Abstract
Aims
Left ventricular (LV) mechanical dyssynchrony has been described in heart failure with preserved ejection fraction (HFpEF), but its prognostic significance is not known.
Method and Results
Of 3445 patients with HFpEF enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist TOPCAT trial, dyssynchrony analysis was performed on 424 patients (12%) by multiple speckle-tracking echocardiography strain-based criteria. The primary dyssynchrony analysis was the standard deviation of the time to peak longitudinal strain (SD T2P LS). Cox proportional hazards models assessed the association of dyssynchrony with the composite outcome of cardiovascular death or HF hospitalization.
Mean age was 70±10 years, LV ejection fraction (LVEF) was 60±8%, and QRS duration was 101±27 ms. Worse dyssynchrony, reflected in SD T2P LS, was associated with wider QRS, prior MI, larger LV volume and mass, worse systolic (lower LVEF and GLS) and diastolic (lower e′, higher E/e′) function. During a median follow-up of 2.6 [IQR 1.5–3.8] years, 107 patients experienced the composite outcome. Worse dyssynchrony was associated with the composite outcome in unadjusted analysis (HR 1.04 (1.01–1.07); p=0.021, per 10 ms increase), but not after adjusting for clinical characteristics, or after further adjustment for LVEF, atrial fibrillation, NYHA class, stroke, heart rate, creatinine, hematocrit and QRS duration (HR 1.03 (0.99–1.06); p=0.16, per 10 ms increase).
Conclusion
Worse LV mechanical dyssynchrony, assessed by speckle-tracking echocardiography, is not an independent predictor of adverse outcomes in HFpEF, suggesting that mechanical dyssynchrony is unlikely to be an important mechanism underlying this syndrome. These findings warrant validation in an independent study specifically designed to assess the prognostic utility of mechanical dyssynchrony in HFpEF.
Keywords: clinical trial, heart failure, heart ventricles, preserved left ventricular function, spironolactone, dyssynchrony
Introduction
Left ventricular (LV) electrical dyssynchrony is a strong prognostic marker of adverse outcomes in heart failure with reduced ejection fraction (HFrEF)1, and resynchronization therapy targeting electric dyssynchrony reduces HF morbidity and mortality2. LV mechanical dyssynchrony, detected by non-invasive imaging such as echocardiography, is similarly associated with worse outcomes in HFrEF – even when occurring in the absence of concomitant electrical dyssynchrony3. HF with preserved LVEF (HFpEF) accounts for approximately half of HF cases in the community and causes substantial morbidity and mortality4,5. While LV diastolic dysfunction is accepted as the primary cardiac perturbation underlying this heterogeneous syndrome, several other cardiovascular and non-cardiovascular abnormalities also appear to contribute. As in HFrEF, LV electrical dyssynchrony – reflected in prolonged QRS duration – is an independent predictor of HF hospitalization and cardiovascular death in HFpEF6. Additionally, detailed echocardiographic characterization has demonstrated greater degrees of LV mechanical dyssynchrony in HFpEF compared to asymptomatic controls, even in the absence of QRS prolongation7,8. However, whether the presence of LV mechanical dyssynchrony is simply a marker of worse cardiac function or a central pathophysiologic mechanism independently associated with worse prognosis in HFpEF is unknown.
The aim of this study was to determine the prognostic relevance of LV mechanical dyssynchrony for incident cardiovascular morbidity (HF hospitalization) and mortality in HFpEF. We studied HFpEF patients enrolled in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial who were included in the echocardiography study and had adequate images for quantitative assessment of indices of LV mechanical dyssynchrony prior to randomization.
Methods
Patient Population
As previously described in detail9, the TOPCAT trial was a multicenter, international, randomized, double-blind, placebo-controlled trial testing the aldosterone antagonist spironolactone to reduce cardiovascular (CV) morbidity and mortality. In total, 3445 adults at least 50 years old with signs and symptoms of HF and an LVEF ≥ 45 % per local site reading were included. Randomization was stratified by the presence of one of the following inclusion criteria: At least 1 hospitalization in the previous 12 months for which HF was a major component of the hospitalization or, if no qualifying hospitalization, a B-type natriuretic peptide (BNP) in the previous 60 days ≥100 pg/mL or N-terminal pro-BNP (NT-proBNP) ≥360 pg/mL. Detailed baseline and clinical characteristics of the trial population10 and the primary trial results11 have previously been published. Randomization to spironolactone did not reduce the composite endpoint of death or heart failure hospitalization but was associated with a lower incidence of HF hospitalization11.
The design and baseline findings of the TOPCAT echocardiographic substudy, including reproducibility metrics for conventional echocardiographic measures, have previously been described in detail12. Dyssynchrony was assessed by strain analysis, which was performed on digitally acquired images in DICOM (digital imaging and communications in medicine) format with acceptable quality. Of 935 patients in the TOPCAT echocardiography study, 663 (71%) were in DICOM format. Of those in DICOM format, 424 (64%) had adequate image quality for strain analysis in the apical 4-chamber view by B-mode speckle tracking echocardiography (STE) as previously described13. Unacceptable image quality was defined as missing view, lack of a full cardiac cycle, >2-segment dropout, or significant foreshortening of the LV. Mechanical dyssynchrony was assessed at baseline in 12% of the TOPCAT study population overall. Of the 935 patients, 305 were enrolled in the dedicated sub-study, 244 of whom underwent follow-up echocardiography at 12 months to 18 months as previously described12. Of patients with feasible strain-based dyssynchrony analysis at baseline, 160 had follow-up studies13. All patients provided written informed consent, and the study was approved by the local Institutional Review Board.
