Abstract
A subset of non-small cell lung cancers (NSCLC) are dependent upon oncogenic driver mutations including the most frequently observed driver mutant KRAS which is associated with a poor prognosis. As direct RAS targeting in the clinic has been unsuccessful to date, use of Heat shock protein 90 (Hsp90) inhibitors appeared to be a promising therapy for KRAS mutant NSCLC, however limited clinical efficacy was observed due to rapid resistance. Furthermore, the combination of the Hsp90 inhibitor (Hsp90i), ganetespib and docetaxel was tested in a phase III clinical trial and failed to demonstrate benefit. Here, we investigated the mechanism(s) of resistance to ganetespib and explored why the combination with docetaxel failed in the clinic. We have not only identified a critical role for the bypass of the G2/M cell cycle checkpoint as a mechanism of ganetespib resistance (GR) but have also found that GR leads to cross-resistance to docetaxel. Reactivation of p90RSK and its downstream target, CDC25C was critical for GR and mediated the bypass of a G2/M arrest. Overexpression of either p90RSK or CDC25C lead to bypass of G2/M arrest and induced ganetespib resistance in vitro and in vivo. Moreover, resistance was dependent on p90RSK/CDC25C signaling, as synthetic lethality to ERK1/2, p90RSK or CDC25C inhibitors was observed. Importantly, the combination of ganetespib and p90RSK or CDC25C inhibitors was highly efficacious in parental cells. These studies provide a way forward for Hsp90 inhibitors through the development of novel rationally designed Hsp90 inhibitor combinations that may prevent or overcome resistance to Hsp90i.
Keywords: KRAS, Hsp90 Inhibitor, Ganetespib, p90RSK, CDC25C, drug resistance, BI-D1870, SCH772984, NSC-663284
INTRODUCTION
Despite recent advances in lung cancer treatment, it remains the leading cause of cancer-related mortality worldwide with nearly 1.6 million deaths and the five-year survival rate is still below 20% (1,2). Almost 30% of adenocarcinomas and 4% of squamous cell carcinomas of the lung (3,4), are driven by presence of an activating KRAS mutation. Although KRAS was one of the earliest oncogenic drivers discovered (5), effective KRAS targeted therapies still remain elusive. Furthermore, KRAS mutant lung cancers have worse outcomes in both early stage and advanced metastatic settings (6). Clearly, there is a critical need for novel agents targeting KRAS mutant NSCLC.
Attempts to directly target RAS in the clinic with small molecules have failed to date (7), which has prompted the development of novel approaches attempting to inhibit signaling molecules downstream of KRAS. Of note, KRAS mutant cells show increased dependence on the heat shock protein 90 (Hsp90) (4,8), which is an ATP-dependent molecular chaperone required for the stability of its ‘client’ oncoproteins, many of which are effectors of KRAS, such as members of RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways (4,9). Several 1st and 2nd generation Hsp90 inhibitors (Hsp90i) have demonstrated promising responses, especially in oncogene driven cancers such as HER2+ breast cancer (10). Hsp90is’, including the most potent 2nd generation inhibitor, ganetespib, have shown significant single agent activity in ALK-driven disease, but only transient, unconfirmed responses in patients with KRAS mutant tumors due to the rapid development of resistance (11,12). Therefore, identifying the acquired resistance mechanism(s) to ganetespib in KRAS mutant NSCLC is critical in order to design effective Hsp90i therapeutic combinations for KRAS mutant NSCLC.
Here, we have utilized multiple ganetespib resistant (GR) KRAS mutant NSCLC cell lines and observed that bypass of ganetespib induced G2/M arrest is an indispensable component of acquired ganetespib resistance. Furthermore, ganetespib resistance led to cross resistance to the anti-microtubule agent, docetaxel which was recently tested in combination with ganetespib in a negative phase III lung cancer trial. These results suggest that this bypass of G2/M arrest is mediated by the hyperactivation of p90RSK and its downstream target CDC25C, which is required for G2/M progression (13–15). Not only are p90RSK or CDC25C hyperactivated during ganetespib resistance, these resistant cells become dependent on p90RSK-CDC25C signaling. In addition, the combination of ganetespib with inhibitors of p90RSK or CDC25C had significant activity in the de novo setting as well. In summary, this preclinical data provides both an explanation for why both previous combinations with docetaxel failed and a justification to pursue clinical trials involving rationally designed Hsp90i combinations that may be effective against KRAS mutant NSCLC.
MATERIALS AND METHODS
Cell lines and Reagents
All human KRAS mutant NSCLC cell lines (A549, H460, and H358), and embryonic kidney cell line HEK 293T were obtained in 2013 from the American Type Culture Collection (ATCC) and maintained in ATCC-specified growth medium. Derivation of ganetespib resistant KRAS mutant NSCLC cell lines (A549-GR100, H460-GR10, and H358-GR10) is described elsewhere (16). Cell line authentication was performed by autosomal STR (short tandem repeat) profiling done at University of Arizona Genetics Core (UAGC). Ganetespib was generously gifted by Synta Pharmaceutical Corp. (Lexington, MA). SCH772984 and BI-D1870 were purchased from Selleck Chemicals; docetaxel from Sigma-Aldrich; NSC-663284 and NSC-95397 from Tocris.
Cell proliferation assays
Cell viability following specific drug treatment was assessed by CellTiter96® Aqueous One Solution Cell Proliferation Assay kit (Promega) according to manufacturer’s protocol. Quadruplets were used for each treatment group and data were normalized to percentage of controls. IC50 values were calculated using Prism V5.0 (GraphPad software). Each cell proliferation assay was performed at least three times independently i.e. biological repeat. In each independent (biological) experiment, quadruplets were used for each condition in order to perform statistical analysis. Colony formation assays were performed three times as previously described (17).
Cell-cycle analysis
Cells were seeded in 25-cm2 flasks at 2 × 105 cells/ml followed by harvesting at noted time points. Cells were washed, fixed, stained with propidium iodide, and analyzed by flow cytometry as previously described (18). Approximately 20,000 gated events were collected and the cell cycle distributions were analyzed using BD Accuri™ C6 software (BD Biosciences). Each flow cytometry analysis was performed three times and statistical significance was determined performing unpaired t-test between groups.
Western blot and antibodies
After being treated with specific drugs for defined periods of times, cell collection, protein preparation, concentration measurements, and western blotting were performed as previously described (17). Information on all antibodies used in this report is provided in supplementary table S1.
Lentiviral production and infection
4 × 106 293T cells were seeded in 25-cm2 flasks. Transfection was performed to generate lentiviral particles using a four-plasmid system as per the TRC Library Production and Performance protocols, RNAi Consortium, Broad Institute (19) and as previously described (17). CDC25C shRNA constructs were purchased from the Sigma MISSION® shRNA Library, and their respective sequences are listed in supplementary table S2. pLKO.1-shRNA scramble (SCR) vector was obtained from Dr. David M. Sabatini through Addgene (plasmid 1864) as previously described (20). The pLenti CMV Puro DEST (w118-1) vector was obtained from Eric Campeau through Addgene (plasmid 17452). The Ultimate™ ORFs (Invitrogen) for CDC25C and RSK isoforms was obtained from the Johns Hopkins University HiT Center and an LR reaction (Invitrogen) was performed to construct pLenti Puro DEST (w118-1)-CDC25C. All constructs were sequence verified. The ORF clone IDs of the constructs are – IOH14569 (RSK1.a, variant 1), IOH12130 (RSK1.b, variant 2), IOH63248 (RSK2), IOH3648 (RSK3), IOH36120 (RSK4), and IOH14569 (CDC25C).
In vivo experiments
For H460 xenografts, a total of 5×106 viable cells were suspended in equal volumes of PBS and Matrigel and subcutaneously injected in adult 6 – 8 weeks old Athymic Nude Mice [Crl:NU (NCr)-F] (Charles Rivers). One tumor per mouse was implanted. Once the tumors reached an average size of ≥150 mm3 (range 100–250 mm3), mice were distributed among control and treatment arms and were intravenously (i.v.) dosed with either vehicle control or ganetespib at 50 mg/kg once a week. In addition, we used a KRAS G12C mutant human NSCLC PDX model established from a brain metastasis (BM012–15). 2 mm2 tumor tissues cut with sterile blade were implanted subcutaneously. Once reaching ≥150 mm3, animals were randomized to four arms and i.v. dosed with vehicle control, ganetespib (50 mg/kg) every 8th day, NSC-663284 (3 mg/kg) (21) every 4th day, or combination of ganetespib and NSC-663284. Animals were sacrificed once tumors reached ~ 2000 mm3. In both experiments, tumor sizes [1/2(length × width 2)] were measured by digital caliper thrice a week. Animals were sacrificed when tumors reached ~ 2000 mm3.
