Abstract
Introduction
New phage-directed nanomedicines have emerged recently as a result of in-depth study of the genetics and structure of filamentous phage and evolution of phage display and phage nanobiotechnology. This review focuses on the progress made in the development of the cancer-targeted nanomaterials and discusses the trends in using phage as a bioselectable molecular navigation system.
Areas covered
The merging of phage display technologies with nanotechnology over the past years has proved promising in different areas of medicine and technology, such as medical diagnostics, molecular imaging, vaccine development and targeted drug/gene delivery, which is the focus of this review. The author used data obtained both in his research group and sourced using Science Citation Index (Web of Science) and NCBI PubMed search resources.
Expert opinion
First attempts of adapting traditional concepts of direct targeting of tumor using phage-targeted nanomedicines has shown minimal improvements. With discovery and study of biological and technical barriers that prevent anti-tumor drug delivery, a paradigm shift from traditional drug targeting to nanomedicine navigation systems is required. The advanced bacteriophage-driven self-navigation systems are thought to overcome those barriers using more precise, localized phage selection methods, multi-targeting “promiscuous” ligands and advanced multifunctional nanomedicine platforms.
Keywords: cancer, nanomedicines, drug delivery, gene delivery, landscape phage, nanobiotechnology, phage display, phage proteins
1. Introduction
Development of precision medicines that provide their effect specifically at the site of disease has been a long sought goal for the last century, starting from pioneering works of Paul Ehrlich, who suggested the concept of site-directed chemotherapeutics, or “magic bullets” [1]. Although this revolutionary concept opened a new, rational way to developing antibacterial and anti-parasite chemotherapeutics, such as Salvarsan and Trypan Red, it has been hardly adapted for the treatment of mammalian organismic diseases, such as cancer. With development of nanomedicines, significant progress towards this goal have been achieved, mostly through exploring passive targeting based on Enhanced Permeability and Retention (EPR) effect [2]. Following the traditional Ehrlich’s concept, the targeting of nanomedicines has been recently proposed to enhance their therapeutic efficacy and decrease non-related side effects [3]. However, it has been shown that the tumor microenvironment is much more complex than previously believed and active targeting of tumor has shown minimal improvements in comparison with EPR-based passive targeting [4]. With discovery and identification of biological and technical barriers that prevent anti-tumor drug delivery [4, 5, 12], a paradigm shift from traditional drug targeting to nanomedicine navigation systems is required. The advanced bacteriophage-driven self-navigation systems are thought to overcome those barriers using more precise, localized phage selection methods, multi-targeting “promiscuous” ligands and advanced multifunctional nanomedicine platforms.
2. Evolution of the drug targeting concept
It is common for traditional cancer chemotherapeutics to have a low therapeutic index because of their non-specific cell accumulation, rapid blood clearance, poor aqueous solubility of hydrophobic drugs, susceptibility to ATP-binding cassette (ABC) transporters, and rapid degradation/modification into a non-active metabolite [6]. Therefore, there is a critical need in advanced cancer drug delivery systems that would improve patient care with increased post-treatment survival time. In the early 1900s Paul Ehrlich pioneered the concept of a therapeutic “magic bullet” and ushered in the era of modern chemotherapy research [1]. The idea that a therapeutic molecule could be delivered specifically to diseased cells within an organism, was the “Holy Grail” for several generations of researchers for the past hundred years. Once delivered, the therapeutic molecule was envisioned to provide curative treatment of the disease by either correction of the disease state or removal of the diseased cells while leaving the healthy cells unharmed. Ehrlich proposed that the specific curative effect could be achieved through specific interactions of the targeted drug with molecular receptors expressed on the cell of interest. The first practical realization of the “magic bullet” concept has been seen in development of antibody-drug conjugates (ADCs) that combine the selectivity of targeted treatment with the cytotoxic effect of chemotherapy drugs. There are several ADCs approved for haematological and non- haematological malignancies treatment and about fifty ADCs under investigation, with more than a half of which investigated in solid tumors [7]. Some of inherited limitations of ADCs include a limited number of conjugated drugs that results in minimal concentration of the cytotoxic payload in tumor cells (1–2% of the administered dose reaching the tumor, according to [7]).
With widespread applications of phage display methods in biological systems, a novel class of “magic bullets”, peptides with antitumor activity, has been discovered recently [8]. Differing from peptides that serve as targeted entities for the delivery of conjugated medicines, these peptides themselves exhibit antitumor activity. Examples of antitumor peptides include peptide LyP-1 that inhibits the growth of human breast cancer MDA-MB-435 in mice; peptide CIGB-300 that exhibits antitumor effect in mice with syngeneic tumors and human tumors; peptide SMSIASPYIALE that impedes the development of metastasis and increases the lifespan of mice with a gastric cancer graft; GMBP1 peptide that specifically binds to the receptors of gastric cancer cells, and contributes to restoration of drug sensitivity, and other peptides described in review [8].
A number of bifunctional peptides has been designed as a reflection of a common trend in the development of multifunctional medicines (Subsection 7.1). Representative examples include: a fusion protein composed of the pro-apoptotic peptide KLAK and peptide RGD, which specifically binds to tumor endothelial cells, penetrates into the cells, and induces their apoptosis through the mitochondrial pathway; the protein M2pepKLA inhibiting tumor growth through reducing the population of tumor-associated macrophages; peptide CRGDKGPDC (iRGD) combining the properties of two peptides, RGD (integrin-binding) and R/KXXR/K (neuropilin-binding). Peptides that have targeting properties and at the same time are able to penetrate into tumor parenchyma form a separate class of “Tumor penetrating peptides” [41]. Encouraging results have been obtained in clinical trials of medicines targeted with tumor-specific peptides [8], for example a cyclic peptide [Arg-Gly-Asp-Dphe-(NMeVal)] containing an RGD as the basis for the Cilengitide—a potential drug for a variety of solid tumors (phase I/II of clinical trials); or anticancer drug NGR-hTNF for pleural mesothelioma, liver cancer, ovarian cancer, colorectal cancer, and small-cell lung cancer (phase I/II).
The steady trend in making antibodies much smaller, “mini-antibodies”, to penetrate the tissues more effectively, and designing multifunctional fusion peptides and their grafting into non-antibody scaffolds to increase their therapeutic efficacy, could make the difference between anticancer antibodies and anticancer peptides less visible [9, 101].
3. Nanomedicines as drug delivery systems
One of the most promising concepts in cancer treatment relies on the use of cancer drugs encapsulated in nanocarriers. These medications, called “nanomedicines”, are composed primarily of two major components: a therapeutic molecule and a drug delivery nanocarrier, and can be modified to introduce additional constituents including a polymer coating, a targeting ligand, or a linker molecule. A common favourable characteristic of nanomedicines in comparison with non-encapsulated drugs is their ability to improve the systemic bioavailability of small molecule drugs and subsequently enhance their therapeutic effect by providing prolonged circulation in a patient [12].
The concept of drug targeting has been adapted without essential revision for the targeted nanomedicine development. It was hypothesized that nanomedicines could deliver high therapeutic doses of the drugs specifically to cancer cells through “active targeting” due to cell-specific, ligand-receptor interactions. The concept of nanomedicine targeting was initially based on the hypothesis that a vascular-specific targeting ligand would allow selective partitioning of nanomedicines out of the central blood flow towards the outer edges of the tumor capillary walls. Once nanomedicines are sidelined, the number of interactions with the tumor vasculature is increased resulting in accumulation of particles by the EPR effect [2]. It was also expected that nanomedicine targeting can help them to carry the drug cargo through the vasculature directly to the tumor cells [13].
4. Biological barriers preventing nanomedicine tumor targeting
With intensive study of tumor physiology, a number of biological barriers has been revealed that protect tumor cells from targeted drug invasion [4].
4.1 Circulatory system
Following infusion of a targeted nanomedicine in the blood stream, any exposed targeting ligands, such as peptides, antibodies or RNA/DNA-aptamers, will be immediately subjected to serum proteases, DNAses, and RNAses. They can also be susceptible to certain chemical reactions that modify the final binding interactions with the desired cell targets [15].