Echocardiographic Methods
Quantitative measures on all study echocardiograms were performed according to the American Society of Echocardiography recommendations by dedicated analysts at the core laboratory who were blinded to clinical information and randomized treatment assignment, as previously described12,13. Digitally acquired echocardiography images in DICOM format with acceptable image quality were uploaded to TomTec software (Munich, Germany) for deformational analyses (2D Cardiac Performance Analysis) as previously described14. For deformation analysis, in the apical views and parasternal views, endocardial borders were traced at the end-diastolic and end-systolic frame respectively as previously described14. The software tracks speckles along the endocardial border throughout the cardiac cycle. Peak strain was computed automatically, generating regional data from 6 segments and an average value for each view. For patients in sinus rhythm, analyses were performed on a single cardiac cycle, whereas for patients in atrial fibrillation, strain values were calculated as the average of 3 selected cardiac cycles. Mechanical dyssynchrony was assessed primarily by the standard deviation of the time to peak (SD T2P) longitudinal strain (LS) in the apical 4-chamber view. Additional echocardiographic measures of LV dyssynchrony assessed included: SD T2P LS of 12 segments from the apical 4- and 2-chamber views, SD T2P transverse strain (TS) obtained from the apical 4-chamber view (6 segments), SD T2P circumferential strain (CS) and radial strain (RS) from the parasternal short axis view at the mid-ventricular level. Peak LS was measured in the apical 4-chamber and apical 2-chamber views (in 6 segments from each view) and averaged to calculate GLS13. All strain measures were performed by a single reader at the echocardiography core laboratory blinded to patient characteristics or treatment assignment. Reproducibility of SD T2P LS obtained from the 4-chamber view was obtained in 20 patients with sinus rhythm and in 20 patients with atrial fibrillation, and expressed as the mean bias and standard deviation using the Bland-Altmann method15. For intraobserver reproducibility, mean bias was 25±38 ms for patients with sinus rhythm, and 9±70 ms for patients with atrial fibrillation. For interobserver reproducibility, mean bias was 23±78 ms for patients with sinus rhythm, and 4±52 ms for patients with atrial fibrillation.
Outcomes
Clinical outcomes included CV death and HF hospitalization during the follow-up period. All events were reported by the primary site investigator and independently adjudicated by the Clinical Endpoints Center. Definitions of these end points have been previously published 9.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed variables, and median and interquartile range for non-normally distributed variables. Categorical variables are expressed as number of subjects and proportion. Clinical characteristics and conventional echocardiographic measures are presented by quartiles of SD T2P LS, with p-values for trend across quartiles calculated using linear regression for continuous normally distributed variables and on an extension of the Wilcoxon rank-sum test16 for continuous non-normally distributed variables. The association between SD T2P LS and GLS, E/e′ and QRS duration was assessed using cubic spline regression models.
The prognostic relevance of measures of LV mechanical dyssynchrony for the composite of HF hospitalization or CV death was assessed by time-to-event analysis using univariable and 2 additive multivariable Cox proportional hazards models whose derivation has been previously described in detail12. Model 1 was adjusted for age, sex, race, randomized treatment assignment (spironolactone versus placebo), randomization strata (qualifying hospitalization or elevated natriuretic peptide level) and enrollment region (the Americas versus Russia/Georgia). Model 2 additionally adjusted for history of atrial fibrillation, core laboratory LVEF, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit and QRS duration. The relationship between baseline dyssynchrony and changes in LV volumes, mass, LVEF, and LA size from baseline to 12 or 18 months was assessed in the 160 patients in whom follow-up echocardiograms were available using linear regression adjusting for the baseline measure and randomized treatment assignment.
As prominent differences in participant characteristics and event rates were noted between patients enrolled in the Americas compared with Russia and Georgia11, we also performed a sensitivity analysis restricted to patients enrolled in the Americas (n=319). A two sided p-value <0.05 was considered significant. Statistical analysis was performed using Stata software Version 12.1 (Stata Corp LP, College Station, TX, USA).
Results
Mechanical dyssynchrony was assessed in 424 patients in TOPCAT (45% of the TOPCAT echocardiographic study; 12% of the overall study population). Strain analysis was not feasible in 55% of the TOPCAT echocardiographic studies because of non-DICOM imaging format, missing views, and poor image quality. Furthermore, due to variable missing data, a sizeable proportion of the study sample did not have deformation data from both apical 4- and 2-chamber view strain, or strain data from the parasternal short axis view. Table 1 compares baseline characteristics between patients enrolled in the overall TOPCAT trial included in this analysis (SD T2P LS measured) compared to those not included. TOPCAT participants including in this analysis tended to be older, more frequently non-white, more frequently enrolled in the Americas, had a higher prevalence of co-morbidities including CAD, previous strokes, atrial fibrillation, and diabetes, and had higher NYHA functional class.
Table 1.