Statistical analyses
For all the dose-response MTS assays, we have shown one representative curve which was derived from one experiment. We determined the 50% inhibition concentration (IC50) values for three biological repeats of each experiment using GraphPad Prism V5.0 Software and generated the average IC50 values for each cell lines from each experiment and finally performed unpaired t-test to determine the level of significance (Supplementary table S3). Unpaired t-test was performed to determine the level of significance in all the cell-cycle analyses. Growth patterns in animal studies were summarized graphically by plotting the mean and standard error for each treatment group at each tumor assessment time. Unpaired t test and ANOVA were performed to determine whether the difference between the control and the treatment groups were significant.
RESULTS
Acquired resistance to ganetespib leads to loss of the ganetespib induced G2/M arrest and cross-resistance to docetaxel
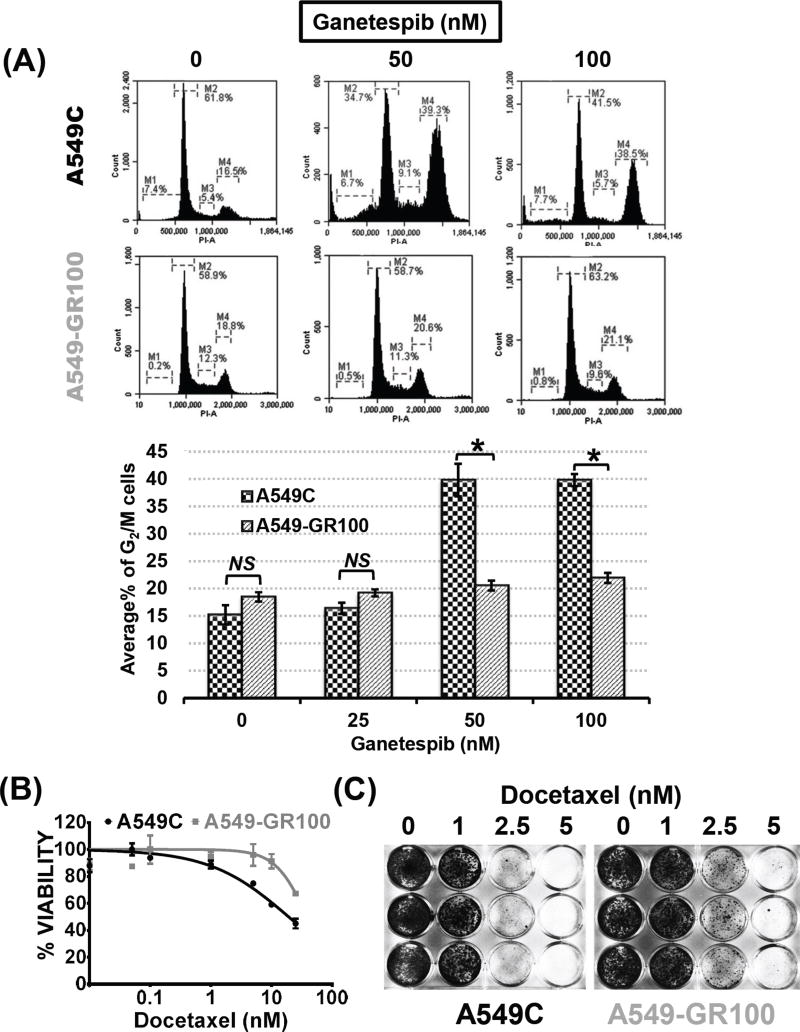
Hsp90 inhibition has been demonstrated to induce a substantial G2/M arrest in a variety of tumor types (22–24). Our group and others have previously shown that ganetespib treatment results in significant growth inhibition in KRAS mutant NSCLC (16,25–27). To determine whether the observed growth inhibition was due to a cell cycle arrest and whether loss of a cell cycle checkpoint was important for ganetespib resistance, we investigated the cell cycle profiles of KRAS mutant NSCLC cells following 48 hours of ganetespib treatment. We utilized our established ganetespib resistant (GR) cells and parental cells (16) to determine whether the G2/M checkpoint was intact in the GR cells. As expected, a significant G2/M arrest was observed in the parental A549 cells with only 16.5% of G2/M phase in DMSO control compared to ganetespib induced accumulation of 39.3% and 38.5% of cells in the G2/M phase at 50 and 100 nM doses, respectively. Surprisingly, ganetespib induced G2/M arrest was significantly diminished in the A549-GR100 cells (Fig. 1A). A similar effect was also observed in a second KRAS mutant cell line H460 with loss of this ganetespib induced arrest in the H460-GR10 cells (Supplementary Fig. S1). Since docetaxel has previously been demonstrated to synergize with ganetespib, induce a G2/M arrest (28) and was tested in combination with ganetespib in a large phase III trial in advanced lung cancer (29), we examined whether the GR cells would be cross resistant to docetaxel (30). Indeed, we observed that the resistant cell line showed cross-resistance to docetaxel with a statistically significant two fold increase in IC50 in short term assays as well as sustained resistance in long term assays (Fig. 1B, C and Supplementary Table S3).
Figure 1. Acquired resistance to ganetespib in KRAS mutant NSCLC leads to loss of ganetespib induced G2/M arrest and docetaxel resistance.
(A) Representative cell cycle analysis at 48 hours after treatment with ganetespib in parental (A549C) and A549-GR100 cells (top). For each group, triplicates were used and average % of G2/M arrested cells was compared between control and GR100 cells in the bar graph (bottom). The two-tailed P values for A549C and A549-GR100 at 50 and 100 nM were < 0.001, and were considered extremely significant (***, p < 0.001) [NS = Not Significant]. (B) Representative MTS assay at 72 hours after treatment with docetaxel in A549C and A549 GR-100. (C) Long term colony formation assay in A549C and A549 GR-100 cells grown in the docetaxel at the indicated doses for 3 days. On day 10, cells were fixed and stained with crystal violet.
