Abstract
Background and objectives
The last 15 years has seen growth in home hemodialysis (HD) utilization in Canada owing to reports of improved outcomes relative to patients on conventional in-center HD. What effect growth has had on home HD technique and patient survival during this period is not known.
Design, settings, participants, & measurements
We compared the risk of home HD technique failure, mortality, and the composite outcome among three incident cohorts of patients on home HD in Canada: 1996–2002, 2003–2007, and 2008–2012. A multivariable piece-wise exponential model was used to evaluate all outcomes using inverse probability of treatment and censoring weights.
Results
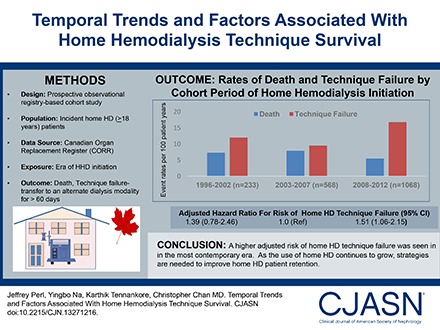
A total of 1869 incident patients on home HD were identified from the Canadian Organ Replacement Register. Relative to those treated between 2003 and 2007 (n=568), the risk of home HD technique failure was similar between patients treated between 1996 and 2002 (n=233; adjusted hazard ratio [AHR], 1.39; 95% confidence interval [95% CI], 0.78 to 2.46) but higher among incident patients on home HD treated between 2008 and 2012 (n=1068; AHR, 1.51; 95% CI, 1.06 to 2.15). Relative to patients treated between 2003 and 2007, adjusted mortality was similar among those treated between 2008 and 2012 (AHR, 0.83; 95% CI, 0.58 to 1.19) and those treated between 1996 and 2002 (AHR, 0.67; 95% CI, 0.38 to 1.21). The risk of the composite outcome of death and technique failure was similar across cohorts, as was the risk of receiving a kidney transplant. Increasing age, diabetes as a comorbidity, and smoking status were associated with an increased risk of death as well as the composite outcome. Medium-sized facilities had a lower risk of death, technique failure, and the composite outcome compared with larger facilities.
Conclusions
A higher risk of technique failure was seen in the most contemporary era. Further characterization of the risk factors for, and causes of technique failure is needed to develop strategies to improve patient retention on home HD.
Keywords: Hemodialysis; survival; technique survival; home hemodialysis; short daily hemodialysis; slow nocturnal hemodialysis; Canadian Organ Replacement Register; Technique Failure; Dialysis Modality Discontinuation; Home Dialysis Discontinuation; Canada; Comorbidity; Death; diabetes mellitus; Hemodialysis, Home; Humans; kidney transplantation; Proportional Hazards Models; risk factors; Smoking
Introduction
In Canada, in-center hemodialysis (HD) continues to remain the predominant form of RRT (1). Born out of the poor outcomes and escalating treatment costs for patients treated with in-center HD, there is increasing interest in achieving greater utilization of home HD both by policymakers and providers. For patients, home HD may offer several clinical benefits, owing largely to dialysis intensification and the ability to more easily increase dialysis frequency and duration (2). Cardiovascular benefits include improvements in BP control, endothelial function, and left ventricular geometry, whereas other clinical benefits of home HD have included enhanced clearance of middle-molecular uremic toxins, superior anemia, and phosphate control, and improvements in sleep disorders, fertility, and pregnancy outcomes (3–7). Home HD has been associated with a superior quality of life and greater cost utility when compared with in-center HD, where mean annual costs for home HD are approximately $10,000 greater (8). When one also accounts for the fact that, in the same study, individuals undergoing home HD were able to rejoin the workforce, reduced costs are also realized for society at large while simultaneously enhancing dialysis patients’ quality of life.
Taken together, as a result, an increasing number of patients are receiving treatment with home HD in Canada. The last 15 years has seen unprecedented growth in the absolute numbers of patients treated with home HD in Canada (1,9). Therefore, a critical and contemporary evaluation of trends in home HD technique and patient survival is needed. In this report, our primary objective was to examine national trends over time in home HD technique survival among incident Canadian patients on home HD. Secondary objectives were to evaluate trends over time in patient survival on home HD and the composite outcome of patient and technique survival, as well as to explore additional predictors of patient and technique survival.
Materials and Methods
Study Design
This was a prospective observational cohort study of consecutive adult patients (aged ≥18 years at the start of home HD) who were registered in the Canadian Organ Replacement Register (CORR) and who initiated home HD between January 1, 1996 and December 31, 2012.
Data Source, Definitions, and Collection
Patients were identified from CORR, a national registry that captures the incidence, prevalence, treatment changes, and outcomes of >99% of patients on chronic dialysis and solid organ transplant recipients in Canada (10). Data are collected by completion of a registration form by the dialysis provider on each patient at dialysis initiation, and yearly thereafter. A change of status form is completed to document patient death, transplantation, or a switch in dialysis modality. CORR data have recently been validated (11). Data from the province of Quebec were not included because of the need for additional ethics and data permissions. We included all submodalities of home HD (conventional home HD, short daily home HD, and slow nocturnal home HD). Information on specific home HD technology was not available within CORR; however, with regards to the NxStage system, this was not widely used during any cohort period of the study as it was broadly introduced into Canada in mid-to-late 2013 (personal communication, Jeffrey Perl, NxStage Medical, Inc.).
The era of home HD initiation was the primary exposure of interest. Other predictors were also explored in secondary analyses. Eligible patients included those who initiated home HD as either as an incident dialysis modality or who transitioned to home HD from a prior RRT. In order to examine trends over time, three cohorts of patients who initiated home HD were established: 1996–2002, 2003–2007, and 2008–2012. The rationale for the selection of these three cohort periods was on the basis of changes in home HD utilization that took place during each of these three periods.