Baseline characteristics of patients enrolled in the TOPCAT trial included versus not included in this analysis.
| Nonecho n=3021 | Echo including T2P LS n=424 | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 68.3 ± 9.5 | 70.2 ± 9.8 | <0.001 |
| Women, n (%) | 1542 (51.1%) | 231 (54.5%) | 0.19 |
| White, n (%) | 2721 (90.2%) | 339 (80.0%) | <0.001 |
| Enrollment in Russia/Georgia, n (%) | 1573 (52.1%) | 105 (24.8%) | <0.001 |
| Clinical | |||
| Enrollment strata: previous hospitalization, n (%) | 2192 (72.6%) | 271 (63.9%) | <0.001 |
| Myocardial Infarction, n (%) | 782 (25.9%) | 111 (26.2%) | 0.89 |
| Coronary revascularization, n (%) | 687 (22.8%) | 126 (29.8%) | 0.001 |
| Stroke, n (%) | 222 (7.4%) | 43 (10.2%) | 0.043 |
| Atrial fibrillation, n (%) | 1037 (34.4%) | 176 (41.6%) | 0.004 |
| Diabetes mellitus, n (%) | 957 (31.7%) | 161 (38.1%) | 0.009 |
| Hypertension, n (%) | 2756 (91.4%) | 389 (92.0%) | 0.69 |
| NYHA functional class (3 & 4), n (%) | 974 (32.3%) | 161 (38.2%) | 0.017 |
| QRS Duration (ms) | 99.4 ± 28.4 | 100.9 ± 27.1 | 0.32 |
| QRS duration > 120 ms | 552 (18.3%) | 84 (19.8%) | 0.44 |
| BMI (kg/m2) | 32.1 ± 7.3 | 32.5 ± 6.9 | 0.30 |
| Heart Rate (beats per minute) | 69.1 ± 10.3 | 68.7 ± 10.8 | 0.41 |
| Systolic blood pressure (mmHg) | 129.6 ± 13.7 | 126.5 ± 15.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 76.2 ± 10.5 | 72.6 ± 10.8 | <0.001 |
| Lab work | |||
| Creatinine (mg/dL) | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.002 |
| Z score BNP | 0.0 ± 1.0 | −0.1 ± 1.0 | 0.30 |
The median SD T2P LS obtained from the 4-chamber view was 59.0 ms (IQR 39.5 to 88.5 ms). Greater SD T2P LS – indicating greater mechanical dyssynchrony – was associated with older age, previous myocardial infarction, greater QRS duration, randomization through the prior HF hospitalization stratum, and higher natriuretic peptide levels among those with available measures (Table 2 and Figure 1). Greater SD T2P LS was also associated with greater LV size, wall thickness, and mass, worse systolic function as determined by lower LVEF and GLS, and worse diastolic function reflected in lower e′ and higher E/e′ ratio (Table 3 and Figure 1).
Table 2.
Clinical parameters by quartiles of Mechanical Dyssynchrony
| All | Dyssynchrony as assessed by SD T2P LS in the 4CH view T2P quartiles
|
|||||
|---|---|---|---|---|---|---|
| n=424 | Quartile 1 n=106 | Quartile 2 n=109 | Quartile 3n=103 | Quartile 4 n=106 | P for trend | |
|
| ||||||
| T2P (ms) | 59.0 [39.5, 88.5] | <39.5 | 39.5–59.0 | 59.1–88.5 | >88.5 | |
| Demographics | ||||||
| Age (years) | 70.2 ± 9.8 | 69.8 ± 9.5 | 69.0 ± 10.4 | 69.7 ± 9.2 | 72.4 ± 9.7 | 0.044 |
| Women, n (%) | 231 (54.5%) | 55 (51.9%) | 71 (65.1%) | 55 (53.4%) | 50 (47.2%) | 0.23 |
| White, n (%) | 339 (80.0%) | 88 (83.0%) | 87 (79.8%) | 77 (74.8%) | 87 (82.1%) | 0.66 |
| Enrollment in Russia/Georgia, n (%) | 105 (24.8%) | 32 (30.2%) | 29 (26.6%) | 18 (17.5%) | 26 (24.5%) | 0.17 |