p90RSK mediates bypass of a ganetespib induced G2/M arrest in KRAS mutant NSCLC
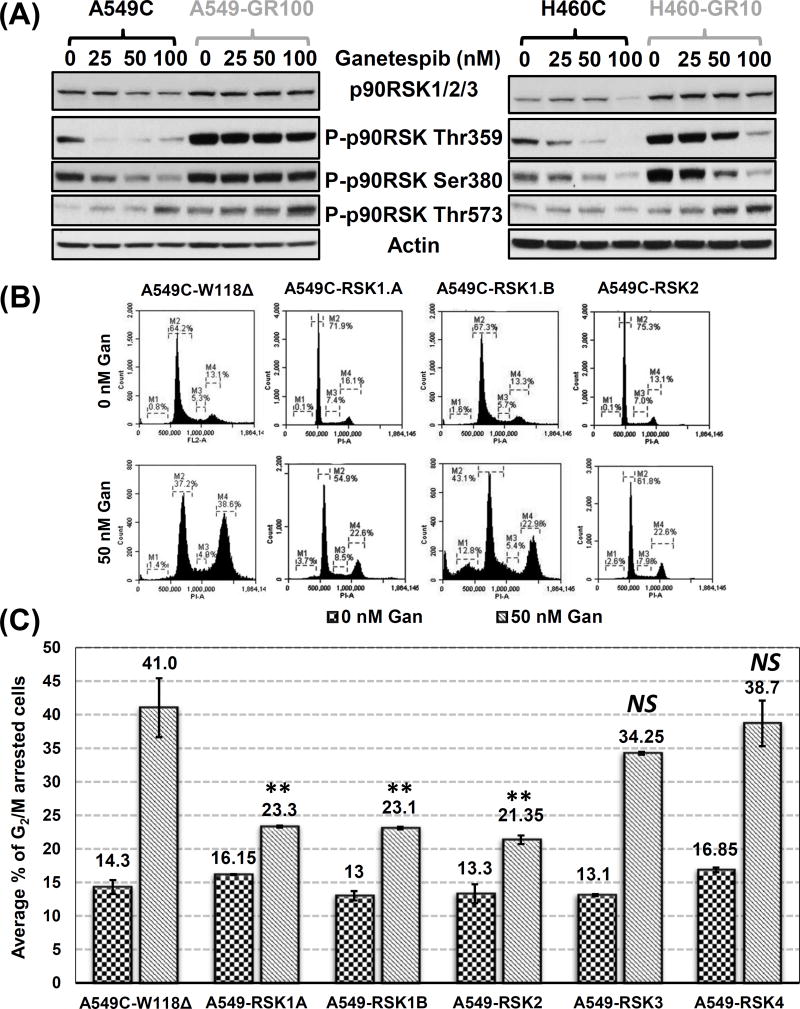
The p90RSK family of kinases has been implicated in promoting nuclear signaling, cell growth and protein synthesis, cell migration and survival, cell proliferation, and more importantly, for the current study, G2/M cell-cycle progression (31,32). We have previously shown that these ERK1/2 targets were hyperactivated in the GR cells and can induce ganetespib resistance in KRAS mutant NSCLC (16) (Fig. 2A). Therefore, given its established role in G2/M cell cycle progression (33), we examined whether p90RSK overexpression was responsible for the observed bypass of G2/M arrest in the GR cells. As the RSK isoform specific functions in lung cancer have not be previously elucidated and our previous studies have suggested that the activity of multiple isoforms is increased in GR cells and required for ganetespib resistance (16), we decided to investigate roles of all isoforms in G2/M regulation. Using these previously established cell lines (16) (Supplementary Fig. S2A), we found that overexpression of p90RSK was sufficient to bypass the ganetespib induced G2/M arrest in an isoform dependent manner (Fig. 2B, 2C and S2B). Compared to 41% of cells arrested at G2/M in control A549-W118Δ cells, A549 cells overexpressing isoforms 1A, 1B, and 2 showed significant reduction in % of G2/M arrested cells, 23.3, 23.1 and 21.35% respectively, in response to 50 nM ganetespib treatment for 48 hours. However, overexpression of isoforms 3 and 4 failed to prevent the ganetespib induced G2/M checkpoint (Fig. 2C and Supplementary S2B). To further support the role of p90RSK in the bypass of G2/M arrest in KRAS mutant NSCLC cells, we asked if treatment with the p90RSK inhibitor, BI-D1870 would lead to G2/M arrest. Notably, BI-D1870 treatment led to a significant G2/M arrest in a dose and time dependent manner (Supplementary Fig. S3A and B).
Figure 2. p90RSK activity is markedly upregulated in GR cells at baseline and after ganetespib treatment and overexpression of p90RSK leads to bypass of the ganetespib induced G2/M arrest.
(A) Western blot analysis of p90RSK activity after ganetespib treatment (48 hours) in A549 and H460, C vs. GR cells. (B) Representative cell cycle analysis at 48 hours after ganetespib (Gan) in parental A549C cells with individual p90RSK isoform overexpression. (C) Bar graph represents a comparative display of % of G2/M cells corresponding to (B). The average % of G2/M cells were compared between control cells expressing empty vector W118Δ and RSK1.A, RSK1.B, RSK2, RSK3 or RSK4 by performing unpaired t-test, and the level of significance were denoted as **, p < 0.01; NS = not significant.
CDC25C, a p90RSK downstream target and an important inducer of G2/M progression, is hyperactivated in GR cells and its overexpression can lead to ganetespib resistance
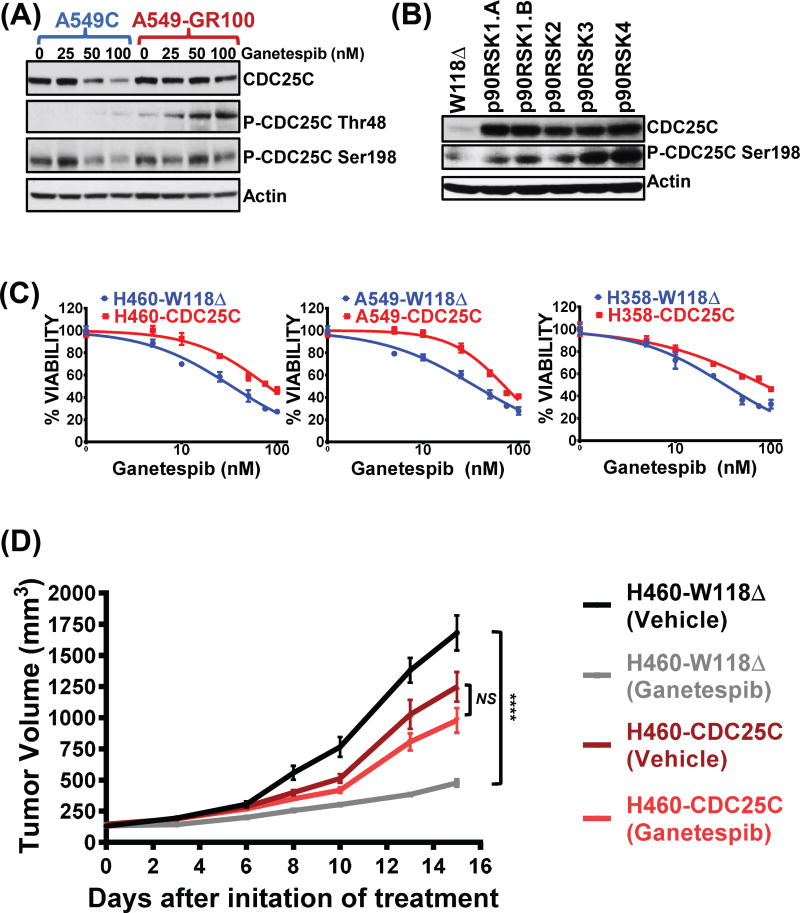
As p90RSK expression mediated bypass of G2/M arrest and led to ganetespib resistance in KRAS mutant NSCLC cells, we wanted to determine the key p90RSK substrate(s) that were responsible for the observed resistance. We therefore performed a systematic protein expression analysis by western blotting of many of the known mediators of G2/M progression in parental and GR cells after ganetespib treatment (Supplementary Fig S4 and Fig 3A). We observed that the expression of the total protein or phosphorylated forms of many of the G2/M regulators such as the mitosis-specific phosphatase CDC20, the dual-specificity phosphatases of the CDC25 family, cyclins B1 and D3, and the cyclin-dependent kinase, cdc2 were upregulated in at least a subset if not all of the GR cells compared to the control parental cells (Supplementary Fig. S4). Since CDC25C is both a known Hsp90 client protein (22,23) and a p90RSK target (33,34), which plays a key role in G2/M progression (35), we examined whether its expression and activity were altered in GR cells. Similar to the observed increase in p90RSK expression in GR cells (Fig 2A), we observed increased expression of the p90RSK phosphorylation target, CDC25C. Most notably, we observed increased expression of the CDC25C phosphorylation at Thr48 and Ser198 residues that correlate with CDC25C activity (14,15,36) (Fig. 3A and Supplementary S5A). Furthermore, p90RSK overexpression was sufficient to induce expression and activation of CDC25C in parental cells (Fig. 3B). Of note, p90RSK induced CDC25C phosphorylation has already been reported to be involved in G2/M checkpoint progression (33,34). These results suggested that CDC25C may be a critical p90RSK target for mediating the bypass of G2/M arrest and ganetespib resistance. To test whether CDC25C was responsible for the observed ganetespib resistance, we overexpressed CDC25C in KRAS mutant NSCLC cell lines and found that CDC25C expression was sufficient to induce ganetespib resistance in vitro as IC50 values of these CDC25C overexpressing A549, H460 and H358 cells increased notably to 74.5, 86, and 79.7 compared to 38.7, 34.1, and 36.3 respectively in their controls expressing empty vector, W118Δ (Fig. 3C; Supplementary table S3). We next examined whether CDC25C overexpression was also sufficient to induce ganetespib resistance in vivo. Although 50 mg/kg (once a week) dosing of ganetespib was sufficient to reduce the tumor growth significantly in W118Δ control group compared to DMSO vehicle (72 % growth inhibition, p < 0.0001), no significant growth inhibition was induced by ganetespib in CDC25C overexpressing cell lines compared to DMSO vehicle (21 % growth reduction, p = NS, not significant) (Fig. 3D). This suggests that CDC25C expression is sufficient to induce ganetespib resistance in vivo.
