Abstract
Condensed abstract
Women with newly developed depression before the diagnosis of breast cancer had a modestly but significantly increased risk for death from any cause and death from breast cancer if diagnosed at late stage. The results also highlight the importance of prevention efforts to support mental health and prevent initial emergence of depression among older women.
Background
Few previous studies investigating depression before breast cancer diagnosis and breast cancer specific mortality have examined depression measured at more than one time point. This study aims to investigate the effect of depression (combining depressive symptoms alone and with antidepressant use) measured at two time points before breast cancer diagnosis on all-cause mortality and breast cancer-specific mortality among older postmenopausal women.
Methods
A large prospective cohort, the Women's Health Initiative (WHI), was used. The study included 3095 women with incident breast cancer who had measures of depressive symptoms and antidepressant use before diagnosis at baseline and at Year three. Multivariable Cox proportional hazards regression was used to estimate adjusted hazard ratios between depression at baseline, depression at Year three, and combinations of depression at these time points, and all-cause mortality and breast cancer-specific mortality.
Results
Depression at Year three before breast cancer diagnosis was associated with higher all-cause mortality after adjusting for multiple covariates (HR, 1.35; 95% CI, 1.02-1.78). There was no statistically significant association of baseline depression and all-cause mortality or breast cancer-specific mortality, whether or not depression was also present at Year three. In women with late-stage (regional or distant stage) breast cancer newly developed depression at Year three was significantly associated with both all-cause mortality (HR, 2.00; 95% CI, 1.13-3.56) and breast cancer specific mortality (HR, 2.42; 95% CI, 1.24-4.70).
Conclusions
Women with newly developed depression before the diagnosis of breast cancer had a modestly but significantly increased risk for death from any cause and death from breast cancer in late stage.
Keywords: depression, breast cancer, mortality, postmenopausal women, Cox proportional hazard regression model
Background
Breast cancer is one of the most common cancers, representing 14% of new cancer cases in the United States 1. Stage of cancer, comorbid conditions, age, and socioeconomic status are well-known prognostic factors for breast cancer specific mortality 2.
The effect of depression before breast cancer diagnosis on all-cause mortality has been of interest in several studies from different countries 3-5. In a national cohort study in Denmark, Hjerl et al. indicated that depression before breast cancer diagnosis was associated with significantly higher mortality among women with breast cancer (hazard ratio (HR): 1.30; 95% confidence interval (CI):1.13–1.49) 4. Similarly, among women with breast cancer aged 67 to 90 in the USA, a prior diagnosis of depression in the 2 years before diagnosis of breast cancer was associated with significantly higher all-cause mortality (HR: 1.42; 95% CI:1.13–1.79) 5. In a recently published study from the UK, patients with a record of depression before breast cancer diagnosis had a higher mortality than women without depression (HR=1.23, 95% CI : 1.07-1.42) 3.
Previous studies measured depression or depressive symptoms before breast cancer diagnosis only at one time point, that is, at baseline. Other studies followed women with depression after breast cancer diagnosis over time and showed a decreasing strength of association between depression and all-cause mortality with longer follow-up time. For example, in a cohort study in the UK, breast cancer survivors with depressive symptoms had a higher risk of death over a 5-year follow-up 6, but this association became non-significant at the 10-year follow-up 7. The single rating of depressive symptoms at baseline loses predictive validity over time 8. However, no prospective observational study with at least two repeated measurements of depressive symptoms and long follow-up has been carried out to examine the relationship between persistent depression and all-cause death or breast cancer specific death. A study with this design could also determine whether the proximity of depression to breast cancer diagnosis influences breast cancer prognosis.
The Women's Health Initiative (WHI) is a large prospective cohort study with extensive data on co-morbid conditions and risk factors, and long follow-up. Thus, we took advantage of the rich WHI data and utilized the depressive symptom data at baseline and the three-year follow-up point. We assessed the effect of depressive symptoms and antidepressant treatment measured at these two time points on all-cause and breast cancer-specific mortality in women who developed breast cancer after the Year three follow-up.
Methods
Data source
The WHI program was launched in 1993, and postmenopausal women aged 50 to 79 (n=161,808) were recruited from 40 US nationwide clinical centers into either three clinical trials (CT) or an observational study (OS) 9.