| Clinical | ||||||
| Enrollment strata: previous hospitalization, n (%) | 271 (63.9%) | 76 (71.7%) | 69 (63.3%) | 65 (63.1%) | 61 (57.5%) | 0.041 |
| Myocardial Infarction, n (%) | 111 (26.2%) | 22 (21.0%) | 24 (22.0%) | 28 (27.2%) | 37 (34.9%) | 0.014 |
| Coronary revascularization, n (%) | 126 (29.8%) | 27 (25.7%) | 35 (32.1%) | 22 (21.4%) | 42 (39.6%) | 0.12 |
| Stroke, n (%) | 43 (10.2%) | 8 (7.6%) | 15 (13.8%) | 9 (8.7%) | 11 (10.4%) | 0.81 |
| Atrial fibrillation, n (%) | 176 (41.6%) | 46 (43.8%) | 47 (43.1%) | 39 (37.9%) | 44 (41.5%) | 0.57 |
| Diabetes mellitus, n (%) | 161 (38.1%) | 39 (37.1%) | 41 (37.6%) | 37 (35.9%) | 44 (41.5%) | 0.59 |
| Hypertension, n (%) | 389 (92.0%) | 96 (91.4%) | 100 (91.7%) | 94 (91.3%) | 99 (93.4%) | 0.65 |
| NYHA functional class (3 & 4), n (%) | 161 (38.2%) | 43 (41.0%) | 45 (41.7%) | 34 (33.0%) | 39 (36.8%) | 0.32 |
| QRS Duration (ms) | 100.9 ± 27.1 | 90.8 ± 17.3 | 98.5 ± 22.9 | 102.6 ± 28.3 | 111.4 ± 33.2 | <0.001 |
| QRS duration > 120 ms | 84 (19.8%) | 8 (7.5%) | 18 (16.5%) | 21 (20.4%) | 37 (34.9%) | <0.001 |
| BMI (kg/m2) | 32.5 ± 6.9 | 32.1 ± 6.3 | 33.6 ± 7.7 | 33.1 ± 6.8 | 31.1 ± 6.6 | 0.26 |
| Heart Rate (beats per minute) | 68.7 ± 10.8 | 68.7 ± 10.3 | 69.7 ± 11.6 | 67.3 ± 10.6 | 69.0 ± 10.6 | 0.75 |
| Systolic blood pressure (mmHg) | 126.5 ± 15.2 | 124.3 ± 13.3 | 127.1 ± 14.3 | 128.4 ± 17.5 | 126.3 ± 15.3 | 0.27 |
| Diastolic blood pressure (mmHg) | 72.6 ± 10.8 | 72.7 ± 11.2 | 73.5 ± 9.8 | 71.0 ± 11.0 | 73.1 ± 11.0 | 0.79 |
| Lab work | ||||||
| Creatinine (mg/dL) | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.47 |
| Z score BNP | −0.1 ± 1.0 | −0.1 ± 1.2 | −0.2 ± 0.9 | 0.1 ± 0.9 | 0.2 ± 0.9 | 0.041 |
Figure 1. Association between the myocardial dyssynchrony and LS, E/e′ and QRS duration.
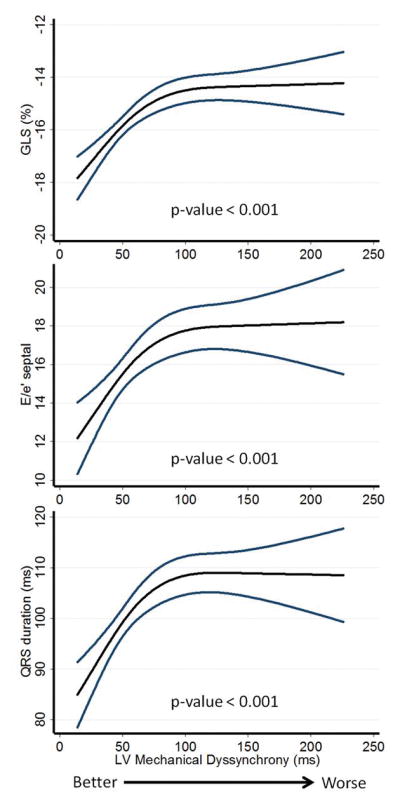
Cubic splines regression models with 95% confidence intervals for the association between mechanical dyssynchrony as assessed by SD T2P LS and LS (A), E/e′ (B) and QRS duration (C), respectively.
LS – Global Longitudinal Strain; SD T2P LS – Standard Deviation Time To Peak LS.
Table 3.
Echocardiographic parameters by quartiles of Mechanical Dyssynchrony
| All | Dyssynchrony as assessed by SD T2P LS in the 4CH view T2P quartiles
|
|||||
|---|---|---|---|---|---|---|
| n=424 | Quartile 1 n=106 | Quartile 2 n=109 | Quartile 3 n=103 | Quartile 4 n=106 | P for trend | |
|
| ||||||
| T2P (ms) | 59.0 [39.5, 88.5] | <39.5 | 39.5–59.0 | 59.1–88.5 | >88.5 | |
| LVEDV (mL) | 98.5 ± 35.1 | 93.0 ± 28.8 | 95.5 ± 35.5 | 101.6 ± 35.1 | 104.1 ± 39.5 | 0.009 |
| LVEDD (cm) | 4.8 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.6 | 4.8 ± 0.5 | 4.9 ± 0.7 | 0.011 |
| Mean wall thickness (cm) | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.3 | <0.001 |
| LV mass (g) | 215.4 ± 68.2 | 194.4 ± 56.9 | 202.6 ± 64.0 | 223.5 ± 65.7 | 241.9 ± 75.5 | <0.001 |