Figure 3. CDC25C, a p90RSK downstream target and an important inducer of G2/M progression, is hyperactivated in GR cells and its overexpression can lead to ganetespib resistance.
(A) Western blot analysis of the downstream p90RSK target, CDC25C, after ganetespib treatment (48 hours) in A549 C vs. GR cells. (B) Western blots showing overexpression of p90RSK isoforms leading to hyperactivation of CDC25C in parental A549 cells. (C) CDC25C was overexpressed in parental KRAS mutant NSCLC cell lines and percent viability were assessed by MTS assays after 72 hours treatment with increasing ganetespib doses. Cells expressing empty vector – W118Δ were used as a negative control. Unpaired t-test was performed on the IC50 values from three biological repeats to determine the level of significance and denoted as ***, p < 0.001. See Supplementary Table S3 for further details. (D) Ganetespib induced tumor regression was compared between vehicle and ganetespib treated H460 xenografts arms either overexpressing empty vector W118Δ or the CDC25C. Tumor growth curves represent mean tumor volume (± SEM). N = 9 mice per treatment arm. The two-tailed P value for H460-CDC25C (Vehicle) and H460-CDC25C (Ganetespib) was 0.1042, and considered not significant (NS). The two-tailed P value for H460-W118Δ (Vehicle) and H460-W118Δ (Ganetespib) was < 0.0001, and was considered extremely significant (****, p < 0.0001).
CDC25C overexpression in KRAS mutant NSCLC cell lines leads to bypass of the ganetespib induced G2/M arrest and docetaxel resistance
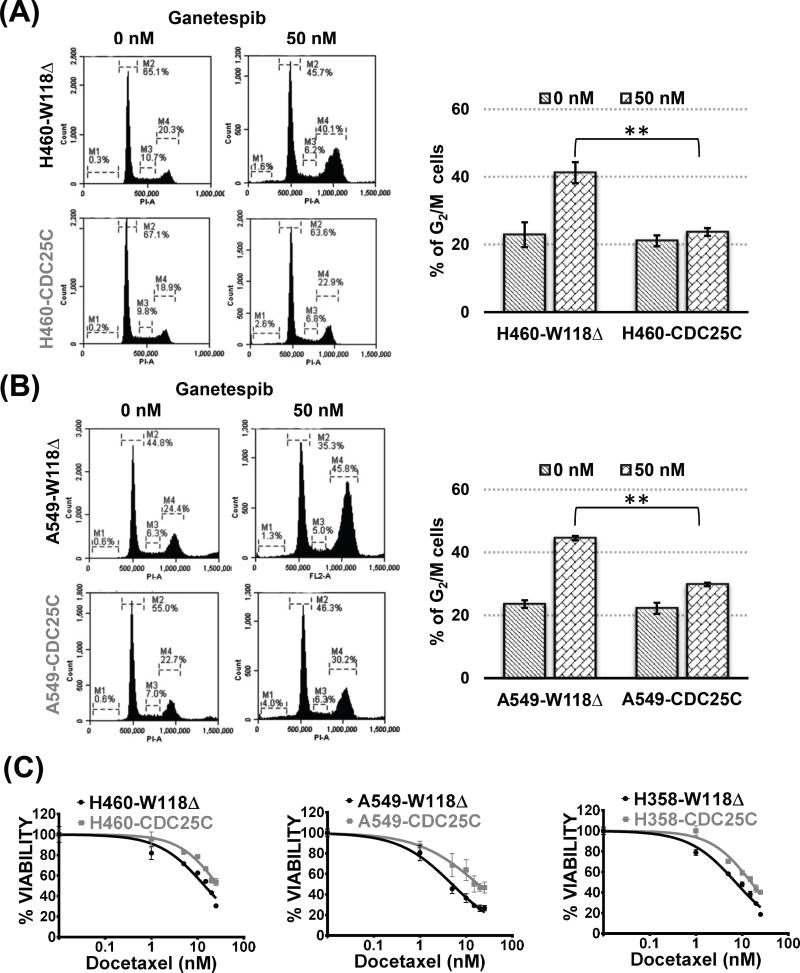
As p90RSK mediated activation of CDC25 phosphatases promotes the G2/M transition leading to entry into M-phase (14,33), we next investigated whether overexpression of CDC25C in parental KRAS mutant NSCLC cells would lead to bypass of the ganetespib induced G2/M arrest, a phenomenon observed in GR cells in response to ganetespib treatment. We observed that CDC25C overexpression was sufficient to phenocopy GR cells as overexpression of CDC25C significantly abrogated the ganetespib induced G2/M arrest (Fig. 4A and B). Given the ability of CDC25C to bypass ganetespib induced G2/M arrest, we next asked whether it could induce docetaxel resistance as well. Remarkably, CDC25C overexpression in all three KRAS mutant NSCLC cell lines, A549, H460, and H358 showed cross-resistance to docetaxel (Fig. 4C) to a similar extent as observed in GR cells (Fig. 1B and C, and Supplementary table S3).
Figure 4. CDC25C overexpression in KRAS mutant NSCLC cell lines leads to bypass of the ganetespib induced G2/M arrest and docetaxel resistance.
Representative cell cycle analysis at 48 hours after treatment with ganetespib in (A) H460 and (B) A549 cells carrying either empty vector W118Δ as control or the CDC25C. Bar graph represents a comparative display of % of G2/M cells corresponding to the flow cytometry data. CDC25C overexpression significantly decreased the % G2/M in response to 50 nM ganetespib as determined by performing unpaired t-test on the % G2/M to obtain the level of significance and denoted as **, p < 0.001(C) Representative MTS assay at 72 hours after treatment with docetaxel in control vs. CDC25C overexpressing KRAS mutant NSCLC cell lines. Unpaired t-test was performed on the IC50 values obtained from three independent biological repeats to determine the level of significance between control and experimental groups and denoted as **, p < 0.01 and ***, p < 0.001. See Supplementary Table S3 for further details.