Participants
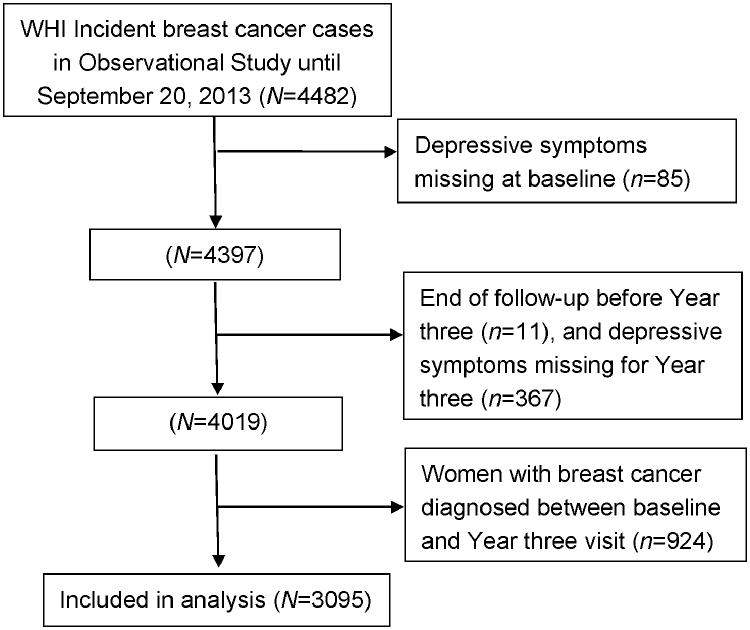
Since diagnosis of breast cancer could affect depressive symptoms, we included only OS women newly diagnosed with invasive breast cancer after the Year three visit (Figure 1). The mean length of time was 8.7 years (range: 3-17.9 years) from baseline to breast cancer diagnosis, 5.7 years (range: 0-14.9 years) from Year three to breast cancer diagnosis and 6.8 years (range: 0-15.2 years) from breast cancer diagnosis to the end of follow-up. Incident invasive breast cancers were initially ascertained based on the participants' self-report and adjudicated by physicians using medical records and pathology reports and then coded according to Surveillance, Epidemiology, and End Results (SEER) Program criteria by the WHI Clinical Coordinating Center 10. Women with a history of cancers other than non-melanoma skin cancers at baseline were excluded. There were 4482 women with incident breast cancer in the OS through September 20, 2013. Women who had missing information on depressive symptoms in either baseline (n=85) or Year three (n=367) or whose follow-up period was less than three years were excluded (n=11). Women with breast cancer diagnosed between baseline and Year three visit were also excluded (n=924). The final sample used for this analysis included 3095 women (Figure 1).
Figure 1. Flow diagram of participants included in the analysis.
Exposures
The main exposures included depressive symptoms and antidepressant use at baseline interview and the third year follow-up.
All women enrolled in the OS were asked to complete a questionnaire on depressive symptoms at baseline and the three-year visit. The depression scores were computed from a short (6-item) form of the Center for Epidemiologic Studies Depression (CES-D) Scale plus 2 questions from the National Institute of Mental Health's Diagnostic Interview Schedule (DIS). This self-report measure departed from traditional depressive symptom screeners as individual items were differentially weighted and included diagnostically-relevant duration of depressed mood 11. The depression score ranged from 0-1. A higher score indicates a greater likelihood of depression. We categorized depressive symptoms as no/yes based on a cutpoint of 0.06 11, which has an established high sensitivity and good positive predictive value for screening depressive disorder.
Use of antidepressants
Treatment for depression was determined from medications brought by the participants in their original pill bottles for all medications to the baseline visit and the Year three follow-up visit. Drug information was entered into a medication database derived from the Master Drug Database (MDDB; Medi-Span, Indianapolis, IN). The National Drug Code (NDC) for Antidepressant Medications Management (AMM) was used to categorize whether the drug was an antidepressant or not 12. In the analysis, trazodone was not considered as an antidepressant due to its common use as a sedative or hypnotic 13. Participants were categorized as antidepressant users and nonusers at either time point.
Definition of depression
Since depressive symptoms and antidepressant use were closely linked, a jointly defined variable was considered in this study, which was referred to as “depression”. The definition of depression in this study combined these two variables, classifying women with either depressive symptoms or antidepressant use as “depressed” and those with neither factor as “non-depressed”.
Combinations of depression at two time points were classified as follows: “no at both time points” if depression was no at baseline and in the third year follow-up, “baseline only” if depression was yes at baseline but no in the third year follow-up, “Year three only” if depression was no at baseline but yes in the third year follow-up, and “yes at both time points” if depression was yes at both time points.
