Abstract
Meningiomas are primary intracranial tumors that are often asymptomatic. To our knowledge, no study has attempted to describe neurocognitive function in patients with incidentally-discovered meningioma. We utilized the Mayo Clinic Study of Aging (MCSA), which is a population-based sample of Olmsted County, Minnesota residents that includes neuropsychological testing and brain MRI approximately every 15 months. Using a text search of radiologists’ notes of 2,402 MCSA individuals (mean age 77 years, scanned between 2004 and 2014) we identified 48 eligible subjects (2%) who had at least one meningioma. Most meningiomas were small (90% < 3cm). We matched each of the 48 subjects to five non-demented MCSA controls (n = 240) on age, sex, and education. Cognitive domains assessed included memory, attention-executive function, language, and visuospatial. More women (67%) had a meningioma than men (33%). Groups did not differ on prevalence of Mild Cognitive Impairment (Meningioma = 19%, Controls = 13%). Across cognitive domains, we observed similar performance for the two groups (p’s ≥ 0.21). Subtle differences emerged in memory and language domains (p = 0.05 and p = 0.11) when we divided the Meningioma group by tumor location, wherein the small group with an infratentorial tumor performed more poorly than controls globally as well as on select memory and language measures. Our findings suggest that small meningiomas are generally cognitively benign, but that may change as the tumor evolves, and might be impacted by other factors such as meningioma location.
Keywords: Meningioma, Cognition, Brain Tumor
Meningiomas are relatively common, accounting for 36.4% of all primary intracranial and CNS tumors [1]. Known risk factors include age, female gender, prior cranial radiation, and certain mutations in the neurofibromatosis gene (NF2) [2]. There are much weaker associations with other factors including lower physical activity, greater BMI, height, and history of uterine fibroids [3].
A clinical work-up for a patient who presents with symptoms such as headache or seizure, may reveal a meningioma that sometimes require surgical resection and/or radiation. More often, though, meningiomas are considered asymptomatic and may be discovered when imaging is conducted for other reasons (i.e., the meningioma is incidentally identified). In fact, the prevalence of meningioma is approximately 1.6% in non-clinical population-based samples [4, 5].
Studies available on cognitive functioning in meningioma have identified cognitive impairment in subjects who are being followed clinically (e.g., as part of a “wait and scan” approach [6], during pre-surgical evaluation [7, 8], or following surgical intervention [9, 10]). Recently, van Nieuwenhuizen et al. studied a sample of 21 individuals with radiologically confirmed WHO Grade I supratentorial meningiomas who were followed with a “wait and scan” approach. The patients had slower psychomotor speed and poorer working memory when compared to healthy matched controls [6].
These aforementioned studies are informative, but rely on clinical samples which are more likely symptomatic. To date, no information is available about cognitive function in individuals with meningioma in the general population. The goal of the present study was to examine characteristics of individuals found to have a meningioma during the routine MRI for a larger epidemiological cohort, and compare neurocognition in this sample to healthy controls.
METHODS
Participants
All patients were from the Mayo Clinic Study of Aging (MCSA), a population-based longitudinal study of Olmsted County, Minnesota residents [11]. Approximately every 15 months a study coordinator interviewed MCSA participants regarding their family and medical history, a physician performed a mental status and neurological examination, and patients completed neuropsychological testing. Participants without a medical contraindication underwent a 3 Tesla brain MRI at the time of or soon after their visit [11, 12]. A radiologist interpreted all MRI scans and entered the findings in the patient record. We completed a text search of the radiologists’ notes of the 2,402 MCSA individuals scanned between 2004 and 2014 using the search terms: meningioma, neoplasm, brain lesion, tumor, intracranial tumor, intracranial mass, intracranial lesion, cystic mass, cyst, extra axial, and dural based. This search resulted in 1050 hits and 786 potential subjects. We reviewed all 1050 hits and retained those that identified a meningioma. We reviewed scans with a “probable” or “possible” meningioma and a radiation-oncologist (PDB) verified the decision. This process yielded 52 subjects who had at least one meningioma. We excluded four of these subjects from our analysis because they received treatment for meningioma prior to study entry, resulting in a final patient group of 48 subjects (2%). We matched each subject to 5 non-demented MCSA controls (n = 240) on age (within 1 year), sex, education (after grouping by high school only, college, or post-graduate), and number of exposures to the MCSA neuropsychological battery (1, 2, >3). We classified tumor location into one of four categories: convexity, tentorium/falx, skull base, or infratentorial [6].