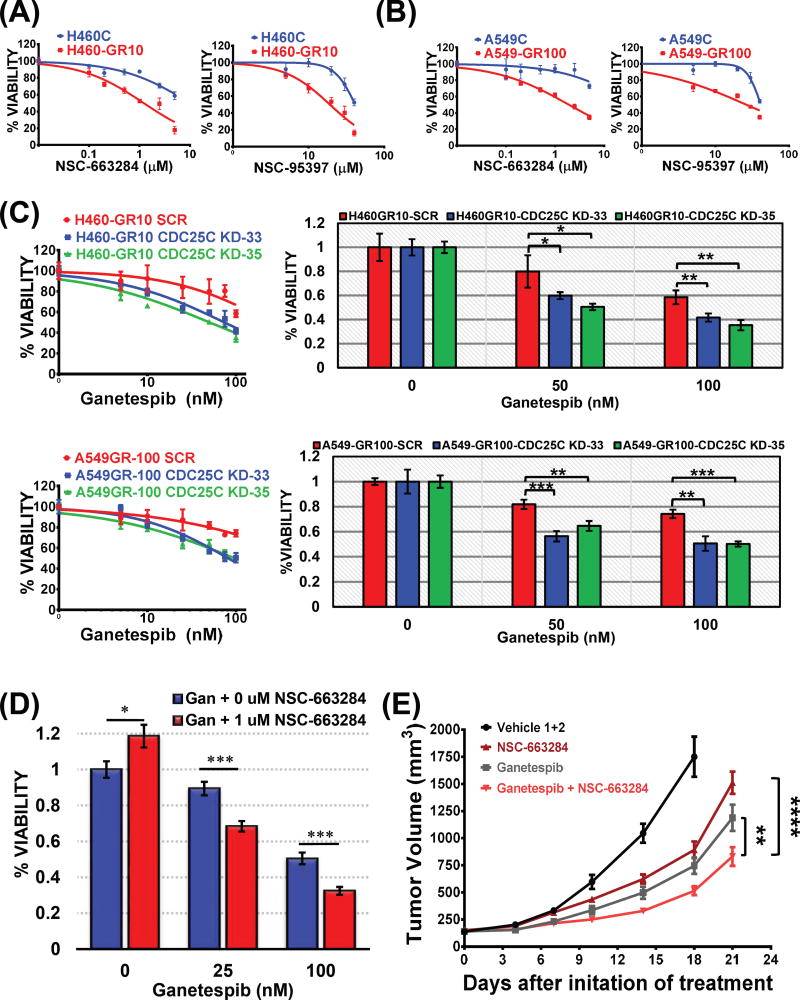
Inhibition of CDC25C, pharmacologically or genetically, induces synthetic lethality in GR cells
The above studies suggested that CDC25C may be required for mediating the observed ganetespib resistance. In order to examine the hypothesis, we first treated GR cells with two well characterized and specific CDC25C inhibitors, NSC-663284 (21,37,38) and NSC-95397 (39,40). Interestingly, both inhibitors demonstrated significant cytotoxicity in KRAS mutant GR NSCLCs compared to control parental cells (Fig. 5A and B, and Supplementary table S3). We observed a striking reduction in IC50 from 9.7 µM in H460 control cells to 1.1 µM in H460-GR10 cells in response to 72 hours of graded NSC-663284 treatment (Fig. 5A, left panel). A similar sensitization to CDC25C inhibition was observed with NSC-95397 in the GR cells compared to parental cells (Fig. 5A, right panel). Likewise, the A549-GR100 cells were significantly more sensitive to these inhibitors compared to the control parental cells (Fig. 5B, and Supplementary table S3). These studies suggest that ganetespib resistance lead to an increased dependence on CDC25C leading to synthetic lethality with CDC25C inhibition. To further validate the requirement of CDC25C for ganetespib resistance and control for off target effects of the inhibitors, we silenced CDC25C in GR cells (Fig. 5C) and examined whether this could resensitize the GR cells to ganetespib. These results show that knockdown of CDC25C was sufficient to induce significant cytotoxicity in GR cells compared to controls (Fig. 5C), suggesting that CDC25C is one of the key factors mediating the acquired resistance to ganetespib. Conversely, when the same shRNA constructs were expressed in parental KRAS mutant NSCLC cells (Supplementary Fig. S6A) which do not overexpress CDC25C, we failed to observe a notable difference in cytotoxicity between the control and the experimental cells (Supplementary Fig. S6C). These experiments suggest that increased CDC25 activity is required for ganetespib resistance and sensitizes to CDC25C inhibition.
Figure 5. GR cells are dependent upon sustained CDC25C signaling as pharmacologic or genetic inhibition of CDC25C induce synthetic lethality.
Percent viability of control and GR (A) H460 and (B) A549 cells after a 72 hours of treatment with the CDC25C inhibitors, NSC-663284 (0 – 10 µM) (left) and NSC-95397 (0 – 40 µM) (right) was determined by MTS assay and level of significance was determined performing unpaired t-test on IC50 values obtained from three independent biological repeats and denoted as **, p < 0.01 and ***, p < 0.001. See Supplementary Table S3 for further details. (C) CDC25C was silenced in KRAS mutant H460 and A549 GR cells by expressing specific shRNA and percent viability was measured by MTS assays after 72 hours treatment with increasing concentrations of ganetespib. A549 or H460 GR cells expressing scramble shRNA (SCR) were used as a negative control. Bar graphs represent a comparative display of the respective dose response curves showing level of significance at 50 nM and 100 nM doses of ganetespib which were determined by performing unpaired t-test between the groups (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (D) The combination of ganetespib and CDC25C inhibitor, NSC-663284 was assessed in A549, where cells were treated with increasing concentrations of ganetespib (0, 25 and 100 nM) in absence or in presence of 1 µM NSC-663284 for 48 hours followed by MTS assay to determine the percent viability of the cells. Bars represent the mean percent cell viability (± SD) relative to the mean of control cells (DMSO). Each cell type in each experiment included at least 4 replicates. Statistical significance by unpaired student t-Test are denoted as *, p < 0.05, and ****, p < 0.0001(E) KRAS mutant NSCLC PDX model. Athymic nude mice bearing the indicated tumors (n = 8 mice per group) were i.v. dosed with either vehicle or ganetespib (50 mg/kg) or NSC-663284 (3 mg/kg) or both. Data are presented as mean ± SD. Ganetespib vs. Ganetespib + NSC-663284, **, p < 0.01; NSC-663284 vs. Ganetespib + NSC-663284, ****, p < 0.0001).
We next asked whether CDC25C overexpression was sufficient to induce dependence on this pathway. Interestingly, both CDC25C inhibitors demonstrated significant cytotoxicity in all three CDC25C overexpressing KRAS mutant NSCLC cell lines compared to controls expressing W118Δ (Supplementary Fig. S7 and Supplementary table S3). Finally, we investigated whether the combination of Hsp90 and CDC25C inhibition in parental cells would lead to a synergistic growth inhibition. Although, we could not detect a synergistic interaction, a significant additive combinatorial activity was observed in Loewe excess matrices for ganetespib with NSC-663284 (Supplementary Fig. S8). To validate the combinatorial activity of ganetespib and NSC-663284, we selected specific dose combinations on the basis of the Loewe excess matrices results to perform MTS assay. We observed a strong additive effect in A549 parental cells for the combination of 1 µM NSC-663284 and 25 or 100 nM ganetespib after 48 hours of treatment as compared to ganetespib or NSC-663284 treatment alone (Fig. 5D; ***, p < 0.001). Finally, we examined this combinatorial cytotoxicity in a KRAS mutant patient derived xenograft (PDX) model. Compared to the individual monotherapy of the two drugs, combination of the drugs induced significant tumor shrinkage (Fig. 5E; Ganetespib vs. Ganetespib + NSC-663284, **, p < 0.01; NSC-663284 vs. Ganetespib + NSC-663284, ****, p < 0.0001).
Acquired resistance to ganetespib mediated by CDC25C hyperactivation is also dependent upon upstream ERK/p90RSK signaling
As we have previously shown that hyperactivation of ERK/p90RSK pathway (Supplementary Fig. S9A and 2A) (16) is an important determinant of acquired resistance to ganetespib in KRAS mutant NSCLC, we wanted to examine whether the ganetespib resistance via CDC25C overexpression was dependent upon upstream ERK1/2 and p90RSK signaling. In order to investigate this hypothesis, we first treated a panel of KRAS mutant NSCLC cells expressing either W118Δ or CDC25C with the potent, ATP competitive and non-competitive ERK1/2 inhibitor, SCH772984 (41,42). All three cell lines expressing CDC25C, when treated with increasing concentration of the drug, compared to cells expressing the control vector, W118Δ, showed significantly greater sensitivity to SCH772984 (Supplementary Fig. S9B, and Supplementary table S3). These results suggest that ganetespib resistance induced by CDC25C overexpression leads to increased dependence on ERK signaling. Of note, we had previously demonstrated that GR cells were more sensitive to this ERK inhibitor (16). Similarly, in order to investigate the dependence of CDC25C induced GR on p90RSK signaling, we treated the same panel of cells with the well characterized and specific p90RSK inhibitor, BI-D1870 (43). While BI-D1870 had little activity as monotherapy in the control cells, it showed significant cytotoxicity in CDC25C overexpressing cells compared to their respective control cells expressing empty vector (W118Δ) (Supplementary Fig. S9C and Supplementary table S3). As the panel of CDC25C overexpressing cells showed synthetic lethality to this drug, we decided to further analyze the efficacy of this drug in combination with ganetespib in the treatment naïve parental cells. Based on the preliminary results of Loewe excess matrices (Supplementary Fig. S10A for H460 and S11A for A549) for ganetespib with BI-D1870, we tested the efficacy of specific dose combinations in H460 and A549 parental cells. These results suggest significant additive effects of 1 µM BI-D1870 in combination with either of 25, 50 or 75 nM ganetespib at 48 hours (*, p < 0.5), compared to ganetespib treatment alone in H460 cells (Fig. S10B). In A549 cells, 2.5 µM BI-D1870 in combination with 25 or 50 nM ganetespib was sufficient to produce significant additive effects after 48 hours of treatment (*, p < 0.5), compared to ganetespib treatment alone (Supplementary Fig. S11B). These results suggest that targeting the ERK/p90RSK/CDC25C pathway in combination with Hsp90i would be an effective therapeutic strategy. As previous studies have suggested that dual pathway inhibition maybe more effective than sequential or parallel pathway inhibition (44–46), we tested if dual pathway inhibition would be more effective in GR cells. We tested the efficacy of specific combinations in the GR cells that inhibited ERK1/2 (SCH772984), p90RSK (BI-D1870) and CDC25C (NSC-663284) (Supplementary Fig. S12 and S13). All combinations tested produced significantly higher growth inhibition compared to individual drug monotherapies at specific dose combinations. Interestingly, the combination of dual ERK and CDC25C inhibition appears to be the most effective (Supplementary Fig. S12C and S13C). In summary, dual pathway inhibition was additive for the combinations examined and may be worth testing in the clinic if response to ganetespib with an ERKi, RSKi or CDC25Ci is not sufficiently efficacious.