Outcomes
The primary outcomes of interest were all-cause mortality and breast cancer-specific mortality. The WHI data was linked with the National Death Index (NDI) of the National Center for Health Statistics. All causes of death were centrally adjudicated by physicians using available medical records, death certificates, and informant interviews 10. Incident invasive breast cancer was coded for diagnosis date, stage, grade, and estrogen and progesterone receptors, and was ascertained and adjudicated. The end of follow-up time was based on the last period covered by NDI review.
Covariates
Race/ethnicity was categorized as White (not of Hispanic origin) or other. Body mass index (BMI) was calculated as weight (kg)/height (m2). Smoking status included never smokers, former smokers and current smokers (never smokers as the reference). History of postmenopausal hormone therapy (treatment of postmenopausal women with estrogen-alone or estrogen plus progestin) was categorized as ever use or never. A comorbidity index was measured by combining 10 co-occurring self-reported conditions at baseline including glaucoma, high cholesterol requiring pills, asthma, emphysema or chronic bronchitis, stomach or duodenal ulcer, osteoporosis (weak, thin, or brittle bones), cardiovascular disease, arthritis, thyroid gland problem other than thyroid cancer, and hypertension. Comorbidities were categorized as 0 (no comorbidity beyond breast cancer), 1 (one comorbid condition), 2 (two comorbid conditions) and 3 (three or more comorbid conditions). Mammography use during last two years was categorized as yes or no. Breast tumors were characterized according to stage (localized stage as the reference; the regional and distant stages were grouped as “late stage” and the localized stage was “early stage”), grade (low grade as the reference) and estrogen receptor (positive/not positive) and progesterone receptor (positive/not positive) status. There was treatment information only among women aged 65 or above, and surgery was classified as no, mastectomy, or lumpectomy/breast-conserving, chemotherapy were classified as yes or no, and radiation was also classified as yes or no.
Statistical Analysis
Participants' characteristics and tumor information were compared between women with and without depression at baseline and at two time points. Mean ± standard deviation was used to describe continuous characteristics at baseline, while proportion was used for categorical variables. T-test and ANOVA were used for continuous variables, and Chi-square test was used to analyze categorical variables.
All-cause mortality and breast cancer-specific mortality stratified by depression were estimated with the Kaplan-Meier method first. Multivariable Cox proportional hazards regression was used to estimate adjusted hazard ratios for each outcome (all-cause mortality and breast cancer-specific mortality). We analyzed depression at baseline and at the Year three visit, and combinations of depression at the two time points. In the Cox models, potential confounders included: age, race, BMI, smoking, history of postmenopausal hormone therapy, comorbidity at baseline, and tumor stage, tumor grade, and estrogen receptor and progesterone receptor status at date of breast cancer diagnosis.
Survival time was measured as the date from breast cancer diagnosis until death or end of follow-up, whichever came first. For all-cause mortality analyses, persons alive at the end of follow-up were treated as censored observations. For breast cancer-specific mortality, we classified cancer-specific deaths using underlying cause of death, and persons who were alive at the end of follow-up or who died from non-cancer causes were treated as censored observations.
We evaluated the effect modification by stage (localized, and regional and distant stage), grade (low, intermediate, and high grade) and estrogen receptor (positive/not positive) and progesterone receptor (positive/not positive) status.
We performed four sensitivity analyses to assess the robustness of our findings. First, since proximity of depression may be important, we added the duration from Year three to breast cancer diagnosis into analysis models. Second, we replaced the variable combining depressive symptoms and antidepressant use by a variable based on depressive symptom alone in the models. Third, we included only incident breast cancer diagnosed after Year 4 to account for depression related to as-yet undiagnosed breast cancer. Four, we did a sensitivity analysis by further adjusting for treatment information among women with age of 65 or older.
Results
Of the total 3095 women, the prevalence of depression at baseline was 11.5% (n=357) (Table 1). The proportion of women with depressive symptoms alone and antidepressant use at baseline was 9.4% (n=290) and 2.8% (n=87), respectively. The prevalence of depression at Year three was 12.5% (n=386). The proportion of women with depressive symptoms alone and antidepressant use at Year three was 8.8% (n=272) and 4.8% (n=149), respectively. Compared to women without depression at baseline, women with depression were more likely to be younger, have higher BMI, be physically inactive, be current smokers, have a history of postmenopausal hormone therapy, and have more comorbid conditions (all p's<0.05). Alcohol intake, mammography history and tumor characteristics did not differ between women with and without depression at baseline, but alcohol intake and mammography history differ among women with and without depression at two time points.