4.2 Immune Reactivity and Clearance
Nanomedicines can promote a number of immune responses in vivo because they might be recognized as foreign agents that must be eliminated to prevent damage to the host. Generating antibodies to a targeted nanomedicine can cause rapid clearance of the product, or cross-reaction with the host’s proteins, that might be harmful to the host [16]. Targeted nanomedicines are subject to the same general problems of immunogenicity as untargeted nanomedicines. However, targeting ligands themselves, considering their potential immunogenicity, are more of a concern in nanomedicine design aimed at improving bioavailability and preventing nanomedicine clearance with repeated doses. The role of immunogenicity as a critical factor hindering the efficacy of targeted nanomedicines is described in the recent comprehensive review [16].
4.3 Diseased vasculature
To exert their biological effect, nanomedicines must escape from circulation and enter into the cancer microenvironment. Physiologically normal capillaries are composed of three major components: endothelial cells, pericytes, and a basement membrane with tight endothelial junctions between neighboring cells [17]. Their architecture, molecular composition and role in homeostasis are studied in much detail elsewhere. In contrast, capillaries within a tumor tissue are more disorganized and contain large gaps between neighboring endothelial cells. Accumulation of drug carriers in the areas with leaky vasculature (EPR effect), depends on “physiological” properties of each particular tumor, its vasculature cut-off size being the most important parameter controlling penetration of drugs and drug carriers through the endothelium into the interstitium [2]. Nanomedicines designed to rely on passive accumulation within the tumor microenvironment by virtue of the EPR effect showed promising results in the clinic include liposome drug delivery systems such as DOXIL, DaunoXome, MYOCET, LLC, DepoCytand MARQIBO.; albumin-based nanoparticles ABRAXANE; micelles Genexol-PM, and a number of polymeric and inorganic nanoparticles, approved individually in USA, Europe, Canada, Japan and South Korea for control of cancer diseases (comprehensive review of nanomedicines applied to oncology, including a description of 23 nanomedicines under clinical trials at different stages, can be found in the review[12]).
4.4 Tumor microenvironment
It was assumed that the failure of numerous nanomedicines in biological experiments could be associated with their inability to penetrate through the complex tumor microenvironment into the core of tumors [4, 18]. The tumor microenvironment is composed of three major compartments: the interstitial fluid; the non-cellular interstitial components, such as the extracellular matrix (ECM); and the cellular components or stroma that contain several cell types in addition to endothelium and cancerous cells, such as macrophages and fibroblasts. The surrounding stroma can have a large impact on blood vessel permeability and diffusivity through the extracellular matrix [19]. It is commonly observed that large nanomedicines fail to penetrate into the tumor and hardly can reach the first few layers of tumor cells [20]. That can result in releasing the therapeutic drug cargo by nanomedicines within tumor environment, or the first layer of cells that results in minimal objective response to the treatment. The affinity of targeted ligands in nanomedicines can play a critical role in their micro-distribution and retention in a tumor. Experimental and theoretical analyses of antibody distribution in the tumor demonstrated a more homogeneous distribution of low affinity antibodies. This effect is attributed to the antibody’s ability to dissociate from the tumor antigens after initial binding and subsequently diffuse further into the tissue [10, 14].
5. Trends in actively targeted nanomedicines
Following the traditional “magic bullet” concept, the targeting of nanomedicines has been proposed to enhance their therapeutic efficacy and decrease non-related side effects in patients. To achieve this goal, nanomedicines were modified with variety of different reactive moieties including small molecules, peptides, proteins, DNA and RNA aptamers, antibodies and their fragments using chemical conjugation, hydrophobic interaction, non-covalent streptavidin-biotin interaction, or by specific association with fusion phage proteins [45]. The ligands can be generated from a number of sources including naturally derived products, phage display libraries [21], aptamer libraries [22], or engineered antibodies [23]. Regardless of the type of ligand used, there are a number of factors that affect the delivery of a nanocarrier, such as ligand density, ligand charge and ligand orientation/availability [3].
5.1. Using defects in the tumor vasculature
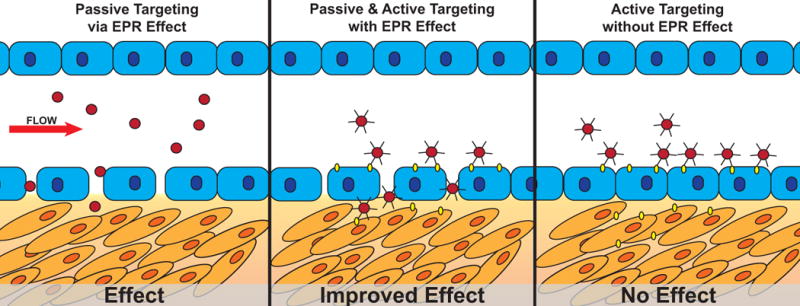
Currently accepted active targeting mechanisms rely heavily on the EPR effect to promote primary accumulation of the nanomedicines within the tumor microenvironment, combined with specific interaction of targeted nanomedicines with tumor-specific receptors (Figure 1A, B). Alternatively, if the actively targeted nanomedicine is applied to a biological system, in which there are no leaky blood vessels, the nanomedicines would likely marginalize but fail to cross the endothelial cell barrier, reach the tumor microenvironment, and induce a biologic response (Figure 1C). Since the accumulation of nanomedicines within the tumor depends on blood circulation, the presence of antibody or ligand on nanoparticle surface may be irrelevant in determining its biodistribution. In fact, the pharmacokinetics of targeted nanomedicines are shown to be the same as for non-targeted nanomedicines [4, 77] because their transport depends on blood circulation and the EPR effect to arrive at the target site. The targeting ligand can affect the “micro-biodistribution” of the nanomedicines if they reach the tumor tissue and may be internalized into the cells, while the non-targeted nanomedicines stay in the tumor interstitium. In this scenario, the presence of antibody or ligand on the nanoparticle surface may increase the cellular uptake if the cells in contact with nanoparticles have overexpressed receptors. This hypothetic mechanism of nanomedicine active targeting can explain some of the discrepancies observed in in vitro versus in vivo studies [4, 24].
Figure 1. Schematic of nanomedicine deposition by passive and/or active targeting in the presence or absence of an observable EPR effect (leaky vasculature).
Left: Passive targeting of non-ligand targeted nanomedicines results in delivery and a biologic effect. Center: Passive targeting of an actively targeted nanomedicine results in enhanced delivery and improved biologic effect. Right: Active targeting of a nanomedicine in the absence of leaky vasculature results in marginalization of nanomedicines without delivery into the tumor microenvironment resulting in no biologic effect.
5.2. Using natural mechanisms of extravasation, transcytosis and paracellular transport
The vascular bed of normal and diseased tissues is highly heterogeneous and expresses a number of different cell surface receptors based on the tissue site and disease state [25, 26]. Targeted nanomedicines may be recruited to the vasculature around the tumor microenvironment through a mechanism similar to that observed with leukocyte accumulation at specific sites, which rolling adhesion interactions are formed between their surface ligands and specific integrins or selectins expressed on endothelial cell surfaces [27]. Similar mechanisms can be exploited to deliver therapeutics specifically to a diseased site by attachment of a homing peptide [25] binding to cancer vasculature-specific proteins, such as aminopeptidase N, integrins, and cell surface expressed nucleolin [28, 51]. The endothelial cell barrier can be crossed using mechanisms that may enable nanomedicines within a blood capillary to extravasate (i.e., cross the vascular walls) into the tumor microenvironment, which include: a) modulation of the EPR effect; b) transcytosis through the intact endothelial cell layer; and c) paracellular transport between the tight junctions of neighboring endothelial cells as seen during leukocyte extravasation.