DISCUSSION
KRAS is mutated in ~30% of lung adenocarcinomas (47) and ~5% of squamous cell carcinomas. Given its high mutation frequency in NSCLC, KRAS is an appealing target, however; no approved therapies exist for these patients. Development of direct KRAS inhibitors has been limited by the complex KRAS biochemistry and these inhibitors have not been successfully translated to the clinic to date (48,49). Targeted inhibition of Hsp90 is quite effective in preclinical studies involving KRAS mutant NSCLC cell lines and mouse models (9,27,50). However, Hsp90i such as ganetespib, which showed strong preclinical efficacy, both in monotherapy or with chemotherapy (9,27,51), had little activity in patients with KRAS mutant tumors (11,12). Furthermore, the combination of ganetespib and docetaxel, which was recently tested in a large phase III clinical trial (Galaxy-2) in advanced lung cancer, failed to demonstrate either a PFS or OS benefit in either KRAS mutant or KRAS wild type NSCLC patients (29). Unfortunately, this current study suggests that this combination was unlikely to succeed due to the development of cross resistance to docetaxel with the development of ganetespib resistance. In addition, our study suggests that simply demonstrating in vitro or in vivo synergy with a combination is insufficient to predict clinical response. Rather it is critical to understand the mechanisms of resistance to targeted agent a priori and then develop rational combinations which not only demonstrate activity but prevent or overcome acquired resistance.
In this report, the goal was to identify the mechanism(s) of acquired resistance to ganetespib in KRAS mutant NSCLC in order to offer a way forward for ganetespib or other newly emerging Hsp90i’s through the rational design of Hsp90 inhibitor combinations that may prevent and/or overcome resistance to Hsp90i’s. These rationally designed combinations would in turn serve as the scaffold for an effective therapeutic strategy for KRAS mutant NSCLC. We have already established hyperactivation of RAF/MEK/ERK and PI3K/AKT/mTOR signaling as the foundation of the acquired ganetespib resistance mechanism providing us with a number of specific inhibitors that could be combined with ganetespib monotherapy to overcome resistance (16). In this report, we unexpectedly found that bypass of the G2/M checkpoint through the p90RSK-CDC25C pathway is a key determinant of resistance to ganetespib as well as leading to cross resistance to docetaxel. These findings will have substantial impact on future trials with emerging Hsp90 inhibitors such as TAS-116 as the critical role of the G2/M checkpoint in determining response to Hsp90 inhibitors has previously been unrecognized. Furthermore, the key role of CDC25C in mediating bypass of the G2/M checkpoint after Hsp90 inhibition provides a novel target to restore this checkpoint and develop for the clinic. Finally, these results could serve as an explanation for the failure of the GALAXY 2 trial (29,52,53).
Our investigation of the role of p90RSK in G2/M regulation also revealed that a subset of p90RSK isoforms were sufficient enough to bypass of G2/M arrest and induce resistance (Fig. 2B, C and Supplementary Fig. S2B). Although all isoforms appear to be active when overexpressed, clearly there is a more subtle difference in either the activity of each isoform or the substrate specificity between those isoforms which can bypass the G2/M arrest (RSK1A, 1B and 2) versus those isoforms which are incapable of overcoming this arrest. As such, our findings represent one of the few examples in the literature and especially in lung cancer to interrogate the isoform specific functions of the p90RSK family of kinases. Our results demonstrating RSK1/RSK2 overexpression leading to G2/M progression are, indeed, in accordance with the previously published reports (31,33,54). As it is beyond the scope of this report future studies will focus on identifying the critical substrates which determine the ability of an individual p90RSK isoform to bypass arrest. Notably, this may be less of an issue in the clinic as the p90RSK inhibitors developed to date have activity against multiple isoforms and can induced marked G2/M arrest (Supplementary Fig. S3). These findings strongly suggest that the p90RSK inhibitor, BI-D1870 is a potential candidate to combine with ganetespib. In addition, to potentially targeting p90RSK directly or upstream with an ERK inhibitor, it may also be possible to target the critical downstream p90RSK substrate(s) if we could identify them. In the current study, we have identified CDC25C phosphatase as potentially target (33,34).
The dual specificity phosphatases of cell division cycle 25 (CDC25) family including CDC25A, CDC25B and CDC25C are required at various stages of the cell-cycle(35) and have been implicated in the development of several types of human cancers (55,56). In addition, several small molecule CDC25C inhibitors have been developed and could be brought to the clinic. CDC25C appeared to be a promising candidate for the mediator of the observed resistance as it is a known p90RSK substrate, an Hsp90 client and is a potent inducer of G2/M progression. We have established the critical role of CDC25C in acquired ganetespib resistance both in vitro and in vivo (Fig. 3, Fig. 4, Supplementary Fig. S5 and S6). We have also shown that it is not only CDC25C overexpression, but the p90RSK-CDC25C signaling arc that is responsible for this ganetespib resistance to develop and the mechanism involved was the bypass of G2/M arrest induced by ganetespib (Fig. 4). These results identified CDC25C as a potential therapeutic target to overcome the ganetespib resistance, as both genetic silencing or pharmacological inhibition of CDC25C was able to restore sensitivity to ganetespib (Fig. 4, and Supplementary Fig. S6).
We have previously described that ERK1/2 specific inhibitor - SCH772984 (41,42) could be an effective candidate to combine with ganetespib (16). In the current study, we examined whether the combination of ganetespib and the p90RSK specific inhibitor- BI-D1870 (43) and CDC25C inhibitor, NSC-663284 (21,37,38) and found that it was extremely effective in both parental cells and the GR cell lines (Supplementary Fig. S8D, E, and Fig. 5). Furthermore, we also tested the sensitivity of CDC25C overexpressing cell lines to either SCH772984 or BI-D1870. Surprisingly, both inhibitors were able to induce synthetic lethality in those cells (Supplementary Fig. S9B, and C). These results suggest that not only NSC-663284, but SCH772984 or BI-D1870 could also be combined with ganetespib to block the G2/M progression with maximum efficacy leading to overcome ganetespib resistance. Interestingly, we attempted to generate GR cells resistant to NSC-663284, SCH772984 or BI-D1870, but failed as the GR cells only survived 11–14 days when they were cultured in respective inhibitors at their IC5-IC10 doses. Our conclusion from these experiments is that the GR cells remain sensitive to these above mentioned inhibitors and that development of resistance to the combination is not readily achievable. This further supports that these combinations may be effective in the clinic. The common theme from these studies is that the bypass of the G2/M checkpoint is critical to ganetespib resistance and that an effective combination will target this vulnerability.