| TDI E′ (septal) (cm/s) | 5.9 ± 2.0 | 6.6 ± 2.0 | 6.3 ± 2.1 | 5.6 ± 2.1 | 5.1 ± 1.7 | <0.001 |
| TDI E′ (lateral) (cm/s) | 8.1 ± 3.1 | 8.7 ± 2.8 | 8.5 ± 3.6 | 7.5 ± 2.8 | 7.6 ± 3.0 | 0.005 |
| LAV (mL) | 60.7 ± 27.4 | 58.0 ± 20.4 | 61.9 ± 35.2 | 59.9 ± 20.5 | 63.0 ± 29.9 | 0.28 |
| E/E′ (septal) | 15.9 ± 6.9 | 13.6 ± 5.8 | 15.5 ± 6.3 | 16.8 ± 7.1 | 17.9 ± 7.6 | <0.001 |
| E/E′ (lateral) | 11.8 ± 5.9 | 10.1 ± 4.8 | 12.2 ± 6.0 | 12.5 ± 6.3 | 12.3 ± 6.4 | 0.023 |
| LVEF (%) | 59.9 ± 8.1 | 62.3 ± 5.7 | 60.6 ± 7.0 | 59.7 ± 8.0 | 57.0 ± 10.1 | <0.001 |
| GLS (%) | −15.6 ± 3.5 | −17.1 ± 2.8 | −15.5 ± 3.4 | −15.6 ± 3.5 | −14.1 ± 3.6 | <0.001 |
During a median follow-up of 2.6 (IQR 1.5–3.8) years, 107 patients (25%) experienced the composite outcome of HF hospitalization or CV death. HF hospitalization occurred in 73 (17%) patients, and CV death occurred in 51 (12%). In unadjusted analysis, greater SD T2P LS was associated with a higher risk of the composite outcome (Figure 2, Table 4). Patients in the highest quartile of SD T2P LS had approximately 2 times higher risk compared to patients in the lowest quartile (4th quartile vs. 1th quartile: HR 2.03, 95% CI 1.17 to 3.50, p=0.011). After multivariable adjustment for clinical characteristics (age, sex, race, randomization strata, region of enrollment, treatment assignment), SD T2P LS was no longer an independent predictor of the composite outcome (Table 4). The same result was found after further adjustment for LVEF, atrial fibrillation, NYHA class, stroke, heart rate, creatinine, hematocrit and QRS duration (4th quartile vs. 1th quartile: HR 1.56, 95% CI 0.85 to 2.86, p=0.15)(Table 4). In addition, in models adjusting only for either GLS or E/e′, SD T2P LS did not retain statistical significance (HR 1.02, 95% CI 0.98–1.06, p=0.21, adjusting only for LS; HR 1.03, 95% CI 1.00–1.07, p=0.060, adjusting only for E/e′). The SD T2P LS derived from the 4- and 2-chamber views (12 segments) demonstrated similar results to SD T2P LS from the 4-chamber view (Table 4). Of the other strain-based measures of LV mechanical dyssynchrony assessed, none were significantly associated with the composite outcome even in unadjusted analysis (Table 4). Among the 160 patients with serial echocardiographic data, greater baseline dyssynchrony was not associated with changes in LV volumes, LV mass, LVEF, or LA volume at 12–18 months follow-up (Table 5).
Figure 2. Association of LV mechanical dyssynchrony and incident HF hospitalization or CV death.
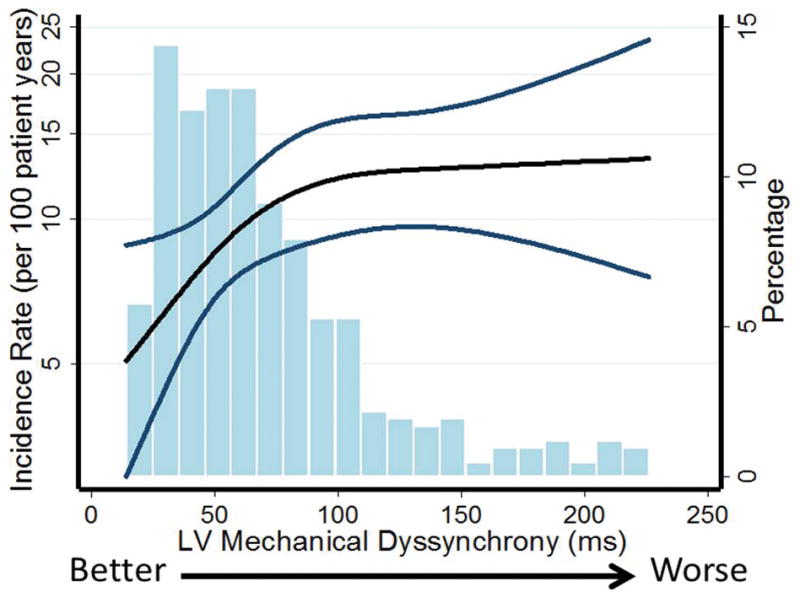
Unadjusted incidence with 95% confidence intervals of composite endpoint per 100 patient years based on LV mechanical dyssynchrony as assessed by SD T2P. A Poisson model was used to estimate the incidence rate. P for overall relationship = 0.021; p for non-linearity = 0.082.
Histograms shows the population distribution of LV mechanical dyssynchrony.
SD T2P LS – Standard Deviation Time To Peak LS.
Table 4.