Transcytosis, which involves the specific transport of molecules across an endothelial cell barrier, occurs in all continuous and fenestrated vascular endothelium [29]. This bidirectional transport mostly proceeds in an apical-to-basolateral direction, favorable for ideal nanomedicine transport from the blood to the tumor interstitium. Although it remains unclear “how does the endothelial cell recognize particles for transcytosis versus degradation”, some progress is observed in using transcytosis for targeted drug delivery [30]. Identification of ligands that promote transcytosis for improved rational design of drug delivery systems are ongoing [31].
The paracellular transport route is normally restricted to molecules < 6 nm in diameter [32]. However, under proper conditions, this barrier can be traversed by much larger objects, as is observed during leukocyte extravasation and diapedesis [33]. Extensive work on the BBB, which is commonly used as a model of endothelial tight junctions, is ongoing with strategies involving direct modification of tight junction integrity or use of positively charged nanoparticles to increase delivery [34]. However, no targeting ligands have been identified to increase paracellular transport in vivo. Recently, peptides were designed to reversibly open tight junctions of polarized intestinal epithelial cells [35, 37]. It is though that targeting with these peptides may increase paracellular transport activity.
5.3. Cancer cell-specific binding and internalization of targeted nanomedicines
Once within the tumor microenvironment, the targeted nanomedicines should navigate through a number of cellular and extracellular components to reach the heterogeneous tumor target cells with the aberrant display of numerous cell surface proteins [36]. Tumor heterogeneity observed in patients (both within the same patient and across different patients) requires identification of a ligand(s) that can be used to target tumors with different cell surface phenotypes, including the tumor-initiating cell population. Following appropriate discrimination of cells with a neoplastic phenotype, nanomedicines must then penetrate through the cytoplasmic membrane and deliver their cargo into the cytoplasm. Endocytosis is the primary mechanism used for penetration of nanomedicines and delivery of therapeutic molecule into the cytoplasm. In the absence of nanomedicine internalization, the extracellular therapeutic molecules are redistributed to surrounding cells within the tissue microenvironment, resulting in failure of cell-specific drug targeting, decrease in overall drug accumulated per cell and increase of drug resistant phenotypes [102]. Alternatively, intracellular delivery of a nanomedicine results in higher doses of therapeutic drug being reached within the desired cell targets, which leads to increased cytotoxicity and improved therapeutic indices [5, 38].
5.4. Stability of targeted nanomedicines
Different approaches have been suggested to increase the stability of nanomedicines in blood stream, enhance their accumulation in tumor vasculature and tumor microenvironment, and provide penetration into cancer cells. It was found that attachment of a high molecular weight PEG to peptide ligand or nanocarrier delivery system results in their increased circulation time and decreased renal clearance [39, 77]. Several strategies have been used to increase resistance of protein ligands to degradative effects of serum proteases including: optimization/selection of cleavage-resistant primary sequences, introduction of secondary structure to reduce substrate recognition, shielding of functionally active domains, derivatization of cleavable sites, or functionalization of peptide termini.
6. Phage-derived targeted nanomedicines
Most targeted drug delivery projects use phage-display systems that explore filamentous phages M13, fd, and f1 as vectors; display systems based on other bacteriophages [40] are also very promising [41] but will not be reviewed here because underlying concepts for their use mostly repeat the principles of filamentous phage use. The filamentous bacteriophages are long, thin viruses, which consist of a single-stranded circular DNA packed in a cylindrical shell composed of the major coat protein p8 (~90% of the virion mass and 98% of the protein mass), and a few copies of the minor coat proteins capping the ends of the phage particle. (Figure 2). In phage display constructs, a foreign coding sequence is spliced in-frame into one of the phage coat protein genes, so that the “guest” peptide encoded by that sequence is fused to the phage coat protein and thereby displayed on the exposed surface of the virion [42]. A phage display library is an ensemble containing up to ~10 billion such phage clones, each harboring a different foreign coding sequence, and therefore displaying a different guest peptide on the virion surface. Phage display libraries are commonly used to identify proteins and their fusion peptides interacting specifically and selectively with various targets, including tumors and their isolated cells [21].
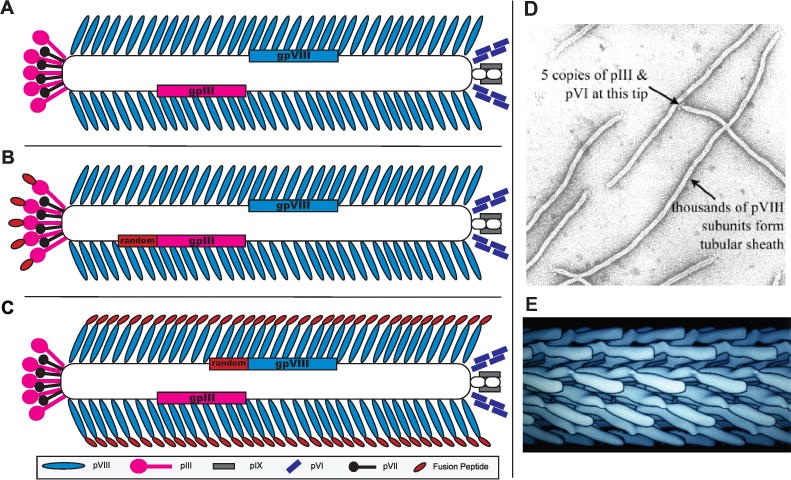
Figure 2. Phage display on filamentous bacteriophages.
Schematic of (A) wild-type fd-tet vectors composed of 4,000 copies of the pVIII major coat protein (blue) encapsulating a single stranded DNA genome at the core of the phage assembly. The ends of the phage particles are closed by minor coat proteins pIII (pink), pVI (black), pIX (gray), and pVII (purple). Schematic of (B) pIII phage display libraries, and (C) pVIII phage display libraries. Each library contains a randomized peptide (red) fused to every copy of either pIII or pVIII proteins, B and C respectively. (D) Transmission election micrograph of negatively stained filamentous bacteriophage fd. (E) Electron density model of the major coat protein pVIII assembly in filamentous bacteriophage fd (D and E) - courtesy of Irina Davidovich, Gregory Kishchenko, and Lee Makowski).
6.1 Two different types of phage display – p3 vs p8
The minor coat protein p3 and the major coat protein p8, commonly used for phage display, are, presented by 5 copies at the phage distal end and up to 2,700 copies all over the virus surface (~4,000 copies in fd-tet type vectors) respectively (Figure 2). P3-displayed libraries are normally used to select individual, high affinity peptides and antibodies in an affinity selection procedure called biopanning [21]. Guest peptides displayed on all five p3 subunits are constrained to lie very close to each other in a ring at one tip of the virion, but their attachment to the virion is probably flexible (Figure 2B). For these reasons, it is likely that such displayed peptides during affinity selection can adapt a configuration allowing multivalent interactions with an immobilized selector or interact monovalently with an isolated receptor (in solution). Multivalent binding leads to an “avidity effect” – a vast increase in overall affinity resulting from the summation of two or more monovalent interactions that are individually weak [43]. The avidity effect is an advantage in some applications, but in others may undermine selection for peptide ligands with highest monovalent affinity for a target receptor. In contrast, the p8-expressing phages allow to select families of peptides with both lower and higher affinities, as their dense constrained arrangement on the virion’s surface results in a stronger binding due to avidity masking, low individual peptide affinity [42]. However, with the goal of using phages as tumor-navigating probes in vivo, p8-fusion phages become invaluable material because they can interact reversibly with cellular receptors using their short low affinity domains accommodated on the fusion peptides [44], rivalling high affinities and binding capacity of p3 expressing phages, which would not support the movement of the phage through different biological barriers, from tumor vasculature to the internal tumor cell compartments, as illustrated in Figure 1. Polyvalent display p8 vectors are also particularly effective for triggering receptor-mediated endocytosis [31]. P8-fusion phages have also clear technological advantages as compared to the p3-fusion phages. In contrast to p3-fusion phage approach, requiring chemical or biochemical synthesis of identified peptides and antibodies, followed by their conjugation with the drugs or their nanocarriers [56], p8-fusion phages can be directly used as a source of the peptide-fused phage proteins in targeted nanomedicine constructions [45]. Selected phages can be easily produced in large scale while their fusion peptides remain restrained to their original conformation through phage selection, propagation, protein isolation and nanomedicine design that is crucial for the peptide function.