Baseline comorbidities were documented by the individual facilities using the CORR registration forms. Information on the presence or absence of coronary artery disease (angina, myocardial infarction, and coronary artery bypass surgery), peripheral vascular disease, hypertension, diabetes mellitus, and cerebrovascular disease were categorized as “yes,” “no,” and “unknown.” The unknowns were combined into the no group. Diabetes was classified as a single variable including diabetes as a comorbidity (among those without presumed diabetic kidney disease) or a cause of ESRD. Current smokers were documented as those having smoked in the last 3 months. Comorbidities were included in the determination of a validated comorbidity index for each patient (12). Body mass index was calculated using the height and weight collected at the start of dialysis. Facility size was calculated as the cumulative number of new patients initiated on home HD over the study period and was divided into tertiles as small, medium, and large facilities. Vascular access within 90 days of home HD initiation, if available, was collected from either baseline CORR registration forms or captured from the annual cross-sectional facility survey as previously described. However, information on vascular access type was not formally captured on CORR forms until 2001.
Outcomes
The primary outcome was time to home HD technique failure. Secondary outcomes included overall mortality and the composite outcome of time to home HD technique failure and/or mortality. Home HD technique failure was defined as a transfer to an alternate dialysis modality for ≥60 days. For analyses examining time to home HD technique failure, patients were censored at death, kidney transplantation, and loss to follow-up or at the end of the observation period (December 31, 2014). For the secondary analysis examining time to death, analyses were performed censoring patients at technique failure. However, all deaths within 60 days of technique failure were included. In all analyses of time to death, patients were censored at kidney transplantation.
Statistical Analyses
Categorical variables were compared using the chi-squared test. The Kruskal–Wallis test was used to analyze differences between continuous variables.
We compared survival by era of home HD initiation and additional predictors of outcomes using a marginal structural model with inverse probability of treatment and censoring weighting (IPTCW). This method was used as previously described to account for the competing risks of kidney transplantation in all analyses and the competing risks of death in analyses of time to technique failure. Use of the IPTCW technique (13–15) allowed us to adjust for measured covariates in a single summary propensity score and simultaneously adjust for the effect of informative censoring because of potential differences in the rates of death and kidney transplantation between patients on home HD over the three cohort periods. In the first step, propensity scores (PS) were determined as an estimate of each study subject’s probability of initial cohort period assignment on the basis of available covariates: age, sex, race, cause of ESRD), weighting of comorbidities (diabetes mellitus, coronary artery disease, peripheral vascular disease, malignancy, lung disease, pulmonary edema) on the basis of a validated ESRD comorbidity index (12), body mass index, province of treatment, home HD center size (as previously described), vascular access type, and distance from the HD facility. Because our exposure of interest was not binary (i.e., three-level: 1996–2002 versus 2003–2007 versus 2007–2012), we used a generalized logit logistic regression models (1996–2002 versus 2003–2007 and 2007–2012 versus 2003–2007) using all available covariates to calculate our PS. Area under receiver operating characteristic curves were evaluated to test the discriminatory capacity of each model. In the second step, we determined stabilized censoring weights by estimating: (1) the probability of remaining transplant free for each individual in successive 1-year time intervals and (2) the probability of remaining alive in each individual, in successive 1-year time intervals. Each observation was then weighted both by the inverse probability of initial cohort assignment (1/PS) for each individual, and by each of the stabilized censoring weights. In a similar approach among secondary analyses where death was the outcome of interest, IPTCW analyses were weighted both by the propensity to remain transplant free and free of technique failure. Given the high rate of missing information on vascular access type, a sensitivity analysis was performed only on patients who had available information on vascular access type. The study protocol and design was approved by the local institutional research ethics broad. All analyses were performed using SAS software (version 9.1.3; SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
Between 1996 and 2012, 1869 incident patients on home HD were registered in CORR, including 233 patients who initiated home HD from 1996 to 2002, 568 patients from 2003 to 2007, and 1068 patients from 2008 to 2012.
Table 1 lists the baseline characteristics of the study population. Compared with patients who initiated home HD between 1996 and 2002, patients in more contemporary cohorts were more likely to be older, not white, have a higher use of a central venous catheter (CVC) as a HD vascular access, have a higher body mass index, and have a greater use of short daily HD as a home HD modality. Diabetes as a cause of ESRD and as a comorbidity was highest in the most contemporary cohort.
Table 1.