Measures of Mechanical Dyssynchrony and the association with outcome
| N | Events | Hazard Ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Obtained from the 4CH view | ||||
| SD T2P LS per 10 ms increase | ||||
| Unadjusted | 424 | 107 | 1.04 (1.01–1.07) | 0.021 |
| Model 1 | 424 | 107 | 1.03 (1.00–1.06) | 0.055 |
| Model 2 | 410 | 105 | 1.03 (0.99–1.06) | 0.157 |
| SD T2P TS per 10 ms increase | ||||
| Unadjusted | 424 | 107 | 1.00 (0.98–1.03) | 0.785 |
| Model 1 | 424 | 107 | 1.00 (0.98–1.03) | 0.838 |
| Model 2 | 410 | 105 | 1.00 (0.98–1.03) | 0.840 |
| Obtained from the 4CH and 2CH view | ||||
| Average of SD T2P LS per 10 ms increase | ||||
| Unadjusted | 212 | 50 | 1.07 (1.00–1.15) | 0.046 |
| Model 1 | 212 | 50 | 1.06 (0.99–1.14) | 0.083 |
| Model 2 | 202 | 49 | 1.07 (0.99–1.16) | 0.083 |
| Obtained from the psax view | ||||
| SD T2P CS per 10 ms increase | ||||
| Unadjusted | 243 | 69 | 1.05 (0.99–1.11) | 0.094 |
| Model 1 | 243 | 69 | 1.05 (0.99–1.11) | 0.093 |
| Model 2 | 232 | 68 | 1.05 (0.99–1.11) | 0.133 |
| SD T2P RS per 10 ms increase | ||||
| Unadjusted | 243 | 69 | 0.99 (0.95–1.02) | 0.467 |
| Model 1 | 243 | 69 | 1.00 (0.96–1.04) | 0.937 |
| Model 2 | 232 | 68 | 1.00 (0.96–1.04) | 0.925 |
| T2P anteroseptal to posterior difference per 10 ms increase | ||||
| Unadjusted | 243 | 69 | 0.99 (0.97–1.01) | 0.495 |
| Model 1 | 243 | 69 | 1.00 (0.97–1.02) | 0.784 |
| Model 2 | 232 | 68 | 0.99 (0.97–1.02) | 0.499 |
| T2P anteroseptal to posterior difference > 130 ms | ||||
| Unadjusted | 243 | 69 | 1.05 (0.56–1.95) | 0.889 |
| Model 1 | 243 | 69 | 1.12 (0.60–2.11) | 0.719 |
| Model 2 | 232 | 68 | 1.01 (0.52–1.97) | 0.970 |
Model 1 is adjusted for age, sex, race, randomization strata (previous HF hospitalization or biomarker criteria), region of enrollment (Americas versus Russia/Georgia), randomized treatment. Model 2 includes the same variables as Model 1 + core laboratory left ventricular LVEF, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit and QRS duration.
Table 5.
Mechanical Dyssynchrony at baseline and LV remodeling at 12–18 months (n=160)
| Dyssynchrony as assessed by SD T2P LS in the 4CH view T2P quartiles
|
|||||
|---|---|---|---|---|---|
| Quartile 1 n=41 | Quartile 2 n=41 | Quartile 3 n=38 | Quartile 4 n=40 | P for trend | |
|
|
|||||
| T2P (ms) | <36 | 36–58 | 58–86 | >86 | |
|
|
|||||
| LVEDV Baseline (mL) | 88.1 ± 24.9 | 99.4 ± 37.2 | 103.9 ± 37.7 | 101.7 ± 36.8 | 0.06 |
| LVEDV Change (mL) | 2.9 ± 14.0 | −1.8 ± 12.1 | 2.0 ± 16.4 | 4.9 ± 14.0 | 0.32* |
| LVESV Baseline (mL) | 32.7 ± 10.0 | 41.1 ± 23.8 | 45.5 ± 25.7 | 46.1 ± 24.3 | 0.004 |
| LVESV Change (mL) | 1.8 ± 9.5 | −0.8 ± 6.2 | 1.3 ± 9.9 | 4.0 ± 8.7 | 0.22* |
| LV mass Baseline (g) | 181.4 ± 50.3 | 197.7 ± 58.4 | 209.4 ± 65.4 | 234.4 ± 73.3 | <0.001 |
| LV mass Change (g) | −1.1 ± 11.8 | 1.0 ± 23.9 | −3.4 ± 30.1 | −2.6 ± 17.8 | 0.84* |
| LAV Baseline (mL) | 62.8 ± 22.0 | 59.5 ± 21.9 | 60.6 ± 23.5 | 61.7 ± 21.8 | 0.91 |
| LAV Change (mL) | 0.1 ± 9.4 | −0.8 ± 13.1 | 3.3 ± 15.4 | 4.3 ± 17.9 | 0.10* |
| LVEF Baseline (%) | 62.7 ± 4.8 | 60.3 ± 7.5 | 58.2 ± 9.4 | 56.7 ± 10.2 | <0.001 |
| LVEF Change (%) | −0.7 ± 6.1 | 0.6 ± 5.9 | −0.7 ± 5.2 | −0.4 ± 6.4 | 0.41* |
adjusted for the baseline measure and randomized treatment assignment.
Neither QRS duration (120 msec < versus ≥ 120 msec; p for interaction = 0.79), LVEF (60% < versus ≥ 60%; p for interaction = 0.27), nor abnormal GLS (−15% < versus ≥ −15%; p for interaction = 0.27) modified the association between mechanical dyssynchrony and the composite outcome. In analyses restricted to patients enrolled in the Americas, no echocardiographic measures of dyssynchrony significantly predicted the composite outcome (Supplemental Tables 1). LV mechanical dyssynchrony, as assessed by SD T2P LS obtained from the 4-chamber view, did not modify the relationship between randomization to spironolactone and the composite outcome (p for interaction 0.71). The prognostic relevance of dyssynchrony was similar among patients in sinus rhythm (n=247) and those in atrial fibrillation (n=176), and rhythm did not modify the relationship between dyssynchrony and the composite outcome (p for interaction = 0.94; Table 6).