Table 1. Baseline and tumor characteristics of participants by depression at WHI baseline and Year three (n=3095).
| Variable | Depression at baseline | Depression at baseline and Year three | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Depression | No depression | P value | No at both time points | Baseline only | Year three only | Yes at both time points | P value | |
| Total number of women | 357 | 2738 | 2533 | 176 | 205 | 181 | ||
| Characteristics of participants | ||||||||
| Age at baseline (mean±SD, yrs) | 61.2±6.8 | 63.4±7.0 | <0.001 | 63.5±7.0 | 62.3±6.7 | 61.8±7.0 | 60.2±6.7 | <0.001 |
| White, non-Hispanic-ethnicity (%) | 90.2 | 90.4 | 0.887 | 90.5 | 90.3 | 89.8 | 90.1 | 0.986 |
| Body mass index (mean±SD, kg/m2) | 28.7±6.6 | 27.0±5.4 | <0.001 | 26.9±5.4 | 28.1±6.5 | 28.0±5.9 | 29.3±6.7 | <0.001 |
| Physical inactivity (mean±SD, METs/wk) | 11.9±12.8 | 13.9±13.1 | 0.006 | 14.0±13.1 | 11.5±12.5 | 12.5±12.6 | 12.3±13.2 | 0.002 |
| Smoking status (%) | 0.008 | 0.021 | ||||||
| Never smokers | 41.4 | 48.2 | 48.5 | 41.5 | 43.9 | 41.2 | ||
| Former smokers | 49.9 | 46.3 | 46.2 | 51.1 | 47.8 | 48.6 | ||
| Current smokers | 8.8 | 5.5 | 5.3 | 7.4 | 8.3 | 10.2 | ||
| Alcohol intake (7+ drinks/wk, %) | 41.7 | 45.5 | 0.176 | 46.2 | 38.1 | 36.6 | 45.3 | 0.013 |
| History of postmenopausal hormone therapy (%) | 72.0 | 64.9 | 0.008 | 64.9 | 65.9 | 64.9 | 77.9 | 0.005 |
| Comorbidity index (%) | 0.008 | 0.009 | ||||||
| 0 | 17.1 | 22.0 | 22.1 | 17.1 | 20.5 | 17.1 | ||
| 1 | 26.1 | 30.2 | 30.6 | 30.1 | 25.4 | 22.1 | ||
| 2 | 28.0 | 25.4 | 25.5 | 27.3 | 24.9 | 28.7 | ||
| 3+ | 28.9 | 22.4 | 21.8 | 25.6 | 29.3 | 32.0 | ||
| Mammography during last two years (%) | 88.5 | 91.3 | 0.081 | 91.7 | 89.5 | 86.9 | 87.5 | 0.037 |
| Mammography interval (%) | 0.342 | 0.160 | ||||||
| <1 year | 59.2 | 62.8 | 63.2 | 57.9 | 57.8 | 60.5 | ||
| 1 to <2 years | 27.9 | 27.2 | 27.1 | 31.0 | 27.6 | 24.9 | ||
| 2 to <5 years | 8.3 | 6.8 | 6.6 | 8.2 | 9.1 | 8.5 | ||
| ≥5 years (or never) | 4.6 | 3.3 | 3.1 | 2.9 | 5.5 | 6.2 | ||
| Tumor Characteristics | ||||||||
| Tumor stage (%) | 0.397 | 0.809 | ||||||
| Localized | 74.0 | 77.0 | 77.0 | 76.1 | 76.6 | 71.8 | ||
| Regional | 24.9 | 21.8 | 21.7 | 22.7 | 22.4 | 27.1 | ||
| Distant | 1.1 | 1.2 | 1.3 | 1.1 | 1.0 | 1.1 | ||
| Tumor grade (%) | 0.676 | 0.892 | ||||||
| Low | 30.6 | 29.0 | 28.9 | 31.5 | 30.4 | 29.8 | ||
| Intermediate | 43.5 | 46.1 | 46.2 | 40.6 | 44.5 | 46.4 | ||
| High | 25.8 | 24.9 | 27.9 | 27.9 | 25.1 | 23.8 | ||
| ER and PR status (%) | 0.455 | 0.374 | ||||||
| ER+PR+ | 67.8 | 70.9 | 71.1 | 64.9 | 69.4 | 70.5 | ||
| ER-PR+/ER+PR- | 18.1 | 15.8 | 16.0 | 20.0 | 13.3 | 16.2 | ||
| ER-PR- | 14.2 | 13.3 | 13.0 | 15.2 | 17.4 | 13.3 | ||
WHI: Women's Health Initiative; SD: Standard deviation; ER: estrogen receptor; PR: progesterone receptor
Among women with invasive breast cancer, there was no association of baseline depression and all-cause mortality (Table 2). However, the association of Year three depression and subsequent all-cause mortality remained significant in four models adjusting for different covariates (HR, 1.35-1.49; all p's<0.05). Although point estimates of the HR for breast cancer specific mortality were similar to those for overall mortality, there were few cases and wide confidence intervals, so there was not a significant association of depression at the Year three visit on breast cancer specific mortality in models after adjusting for age or multiple covariates (Table 2).