Patient characteristics at home hemodialysis initiation in Canada during the period 1996–2012
| Variable | Yr of Dialysis Initiation | P Value | ||
|---|---|---|---|---|
| 1996–2002 (n=233) | 2003–2007 (n=568) | 2008–2012 (n=1068) | ||
| Age, yr, mean±SD | 49±13 | 52±14 | 53±14 | <0.001 |
| Race, n (%) | ||||
| White | 175 (75) | 392 (69) | 740 (69) | 0.001 |
| Asian | 9 (4) | 49 (9) | 63 (6) | |
| Black | 12 (5) | 25 (4) | 71 (7) | |
| Other | 15 (6) | 61 (11) | 139 (13) | |
| Unknown | 22 (9) | 41 (7) | 55 (5) | |
| Women, % | 72 (31) | 182 (32) | 374 (35) | 0.2 |
| Primary diagnosis, n (%) | ||||
| GN | 48 (21) | 109 (19) | 176 (17) | 0.01 |
| Diabetes | 49 (21) | 119 (21) | 292 (27) | |
| Renal vascular disease | 23 (10) | 69 (12) | 111 (10) | |
| Polycystic kidney disease | 37 (16) | 57 (10) | 120 (11) | |
| Failed kidney transplant | 8 (3) | 41 (7) | 83 (8) | |
| Other | 45 (19) | 99 (17) | 175 (16) | |
| Unknown | 23 (10) | 74 (13) | 111 (10) | |
| Comorbid conditions, n (%) | ||||
| Diabetesa | 12(5) | 37 (7) | 97 (9) | 0.05 |
| Coronary artery disease | 84 (36) | 174 (31) | 281 (26) | 0.04 |
| Peripheral vascular disease | 24 (10) | 49 (9) | 69 (7) | 0.07 |
| Previous CVA | 14 (6) | 27 (5) | 58 (5) | 0.6 |
| Smokerb | 24 (10) | 46 (8) | 108 (10) | 0.4 |
| Vascular access type, n (%)c | ||||
| AV access | 50 (22) | 214 (38) | 458 (43) | <0.001 |
| CVC | 24 (10) | 149(26) | 415(39) | |
| Unknownc | 159 (68) | 205 (36) | 195 (18) | |
| BMI, median (IQR), kg/m2 | 25.1 (22.5–29.6) | 26.6 (22.7–31.5) | 27.9 (23.8–33.7) | <0.001 |
| ESRD vintage, yr, median (IQR) | 0.8 (0.3–1.9) | 1.3 (0.3–3.1) | 0.9 (0.2–3.1) | 0.003 |
| Prior RRT, n (%) | ||||
| None | 7 (3) | 50 (9) | 113 (11) | <0.01 |
| In-center HD | 207 (89) | 487 (86) | 880 (82) | |
| PD | 18 (8) | 24 (4) | 65 (6) | |
| Kidney transplant | 1 (0.4) | 7 (1) | 10 (1) | |
| Facility size n (%) | ||||
| Small (≤1–4 patients) | 34 (15) | 23 (4) | 47 (4) | <0.001 |
| Medium (5–9 patients) | 68 (29) | 93 (16) | 240 (23) | |
| Large (≥9 patients) | 131 (56) | 452 (80) | 781 (73) | |
| Home hemodialysis modality, n (%) | ||||
| Conventional | 181 (78) | 216 (38) | 532 (50) | <0.001 |
| Short daily | 5 (2) | 104 (18) | 166 (16) | |
| Slow nocturnal | 47 (20) | 248 (44) | 370 (35) | |
| Neighborhood income quintile, n (%) | ||||
| 1 | 43 (19) | 73 (13) | 156 (15) | 0.02 |
| 2 | 41 (18) | 96 (17) | 180 (17) | |
| 3 | 49 (21) | 124 (22) | 213 (20) | |
| 4 | 29 (12) | 132 (23) | 257 (24) | |
| 5 | 63 (27) | 134 (24) | 244 (23) | |
| Unknown | 8 (3) | 9 (2) | 18 (2) | |
| Distance (in km) from the dialysis facility, n (%) | ||||
| <15 | 109 (47) | 260 (46) | 536 (50) | 0.06 |
| 15–25 | 20 (9) | 67 (12) | 146 (14) | |
| 25–50 | 30 (13) | 78 (14) | 145 (14) | |
| 50–75 | 13 (6) | 35 (6) | 52 (5) | |
| 75–100 | 14 (6) | 35 (6) | 39 (4) | |
| >100 | 40 (17) | 86 (15) | 133 (13) | |
| Unknown | 7 (3) | 7 (1) | 17 (2) | |
CVA, cerebrovascular accident; AV, arteriovenous; CVC, central venous catheter; BMI, body mass index; IQR, interquartile range.
Diabetes as a comorbidity among those without presumed kidney disease.
Smoking status at ESRD initiation.
Data on vascular access type were not collected until 2001.
All-Cause Home HD Technique Failure by Era of Dialysis Initiation
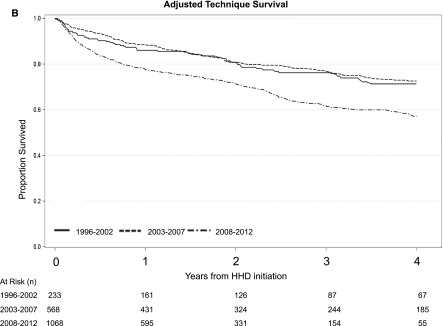
During follow-up, a total of 503 (27%) patients experienced home HD technique failure. Among these patients, the median time to technique failure was 9.3, 6.7, and 4.6 years for patients initiating home HD in 1996–2002, 2003–2007, and 2008–2012, respectively. Table 2 summarizes the results from the IPTCW analyses. The overall rate of home HD technique failure was 13.2 per 100 patient-years for the entire cohort over the entire period of follow-up. When patients on home HD were stratified by the period of dialysis initiation, the unadjusted rates of technique failure were 12.0 per 100 patient-years, 9.5 per 100 patient-years, and 16.8 per 100 patient-years for patients in the 1996–2002, 2003–2007, and 2008–2012 cohorts, respectively. Patients initiating home HD between 2008 and 2012 had a higher adjusted risk of technique failure (adjusted hazard ratio [AHR], 1.51; 95% confidence interval [95% CI], 1.06 to 2.15) compared with patients initiating home HD between 2003 and 2007. The risk was significantly highest in the first year of home HD (AHR, 2.45; 95% CI, 1.78 to 3.39). Data across other time intervals are in Supplemental Tables 1 and 2. Patients initiating home HD between 1996 and 2002 had a similar risk of technique failure compared with patients initiating home HD between 2003 and 2007 (AHR, 1.39; 95% CI, 0.78 to 2.46). Results were similar when restricted only to those patients with available information on vascular access subtype (see Supplemental Table 3).