Table 6.
Mechanical Dyssynchrony as assessed by SD T2P LS and the association with outcome in patients with and without atrial fibrillation
| N | Events | Hazard Ratio (95% CI) | P-value | |
|---|---|---|---|---|
| In patients with sinus rhythm | ||||
| SD T2P LS obtained from the 4CH view per 10 ms increase | ||||
| Unadjusted | 247 | 60 | 1.04 (0.99–1.08) | 0.096 |
| Model 1 | 247 | 60 | 1.02 (0.98–1.07) | 0.273 |
| Model 2 | 239 | 58 | 1.03 (0.98–1.08) | 0.275 |
| In patients with atrial fibrillation | ||||
| SD T2P LS obtained from the 4CH view per 10 ms increase | ||||
| Unadjusted | 176 | 47 | 1.04 (0.99–1.08) | 0.098 |
| Model 1 | 176 | 47 | 1.03 (1.00–1.08) | 0.129 |
| Model 2 | 171 | 47 | 1.02 (0.96–1.07) | 0.526 |
| Interaction between SD T2P LS and atrial fibrillation | ||||
| Unadjusted | 0.940 | |||
| In fully adjusted model (Model 2) | 0.154 |
Model 1 is adjusted for age, sex, race, randomization strata (previous HF hospitalization or biomarker criteria), randomized treatment. Model 2 includes the same variables as Model 1 + core laboratory left ventricular LVEF, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit and QRS duration.
Discussion
While LV mechanical dyssynchrony has previously been described in HFpEF, its prognostic relevance in this syndrome is unknown. We report that greater mechanical dyssynchrony as assessed by the SD T2P LS is significantly associated with the composite of HF hospitalization or CV death in unadjusted analysis, but not after adjusting for clinical characteristics. Other strain-based measures of mechanical dyssynchrony are not associated with the composite outcome even in unadjusted analyses. Together, these data suggest that the presence of mechanical dyssynchrony as assessed by STE does not provide independent prognostic information in HFpEF.
LV electrical dyssynchrony, reflected in prolonged QRS duration, is prognostic in both HFrEF1 and HFpEF6, and is a validated treatment target in HFrEF2. LV mechanical dyssynchrony, detected by direct imaging of the LV contraction pattern, is also prognostic of adverse outcomes in HFrEF17 even in the absence of electrical dyssynchrony3. Improving mechanical dyssynchrony by CRT in HFrEF patients with concomitant electrical dyssynchrony (prolonged QRS duration) has been associated with improved outcome17. Importantly, however, isolated mechanical dyssynchrony does not appear to be an effective treatment target as evidenced by the neutral results of the recent Echocardiography-Guided Cardiac Resynchronization Therapy (EchoCRT) trial3,18 which tested the efficacy of resynchronization therapy in HFrEF patients with echocardiographic evidence of mechanical dyssynchrony and a narrow QRS. Several studies have demonstrated greater mechanical dyssynchrony in HFpEF compared to asymptomatic controls14,19, although this has not been a universal finding8. The mechanisms responsible for mechanical dyssynchrony in HFpEF are unclear. Elevated LV afterload, as seen in hypertension, is associated with lower average LV LS due to lower regional strain in certain segments of the LV,20,21 with a concomitant increase in mechanical dyssynchrony21. Indeed, a prior study demonstrated a similar magnitude of dyssynchrony in asymptomatic persons with hypertension and HFpEF8.
The magnitude of mechanical dyssynchrony in this TOPCAT sample was similar to that in the Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection fraction (PARAMOUNT) HFpEF trial7,22 but considerably less than in HFrEF patients with prolonged QRS enrolled in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial23,24 and in post-MI patients25. Despite a similar magnitude of mechanical dyssynchrony as other HFpEF studies, mechanical dyssynchrony did not provide independent prognostic information in HFpEF patients beyond basic clinical characteristics. The absence of independent prognostic value of mechanical dyssynchrony in HFpEF is important, as the prospect of treating HFpEF patients with mechanical dyssynchrony using CRT has been discussed26. The reason for the lack of independent prognostic value is unclear. Given the smaller magnitude of dyssynchrony in HFpEF compared to HFrEF, mechanical dyssynchrony in HFpEF may not be of sufficient magnitude to cause substantive LV inefficiency. Alternatively, greater mechanical dyssynchrony was significantly associated with worse systolic and diastolic function (GLS and E/e′) and worse electrical dyssynchrony (QRS duration), each of which is a strong independent predictor of outcome in HFpEF6,12,13,27. The observed univariable association of mechanical dyssynchrony with outcomes in HFpEF may therefore simply be secondary to the association of mechanical dyssynchrony with worse systolic and diastolic function6,12,13. This is supported by our observation that the association of SD T2P LS with CV death or HF hospitalization was no longer significant after adjusting for GLS or for E/e′.