Table 2. Hazard ratios (HRs) and 95% confidence interval (CIs) for all-cause mortality and breast cancer specific mortality in relation to depression*.
| n | Cases | Model 1a | Model 2b | Model 3c | Model 4d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| All-cause mortality | ||||||||||
| Baseline analysis | ||||||||||
| Depression | ||||||||||
| No | 2738 | 476 | 1 | 1 | 1 | 1 | ||||
| Yes | 357 | 66 | 1.31 | 0.98-1.74 | 1.22 | 0.91-1.62 | 1.13 | 0.84-1.52 | 1.07 | 0.79-1.44 |
| Year three analysis | ||||||||||
| Depression | ||||||||||
| No | 2709 | 469 | 1 | 1 | 1 | 1 | ||||
| Yes | 386 | 73 | 1.49 | 1.13-1.95 | 1.37 | 1.04-1.80 | 1.32 | 1.00-1.75 | 1.35 | 1.02-1.78 |
| Breast cancer mortality | ||||||||||
| Baseline analysis | ||||||||||
| Depression | ||||||||||
| No | 2738 | 165 | 1 | 1 | 1 | 1 | ||||
| Yes | 357 | 22 | 1.03 | 0.62-1.70 | 1.01 | 0.60-1.67 | 0.87 | 0.50-1.50 | 0.76 | 0.44-1.32 |
| Year three analysis | ||||||||||
| Depression | ||||||||||
| No | 2709 | 158 | 1 | 1 | 1 | 1 | ||||
| Yes | 386 | 29 | 1.48 | 0.95-2.30 | 1.45 | 0.93-2.26 | 1.39 | 0.87-2.19 | 1.39 | 0.87-2.20 |
Results were adjusted for age.
Results were adjusted for age, race, body mass index, smoking.
Results were adjusted for age, race, body mass index, smoking, history of postmenopausal hormone therapy, comorbidity, mammography use, tumor stage.
Results were adjusted for age, race, body mass index, smoking, history of postmenopausal hormone therapy, comorbidity, mammography use, tumor stage, tumor grade, and estrogen receptor & progesterone receptor status.
“Depression” includes depressive symptoms or anti-depressant use.
We next examined combinations of baseline and Year three depression (Table 3). Neither newly developed depression (present at Year three but not baseline) nor persistent depression (present at baseline and Year three) were associated with all-cause mortality after adjusting for multiple covariates. The point estimates for breast cancer specific mortality were similar to those for all-cause mortality, but statistically weaker due to larger confidence intervals.
Table 3. Hazard ratios (HRs) and 95% confidence interval (CIs) for mortality in relation to depression and depressive symptoms.