Table 2.
Associations of era of home hemodialysis initiation with all-cause mortality, technique failure, and the composite of death and technique failure
| Death | Technique Failure | Death and Technique Failure | ||||
|---|---|---|---|---|---|---|
| Incident Cohort Period | Events | Event Rates/100 Patient-Yr | Events | Event Rates/100 Patient-Yr | Events | Event Rates/100 Patient-Yr |
| 1996–2002 | 51 | 7.3 | 74 | 12.0 | 125 | 19.3 |
| 2003–2007 (ref) | 127 | 7.9 | 158 | 9.5 | 285 | 17.4 |
| 2008–2012 | 91 | 5.5 | 271 | 16.8 | 362 | 22.3 |
| Unadjusted Hazard Ratio (95% CI) | ||||||
| Overall | ||||||
| 1996–2002 | 0.94 (0.68 to 1.31) | 1.10 (0.93 to 1.45) | 1.03 (0.83 to 1.27) | |||
| 2003–2007 (ref) | 1 | 1 | 1 | |||
| 2008–2012 | 0.75 (0.56 to 0.99) | 1.51 (1.23 to 1.86) | 1.19 (1.01 to 1.40) | |||
| Adjusted Hazard Ratio (95% CI) | ||||||
| Overalla | ||||||
| 1996–2002 | 0.67 (0.38 to 1.21) | 1.39 (0.78 to 2.46) | 1.11 (0.71 to 1.74) | |||
| 2003–2007 (ref) | 1 | 1 | 1 | |||
| 2008–2012 | 0.83 (0.58 to 1.19) | 1.51 (1.06 to 2.15) | 1.25 (0.98 to 1.61) | |||
| Yr 1a | ||||||
| 1996–2002 | 0.67 (0.31 to 1.49) | 1.49 (0.69 to 3.21) | 1.19 (0.62 to 2.26) | |||
| 2003–2007 (ref) | 1 | 1 | 1 | |||
| 2008–2012 | 0.71 (0.43 to 1.20) | 2.45 (1.78 to 3.39) | 1.84 (1.41 to 2.38) | |||
ref, referent group; 95% CI, 95% confidence interval.
Adjusted for age, sex, race, body mass, vascular access type, dialysis vintage, home hemodialysis modality, region, facility size, distance, income quintile, primary diagnosis, diabetes, coronary artery disease, stroke, peripheral vascular disease, and smoking status.
All-Cause Mortality by Cohort Period of Home HD Initiation and the Composite of Mortality or Technique Failure
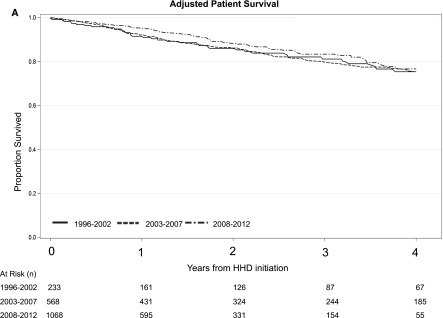
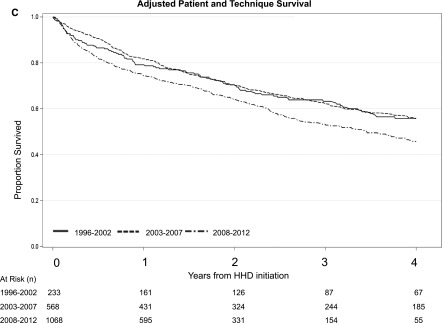
A total of 269 patients (14%) died over the course of follow-up. The rates of death were 7.3 per 100 patient-years, 7.9 per 100 patient-years, and 5.5 per 100 patient-years for the 1996–2002, 2003–2007, and 2008–2012 cohorts, respectively. Table 2 summarizes the results from the IPTCW analyses. When patients on home HD were stratified by the period of dialysis initiation, there were no significant differences in the adjusted risk of death across all three cohort periods. When the composite end point of time to death or technique failure was examined, there were no significant differences in the adjusted risk across all three cohorts (Table 2). Adjusted survival curves for patient, technique survival, and the composite of patient and technique survival is shown in Figure 1.
Figure 1.
Direct adjusted patient survival, technique survival, and the composite of patient and technique survival among patients on home hemodialysis demonstrating a higher risk of technique failure in the 2008–2012 cohort relative to the 2003–2007 cohort. (A) Patient survival, (B) technique survival, and (C) patient and technique survival. 95% CI, 95% confidence interval; HHD, home hemodialysis.
Additional Predictors of Mortality, Technique Failure, and the Composite of Mortality and Technique Failure
Table 3 lists additional predictors of mortality, technique failure, and the composite of death and technique failure. Increasing age, diabetes as a comorbidity, and smoking status were associated with an increased risk of death as well as the composite of death and technique failure. Other race was associated with an increased risk of death and the composite of death and technique failure. Renovascular disease, polycystic kidney disease, and a failed kidney transplant as a cause of ESRD were associated with a lower risk of the composite of death and technique failure compared with diabetes as a cause of ESRD. Compared with the initial use of a CVC, use of an arteriovenous (AV) access was associated with a lower risk of technique failure and the composite of death and technique failure. Increasing ESRD vintage was associated with a greater risk of the composite of death and technique failure. Medium-sized facilities had a lower risk of death, technique failure, and the composite end point compared with larger facilities. There was no significant effect of home HD dialysis modality on the risk of death or technique failure. Higher neighborhood income quintile was associated with a lower risk of death, technique failure, and the composite end point.
Table 3.
