1075–1085, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Havenith SHC, Yong SL, van Donselaar-van der Pant KAMI, van Lier RAW, ten Berge IJM, Bemelman FJ: Everolimus-treated renal transplant recipients have a more robust CMV-specific CD8+ T-cell response compared with cyclosporine- or mycophenolate-treated patients. Transplantation 95: 184–191, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Yakhontova K, Lu M, Manzetti J: BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant 16: 821–832, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim WH, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, Russ GR: A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant 14: 2106–2119, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Cherikh WS, Abbott KC: An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 87: 1019–1026, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Radtke J, Dietze N, Fischer L, Achilles EG, Li J, Scheidat S, Thaiss F, Nashan B, Koch M: Incidence of BK polyomavirus infection after kidney transplantation is independent of type of immunosuppressive therapy. Transpl Infect Dis 18: 850–855, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 6: e1000100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S (editors): Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org. Accessed January 4, 201619845597
- 13.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA: Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ 336: 601–605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ: GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394, 2011 [DOI] [PubMed] [Google Scholar]
- 16.GRADEpro GDT: GRADEpro Guideline Development Tool [Software] McMaster University, 2015 (developed by Evidence Prime, Inc.). Available at: gradepro.org. Accessed January 4, 2016
- 17.Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, Moulin B, Merville P, Hazzan M, Lang P, Touchard G, Hurault deLigny B, Quéré S, Di Giambattista F, Dubois YC, Rondeau E; CERTITEM Study Group : Fibrosis progression according to epithelial-mesenchymal transition profile: A randomized trial of everolimus versus CsA. Am J Transplant 15: 1303–1312, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Budde K, Rath T, Sommerer C, Haller H, Reinke P, Witzke O, Suwelack B, Baeumer D, May C, Porstner M, Arns W: Renal, efficacy and safety outcomes following late conversion of kidney transplant patients from calcineurin inhibitor therapy to everolimus: The randomized APOLLO study. Clin Nephrol 83: 11–21, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Budde K, Sommerer C, Rath T, Reinke P, Haller H, Witzke O, Suwelack B, Baeumer D, Sieder C, Porstner M, Arns W: Renal function to 5 years after late conversion of kidney transplant patients to everolimus: A randomized trial. J Nephrol 28: 115–123, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Wüthrich RP, Mühlfeld A, Heller K, Porstner M, Veit J, Paulus EM, Witzke O; ZEUS Study Investigators : Five-year outcomes in kidney transplant patients converted from cyclosporine to everolimus: The randomized ZEUS study. Am J Transplant 15: 119–128, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Silva HT Jr., Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FLC, de Carvalho DDBM, Pestana JOM: Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant 13: 3155–3163, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento-Reodica N, Najafian N, Skaro A, Levitsky J, Mas V, Gallon L: Impact of calcineurin-inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone-free regimen. Am J Transplant 13: 2902–2911, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V: Deferred pre-emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of T regulatory cell population: A randomized, controlled trial. PLoS One 8: e75591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mjörnstedt L, Sørensen SS, von Zur Mühlen B, Jespersen B, Hansen JM, Bistrup C, Andersson H, Gustafsson B, Undset LH, Fagertun H, Solbu D, Holdaas H: Improved renal function after early conversion from a calcineurin inhibitor to everolimus: A randomized trial in kidney transplantation. Am J Transplant 12: 2744–2753, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Guba M, Pratschke J, Hugo C, Krämer BK, Pascher A, Pressmar K, Hakenberg O, Fischereder M, Brockmann J, Andrassy J, Banas B, Jauch KW; SMART-Study Group : Early conversion to a sirolimus-based, calcineurin-inhibitor-free immunosuppression in the SMART trial: Observational results at 24 and 36 months after transplantation. Transpl Int 25: 416–423, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, Kalil RN, Pearson TC: Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: A randomized, controlled Spare-the-Nephron trial. Kidney Int 79: 897–907, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA, Gonwa TA: Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation 92: 767–773, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, Andrassy J, Reinke P, Pressmar K, Hakenberg O, Fischereder M, Pascher A, Illner WD, Banas B, Jauch KW; SMART-Study Group : Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: One-year analysis of a randomized multicenter trial. Transplantation 90: 175–183, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M: Tubular toxicity in sirolimus- and cyclosporine-based transplant immunosuppression strategies: An ancillary study from a randomized controlled trial. Am J Kidney Dis 55: 335–343, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, Heng AE, Onno C, Buchler M, Girardot-Seguin S, Hurault de Ligny B: Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: Concept study. Am J Transplant 9: 1115–1123, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil-Noble C, Kessler M, Viron B, Thervet E: Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation 85: 486–490, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, Babineau D, Kurian S, Salomon D, Novick AC, Cook DJ: Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 83: 883–892, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Büchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, Hurault de Ligny B, Le Meur Y, Thierry A, Villemain F, Heng AE, Moulin B, Morin MP, Noël C, Lebranchu Y; SPIESSER Group : Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant 7: 2522–2531, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, Nyberg SL, Ishitani MB, Prieto M, Velosa JA: Complete avoidance of calcineurin inhibitors in renal transplantation: A randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6: 514–522, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, Savas K, Cook DJ, Novick AC: Kidney transplantation without calcineurin inhibitor drugs: A prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 74: 1070–1076, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, Campistol JM, Morales JM, Grinyo JM, Mourad G, Berthoux FC, Brattström C, Lebranchu Y, Vialtel P: Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 69: 1252–1260, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, Touraine JL, Claesson K, Campistol JM, Durand D, Wramner L, Brattström C, Charpentier B; Sirolimus European Renal Transplant Study Group : Sirolimus (rapamycin)-based therapy in human renal transplantation: Similar efficacy and different toxicity compared with cyclosporine. Transplantation 67: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Tedesco-Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes-Freitas T, Aguiar W, Campos E, Gerbase-DeLima M, Franco M, Medina-Pestana J: Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant 15: 2655–2664, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Suszynski TM, Gillingham KJ, Rizzari MD, Dunn TB, Payne WD, Chinnakotla S, Finger EB, Sutherland DER, Najarian JS, Pruett TL, Matas AJ, Kandaswamy R: Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. Am J Transplant 13: 961–970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, Cornu-Artis C, Kobayashi E: Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-Month results. Transplant Res 2: 14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cibrik D, Silva HT Jr., Vathsala A, Lackova E, Cornu-Artis C, Walker RG, Wang Z, Zibari GB, Shihab F, Kim YS: Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 95: 933–942, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Bertoni E, Larti A, Rosso G, Zanazzi M, Di Maria L, Salvadori M: Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol 24: 613–618, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Tedesco Silva H Jr., Cibrik D, Johnston T, Lackova E, Mange K, Panis C, Walker R, Wang Z, Zibari G, Kim YS: Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 10: 1401–1413, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Sawinski D, Trofe-Clark J, Leas B, Uhl S, Tuteja S, Kaczmarek JL, French B, Umscheid CA: Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: A systematic review and meta-analysis. Am J Transplant 16: 2117–2138, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.