Standard protocol approvals and consents
The Mayo Clinic Institutional Review Board approved this study, which included review of data collected as part of the Mayo Clinic Study of Aging. All subjects provided written informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Neuropsychological Testing
Patients completed neuropsychological testing conducted by a trained psychometrist prior to the brain MRI. The domains evaluated included (1) attention-executive function (Trail Making Test B [13] and Digit Symbol Substitution Test from WAIS-R [14]); (2) language (Boston Naming Test [15] and Category Fluency [16]); (3) memory [Logical Memory-II (delayed recall) and Visual Reproduction-II (delayed recall) from the WMS-R [17] and Auditory Verbal Learning Test (delayed recall) [18]], and (4) visuospatial (Picture Completion and Block Design from the WAIS-R [14]). We calculated domain z-scores by converting individual test scores to z-scores. The reference population for these z-score calculations consisted of cognitively normal MCSA subjects age 50 and older weighted by age and sex to the 2013 Olmsted County population. We averaged the z-scores for tests within a domain to obtain a domain-level z-score. We then averaged the four domain z-scores to obtain a global z-score. We analyzed both raw scores and domain scores.
Statistical Analysis
We used the neuropsychological assessment closest to when the meningioma was presumed the largest. We used linear regression models to evaluate the mean difference in z-score or individual tests between those with and without meningioma. To account for imperfect matching and possible residual confounding due to age, sex, education, and number of exposures to the MCSA neuropsychological battery, we adjusted for these factors in our regression models. We used transformations to obtain approximate conditional normality for two tests: scores from the Boston Naming Test were modeled as the square root of 60 minus the subject’s total while times from the Trail Making Test B were log-transformed. We used ANCOVA models to evaluate group-wise differences across five groups defined by the no-meningioma group and the meningioma group divided according to meningioma location. These models were adjusted for age, sex, education, and number of neuropsychological test exposures.
RESULTS
Demographics
There were no group differences in number of individuals in each group carrying at least one APOE ε4 allele (32% Controls, 27% Meningioma) or prevalence of MCI (13% Controls, 19% Meningioma; p’s > 0.05, see Table 1). There was a greater prevalence of meningioma in females compared to males (F = 67%, M = 33%). The primary tumor location was in the convexity in 44% (21/48), tentorium/falx in 29% (14/48), skull base in 19% (9/48), and infratentorial for 8% (4/48) of the Meningioma patients. The vast majority of tumors were small (90% < 3cm), with similar frequencies across location. The meningioma was < 3cm in 90% of convexity (18/20), 100% of the tentorium/falx (14/14), 80% of the skull base (8/10), and 75% of the infratentorial (3/4) groups, respectively. Roughly equal proportion of meningiomas were found in the left (21/48, 44%) versus the right (19/48, 40%) hemispheres with a smaller fraction found to be bilateral (8/48, 17%).
Table 1.
Demographics of Meningioma and Control groups
| No meningioma (n=240) |
Meningioma (n=48) |
P | |
|---|---|---|---|
| Sex, n (%) | >0.99 | ||
| Female | 160 (67%) | 32 (67%) | |
| Male | 80 (33%) | 16 (33%) | |
| Age at MRI, years | 0.98 | ||
| Median (IQR) | 80 (73, 84) | 80 (73, 84) | |
| Range | 51 to 94 | 51 to 95 | |
| Education, years | 0.39 | ||
| Median (IQR) | 14 (12, 16) | 15 (12, 16) | |
| Range | 6 to 20 | 8 to 20 | |
| Cycle number, n (%) | 0.62 | ||
| 1 | 42 (18%) | 7 (15%) | |
| 2 | 50 (21%) | 11 (23%) | |
| 3 | 62 (26%) | 9 (19%) | |
| >4 | 86 (36%) | 21 (44%) | |
| APOE ε4, n (%) | 0.47 | ||
| Non-carrier | 161 (68%) | 35 (73%) | |
| Carrier | 77 (32%) | 13 (27%) | |
| Cognitive status, n (%) | 0.29 | ||
| Normal | 209 (87%) | 39 (81%) | |
| MCI | 31 (13%) | 9 (19%) | |
| CDR global score, n (%) | 0.90 | ||
| 0 | 204 (85%) | 41 (85%) | |
| 0.5 | 35 (15%) | 7 (15%) | |
| Short Test of Mental Status | 0.57 | ||
| Median (IQR) | 35 (33, 37) | 35 (32, 36) | |
| Range | 25 to 38 | 23 to 38 |
Neuropsychological Differences
We did not find any association between size and location, nor did we find any association between location and laterality. There were no differences in global z-score or cognitive domain z-scores (p’s > 0.05, see Figure 1). There was, however, a trending difference at the individual test level on Logical Memory performance, in which the Meningioma group had lower scores than the Control group (p = 0.08; see Figure 2). When dividing the Meningioma group into subgroups based on location, additional subtle differences emerged. While the global ANCOVA test was not significant, an exploratory analysis revealed significant differences in the memory domain (p = 0.05; Figure 3), with particular differences observed for the Logical Memory subtest (p = 0.04; Figure 4). Additional exploratory analyses revealed differences in the pair-wise comparisons of global, memory and language domains between those with an infratentorial meningioma compared to controls (p =0.05, p = 0.01 and p = 0.04, respectively; Figure 3). In particular, those with an infratentorial meningioma scored lower on Logical Memory (p = 0.004) and the Auditory Verbal Learning Test (p = 0.08), as well as on the Category Fluency Test (p = 0.06) compared to controls (Figure 4).