Associations of additional covariates with all-cause mortality, technique failure, and the composite of death and technique failure
| Variable | Death | Technique Failure | Death and Technique Failure |
|---|---|---|---|
| No. of events | 269 | 503 | 772 |
| AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | |
| Age, yr | |||
| 18–34 (ref) | 1 | 1 | 1 |
| 35–54 | 3.40 (1.3 to 8.6) | 1.0 (0.65 to 1.56) | 1.17 (0.79 to 1.73) |
| 55–64 | 5.90 (2.30 to 15) | 1.09 (0.68 to 1.76) | 1.49 (0.99 to 2.3) |
| 65–74 | 6.40 (2.40 to 16.9) | 2.34 (1.43 to 3.9) | 2.57 (1.65 to 3.99) |
| >75 | 16.5 (6.10 to 44.70) | 1.47 (0.79 to 2.74) | 2.71 (1.70 to 4.3) |
| Race | |||
| White (ref) | 1 | 1 | 1 |
| Asian | 1.18 (0.60 to 2.30) | 0.88 (0.51 to 1.50) | 0.96 (0.64 to 1.43) |
| Black | 1.90 (0.75 to 4.80) | 1.47 (0.87 to 2.49) | 1.49 (0.92 to 2.30) |
| Other | 0.72 (0.41 to 1.29) | 1.70 (1.16 to 2.50) | 1.43 (1.01 to 2.02) |
| Men | 1.1 (0.76 to 1.50) | 0.85 (0.66 to 1.10) | 0.91 (0.73 to 1.13) |
| Primary diagnosis | |||
| GN | 0.23 (0.12 to 0.45) | 0.60 (0.39 to 0.93) | 0.48 (0.33 to 0.69) |
| Diabetes | 1 | 1 | 1 |
| Renovascular disease | 0.37 (0.21 to 0.65) | 0.89 (0.60 to 1.34) | 0.70 (0.51 to 0.97) |
| Polycystic kidney disease | 0.15 (0.06 to 0.38) | 0.58 (0.35 to 0.97) | 0.42 (0.27 to 0.66) |
| Failed kidney transplant | 0.37 (0.13 to 1.04) | 0.53 (0.27 to 1.06) | 0.47 (0.26 to 0.83) |
| Other | 0.40 (0.23 to 0.70) | 0.99 (0.67 to 1.48) | 0.79 (0.57 to 1.10) |
| Comorbid conditions | |||
| Diabetes | 2.7 (1.60 to 4.40) | 1.23 (0.80 to 1.9) | 1.54 (1.1 to 2.14) |
| CADa | 1.2 (0.80 to 1.70) | 0.95 (0.67 to 1.36) | 1.02 (0.79 to 1.33) |
| PVD | 2.0 (1.30 to 3.10) | 1.0 (0.65 to 1.58) | 1.39 (1.03 to 1.88) |
| Previous CVA | 1.0 (0.60 to 1.70) | 1.24 (0.58 to 2.67) | 1.14 (0.67 to 1.94) |
| Current smokera | 1.8 (0.99 to 3.40) | 1.62 (1.17 to 2.25) | 1.67 (1.27 to 2.21) |
| Vascular access type | |||
| AV access | 0.46 (0.29 to 0.71) | 0.76 (0.52 to 1.10) | 0.65 (0.48 to 0.87) |
| CVC (ref) | 1 | 1 | 1 |
| Body mass index, kg/m2 | |||
| <18.5 | 0.89 (0.4 to 1.97) | 0.70 (0.26 to 1.89) | 0.71 (0.36 to 1.38) |
| 18.5–24.9 (ref) | 1 | 1 | 1 |
| 25–29.9 | 0.64 (0.40 to 1.00) | 1.06 (0.73 to 1.54) | 0.91 (0.68 to 1.22) |
| ≥30 | 0.74 (0.43 to 1.38) | 1.16 (0.83 to 1.60) | 1.03 (0.79 to 1.34) |
| ESRD vintage, per yr | 1.1 (0.99 to 1.18) | 1.04 (0.99 to 1.10) | 1.05 (1.01 to 1.10) |
| Prior RRT | |||
| None | 0.52 (0.18 to 1.44) | 2.09 (1.1 to 3.97) | 1.55 (0.88 to 2.73) |
| In-center hemodialysis | 1 | 1 | 1 |
| PD | 0.82 (0.41 to 1.66) | 0.70 (0.41 to 1.19) | 0.76 (0.49 to 1.17) |
| Kidney transplant | 0.26 (0.03 to 2.30) | 0.29 (0.04 to 2.39) | 0.29 (0.06 to 1.42) |
| Facility size | |||
| Small (1–4) | 0.31 (0.12 to 0.82) | 0.74 (0.39 to 1.43) | 0.60 (0.33 to 1.08) |
| Medium (5–9) | 0.64 (0.41 to 1.00) | 0.66 (0.45 to 0.96) | 0.63 (0.47 to 0.85) |
| Large (>9) | 1 | 1 | 1 |
| Home hemodialysis modality | |||
| Conventional | 1 | 1 | 1 |
| Short daily | 0.69 (0.40 to 1.19) | 1.09 (0.77 to 1.05) | 0.97 (0.72 to 1.30) |
| Slow nocturnal | 1.03 (0.66 to 1.59) | 0.99 (0.70 to 1.42) | 0.98 (0.74 to 1.30) |
| Neighborhood income quintile (per quintile increase) | 0.94 (0.82 to 1.08) | 0.89 (0.81 to 0.97) | 0.90 (0.83 to 0.97) |
| Distance from the dialysis facility, km | |||
| <15 | 1 | 1 | 1 |
| 15–25 | 0.87 (0.51 to 1.49) | 0.74 (0.50 to 1.09) | 0.75 (0.55 to 1.04) |
| 26–50 | 0.64 (0.35 to 1.16) | 0.54 (0.34 to 0.85) | 0.55 (0.37 to 0.80) |
| 51–75 | 1.6 (0.85 to 3.05) | 0.54 (0.27 to 1.09) | 0.74 (0.46 to 1.21) |
| 76–100 | 1.04 (0.51 to 2.10) | 1.32 (0.83 to 2.10) | 1.25 (0.83 to 1.88) |
| >100 | 1.16 (0.68 to 1.96) | 0.72 (0.48 to 1.05) | 0.82 (0.61 to 1.10) |
AHR, adjusted hazard ratio; 95% CI, 95% confidence interval; ref, referent group; CAD, coronary artery disease; PVD, peripheral vascular disease; CVA, cerebrovascular accident; AV, arteriovenous; CVC, central venous catheter; PD, peritoneal dialysis.