6.2 In vivo selection
Given the wide number of platforms being targeted with phage display-derived peptides, there is an increasing need for diverse collections of targeting ligands that possess tumor cell specificity and also capable of intracellular delivery. As the tumor cells were considered the final destination of the targeted drugs and their encapsulated forms, the individual strains of cancer cells have been commonly used for development of tumor-targeted ligands and studying their activity in various drug delivery platforms [44, 46–52]. However, before delivering their load to the tumor cells, the nanomedicines should overcome many obstacles on the way from the vasculature to the tumor cells. Standing at the beginning of this journey, endothelial cells of tumor vasculature have been considered as an attractive target for peptide discovery and targeted drug delivery [25, 26, 53–60] as they are genetically stable, accessible to intravenously administered agents and express distinct patterns of molecular signatures. Discovery of unique, organ vasculature-specific “zip codes” in different tissues opened a new avenue for organ- and tumor-directed drug delivery [53, 63]. During the past 20 years, since the pioneering publication of Pasqualini and Ruoslahti [57], a number of specific receptors expressed on the surfaces of tumor cells, tumor endothelial and perivascular cells, the extracellular matrix and stromal cells have been identified by in vivo selection using combinatorial phage displayed peptide libraries [18, 64]. These tumor-homing peptides have valuable potential as targeting probes for tumor molecular imaging and drug delivery [60]. The most advanced in vivo phage display technology, combining Next Generation Sequencing (NGS) and laser capture microdissection (LMD) techniques allow isolation and identification of phage displayed peptides homing to various tumor blood vessels and their microenvironment [65–68].
6.3 Targeting by peptides revealed through p3 display
Peptide-based ligands are emerging as attractive alternative targeting molecules due to their small size, high stability, and relatively low immunogenicity as compared with many proteins. Like monoclonal antibodies and aptamers, peptides can bind to several molecular targets with a high degree of affinity and specificity. Thousands of biologically active peptides have been selected in vitro and in vivo during the last 20 years using mostly p3-type phage displayed peptide libraries [56, 69]. To reveal their drug-targeting potential, diverse chemical methods of peptide ligand synthesis and conjugation with drugs and their encapsulated forms have been developed [70]. However, while sophisticated peptide and antibody conjugation procedures allow confirmation of the concept of targeted drug delivery and can be used for preparation of targeted nanomedicines in small scale—for their preliminary laboratory and preclinical studies, their use may be technically challenging and less cost effective at the industrial scaling step, when very standardized and pharmaceutically acceptable preparations are required [71]. Thus, despite its promise, targeted nanomedicine technology is not without difficulties. Preparation of the targeting ligands, such as synthetic peptides, aptamers and antibodies, and their conjugation to make usable quantities of the addressed vesicles, has proven troublesome, differing idiosyncratically from one targeted particle to another [73]. These considerations and others have led researchers to cast about intact fusion phage proteins as easily available alternative targeting components of the drug carriers [74].
6.4 Targeting by fusion phage proteins obtained through p8 display
Landscape phage libraries of type f8 designed on the filamentous bacteriophage vector fd-tet [45] consist of a collection of phage with random inserts introduced at the N-terminal end of every copy of the p8 major coat protein of the phage vector and are subsequently expressed in ~4,000 copies across the length of the phage particle [72] (Figure 2C). This layout dramatically alters the physical properties of the phage surface so that each phage particle is structurally unique compared to the remainder of the library and each phage possesses a specific ability to interact with the surrounding environment. Landscape phages are an ideal targeting material compared to traditional antibodies and synthetic peptides as high purity phage proteins can be produced rapidly and inexpensively while retaining shelf-storage stability.
Several nanomedicine platforms derived from the cancer cell-selective phage fusion proteins have been developed for various diagnostic and therapeutic applications, described and many detail in our previous review summarizing and justifying the use of p8 fusion phage proteins for nanomedicine targeting [45] (Figure 3). For selection of p8 fusion phage proteins, various specifically designed selection schemes can be explored, based on the desired outcome, for example, ability to bind cancer cellular receptors, penetrate into the cells, accommodate at specific cellular compartments, and ultimately—produce expected cytotoxic effect of the phage protein-targeted nanomedicines. To prepare a targeted nanomedicines, phages selected from the multibillion landscape phage libraries are converted to the liposome, micelles or RNA/DNA complexes exploring intrinsic amphiphilic and dipolar properties of the phage protein [42]. As a result, the targeting probe—the tumor-specific peptide fused to the major coat protein is exposed on the shell of the drug-loaded vesicle. The major principle of the p8-based approach is that targeted drug-loaded nanoparticles—micelles, liposomes and complexes with nucleic acids recognize the same receptors, cells, tissues and organs that have been used for selection of the precisely targeting landscape phage. In opposite to the sophisticated and poorly controllable conjugation procedures used for coupling of antibodies and synthetic peptides to the nanocarrier [73], the landscape phage-based approach relies on very powerful and precise mechanisms of selection, biosynthesis and self-assembly. The major coat protein constitute 98% of the total protein mass of the virion and can be easily isolated with the purity hardly reachable in normal synthetic and bioengineering procedures. As a normal intestinal inhabitant, wild-type phage itself and its p3-fusion proteins are not toxic and have been already tested for safety in preclinical trials [75, 76].
Figure 3. Nanomedicine platforms derived from phage fusion proteins.
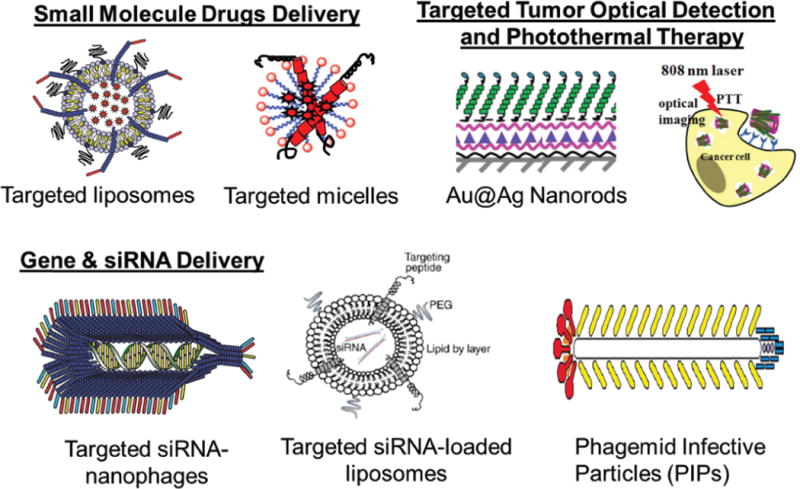
Examples include: targeted liposomes, targeted micelles, Au@Ag Nanorods, targeted siRNA-nanophages, targeted siRNA-loaded liposomes, and phagemid infective particles (PIPs) [45, 50, 52, 74, 103].
Some challenges in using multivalent p8-type landscape libraries, in comparison with p3-type libraries, such as their commercial unavailability, limitations in the length of the inserted peptides and low infectivity (5–10% for fd-tet-derived libraries in comparison with ~50% for M13-derived p3-type), can be accepted during their further study and modification. Regarding potential toxicity of the p8-fusion phage proteins, it should be established for each selected clone and for the whole library in standard animal experiments. The largest concern for using landscape phage in biological experiments is their potential for high immunogenicity, which is routinely exploited for the construction of vaccines. For studies involving multiple injections and subjects with intact immune systems, two strategies have been identified which overcome rapid phage clearance or adverse immune responses: injection of all phage within several days, and/or iterative injection scheme using immunologically different phages that have similar targeting and navigating properties.