Figure 1.
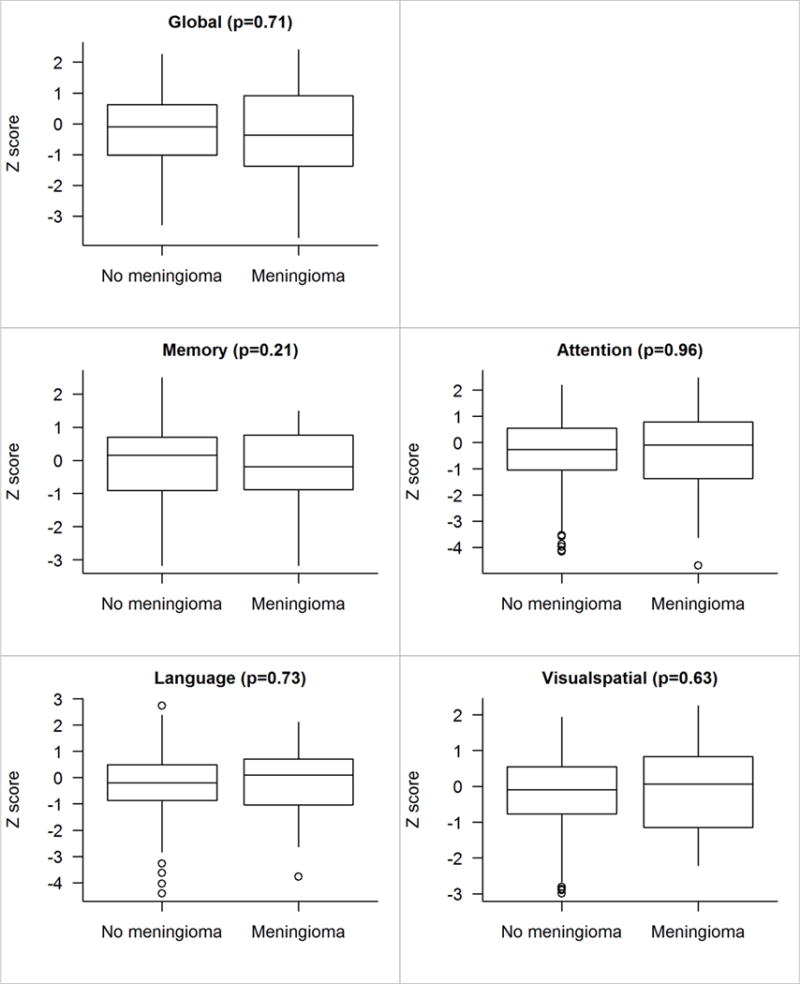
Z score comparisons for those without versus with meningioma findings. The p-value shown is based on a linear regression model including age, sex, education, and cycle number as covariates. Adjusting for matching variables is generally a good idea and sometimes given the name “doubly robust”.
Figure 2.