| n | Cases | Model 1a | Model 2b | Model 3c | Model 4d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| All-cause mortality | ||||||||||
| Depressione | ||||||||||
| No at both points | 2533 | 437 | 1 | 1 | 1 | 1 | ||||
| Baseline only | 176 | 32 | 1.20 | 0.82-1.76 | 1.15 | 0.78-1.70 | 1.06 | 0.71-1.60 | 0.94 | 0.62-1.42 |
| Year three only | 205 | 39 | 1.47 | 1.04-2.09 | 1.39 | 0.98-1.96 | 1.37 | 0.96-1.97 | 1.35 | 0.94-1.94 |
| Yes at both points | 181 | 34 | 1.56 | 1.04-2.33 | 1.38 | 0.92-2.07 | 1.28 | 0.85-1.93 | 1.32 | 0.88-1.99 |
| Depressive symptomsf | ||||||||||
| No at both points | 2642 | 456 | 1 | 1 | 1 | 1 | ||||
| Baseline only | 181 | 31 | 1.18 | 0.80-1.74 | 1.13 | 0.77-1.68 | 1.08 | 0.71-1.63 | 0.97 | 0.64-1.47 |
| Year three only | 163 | 30 | 1.44 | 0.97-2.13 | 1.38 | 0.94-2.05 | 1.31 | 0.87-1.97 | 1.33 | 0.88-2.00 |
| Yes at both points | 109 | 25 | 1.73 | 1.07-2.78 | 1.51 | 0.94-2.44 | 1.49 | 0.92-2.42 | 1.61 | 1.00-2.62 |
| Breast cancer mortality | ||||||||||
| Depressione | ||||||||||
| No at both points | 2533 | 150 | 1 | 1 | 1 | 1 | ||||
| Baseline only | 176 | 8 | 0.82 | 0.38-1.75 | 0.82 | 0.38-1.76 | 0.66 | 0.27-1.61 | 0.53 | 0.21-1.30 |
| Year three only | 205 | 15 | 1.53 | 0.88-2.67 | 1.54 | 0.88-2.68 | 1.57 | 0.88-2.81 | 1.58 | 0.88-2.83 |
| Yes at both points | 181 | 14 | 1.37 | 0.71-2.62 | 1.31 | 0.68-2.52 | 1.14 | 0.58-2.21 | 1.08 | 0.55-2.12 |
| Depressive symptomsf | ||||||||||
| No at both points | 2642 | 158 | 1 | 1 | 1 | 1 | ||||
| Baseline only | 181 | 9 | 0.92 | 0.45-1.89 | 0.92 | 0.45-1.89 | 0.77 | 0.34-1.76 | 0.63 | 0.28-1.45 |
| Year three only | 163 | 11 | 1.55 | 0.83-2.86 | 1.57 | 0.84-2.90 | 1.41 | 0.73-2.70 | 1.51 | 0.78-2.91 |
| Yes at both points | 109 | 9 | 1.14 | 0.47-2.80 | 1.04 | 0.42-2.56 | 0.97 | 0.39-2.40 | 1.08 | 0.44-2.69 |
Results were adjusted for age.
Results were adjusted for age, race, body mass index, smoking.
Results were adjusted for age, race, body mass index, smoking, history of postmenopausal hormone therapy, comorbidity, mammography use, tumor stage.
Results were adjusted for age, race, body mass index, smoking, history of postmenopausal hormone therapy, comorbidity, mammography use, tumor stage, tumor grade, and estrogen receptor & progesterone receptor status.
“Depression” includes depressive symptoms or anti-depressant use.
“Depressive symptoms” refers to depressive symptoms only.
Effect modification analysis by tumor characteristics showed that the greatest all-cause mortality risk was for women with late-stage cancer and with newly developed depression (HR, 2.00; 95% CI, 1.13-3.56) (Table 4). A similar effect was found on breast cancer-specific mortality (HR, 2.42; 95% CI, 1.24-4.70).
Table 4. Hazard ratios (HRs) and 95% confidence interval (CIs) for breast cancer outcome in relation to depression and depressive symptoms stratified by tumor stage*.
| Variable | n | All-cause mortality | Breast cancer mortality | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Cases | HR | 95%CI | Cases | HR | 95%CI | ||
| Depressiona | |||||||
| Tumor stage | |||||||
| Localized | |||||||
| No at both points | 1951 | 287 | 1 | 63 | 1 | ||
| Baseline only | 134 | 19 | 0.93 | 0.56-1.55 | 3 | 0.74 | 0.23-2.40 |
| Year three only | 157 | 22 | 1.05 | 0.66-1.69 | 2 | 0.46 | 0.11-1.89 |
| Yes at both points | 130 | 17 | 1.46 | 0.82-2.60 | 3 | 0.90 | 0.21-3.77 |
| Regional or distant | |||||||
| No at both points | 582 | 150 | 1 | 87 | 1 | ||
| Baseline only | 42 | 13 | 1.09 | 0.54-2.19 | 5 | 0.43 | 0.10-1.79 |
| Year three only | 48 | 17 | 2.00 | 1.13-3.56 | 13 | 2.42 | 1.24-4.70 |
| Yes at both points | 51 | 17 | 1.23 | 0.67-2.24 | 11 | 1.28 | 0.59-2.78 |
| Depressive symptomsb | |||||||
| Tumor stage | |||||||
| Localized | |||||||
| No at both points | 2034 | 297 | 1 | 65 | 1 | ||
| Baseline only | 140 | 19 | 0.95 | 0.57-1.59 | 4 | 0.96 | 0.34-2.70 |
| Year three only | 118 | 14 | 0.93 | 0.52-1.67 | 0 | ||
| Yes at both points | 80 | 15 | 1.92 | 1.03-3.58 | 2 | 0.82 | 0.11-6.08 |
| Regional or distant | |||||||
| No at both points | 608 | 159 | 1 | 93 | 1 | ||
| Baseline only | 41 | 12 | 1.08 | 0.52-2.25 | 5 | 0.42 | 0.10-1.74 |
| Year three only | 45 | 16 | 2.14 | 1.18-3.88 | 11 | 2.46 | 1.23-4.90 |
| Yes at both points | 29 | 10 | 1.24 | 0.57-2.70 | 7 | 1.27 | 0.45-3.55 |
Adjusted for age, race, body mass index, smoking, history of postmenopausal hormone therapy, comorbidity, mammography use, tumor grade, and estrogen receptor & progesterone receptor status.