7. New trends in phage-driven targeted drug delivery
7.1 Multiple targeting
The use of multiple, unique ligands decorated on the surface of a nanomedicine core was shown to result in synergistic targeting, in which the combination of targeting ligands outperforms the efficiency of the individual targeting ligands alone [77–91]. For example, much interest has also generated recently in using active targeting in combination with cell penetrating peptides (CPP) (reviewed in [77]), which translocate through the cell membranes and facilitate the transport of associated cargo. Similarly, bispecific antibodies, in a number of formats, have been developed to specifically target two different targets with a single targeting ligand [82, 92]. This approach can suggest a new strategy to drug targeting based on navigation of nanomedicines towards the target cancer cells by a variety of ligands binding different components of tumor vasculature and its microenvironment.
Another class of multifunctional antibody-drug conjugates—bispecific T-cell engager (BiTE) antibodies—emerged recently as an attempt to increase the clinical performance of gemtuzumab-ozogamicin and other anti-acute myeloid leukemia (AML) antibody-drug conjugates. The major principle of their action does not relate to the drug navigation problem, discussed in this review, but instead uses T-cell specific anti-CD3 antibody component to harness a polyclonal population of cytotoxic T-cells in the elimination of cancer cells (reviewed in [61]).
7.2 Addressed drug navigation with “promiscuous” phages and their proteins
It was observed in our and other’s experiments, that some nanomedicine-linked phage protein specifically interacting with cancer cells in vitro don’t show significant changes in the effect of their parental nanomedicines in vivo. These results enforced us to modify the traditional concept of drug targeting and suggest a novel paradigm called “addressed drug self-navigation”. In this concept, the targeting phage proteins are selected by their ability to get through the molecular/cellular barriers surrounding tumors, and then penetrate into the tumor mass, thus effecting the diverse tumor cell population. In this way, the migration of the phage particles is thought to mimic the mechanism of leucocytes extravasation from the blood stream through the vasculature to the site of disease. In this concept, fusion phage proteins recognize not only receptors of the target tumor cells, but also specifically interact with the cells of tumor vasculature and tumor microenvironment. It is conceivable that such multi-targeted nanoparticles could be constructed by conjugation with a variety of differently targeted peptides selected in vivo in animal models or in human patients [48, 64, 67, 93–95].
7.3 Molecular selection during phage migration
The rational design of efficient multi-targeted self-navigating nanomedicines is still difficult, because the migration of such multifunctional particle towards the tumor is subject to many hardly controlled and variable conditions [14]. Pursuing the addressed drug delivery concept, we suggested using “molecular self-navigating ligands”, obtained through “micro-biopanning” of landscape phage display libraries as conceptualized in Figure 4. This approach was inspired by the recent discovery of “promiscuous” multi-domain phage proteins targeted to different cellular receptors [44].
Figure 4. Molecular selection of cancer cell-specific phage particles during their migration through complex tumor microenvironments.
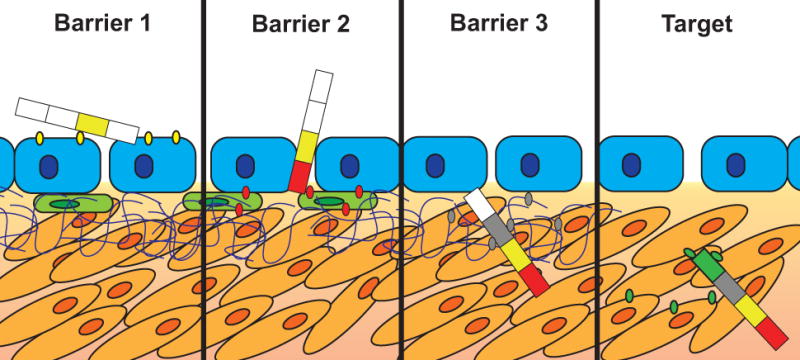
Enrichment of peptide segments within a multi-domain phage-displayed protein leads to the accumulation of discreet functional domains that enhance penetration of phage through different physiological barriers towards the target cancer cells. Here, the first domain (yellow) is selected against tumor-specific antigens expressed on tumor endothelial cells in the blood vessel [53, 58]. The next domains (red and grey) are enriched to allow penetration through additional barriers such as stromal cells and the extracellular matrix, as discussed in Subsection 4. A finally selected domain (green) is responsible for specific interaction with the target cancer cells and penetration into the correct subcellular compartment [5, 48].
In phage display technique, selection of the phage of interest relies on separating an initial population of phages to enrich a specific subpopulation with increased “fitness” according to user-defined criterion. The most common selection pressure imposed on phage displayed peptide populations is affinity for a target receptor [21]. However, it was shown that phages might be selected on the basis of fitness criteria other than affinity for a target receptor. Thus, for example, f8-type landscape phage clones have been selected that are resistant to chloroform and high temperature, or migrate differently in electrical field [72]. Keeping in mind these remarkable properties of multivalent f8-type landscape phages, we envision development of the “self-navigating phages” by their selection during migration from blood stream towards the tumor cells in cancer patients, a process conceptually similar to selection of viruses during their migration from one host to another [62]. To justify this novel navigating paradigm as opposed to the traditional “point-to-point magic bullet” targeting concept, we use the spoke-hub distribution model, in which the traffic moves along spokes to interconnected hubs in a “networked” system. “Promiscuous” phages and their proteins, containing a set of sub-domains are envisioned to be independently responsible for phage extravasation from tumor vasculature (serving as the first temporary phage accumulating hub), and migration through tumor microenvironment components (serving also as consecutive phage-accumulating hubs during phage transportation towards cancer cells) will be selected from multivalent phage display libraries using an advanced “Micro-Biopanning” approach combining, in vivo selection, laser capture microdissection (LMD), Next Generation Sequencing (NGS) and “phage rescue” techniques. Starting from multibillion clone libraries containing multiple mutations in the major coat protein p8, we expect to identify variants collecting in different compartments of tumor microenvironment (serving as hubs), along with “champions” able to rich and penetrate into the diverse target tumor cell population. The advantage of this approach in comparison with rational design of multifunctional compositions is emergence of entirely unexpected motifs as a result of selection from random peptide libraries and testimony to the power of molecular evolution to reveal bioactive structures that could not be discovered by other means. We believe that the replacement of traditional drug targeting concept for the novel drug self-navigation paradigm can result in new horizons for delivery of any drug to any site of the body.
8. Clinical progress in actively targeted nanomedicines
While the potential benefit of targeted nanomedicine is widely proved in animal models, their conversion into clinically accepted preparations has been slower than expected. For 35 years, since the first targeted nanomedicine was designed, a great number of actively targeted nanoparticles have been developed for drug delivery and imaging. However, only eight of these actively targeted nanomedicines have progressed into phase I (six) or II (two) clinical trials [12, 59, 100] and no actively targeted nanomedicines have been approved for clinical use. The most commonly used ligands for actively targeting nanomedicines to tumor cells are transferrin and folate. For example, MBP-426, recently completed a phase-1 clinical trial in patients with advanced or metastatic solid tumors, is a liposome targeted with transferrin. Meantime, a new generation of nanomedicines targeted with products of combinatorial chemistry showed up recently, such as BIND-014 nanodrug candidate, currently in phase-2 clinical trials for NSCLC and prostate cancer. BIND-14 is a polymeric nanoparticle that delivers docetaxel by actively targeting the prostate-specific membrane antigen (PSMA), expressed on prostate-cancer cells and in the vasculature of most solid tumors. Tumor targeting is achieved through an RNA aptamer, which binds specifically to PSMA on the surface of cancer cells.