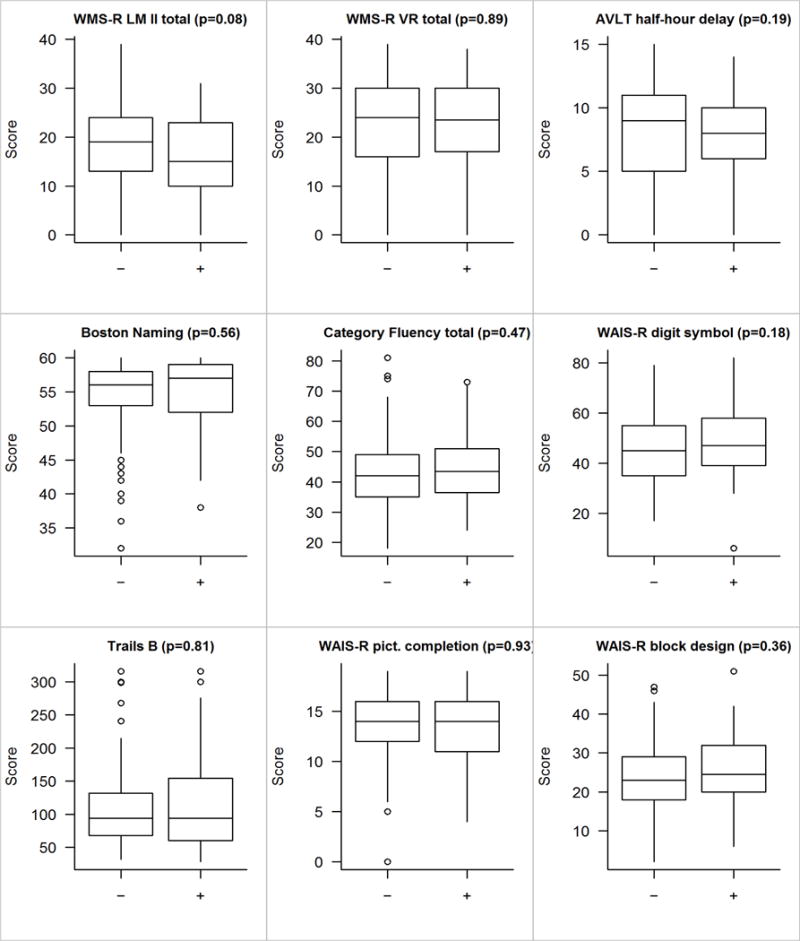
Individual test score comparisons for those without (“−”) versus with (“+”) those meningioma. The p-value shown is based on a linear regression model including age, sex, education, and cycle number as covariates. Adjusting for matching variables is generally a good idea and sometimes given the name “doubly robust”.
Figure 3.
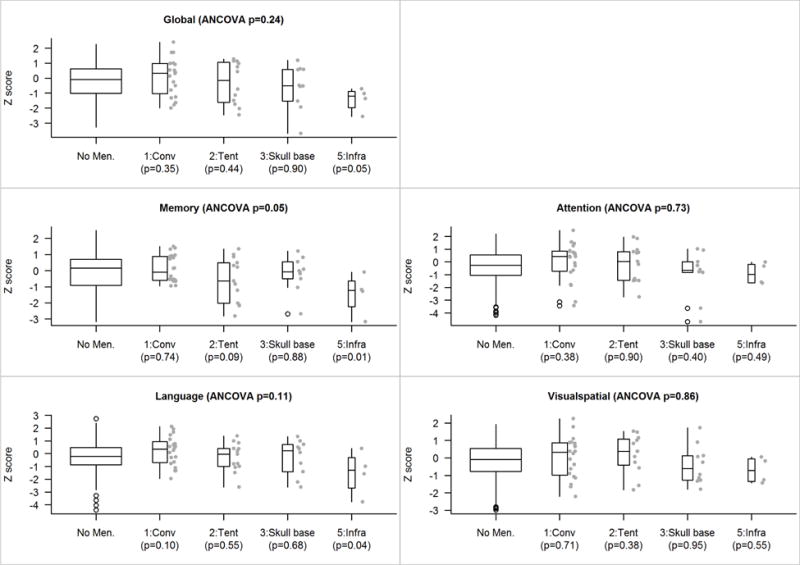
Z score comparisons accounting for first/primary location. The p-value shown at the top of each panel is an based on a four-degree-of-freedom test of the five-level location variable (which includes a level for those with no meningioma) after adjusting for age, sex, education, and cycle number as covariates. Pairwise p-values versus those with no meningioma are shown along the bottom of each panel. Because there are few subjects in each location group we show their individual values beside the box plots.
Figure 4.