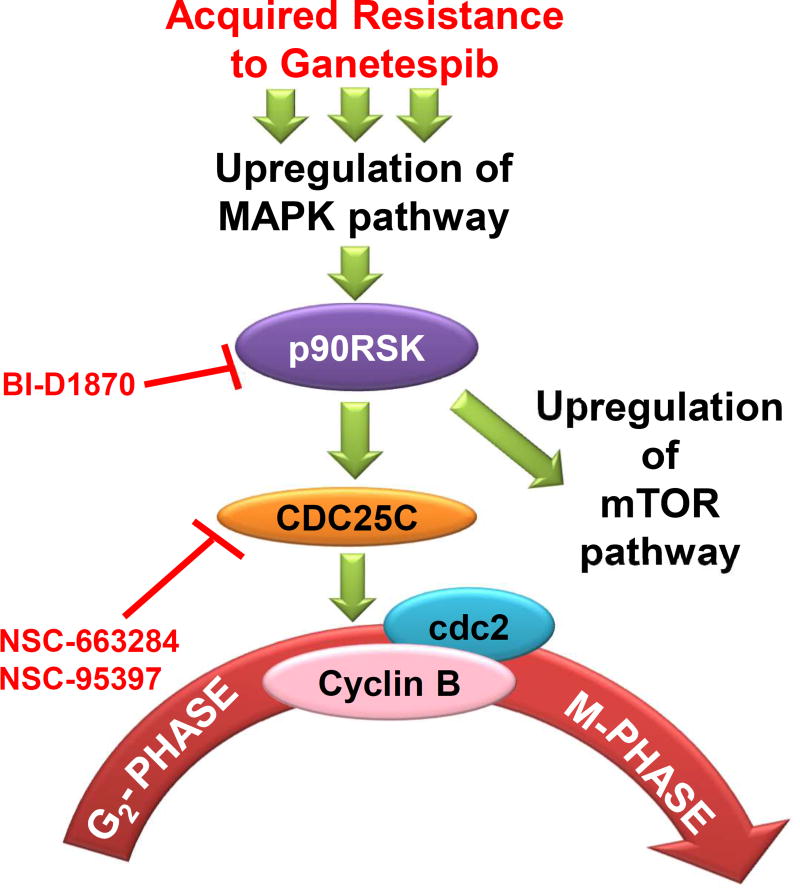
Based on these results, we propose the following model for ganetespib resistance as depicted in Fig. 6. Activation of p90RSK by the MEK/ERK and PDK signaling pathways lead to upregulation and activation of its downstream target CDC25C leading to bypass of the G2/M arrest and ganetespib resistance. This hyperactivation of CDC25C then drives the GR cells to bypass the G2/M arrest via regulation of Cyclin B/cdc2 signaling (Supplementary Fig. S4), a well-established mechanism that is involved in G2/M progression (14,22,33,57). These preclinical studies strongly advocate that the combination of ganetespib with an ERK1/2 inhibitor, a p90RSK inhibitor or a CDC25C inhibitor would be an effective strategy to test in the clinic (Fig. 6). Combination of any one of the inhibitors tested in this study with ganetespib or even any other Hsp90i currently under clinical evaluation (e.g., AT13387, NCT01712217 and TAS-116, NCT02965885) may well prevent Hsp90i resistance and/or help overcome the resistance after single agent treatment. Finally, our results further suggest that dual pathway inhibition may be worth testing in the clinic if response to ganetespib with an ERKi, RSKi or CDC25Ci is not efficacious. In the short term, a combination of a Hsp90 inhibitor with an ERK inhibitor would be the most practical to bring to the clinic as 1) ERK1/2 resides at the top of the ERK-RSK-CDC25C signaling cascade currently and therefore targeting ERK1/2 should have effects potentially of larger magnitude as it will not only affect the functions of p90RSK or CDC25C, but others critical for cellular function and 2) Although SCH772984 is not part of any on-going clinical investigation, there are other ERK1/2 inhibitors that are currently being evaluated in the clinic such as ulixertinib (BVD-523) that is currently under clinical investigation involving patients with BRAF and NRAS mutant melanoma. In the long term, the combination of Hsp90 inhibitors and CDC25C inhibitors would more specifically target the bypass of the G2/M checkpoint that is seen with ganetespib resistance. As such, our findings presented here serve as the preclinical rationale for a future Phase I/II trial in KRAS mutant NSCLC testing these therapeutic combinations.
Figure 6. Acquired resistance to ganetespib in KRAS mutant NSCLC is mediated through bypass of ganetespib induced G2/M arrest in response to hyperactivation of p90RSK-CDC25C signaling pathway.
In KRAS mutant NSCLC, acquired resistance to ganetespib is initiated by hyperactivation of ERK, which in turn augments the expression of master switch, p90RSK. p90RSK hyperactivation then leads to hyperactivation of the mTOR pathways as well as induces G2/M progression via overexpressing and activation of CDC25C and subsequently its targets – Cyclin B1 and cdc2. Targeted inhibition of p90RSK or CDC25C induced synthetic lethality in GR cells. Based on these results, this model illustration proposes that combination of HSP90i, ganetespib with inhibitors of p90RSK (BI-D1870), or CDC25C (NSC-663284 or NSC-95397) may prevent ganetespib resistance and/or help overcome the resistance after single agent treatment.
Supplementary Material
Acknowledgments
The authors thank Mark Socinski, MD, James G. Herman, MD, Laura Stabile, PhD, Deborah L. Galson, PhD, Frank P. Vendetti, PhD, Juraj Adamik, PhD, Zachary A. Yochum, B.S., at the University of Pittsburgh and David Proia formerly of Synta Pharmaceuticals for the discussion, advice regarding this work and supply of resources when applicable. They also thank Synta Pharmaceuticals Corp. (Lexington, MA) for the constant supply of ganetespib. T.F.B. and S.C. have received research funding for this project from a LUNGevity Foundation Career Development Award and National Cancer Institute SPORE IN LUNG CANCER Career Development award (P50CA090440). This project used the UPCI Animal and Cytometry Facilities that are supported in part by award P30CA047904. We apologize to the authors whose work was not cited due to space limitations.