“Depression” includes depressive symptoms or anti-depressant use.
“Depressive symptoms” refers to depressive symptoms only.
Results of the sensitivity analyses were generally similar to main findings when the follow-up duration from Year three to breast cancer diagnosis was added into analysis models, when the variable combining depressive symptoms and antidepressant use was replaced by a variable based on depressive symptoms alone in the models, or only incident breast cancer diagnosed after Year 4 was included. For the sensitivity analysis by further adjusting for treatment information among women aged 65 or older, we observed similar magnitudes of HRs as in the main results, but due to reduced sample size (half of women have treatment information), none of them reached statistical significance.
Discussion
This is the first prospective observational study to explore the effect of depression at two time points before breast cancer diagnosis on all-cause mortality and breast cancer-specific mortality. The results indicate that newly developed depression (present at Year three but not at baseline) before breast cancer diagnosis was associated with higher all-cause mortality and breast cancer-specific mortality among late-stage breast cancer survivors.
With the WHI data, Brown et al. found that neither depression nor antidepressant use were associated with increased breast cancer risk 14, but no WHI study has been done to explore the effect of depression on breast cancer mortality. Several other studies have demonstrated that for breast cancer survivors, a prior diagnosis of depression was associated with higher mortality 3-5, which is consistent with our study findings. Few studies have evaluated whether depression at two or more time points before breast cancer diagnosis is associated with the risk for death from all cause or breast cancer, with the exception of several randomized trials for breast cancer survivors, which have demonstrated improved depressive symptoms after psychiatric group intervention (enhancing of coping skill, psychological support, etc.) increased survival 15, 16. Whereas, in this study neither depression at baseline only or depression both at baseline and Year three were associated with increased all-cause mortality.
Hjerl et al. indicated that depression before breast cancer diagnosis was associated with significantly increased mortality for late-stage breast cancer survivors, but not for early-stage breast cancer survivors 4, which is also similar to our findings. The current study showed that among women with subsequent late-stage breast cancer, newly developed depression at Year three was associated with the risk of both all-cause mortality and breast cancer specific mortality. Possible explanations for the particular association of newly developed but not persistent depression may be explained by the difficulty in dealing with new depression compared to having coped with depression for longer times. This new depression may develop from changes in life circumstances, other unmeasured illness or stress, which may have physiological impacts on other disease mechanisms, or lead to different screening behaviors.
Although mechanisms between depression and breast cancer survival are not very clear, discussion of causal links usually include two broad categories of explanation. The first category sees depression as an indication of global brain dysfunction 5. Depression influences neuro-endocrine axis, neuro-immunological function, and other central nervous system activities. This central nervous system dysregulation puts the organism at greater risk for morbidity and mortality 17. The second category indicates that depression renders breast cancer survivors less capable of functioning successfully in modern society 16. People with depression are less likely to receive appropriate and correct health screening, although our results do not indicate any differences in breast cancer screening between women with and without depression at baseline. Some studies suggest cancer survivors may be faced with adherence difficulties to chemotherapy and other complementary treatment 17, which can lead to faster cancer progression. Therefore, depressive symptoms may impair the effect of conventional medical treatment 17, although our results from the sensitivity analysis do not show the difference in treatment between women aged 65 or above with and without depression at baseline.