All models adjusted for age, sex, race, body mass, vascular access type, dialysis vintage, home hemodialysis modality, region, facility size, distance, income quintile, primary diagnosis, diabetes, coronary artery disease, stroke, peripheral vascular disease, and smoking status.
Transplantation Events
Transplantation events occurred in 92 (40%), 203 (36%), and 151 (14%) individuals in the 1996–2002, 2003–2007, and 2008–2012 cohorts, respectively. There were no differences in the adjusted time to kidney transplantation among all three cohorts. Compared with the 2003–2007 cohort, the adjusted relative risk to kidney transplantation was 1.09 for the 2008–2012 cohort (95% CI, 0.83 to 1.43) and 1.12 for the 1996–2002 cohort (95% CI, 0.78 to 1.61).
Discussion
In this nationally representative cohort of patients on home HD, our main finding was that during a significant increase in home HD utilization in Canada between 1996 and 2012, we demonstrated a 51% higher relative risk of home HD technique failure compared with the earliest cohort. We also demonstrated no difference in the risk of death or in the composite end point of death and technique failure across all three incident cohorts.
Our findings build upon previous reports examining patient and technique survival on home HD in Canada. The Canadian Slow Long Nightly Extended Dialysis Programs (CAN-SLEEP) collaborative group demonstrated unadjusted 1- and 5-year adverse event–free (the composite of death and technique failure) survival of 95% and 80% among a cohort of 247 patients undergoing nocturnal home HD from three home HD sites in Canada between 1994 and 2006 (16). In contrast, in the most contemporary cohort in this study, we observed 1- and 4-year event-free survival rates that were significantly lower at 79% and 63%. It is possible that the superior outcomes in the CAN-SLEEP cohort may relate to the inclusion of only selected centers that tended to have subspecialized expertise in home HD and patients that were exclusively treated with nocturnal home HD.
Our rates of failure are significantly lower than those reported among patients on home HD in the United States across all eras. We observed an 18% risk of technique failure within the first year of home HD initiation in the most recent cohort. However, in a recent analysis by Seshasai et al., among 2840 patients in the United States who initiated home HD between 2007 and 2009, 25% switched to an alternate dialysis modality by the first year of follow-up (17). Moreover, a recent interim analysis of the Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements Study revealed that 22% of patients discontinued home HD therapy by 12 months of treatment (18). Further, a recent study by Weinhandl et al. reported 21.3 discontinuation events per 100 patient-years among 3400 patients on home HD, a rate significantly higher than the rates of 13.2 and 16.8 discontinuation events per 100 patient-years that we observed in the overall cohort and in our most recent cohort, respectively (19).
Differences between discontinuation rates of home HD in Canada and the United States may relate to case-mix differences between the patients selected for and treated with home HD. In this study, we found that increasing age, diabetes as a cause of ESRD, and increasing dialysis vintage were associated with a higher risk of death, technique failure, or the composite end point. In the study by Seshasai et al., patients had a higher median ESRD vintage at home HD initiation of 2.1 years, which was significantly greater compared with the ESRD vintage in our cohort, which ranged between 0.8 and 1.3 years depending on the era of dialysis initiation (17). However, in their analysis, dialysis vintage was not a predictor of home HD discontinuation and the mean age of patients in their cohort was 52 years, with 70% white and 30% with diabetes—patient characteristics that are similar to those seen in our home HD cohort.
It is possible that the trends over time may have related to additional modifiable practices rather than a change in the case-mix of patients over time. Factors such as the duration and quality of home HD training and education, and the nature and degree of post home HD initiation care (including nursing follow-up, availability of respite, and frequency of clinic visits) could not be explored in this analysis. In Canada, approximately 6 weeks is the typical duration of home HD training across facilities, although training periods tends to be shorter in the United States, which may explain between-country differences in outcomes (20). In other countries with longer training times, such as Australia and New Zealand, rates of discontinuation of home HD are significantly lower at 25% after 2 years of follow-up (21). However, this may also relate to the higher rates of AV access use in patients on home HD in Australia and New Zealand compared with those in Canada (22). In Canada, a temporal decrease in AV access use among patients on home HD was observed, and AV access was demonstrated to be associated with a lower risk of adverse events both in this study and in a previous analysis by our group (23). Taken together, this may suggest that reduction of CVC use among patients on home HD may represent an opportunity to improve outcomes among these patients.
In our most recent cohort, >34% of patients were performing slow nocturnal HD at home, which may also have negatively affected rates of home HD discontinuation because of a potentially higher rate of patient burnout from more intensive dialysis (24). Alternatively, one may postulate that given that more intensive dialysis may be associated with improved outcomes, this may be associated with a lower risk of death and technique failure. However, home HD modality is unlikely to have been a factor in our analysis given that the increase in technique failure that we observed in this analysis was adjusted for home HD modality. Furthermore, although our analysis was not specifically designed to address this question, nocturnal home HD was not a significant predictor of patient and technique survival compared with other home HD modality types.