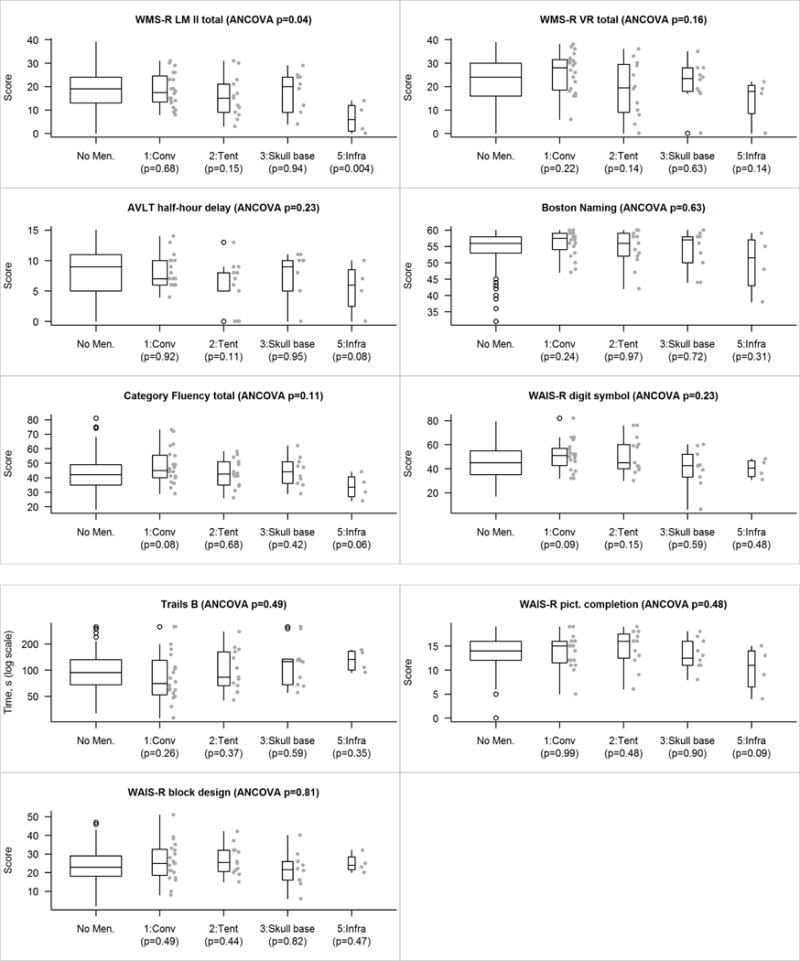
Individual test score comparisons accounting for tumor location. The p-value shown at the top of each panel is an based on a four-degree-of-freedom test of the five-level location variable (which includes a level for those with no meningioma) after adjusting for age, sex, education, and cycle number as covariates. Pairwise p-values versus those with no meningioma are shown along the bottom of each panel. Because there are few subjects in each location group we show their individual values beside the box plots.
DISCUSSION
In this population-based sample, 2% of individuals had at least one meningioma. This is consistent with prevalence rates reported in prior population-based studies [4, 5]. As a group, those with incidentally-identified meningioma did not differ on cognitive tests compared to matched controls. However, subtle cognitive differences emerged when we divided the meningioma group by tumor location. Individuals with infratentorial tumors, in particular, performed worse than controls globally, and on tests of memory and verbal fluency. Taken together, these findings support the conventional thinking that small meningiomas are clinically benign, but that may change as the tumor evolves, and other factors, such as meningioma location, may also have an impact.
To our knowledge, this is the first study to examine neurocognitive function in individuals with incidentally-identified meningioma in a population-based sample. Our findings of generally similar neurocognitive functioning in the meningioma group compared to the control group differs from a recent study finding slower processing speed and poorer working memory in individuals with meningioma followed in a “wait and scan” approach [6]. However, the meningioma group from that study was drawn from patients at a tertiary cancer center, and thus may be much more likely to be symptomatic than a population sample. In that study, there was no association between volume and cognitive functioning [6]. Other studies have reported significant cognitive impairment in meningioma patients either pre- or post- surgery [9, 10], although the average tumor size in both of these studies was much greater than the average size of tumor in our study. Those looking at patients both before and after surgery show mixed findings based on factors such as type of intervention, meningioma size, and patient characteristics [7, 8, 19, 20]. Previous authors have suggested the cognitive impairment could be due to mass effect, edema, and even extra-tumor factors such as seizures and anti-seizure medications. In our study, tumors were small and these factors were rarely present.