9. Conclusion
Targeting of nanomedicines was shown to significantly increase their therapeutic index in model animals. However, there are increasing concerns that actively targeted nanomedicines are not showing significant improvements in tumor drug accumulation compared to their passively targeted counterparts as originally hypothesized. Unfolding the complexity of the tumor microenvironment has revealed additional biological barriers hindering efficacy of the targeted drug delivery. As suggested, combination of multiple ligands with appropriate functions to overcome specific barriers will likely prevail as a necessity for success of actively targeted nanomedicines. Subsequently, the underlying biological and/or physical barriers preventing active nanomedicine targeting and appropriate ligands must be identified to overcome these barriers. Multivalent phage display and “migration” selection in animal models can be used to identify phage proteins harboring two or more functional domains with specificity to a variety of tumor-surrounding cells. Identification of “promiscuous” ligands, navigating phage-derived nanomedicines through various biological barriers, and combining multiple motifs, selected in advanced selection procedures, into a single-targeting ligand can be used to overcome the emerging complexity of rationally design multi-targeted nanomedicines.
10. Expert opinion
Based on our analysis, the low efficacy of actively targeted phage-driven nanomedicines in biological experiments would encourage researchers to challenge the traditional concept of drug targeting and follow a novel concept of “addressed nanomedicine self-navigation”, in which affinity selection (biopanning) of targeting peptides is substituted for migration selection that explores the ability of multi-functional “promiscuous” phages and to extravagate from the vasculature, migrate through the molecular/cellular barriers surrounding tumors, penetrate into the tumor mass and affect the diverse tumor cell population. Thus, we can anticipate a paradigm shift from traditional development of molecular targeting systems based on binding activity of conjugated ligands towards the target cell (“magic bullet”) to the development of nanomedicines driven by a combination of binding entities (peptides, proteins, DNA/RNA aptamers, or antibodies) discovered by precise in vivo selection. In this context, isolation from multivalent phage display libraries of f8 type of self-navigating phages containing multi-targeting promiscuous p8 fusion proteins in a self-contained peptide motif can significantly facilitate preparation of self-navigating nanomedicines that would overcome the homing and targeting challenges. Considering the power of molecular selection and incredible simplicity of phage protein preparation and their assembly into the “self-navigating nanomedicines”, we can envision a principal breakthrough into a novel era of selective, safe, efficient and economical anticancer medicines. Furthermore, the novel paradigm of “self-navigating nanomedicines” can be adapted as well for development of a novel generation of medications for precise and personal medicine.
Highlight box.
With development of nanomedicines, significant progress towards targeted cancer chemotherapy have been achieved, mostly through exploring passive targeting based on Enhanced Permeability and Retention (EPR) effect.
Following the traditional “magic bullet” concept, the active targeting of nanomedicines has been proposed to enhance their therapeutic efficacy and decrease side effects in patients.
The low efficacy of numerous nanomedicines in biological experiments could be associated with inability of nanomedicines to penetrate through the complex tumor microenvironment into the core of tumors.
With the goal of using phages as tumor-navigating probes in vivo, p8 expressing phages become invaluable analytical research and construction material.
“Self-navigating phages” can be selected from multivalent phage display libraries during migration from blood stream towards the tumor cells in cancer patients
Advanced bacteriophage-driven self-navigation systems are thought to overcome organismic biological barriers using more precise, localized phage selection methods, multi-targeting “promiscuous” ligands and advanced nanomedicine platforms.
Acknowledgments
The authors would like to acknowledge the Auburn University Research Initiative in Cancer (AURIC) and the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award numbers [1U54CA151881 & R01CA125063] for financial assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Auburn University.
Footnotes
Declaration of interest
The authors state no conflicts of interest and have received no payment in the preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008 Jun;8(6):473–80. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015 Aug 30;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014 Feb;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012 Dec 10;164(2):108–14. doi: 10.1016/j.jconrel.2012.07.010. A critical review that examines the current misunderstandings and the main difficulties in targeted drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Hu L, Siahaan TJ. Intracellular Delivery of Proteins and Peptides. In: Sarisozen C, Torchilin VP, editors. Drug Delivery: Principles and Applications. Second. John Wiley & Sons, Inc; 2016. pp. 576–622. [Google Scholar]
- 6.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012 Oct;11(10):751–61. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 7.Diamantis N, Banerji U. Antibody-drug conjugates-an emerging class of cancer treatment. Br J Cancer. 2016 Feb 16;114(4):362–7. doi: 10.1038/bjc.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Nemudraya AA, Richter VA, Kuligina EV. Phage Peptide Libraries As a Source of Targeted Ligands. Acta Naturae. 2016;8(1):48–57. The recent review that discusses the technology of phage display as a method for obtaining specific targeted peptide agents and offers examples of their use in diagnostic and clinical practice. [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez-Lombardi R, Phan TG, Zimmermann C, Lowe D, Jermutus L, Christ D. Challenges and opportunities for non-antibody scaffold drugs. Drug Discovery Today. 2015;20(10):1271–1283. doi: 10.1016/j.drudis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Vasalou C, Helmlinger G, Gomes BA. Mechanistic Tumor Penetration Model to Guide Antibody Drug Conjugate Design. Plos One. 2015;10 doi: 10.1371/journal.pone.0118977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015 Apr;21(4):223–32. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomedicine. 2016;12(1):81–103. doi: 10.1016/j.nano.2015.08.006. A critical review that discuss in much detail both clinically available anticancer nanomedicines and those en route to future clinical application. [DOI] [PubMed] [Google Scholar]
- 13.Stylianopoulos T, Jain RK. Design considerations for nanotherapeutics in oncology. Nanomedicine. 2015 Nov;11(8):1893–907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliver Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakankar AA, Borchardt RT. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J Pharm Sci. 2006 Nov;95(11):2321–36. doi: 10.1002/jps.20740. [DOI] [PubMed] [Google Scholar]
- 16.Ilinskaya AN, Dobrovolskaia MA. Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol Appl Pharmacol. 2016;299:70–77.17. doi: 10.1016/j.taap.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008 Mar;1778(3):660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao G, Rodriguez BL. Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int J Nanomedicine. 2013;8:61–71. doi: 10.2147/IJN.S37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobbs S, Monsky W, Yuan F, Roberts W, Griffith L, Torchilin V, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapleton S, Allen C, Pintilie M, Jaffray DA. Tumor perfusion imaging predicts the intra-tumoral accumulation of liposomes. J Control Release. 2013 Nov 28;172(1):351–7. doi: 10.1016/j.jconrel.2013.08.296. [DOI] [PubMed] [Google Scholar]
- 21••.Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997 Apr 1;97(2):391–410. doi: 10.1021/cr960065d. A comprehensive review of phage display technique. [DOI] [PubMed] [Google Scholar]