Other practice differences may relate to facility size. Interestingly in our analysis, we found that patients treated in middle-sized facilities had a lower risk of death and technique failure. Among PD facilities, given that facility size is an important determinant of PD technique failure, we initially postulated that treatment at the largest tertile of home HD facility size would be associated with the longest patient and technique survival (25–28). However, it is possible that among home HD facilities, the positive effect of increased experience offered by larger facilities may have been offset with a higher comfort level with the therapy and less restrictive eligibility criteria for home HD compared with their medium facility counterparts. In this regard, larger facilities may be enriched with potentially sicker and more marginal candidates, leading to higher rates of death and technique failure.
Across programs, home HD technique failure rates are increasingly recognized as an important clinical effectiveness measure and a potentially important quality improvement metric to track. However, similar to other large observational studies, we could not elucidate the causes for discontinuation off home HD. Unlike the causes of PD technique failure that have been better studied, the causes of home HD technique failure have not been as well studied nor captured across large national registries (29). Possible reasons for home HD discontinuation may include patient and/or caregiver choice and burnout, vascular access cannulation difficulties, a change in geographic location or home environment that may no longer support home HD, changes in physical and/or cognitive capacity that may limit the ability to safely perform self-care dialysis, and lastly, a change or absence of a caregiver to aid in performing the home HD treatments. It is tempting to speculate that the observation between higher neighborhood income quintile and a lower risk of adverse outcomes that we observed may relate to differences in access to care and availability of additional supports that may modify the risk of home HD technique failure. In our most recent cohort, we observed an increased risk of technique failure events that tended to occur early in the first year (Table 2). In this regard, early failure events in the first year may be more easily modified than late events where technique failure may be an indicator of a premorbid event stemming from progressive cognitive or physical incapacity to perform home HD (16). Whether novel programs such as increased peer-to-peer support, personal support worker–supported home HD, and increased availability of respite care can affect rates of home HD failure remains to be evaluated via further prospective study.
Although we observed higher rates of home HD technique failure in the most contemporary cohort, this finding needs to be interpreted in the context of the significant growth that home HD utilization has experienced in Canada over the last 20 years. Although the absolute number of patients has increased substantially, this represents a change from <1% of the Canadian ESRD population to approximately 2.5% of the ESRD population over the course of our study (1). With increasing interest from both patients and providers, it is more likely that home HD eligibility is being applied with less stringent or different criteria than have been traditionally applied. In this regard, patients with cardiac disease, large intradialytic weight gains, significant dialysis-related hypotension, or uncontrolled serum phosphorus may be increasingly encouraged to consider home HD because of its purported cardiovascular and biochemical benefits. Although we could not examine the reasons for home HD utilization and dialytic and biochemical treatment parameters before the initiation of home HD, patients in the most contemporary era were indeed older, with a greater burden of diabetes. We believe that case-mix differences between historical cohorts and the most contemporary cohort is the primary reason that we observed the higher rates of home HD technique failure in the most contemporary era. However, it is encouraging that despite the change in case-mix of patients in the most recent era, we did not observe a higher risk of death and the composite of death and technique failure among these patients.
Despite robust multivariable adjustment on the basis of a number of variables, residual confounding may remain on the basis of unmeasured differences between patients and the lack of documented reasons for home HD initiation. Large administrative datasets, such as CORR, are subject to limitations arising from data validity and the availability of data elements that are relevant to the research question, particularly with regards to capture of technique failure events. Comorbidities captured within CORR have been recently validated and are therefore likely to offer reliable information (11). Furthermore, of interest to this analysis would have been important biochemical variables, such as serum albumin, phosphorus, and measurements of residual kidney function, that may have affected the risk of home HD mortality and technique failure; however, these were not available at the time of home HD initiation. There was a significant amount of missing information on vascular access type that was not formally documented until 2001; however, a sensitivity analysis among subjects with information on vascular access type did not appreciably change the direction and magnitude of our primary findings (Supplemental Table 3). Important facility-based characteristics, such as level of expertise in home HD and dedicated resources for home HD, would have similarly been of interest, particularly given a recent survey that demonstrated wide variation in programmatic approaches to the care of patients on home HD in Canada in domains of patient recruitment, patient training, and patient follow-up (30).
In addition, given the small patient numbers by era, we may have not been able to detect differences in patient and technique survival when differences did exist. However, we utilized a nationally representative data source with comprehensive and national patient coverage, with the exception of the province of Quebec. Lastly, the causes of home HD technique failure would have provided important insights in evaluating cause-specific trends over time in patient and technique survival, but these were similarly poorly captured in CORR.
In this analysis, we have provided extensive data exploring patient and technique survival among patients on home HD in Canada over a span of 16 years. In doing so, we have demonstrated encouraging results with regards to the long-term success of home HD in terms of patient and technique survival. With increasing home HD utilization in Canada, there has been no increase in the risk of death or the composite of death and technique failure, but there has been an increased risk of home HD technique failure. In this regard, further prospective research is needed to elucidate the causes of home HD technique failure, and to evaluate novel strategies that may potentially modify the risk of home HD technique failure in an effort to better support patients to continue to receive dialysis treatment at home.