While our main finding is that individuals with small incidentally identified meningioma tend to not have significant cognitive deficits, we did detect subtle differences when we examined neurocognitive functioning by location. Specifically, in our study there were no cognitive difference between the groups with a supratentorial meningioma and controls, which differs from the van Nieuwenhuizen et al. (2013) study that only included subjects with supratentorial meningioma and found better memory performance than controls. We did, however, find poorer memory and language performances in a very small group of individuals with infratentorial meningioma compared to controls. Although this finding is interesting and offers an area warranting further investigation as a potential risk factor for cognitive impairment associated with meningioma, we acknowledge the need for replication of this finding ideally with larger groups given that this may represent a Type 1 error.
Anecdotally, small meningiomas are often presumed to be clinically benign; however, no study has systematically studied this in a sample of individuals with an incidentally identified meningioma. As such, this study provides valuable empirical support for the clinician for use in patient education and clinical decision making. In addition to providing empirical evidence that small supratentorial meningiomas are generally neurocognitively benign, this population-based study also adds data to the epidemiological literature by suggesting those with a small meningioma are not at greater risk of developing Mild Cognitive Impairment. Similar to other epidemiologic studies involving meningioma [2], our study shows a greater prevalence of meningioma in females compared to males.
Future work may benefit from re-analysis with larger group numbers given that this was a relatively small sample size due to the low base rate of meningioma (2% in our sample). Comparing the degree of parenchymal tumor involvement may illuminate an association between region of tumor and cognitive domain affected. Finally, comparing neurocognitive functioning in those who later go on to have intervention (e.g., resection, radiotherapy, etc) relative to those who continue with surveillance could be revealing and help identify baseline neurocognitive markers that predict future need for intervention.
Conclusions
To our knowledge, this is the first study to examine neurocognitive functioning in persons with incidentally-identified meningioma in a population-based sample. These findings support the assumption that small meningiomas are cognitively benign, but tumor location may place the individual at higher risk of certain cognitive deficits. These findings may help inform intervention decision-making.
Acknowledgments
This study was supported by a grant from the National Institute of Health (U01 AG006786, AG041851, AG011378), the Mayo Foundation for Medical Education and Research, and the Mayo Clinic Department of Psychiatry and Psychology Small Grants Program.
References
- 1.Ostrom QT, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. Journal of Neuro-Oncology. 2010;99(3):307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DR, et al. Risk factors for meningioma in postmenopausal women: results from the Iowa Women’s Health Study. Neuro-Oncology. 2011;13(9):1011–9. doi: 10.1093/neuonc/nor081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernooij MW, et al. Incidental findings on brain MRI in the general population. The New England journal of medicine. 2007;357(18):1821–8. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 5.Krampla W, et al. Frequency and risk factors for meningioma in clinically healthy 75-year-old patients: results of the Transdanube Ageing Study (VITA) Cancer. 2004;100(6):1208–12. doi: 10.1002/cncr.20088. [DOI] [PubMed] [Google Scholar]
- 6.van Nieuwenhuizen D, et al. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. Journal of Neuro-Oncology. 2013;113(3):433–40. doi: 10.1007/s11060-013-1132-4. [DOI] [PubMed] [Google Scholar]
- 7.Tucha O, et al. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. Journal of neurosurgery. 2003;98(1):21–31. doi: 10.3171/jns.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 8.Tucha O, Smely C, Lange KW. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. British journal of neurosurgery. 2001;15(2):184–8. doi: 10.1080/02688690151127608. [DOI] [PubMed] [Google Scholar]
- 9.van Nieuwenhuizen D, et al. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. Journal of Neuro-Oncology. 2007;84(3):271–8. doi: 10.1007/s11060-007-9366-7. [DOI] [PubMed] [Google Scholar]
- 10.Dijkstra M, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(8):910–5. doi: 10.1136/jnnp.2007.138925. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RO, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills. 1958;8:271–276. [Google Scholar]
- 14.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 15.Kaplan EF, Goodlass H, S W. The Boston Naming Test. 2. Philadelphia: Lea & Febiger; 1982. [Google Scholar]
- 16.Lucas JA, et al. Mayo’s older Americans normative studies: category fluency norms. Journal of clinical and experimental neuropsychology. 1998;20(2):194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Memory Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 18.Rey A. L’examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 19.Steinvorth S, et al. Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: a prospective 1-year follow-up. Radiother Oncol. 2003;69(2):177–82. doi: 10.1016/s0167-8140(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 20.Meskal I, et al. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. Journal of Neuro-Oncology. 2015;121(3):617–625. doi: 10.1007/s11060-014-1679-8. [DOI] [PubMed] [Google Scholar]