This study's unique contributions include utilizing a large prospective cohort to explore the effect of depression at two time points on all-cause mortality and breast cancer-specific mortality. Our study has limitations. First, as time-dependent variables, depressive symptoms and antidepressant use were only surveyed at baseline and Year three. However, WHI provided a relatively long time interval between the measurement of depression and the assessment of mortality. Second, there is only treatment information for incident breast cancer in the WHI data among women aged 65 or above. This paper did not include information about endocrine therapy. However, it appears that differences in mortality rates are not entirely explained by treatment disparities, and as such depression leading to mortality differences may not be entirely due to difficulty with compliance. The third limitation is that depressive symptoms, assessed via a self-report scale as we had in the WHI, is not equivalent to a clinical diagnosis of depression. However, among WHI participants, the depression-screening method was found to have a sensitivity of 74% and a specificity of 87% compared with clinician diagnosis of depression 18. In addition, women were unlikely to have depressive symptoms if they had depression but were effectively treated. Therefore, antidepressant use was combined into the definition of depressive symptoms, which improved the identification of depressed women. Fourthly, the association between depression and mortality is very much driven by the quality of symptoms—their severity, chronicity, and even symptom type 19. In the WHI, there was no exclusion for women with depression, but severely depressed women probably were less likely to volunteer for the study. Thus, there was a fairly restricted range of depression scores, which could limit, to some extent, generalization of the results. Lastly, white women accounted for 90% of participants in the current study, which could also limit the generalization to more diverse populations.
Further studies should explore breast cancer-specific mortality associated with depression with multiple measurements during follow-up, which could capture a long-term trend in depression. In addition, further study should assess the interactions of depressive symptoms, antidepressant use and breast cancer treatment on the risk of death from breast cancer, especially with consideration of detailed specific medication data.
Conclusions
Women with newly developed depression before the diagnosis of breast cancer had a modestly but significantly increased risk for death from any cause and death from breast cancer if diagnosed at late stage. The results also highlight the importance of prevention efforts to support mental health and prevent initial emergence of depression among older women.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. A short list of WHI investigators appears in the Supplemental Material published online.
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R15CA179463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The study was supported by a grant from the Youth Scholars Program of Beijing Normal University.
Footnotes
Conflict of interest: The authors do not have any conflicts of interest to report.
Author Contributions: Xiaoyun Liang: Conception and design, data analysis and interpretation, drafting, revising and approving the article. Karen L Margolis, Michael Hendryx, Katherine Reeves, Sylvia Wassertheil-Smoller, Julie Weitlauf, Suzanne C. Danhauer, Rowan Chlebowski, Bette Caan, Lihong Qi, Dorothy Lane, and Sayeh Lavasani: Data analysis and interpretation, revising and approving the article. Juhua Luo: Conception and design, data analysis and interpretation, revising and approving the article.
References
- 1.NCI. SEER Stat Fact Sheets: Breast Cancer. [accessed January 23, 2015]; Available from URL: Available at: http://seer.cancer.gov/statfacts/html/breast.html.
- 2.Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ. 2000;320:474–478. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanani R, Davies EA, Hanchett N, Jack RH. The association of mood disorders with breast cancer survival: an investigation of linked cancer registration and hospital admission data for South East England. Psychooncology. 2016;25:19–27. doi: 10.1002/pon.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354:1331–1336. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 7.Watson M, Homewood J, Haviland J, Bliss JM. Influence of psychological response on breast cancer survival: 10-year follow-up of a population-based cohort. Eur J Cancer. 2005;41:1710–1714. doi: 10.1016/j.ejca.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Vodermaier A, Linden W, Rnic K, et al. Prospective associations of depression with survival: a population-based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2014;143:373–384. doi: 10.1007/s10549-013-2795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 11.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 12.NCQA. HEDIS 2013 Final NDC Lists. [accessed September 20 2015]; Available from URL: Available at: http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2013/HEDIS2013FinalNDCLists.aspx.
- 13.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2012;60:854–861. doi: 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown SB, Hankinson SE, Arcaro KF, Qian J, Reeves KW. Depression, Antidepressant Use, and Postmenopausal Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2016;25:158–164. doi: 10.1158/1055-9965.EPI-15-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 16.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 18.Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103:261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 19.Jamison RN, Burish TG, Wallston KA. Psychogenic factors in predicting survival of breast cancer patients. J Clin Oncol. 1987;5:768–772. doi: 10.1200/JCO.1987.5.5.768. [DOI] [PubMed] [Google Scholar]