- 22.Cerchia L, de Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010 Oct;28(10):517–25. doi: 10.1016/j.tibtech.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Attarwala H. Role of antibodies in cancer targeting. J Nat Sci Biol Med. 2010 Jul;1(1):53–6. doi: 10.4103/0976-9668.71675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray BP, McGuire MJ, Brown KC. A liposomal drug platform overrides peptide ligand targeting to a cancer biomarker, irrespective of ligand affinity or density. PLoS One. 2013;8(8):e72938. doi: 10.1371/journal.pone.0072938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998 Jan 16;279(5349):377–80. doi: 10.1126/science.279.5349.377. This was the first development of peptide-conjugated chemotherapies targeted to tumor-specific vasculature. [DOI] [PubMed] [Google Scholar]
- 26.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002 Feb;2(2):83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 27.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, et al. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995 Feb 10;80(3):413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 28.Koutsioumpa M, Papadimitriou E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat Anticancer Drug Discov. 2014 May;9(2):137–52. doi: 10.2174/1574892808666131119095953. [DOI] [PubMed] [Google Scholar]
- 29.Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003 Jul;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanenkov VV, Menon AG. Peptide-mediated transcytosis of phage display vectors in MDCK cells. Biochem Biophys Res Commun. 2000 Sep 16;276(1):251–7. doi: 10.1006/bbrc.2000.3358. [DOI] [PubMed] [Google Scholar]
- 32.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–93. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 33.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009 Jul 31;105(3):223–30. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. 2004;12(9–10):635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 35.Taverner A, Dondi R, Almansour K, Laurent F, Owens SE, Eggleston IM, et al. Enhanced paracellular transport of insulin can be achieved via transient induction of myosin light chain phosphorylation. J Control Release. 2015 Jul 28;210:189–97. doi: 10.1016/j.jconrel.2015.05.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher R, Larkin J, Swanton C. Inter and intratumour heterogeneity: a barrier to individualized medical therapy in renal cell carcinoma? Front Oncol. 2012;2:49. doi: 10.3389/fonc.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science. 2010;328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002 Dec 15;62(24):7190–4. [PubMed] [Google Scholar]
- 39.Pollaro L, Heinis C. Strategies to prolong the plasma residence time of peptide drugs. MedChemComm. 2010;1(5):319–24. [Google Scholar]
- 40.Krumpe LR, Mori T. The Use of Phage-Displayed Peptide Libraries to Develop Tumor-Targeting Drugs. Int J Pept Res Ther. 2006 Mar;12(1):79–91. doi: 10.1007/s10989-005-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2016 Apr 1; doi: 10.1016/j.addr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opella SJ. The Roles of Structure, Dynamics and Assembly in the Display of Peptides on Filamentous Bacteriophage. In: Petrenko VA, Smith GP, editors. Phage Nanobiotechnology. RSC Publishing; 2011. pp. 12–32. [Google Scholar]
- 43.Knez K, Noppe W, Geukens N, Janssen KP, Spasic D, Heyligen J, et al. Affinity comparison of p3 and p8 peptide displaying bacteriophages using surface plasmon resonance. Anal Chem. 2013 Nov 5;85(21):10075–82. doi: 10.1021/ac402192k. [DOI] [PubMed] [Google Scholar]
- 44•.Gross AL, Gillespie JW, Petrenko VA. Promiscuous tumor targeting phage proteins. Protein Eng Des Sel. 2016 Mar;29(3):93–103. doi: 10.1093/protein/gzv064. This is the first demonstration of multidomain p8 structure in promiscuous phages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Petrenko VA, Jayanna PK. Phage protein-targeted cancer nanomedicines. FEBS Lett. 2014 Jan 21;588(2):341–9. doi: 10.1016/j.febslet.2013.11.011. The first review summarizing and justifying the use of p8 fusion phage proteins for nanomedicine targeting in the context of conventional drug targeting concept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996 Mar;2(3):299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 47.Wu CH, Liu IJ, Lu RM, Wu HC. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016;23(1):8. doi: 10.1186/s12929-016-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray BP, Brown KC. Combinatorial peptide libraries: mining for cell-binding peptides. Chem Rev. 2014 Jan 22;114(2):1020–81. doi: 10.1021/cr400166n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedi D, Gillespie JW, Petrenko VA. Selection of pancreatic cancer cell-binding landscape phages and their use in development of anticancer nanomedicines. Protein Eng Des Sel. 2014 Jul;27(7):235–43. doi: 10.1093/protein/gzu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagbohun OA, Kazmierczak RA, Petrenko VA, Eisenstark A. Metastatic prostate cancer cell-specific phage-like particles as a targeted gene-delivery system. J Nanobiotechnology. 2013;11:31. doi: 10.1186/1477-3155-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fagbohun OA, Bedi D, Grabchenko NI, Deinnocentes PA, Bird RC, Petrenko VA. Landscape phages and their fusion proteins targeted to breast cancer cells. Protein Eng Des Sel. 2012 Jun;25(6):271–83. doi: 10.1093/protein/gzs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie JW, Gross AL, Puzyrev AT, Bedi D, Petrenko VA. Combinatorial synthesis and screening of cancer cell-specific nanomedicines targeted via phage fusion proteins. Front Microbiol. 2015;6:628. doi: 10.3389/fmicb.2015.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasqualini R, Moeller BJ, Arap W. Leveraging molecular heterogeneity of the vascular endothelium for targeted drug delivery and imaging. Semin Thromb Hemost. 2010 Apr;36(3):343–51. doi: 10.1055/s-0030-1253456. [DOI] [PubMed] [Google Scholar]
- 54.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, et al. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006 Jan 1;66(1):34–40. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 55.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010 Mar 22;188(6):759–68. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown KC. Peptidic tumor targeting agents: the road from phage display peptide selections to clinical applications. Curr Pharm Des. 2010;16(9):1040–54. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996 Mar 28;380(6572):364–6. doi: 10.1038/380364a0. This was the first use of phage display technique for selective targeting of tumor cells in vivo. [DOI] [PubMed] [Google Scholar]
- 58.Ruoslahti E. Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol. 2000 Dec;10(6):435–42. doi: 10.1006/scbi.2000.0334. [DOI] [PubMed] [Google Scholar]
- 59.Shi JJ, Xiao ZY, Kamaly N, Farokhzad OC. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Accounts of Chemical Research. 2011;44(10):1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 60.Li ZJ, Cho CH. Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J Transl Med. 2012 Sep;19:10. doi: 10.1186/1479-5876-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter RB. Biting back: BiTE antibodies as a promising therapy for acute myeloid leukemia. Expert Review of Hematology. 2014;7(3):317–319. doi: 10.1586/17474086.2014.896190. [DOI] [PubMed] [Google Scholar]
- 62.Worobey M, Han GZ, Rambaut A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature. 2014 Apr 10;508:254–257. doi: 10.1038/nature13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012 Jul 24;24(28):3747–56. doi: 10.1002/adma.201200454. This review discusses the developments in the nanoparticle targeting with peptides that home to vascular “zip codes” in target tissues and provide a tissue- and cell-penetrating function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Babickova J, Tothova L, Boor P, Celec P. In vivo phage display–a discovery tool in molecular biomedicine. Biotechnol Adv. 2013 Dec;31(8):1247–59. doi: 10.1016/j.biotechadv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Matochko WL, Chu K, Jin B, Lee SW, Whitesides GM, Derda R. Deep sequencing analysis of phage libraries using Illumina platform. Methods. 2012 Sep;58(1):47–55. doi: 10.1016/j.ymeth.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Rentero Rebollo I, Sabisz M, Baeriswyl V, Heinis C. Identification of target-binding peptide motifs by high-throughput sequencing of phage-selected peptides. Nucleic Acids Res. 2014 Dec 16;42(22):e169. doi: 10.1093/nar/gku940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dias-Neto E, Nunes DN, Giordano RJ, Sun J, Botz GH, Yang K, et al. Next-generation phage display: integrating and comparing available molecular tools to enable cost-effective high-throughput analysis. PLoS One. 2009;4(12):e8338. doi: 10.1371/journal.pone.0008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kubo N, Akita N, Shimizu A, Kitahara H, Parker AL, Miyagawa S. Identification of oligopeptide binding to colon cancer cells separated from patients using laser capture microdissection. J Drug Target. 2008 Jun;16(5):396–404. doi: 10.1080/10611860802088796. [DOI] [PubMed] [Google Scholar]