Abbreviations
- Gan
Ganetespib
- C
control
- GR
ganetespib resistance/resistant
- NSCLC
non-small cell lung cancer
- Hsp90i
Hsp90 inhibitor
- p90RSK
p90 ribosomal S6 kinase
- CDC25C
cell division cycle 25, isoform C
References
- 1.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Translational lung cancer research. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Guin S, Ru Y, Wynes MW, Mishra R, Lu X, Owens C, et al. Contributions of KRAS and RAL in non-small-cell lung cancer growth and progression. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1492–501. doi: 10.1097/JTO.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer metastasis reviews. 2010;29:49–60. doi: 10.1007/s10555-010-9209-4. [DOI] [PubMed] [Google Scholar]
- 5.Santos E, Martin-Zanca D, Reddy EP, Pierotti MA, Della Porta G, Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984;223:661–4. doi: 10.1126/science.6695174. [DOI] [PubMed] [Google Scholar]
- 6.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proceedings of the American Thoracic Society. 2009;6:201–5. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 7.Riely GJ, Johnson ML, Medina C, Rizvi NA, Miller VA, Kris MG, et al. A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1435–7. doi: 10.1097/JTO.0b013e318223c099. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Kikuchi E, Xu C, Ebi H, Ercan D, Cheng KA, et al. Combined EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of lung cancers codriven by mutant EGFR containing T790M and MET. Cancer research. 2012;72:3302–11. doi: 10.1158/0008-5472.CAN-11-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Bhattacharya S, Socinski MA, Burns TF. HSP90 inhibitors in lung cancer: promise still unfulfilled. Clinical advances in hematology & oncology : H&O. 2016;14:346–56. [PubMed] [Google Scholar]
- 10.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3068–77. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner AK, Reikvam H, Lavecchia A, Bruserud O. Therapeutic targeting the cell division cycle 25 (CDC25) phosphatases in human acute myeloid leukemia--the possibility to target several kinases through inhibition of the various CDC25 isoforms. Molecules (Basel, Switzerland) 2014;19:18414–47. doi: 10.3390/molecules191118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdiguero E, Nebreda AR. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell cycle (Georgetown, Tex) 2004;3:733–7. [PubMed] [Google Scholar]
- 15.Wang R, He G, Nelman-Gonzalez M, Ashorn CL, Gallick GE, Stukenberg PT, et al. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell. 2007;128:1119–32. doi: 10.1016/j.cell.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S, Huang EH, Christie I, Kurland BF, Burns TF. Acquired resistance to the Hsp90 inhibitor, ganetespib in KRAS mutant NSCLC is mediated via reactivation of the ERK-p90RSK-mTOR signaling network. Molecular cancer therapeutics. 2017 doi: 10.1158/1535-7163.MCT-16-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns TF, Dobromilskaya I, Murphy SC, Gajula RP, Thiyagarajan S, Chatley SN, et al. Inhibition of TWIST1 leads to activation of oncogene-induced senescence in oncogene-driven non-small cell lung cancer. Molecular cancer research : MCR. 2013;11:329–38. doi: 10.1158/1541-7786.MCR-12-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozoren N, Fisher MJ, Kim K, Liu CX, Genin A, Shifman Y, et al. Homozygous deletion of the death receptor DR4 gene in a nasopharyngeal cancer cell line is associated with TRAIL resistance. International journal of oncology. 2000;16:917–25. doi: 10.3892/ijo.16.5.917. [DOI] [PubMed] [Google Scholar]
- 19.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 21.Guo J, Parise RA, Joseph E, Lan J, Pan SS, Joo B, et al. Pharmacology and antitumor activity of a quinolinedione Cdc25 phosphatase inhibitor DA3003-1 (NSC 663284) Anticancer research. 2007;27:3067–73. [PubMed] [Google Scholar]
- 22.Garcia-Morales P, Carrasco-Garcia E, Ruiz-Rico P, Martinez-Mira R, Menendez-Gutierrez MP, Ferragut JA, et al. Inhibition of Hsp90 function by ansamycins causes downregulation of cdc2 and cdc25c and G(2)/M arrest in glioblastoma cell lines. Oncogene. 2007;26:7185–93. doi: 10.1038/sj.onc.1210534. [DOI] [PubMed] [Google Scholar]
- 23.Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, et al. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. Journal of cancer research and clinical oncology. 2006;132:150–8. doi: 10.1007/s00432-005-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Marmarelis ME, Hodi FS. Activity of the heat shock protein 90 inhibitor ganetespib in melanoma. PloS one. 2013;8:e56134. doi: 10.1371/journal.pone.0056134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acquaviva J, He S, Zhang C, Jimenez JP, Nagai M, Sang J, et al. FGFR3 translocations in bladder cancer: differential sensitivity to HSP90 inhibition based on drug metabolism. Molecular cancer research : MCR. 2014;12:1042–54. doi: 10.1158/1541-7786.MCR-14-0004. [DOI] [PubMed] [Google Scholar]
- 26.Cercek A, Shia J, Gollub M, Chou JF, Capanu M, Raasch P, et al. Ganetespib, a novel Hsp90 inhibitor in patients with KRAS mutated and wild type, refractory metastatic colorectal cancer. Clinical colorectal cancer. 2014;13:207–12. doi: 10.1016/j.clcc.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acquaviva J, Smith DL, Sang J, Friedland JC, He S, Sequeira M, et al. Targeting KRAS-mutant non-small cell lung cancer with the Hsp90 inhibitor ganetespib. Molecular cancer therapeutics. 2012;11:2633–43. doi: 10.1158/1535-7163.MCT-12-0615. [DOI] [PubMed] [Google Scholar]
- 28.Proia DA, Sang J, He S, Smith DL, Sequeira M, Zhang C, et al. Synergistic activity of the Hsp90 inhibitor ganetespib with taxanes in non-small cell lung cancer models. Investigational new drugs. 2012;30:2201–9. doi: 10.1007/s10637-011-9790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramalingam SS, Zaric B, Ceric T, Ciuleanu TE, et al. Galaxy-2 trial ( NCT01798485): A randomized phase 3 study of ganetespib in combination with docetaxel versus docetaxel alone in patients with advanced lung adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:5s. [Google Scholar]
- 30.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer discovery. 2015;5:860–77. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nature reviews Molecular cell biology. 2008;9:747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 32.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. The Biochemical journal. 2012;441:553–69. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 33.Wu CF, Liu S, Lee YC, Wang R, Sun S, Yin F, et al. RSK promotes G2/M transition through activating phosphorylation of Cdc25A and Cdc25B. Oncogene. 2014;33:2385–94. doi: 10.1038/onc.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun J, Chau AS, Maingat FG, Edmonds SD, Ostergaard HL, Shibuya EK. Phosphorylation of Cdc25C by pp90Rsk contributes to a G2 cell cycle arrest in Xenopus cycling egg extracts. Cell cycle (Georgetown, Tex) 2005;4:148–54. doi: 10.4161/cc.4.1.1323. [DOI] [PubMed] [Google Scholar]
- 35.Galaktionov K, Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–94. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 36.Perry JA, Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell division. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu L, Amoscato AA, Bier ME, Lazo JS. Dual G1 and G2 phase inhibition by a novel, selective Cdc25 inhibitor 6-chloro-7-[corrected](2-morpholin-4-ylethylamino)-quinoline-5,8-dione. The Journal of biological chemistry. 2002;277:46877–85. doi: 10.1074/jbc.M207902200. [DOI] [PubMed] [Google Scholar]
- 38.Vogt A, McDonald PR, Tamewitz A, Sikorski RP, Wipf P, Skoko JJ, 3rd, et al. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Molecular cancer therapeutics. 2008;7:330–40. doi: 10.1158/1535-7163.MCT-07-2165. [DOI] [PubMed] [Google Scholar]
- 39.Larsson DE, Wickstrom M, Hassan S, Oberg K, Granberg D. The cytotoxic agents NSC-95397, brefeldin A, bortezomib and sanguinarine induce apoptosis in neuroendocrine tumors in vitro. Anticancer research. 2010;30:149–56. [PubMed] [Google Scholar]
- 40.Yang Y, Yang WS, Yu T, Yi YS, Park JG, Jeong D, et al. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochemical pharmacology. 2014;88:201–15. doi: 10.1016/j.bcp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer discovery. 2013;3:742–50. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 42.Wong DJ, Robert L, Atefi MS, Lassen A, Avarappatt G, Cerniglia M, et al. Antitumor activity of the ERK inhibitor SCH772984 [corrected] against BRAF mutant, NRAS mutant and wild-type melanoma. Molecular cancer. 2014;13:194. doi: 10.1186/1476-4598-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. The Biochemical journal. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. The New England journal of medicine. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 46.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet (London, England) 2015;386:444–51. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 47.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gysin S, Salt M, Young A, McCormick F. Therapeutic Strategies for Targeting Ras Proteins. Genes & Cancer. 2011;2:359–72. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4944–9. doi: 10.1073/pnas.081441398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharya S, Socinski MA, Burns TF. KRAS mutant lung cancer: progress thus far on an elusive therapeutic target. Clinical and Translational Medicine. 2015;4:35. doi: 10.1186/s40169-015-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazenby M, Hills R, Burnett AK, Zabkiewicz J. The HSP90 inhibitor ganetespib: A potential effective agent for Acute Myeloid Leukemia in combination with cytarabine. Leukemia research. 2015;39:617–24. doi: 10.1016/j.leukres.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Synta Announces Termination for Futility of Ganetespib Phase 3 GALAXY-2 Trial in Lung Cancer. Businesswirecom. 2015 [Google Scholar]
- 53.Ramalingam S, Goss G, Rosell R, Schmid-Bindert G, Zaric B, Andric Z, et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1) Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26:1741–8. doi: 10.1093/annonc/mdv220. [DOI] [PubMed] [Google Scholar]
- 54.Casalvieri KA, Matheson CJ, Backos DS, Reigan P. Selective Targeting of RSK Isoforms in Cancer. Trends in Cancer. 3:302–12. doi: 10.1016/j.trecan.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Kristjansdottir K, Rudolph J. Cdc25 phosphatases and cancer. Chemistry & biology. 2004;11:1043–51. doi: 10.1016/j.chembiol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Lavecchia A, Di Giovanni C, Novellino E. Inhibitors of Cdc25 phosphatases as anticancer agents: a patent review. Expert opinion on therapeutic patents. 2010;20:405–25. doi: 10.1517/13543771003623232. [DOI] [PubMed] [Google Scholar]
- 57.Kumagai A, Dunphy WG. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Molecular biology of the cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.