Disclosures
J.P. has received speaking honoraria from Baxter Healthcare and has received consulting fees from Baxter Healthcare, Fresenius Medical Care Canada, Otsuka, Janssen Ortho, Shire, Takeda, and Boehringer-Ingelheim, as well as research support from Baxter Healthcare and salary support from Arbor Research Collaborative for Health. Y.N. declares no competing interests. K.K.T. has received research funding from Amgen Canada and Astellas Canada. C.T.C. has served as a consultant for NxStage, Intelomed, and Baxter Healthcare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the staff at the Canadian Organ Replacement Register (CORR) for maintaining the database and the dialysis facilities throughout Canada for submitting information to CORR.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Maintaining Patients on Home Hemodialysis: The Journey Matters as Does the Destination,” on pages 1209–1211.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13271216/-/DCSupplemental.
References
- 1.Canadian Organ Replacement Register: Preliminary Statistics for Renal Failure and Solid Organ Transplantation in Canada, Ottawa, ON, Canadian Organ Replacement Register, 2015 [Google Scholar]
- 2.Jayanti A, Morris J, Stenvinkel P, Mitra S: Home hemodialysis: Beliefs, attitudes, and practice patterns. Hemodial Int 18: 767–776, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A: Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61: 2235–2239, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Walsh M, Culleton B, Tonelli M, Manns B: A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 67: 1500–1508, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Manns BJ, Klarenbach S, Quinn R, Tonelli M, Culleton BF: The effects of nocturnal hemodialysis compared to conventional hemodialysis on change in left ventricular mass: Rationale and study design of a randomized controlled pilot study. BMC Nephrol 7: 2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen DA, Kuliszewski MA, Liao C, Rudenko D, Leong-Poi H, Chan CT: Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clin J Am Soc Nephrol 6: 1345–1353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarlane PA, Pierratos A, Redelmeier DA: Cost savings of home nocturnal versus conventional in-center hemodialysis. Kidney Int 62: 2216–2222, 2002 [DOI] [PubMed] [Google Scholar]
- 9.CORR : 2002 CORR Prelimanary Report: Preliminary Statistics For Renal Failure and Solid Organ Transplantation in Canada, Ottawa, ON, CORR, 2002 [Google Scholar]
- 10.Karamadoukis L, Ansell D, Foley RN, McDonald SP, Tomson CRV, Trpeski L, Caskey FJ: Towards case-mix-adjusted international renal registry comparisons: How can we improve data collection practice? Nephrol Dial Transplant 24: 2306–2311, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Canadian Institute for Health Information : Canadian Institute for Health Information, Data Quality Study on the Canadian Organ Replacement Register, Ottawa, ON, Canadian Institute for Health Information, 2009 [Google Scholar]
- 12.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 15.van der Wal WM, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT, Korevaar JC, Geskus RB: Comparing mortality in renal patients on hemodialysis versus peritoneal dialysis using a marginal structural model. Int J Biostat 6: 2, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Pauly RP, Maximova K, Coppens J, Asad RA, Pierratos A, Komenda P, Copland M, Nesrallah GE, Levin A, Chery A, Chan CT; CAN-SLEEP Collaborative Group : Patient and technique survival among a Canadian multicenter nocturnal home hemodialysis cohort. Clin J Am Soc Nephrol 5: 1815–1820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshasai RK, Mitra N, Chaknos CM, Li J, Wirtalla C, Negoianu D, Glickman JD, Dember LM: Factors associated with discontinuation of home hemodialysis. Am J Kidney Dis 67: 629–637, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaber BL, Lee Y, Collins AJ, Hull AR, Kraus MA, McCarthy J, Miller BW, Spry L, Finkelstein FO; FREEDOM Study Group : Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis 56: 531–539, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Weinhandl ED, Gilbertson DT, Collins AJ: Mortality, hospitalization, and technique failure in daily home hemodialysis and matched peritoneal dialysis patients: A Matched Cohort Study. Am J Kidney Dis 67: 98–110, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Rioux JP, Marshall MR, Faratro R, Hakim R, Simmonds R, Chan CT: Patient selection and training for home hemodialysis. Hemodial Int 19[Suppl 1]: S71–S79, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Rioux J-P, Bargman JM, Chan CT: Systematic differences among patients initiated on home haemodialysis and peritoneal dialysis: The fallacy of potential competition. Nephrol Dial Transplant 25: 2364–2367, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Marshall MR, Hawley CM, Kerr PG, Polkinghorne KR, Marshall RJ, Agar JW, McDonald SP: Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis, 58: 782–793, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Perl J, Nessim SJ, Moist LM, Wald R, Na Y, Tennankore KK, Chan CT: Vascular access type and patient and technique survival in home hemodialysis patients: The Canadian Organ Replacement Register. Am J Kidney Dis 67: 251–259, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Agar JW, Hawley CM, Kerr PG: Home hemodialysis in Australia and New Zealand: How and why it has been successful. Semin Dial 24: 658–663, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO: Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 29: 292–296, 2009 [PubMed] [Google Scholar]
- 26.Schaubel DE, Blake PG, Fenton SS: Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 60: 1517–1524, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Guo A, Mujais S: Patient and technique survival on peritoneal dialysis in the United States: Evaluation in large incident cohorts. Kidney Int Suppl 64(88): S3–S12, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Huisman RM, Nieuwenhuizen MG, Th de Charro F: Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 17: 1655–1660, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Mujais S, Story K: Patient and technique survival on peritoneal dialysis in patients with failed renal allograft: A case-control study. Kidney Int Suppl 70(103): S133–S137, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pauly RP, Komenda P, Chan CT, Copland M, Gangji A, Hirsch D, Lindsay R, MacKinnon M, MacRae JM, McFarlane P, Nesrallah G, Pierratos A, Plaisance M, Reintjes F, Rioux JP, Shik J, Steele A, Stryker R, Wu G, Zimmerman DL: Programmatic variation in home hemodialysis in Canada: Results from a nationwide survey of practice patterns. Can J Kidney Health Dis 1: 11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.