- 69.He B, Chai G, Duan Y, Yan Z, Qiu L, Zhang H, et al. BDB: biopanning data bank. Nucleic Acids Res. 2016 Jan 4;44(D1):D1127–32. doi: 10.1093/nar/gkv1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015 Mar;14(3):203–19. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 71.Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, Broadt TL, et al. Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol Prog. 2005 Jan-Feb;21(1):205–20. doi: 10.1021/bp049840y. [DOI] [PubMed] [Google Scholar]
- 72•.Petrenko VA, Smith GP, Gong X, Quinn T. A library of organic landscapes on filamentous phage. Protein Eng. 1996;9(9):797–801. doi: 10.1093/protein/9.9.797. This paper describes the first phage display library with multivalently displayed random peptides fused to all copies of the major coat protein pVIII. [DOI] [PubMed] [Google Scholar]
- 73.Avvakumova S, Colombo M, Tortora P, Prosperi D. Biotechnological approaches toward nanoparticle biofunctionalization. Trends Biotechnol. 2014 Jan;32(1):11–20. doi: 10.1016/j.tibtech.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Petrenko VA, Jayanna PK. Phage-mediated Drug Delivery. In: Petrenko VA, Smith GP, editors. Phage Nanobiotechnology. RSC Publishing; 2011. pp. 55–82. [Google Scholar]
- 75.Krag DN, Fuller SP, Oligino L, Pero SC, Weaver DL, Soden AL, et al. Phage-displayed random peptide libraries in mice: toxicity after serial panning. Cancer Chemother Pharmacol. 2002 Oct;50(4):325–32. doi: 10.1007/s00280-002-0489-4. [DOI] [PubMed] [Google Scholar]
- 76•.Krag DN, Shukla GS, Shen G-P, Pero S, Ashikaga T, Fuller S, et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006;66(15):7724–33. doi: 10.1158/0008-5472.CAN-05-4441. This is the first study of safety and specific targeting activity of phage display libraries in human tumor patients. [DOI] [PubMed] [Google Scholar]
- 77.Pattni BS, Chupin VV, Torchilin VP. New Developments in Liposomal Drug Delivery. Chemical Reviews. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 78.Ringhieri P, Diaferia C, Galdiero S, Palumbo R, Morelli G, Accardo A. Liposomal doxorubicin doubly functionalized with CCK8 and R8 peptide sequences for selective intracellular drug delivery. J Pept Sci. 2015 May;21(5):415–25. doi: 10.1002/psc.2759. [DOI] [PubMed] [Google Scholar]
- 79.Sawant RR, Jhaveri AM, Koshkaryev A, Qureshi F, Torchilin VP. The effect of dual ligand-targeted micelles on the delivery and efficacy of poorly soluble drug for cancer therapy. Journal of Drug Targeting. 2013;21(7):630–638. doi: 10.3109/1061186X.2013.789032. [DOI] [PubMed] [Google Scholar]
- 80.Gray BP, Li S, Brown KC. From phage display to nanoparticle delivery: functionalizing liposomes with multivalent peptides improves targeting to a cancer biomarker. Bioconjug Chem. 2013 Jan 16;24(1):85–96. doi: 10.1021/bc300498d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kluza E, Jacobs I, Hectors SJ, Mayo KH, Griffioen AW, Strijkers GJ, et al. Dual-targeting of alphavbeta3 and galectin-1 improves the specificity of paramagnetic/fluorescent liposomes to tumor endothelium in vivo. J Control Release. 2012 Mar 10;158(2):207–14. doi: 10.1016/j.jconrel.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 82.Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012 Mar-Apr;4(2):182–97. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, et al. A novel DDS strategy, “dual-targeting”, and its application for antineovascular therapy. Cancer Lett. 2010 Jan 28;287(2):165–71. doi: 10.1016/j.canlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Takara K, Hatakeyama H, Ohga N, Hida K, Harashima H. Design of a dual-ligand system using a specific ligand and cell penetrating peptide, resulting in a synergistic effect on selectivity and cellular uptake. Int J Pharm. 2010 Aug 30;396(1–2):143–8. doi: 10.1016/j.ijpharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011 Jul 30;153(2):141–8. doi: 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 86.Sugiyama T, Asai T, Nedachi YM, Katanasaka Y, Shimizu K, Maeda N, et al. Enhanced active targeting via cooperative binding of ligands on liposomes to target receptors. PLoS One. 2013;8(6):e67550. doi: 10.1371/journal.pone.0067550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meng S, Su B, Li W, Ding Y, Tang L, Zhou W, et al. Enhanced antitumor effect of novel dual-targeted paclitaxel liposomes. Nanotechnology. 2010 Oct 15;21(41):415103. doi: 10.1088/0957-4484/21/41/415103. [DOI] [PubMed] [Google Scholar]
- 88.Mei L, Fu L, Shi KR, Zhang QY, Liu YY, Tang J, et al. Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. Int J Pharmaceut. 2014 Jul 1;468(1–2):26–38. doi: 10.1016/j.ijpharm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Hayashi Y, Hatakeyama H, Kajimoto K, Hyodo M, Akita H, Harashima H. Multifunctional Envelope-Type Nano Device: Evolution from Nonselective to Active Targeting System. Bioconjugate Chem. 2015 Jul;26(7):1266–76. doi: 10.1021/acs.bioconjchem.5b00184. [DOI] [PubMed] [Google Scholar]
- 90.Liu YY, Ran R, Chen JT, Kuang QF, Tang J, Mei L, et al. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014 Jun;35(17):4835–47. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 91.Pearce TR, Shroff K, Kokkoli E. Peptide Targeted Lipid Nanoparticles for Anticancer Drug Delivery. Advanced Materials. 2012 Jul 24;24(28):3803–22. doi: 10.1002/adma.201200832. [DOI] [PubMed] [Google Scholar]
- 92.Shin TH, Sung ES, Kim YJ, Kim KS, Kim SH, Kim SK, Lee YD, Kim YS. Enhancement of the Tumor Penetration of Monoclonal Antibody by Fusion of a Neuropilin-Targeting Peptide Improves the Antitumor Efficacy. Molecular Cancer Therapeutics. 2014;13:651–661. doi: 10.1158/1535-7163.MCT-13-0748. [DOI] [PubMed] [Google Scholar]
- 93.D’Onofrio N, Caraglia M, Grimaldi A, Marfella R, Servillo L, Paolisso G, et al. Vascular-homing peptides for targeted drug delivery and molecular imaging: meeting the clinical challenges. Biochim Biophys Acta. 2014 Aug;1846(1):1–12. doi: 10.1016/j.bbcan.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Shukla GS, Krag DN, Peletskaya EN, Pero SC, Sun YJ, Carman CL, et al. Intravenous infusion of phage-displayed antibody library in human cancer patients: enrichment and cancer-specificity of tumor-homing phage-antibodies. Cancer Immunol Immunother. 2013 Aug;62(8):1397–410. doi: 10.1007/s00262-013-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanaka T, Ito T, Furuta M, Eguchi C, Toda H, Wakabayashi-Takai E, et al. In situ phage screening. A method for identification of subnanogram tissue components in situ. J Biol Chem. 2002 Aug 16;277(33):30382–7. doi: 10.1074/jbc.M203547200. [DOI] [PubMed] [Google Scholar]
- 96.Lu H, Jin D, Kapila YL. Application of laser capture microdissection to phage display peptide library screening. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Dec;98(6):692–7. doi: 10.1016/j.tripleo.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Ruan W, Sassoon A, An F, Simko JP, Liu B. Identification of clinically significant tumor antigens by selecting phage antibody library on tumor cells in situ using laser capture microdissection. Mol Cell Proteomics. 2006 Dec;5(12):2364–73. doi: 10.1074/mcp.M600246-MCP200. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y, Shukla GS, Weaver D, Pero SC, Krag DN. Phage-display selection on tumor histological specimens with laser capture microdissection. J Immunol Methods. 2009 Aug 15;347(1–2):46–53. doi: 10.1016/j.jim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun Y, Shukla GS, Kennedy GG, Warshaw DM, Weaver DL, Pero SC, et al. Biopanning Phage-Display Libraries on Small Tissue Sections Captured by Laser Capture Microdissection. J Biotech Res. 2009 Jan 1;1:55–63. [PMC free article] [PubMed] [Google Scholar]
- 100.Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomed. 2014;9:467–83. doi: 10.2147/IJN.S36654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bull C, Bennet G, Fletcha A, Harrison H, Baldassare L, Hamilton B. Time-resolved functional perfusion-based analysis of peptide vs. antibody tumor penetration by photoacoustics. Cancer Research. Aug;75(sup 15) 12015. Abstract 3239. [Google Scholar]
- 102.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 103.Wang F, Liu P, Sun L, Li C, Petrenko VA, Liu A. Bio-mimetic nanostructure self-assembled from Au@Ag heterogeneous nanorods and phage fusion proteins for targeted tumor optical detection and photothermal therapy. Sci Rep. 2014 Oct 28;4:6808. doi: 10.1038/srep06808. [DOI] [PMC free article] [PubMed] [Google Scholar]