Dyssynchrony decreases ventricular contractile efficiency, and progressive LV remodeling – and reverse remodeling with resynchronization therapy – is one recognized mechanism mediating prognosis28. Previous studies in patients with a normal LVEF have suggested that electrical dyssynchrony due to chronic RV pacing is associated with progressive LV enlargement, worsening LV systolic function, and LA enlargement29,30. The randomized controlled Pacing to Avoid Cardiac Enlargement (PACE) trial, which randomized 177 patients with bradycardiac and LVEF >45% to biventricular or right ventricular pacing, demonstrated significant increase in LVEDV and decrease in LVEF at 12 months among patients randomized to RV pacing. Biventricular pacing ameliorated these dyssynchrony-associated changes30. However, the association of mechanical dyssynchrony in the context of a preserved LVEF with adverse LV remodeling has not been defined. Among the 160 TOPCAT participants with baseline dyssynchrony data and follow-up echocardiography at 12–18 months, baseline dyssynchrony was not associated with progressive worsening of LV structure or function, which is concordant with the lack of independent association of dyssynchrony with incident HF or CV death. Additionally, baseline dyssynchrony was not associated with progressive LA enlargement, an important prognostic measure in HFpEF31,32.
While several echocardiographic measures of LV mechanical dyssynchrony have been developed, including M-mode, Doppler, tissue Doppler imaging (TDI) and STE, we primarily evaluated STE-based measures evaluating the temporal dispersion in time to peak regional deformation. It is therefore possible that another imaging-based measure of dyssynchrony not assessed in this study would be independently predictive of adverse outcomes. However, we assessed mechanical dyssynchrony by STE using several different methods including those employed in recent CRT trials in HFrEF, MADIT-CRT2,23 and EchoCRT3,18. While SD T2P LS was the metric most strongly associated with outcomes, there was uniformly no independent association of any of the dyssynchrony measures assessed with outcomes after multivariable adjustment. Metrics of the temporal dispersion in the time to peak segmental deformation assessed by TDI and STE have been the most rigorously studied measures of mechanical dyssynchrony to date3,18,23,33. However, both TDI and STE curves provide a substantial amount of information regarding regional patterns of deformation beyond just the timing of peak deformation. Novel approaches have evaluated differences in the patterns of the complete velocity34 or deformation curves21, based on the concept that these curves can display similar patterns of contraction despite different time to peak values, and may be superior to the time to peak methods to identify responders to CRT34,35.
Previous studies have described SD T2P LS as a strong predictor of ventricular arrhythmias36,37, although recent reports suggest limited utility of this measure to predict arrhythmic events in HFrEF24,38–40. No study has previously assessed whether this measure is associated with ventricular arrhythmias in HFpEF. Data regarding incident ventricular arrhythmias, short of aborted sudden death, are not available in TOPCAT. We were therefore unable to assess the relationship between SD T2P LS and ventricular arrhythmias in this analysis, and the association of this measure with ventricular arrhythmias in HFpEF remains unknown.
Strain analysis was feasible in 45% of patients in the TOPCAT echocardiography study and 12% of the overall TOPCAT study population, limiting statistical power and generalizability for these analyses. In addition, HFpEF is known to be a heterogeneous syndrome41 and we cannot exclude the possibility that in a subset of HFpEF patients, LV mechanical dyssynchrony is particularly relevant to disease pathophysiology and prognosis. Not all previously proposed measures of the LV mechanical dyssynchrony – particularly those based on M-mode imaging or TDI – could be assessed in this study. In the TOPCAT trial, strain was not assessed in the apical long axis view, but only in the apical 4- and 2-chamber views. Dyssynchrony was therefore only calculated from 6-segments (4-chamber view) and 12-segments (4- and 2-chamber views), and was not assessed from 16-segments (4-, 2-, and 3-chamber views)13. Finally, prior studies have suggested that, as opposed to greater resting dyssynchrony, HFpEF is characterized by a failure of exercise associated decrease in LV dyssynchrony19. However, only resting-state imaging was available in this study, so the prognostic relevance of exercise-induced changes in LV synchrony could not be assessed.
Conclusion
Worse LV mechanical dyssynchrony, as assessed by STE, is not an independent predictor of adverse outcomes in HFpEF. These findings suggest that mechanical dyssynchrony is a marker of worse cardiac structure and function in HFpEF, but is unlikely to be an important mechanism underlying this syndrome. These findings warrant validation in an independent study specifically designed to assess the prognostic utility of mechanical dyssynchrony in HFpEF.
Supplementary Material
Acknowledgments
Funding
This work was supported by research grants from the P. Carl Petersen foundation (T.B.S.), The Danish Council for Independent Research Sapere Aude research talent grant (DFF – 4004-00248B; T.B.S.), the National Institutes of Health (grant K08HL116792; A.M.S.), and the American Heart Association (grant 14CRP20380422; A.M.S.). TOPCAT was funded by National Heart, Lung, and Blood Institute, National Institutes of Health (Bethesda, MD), contract HHSN268200425207C. The content of this article does not necessarily represent the views of the sponsor or of the Department of Health and Human Services.
Footnotes
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00094302
Conflict of interest
Dr A. Shah reports receiving research support from Novartis, Gilead, and Myocaria Inc. Dr Pitt reports serving as a consultant for Bayer, Astra Zeneca, Merck, Boehringer Ingelheim, KBP biosciences and Relypsa; and has a patent pending on site-specific delivery of Eplerenone to the myocardium. Dr Sweitzer reports consulting for Medtronic. Dr Pfeffer reports receiving research grants from Amgen, Celladon, Novartis and Sanofi Avantis and serving as a consultant for Amgen, AstraZeneca, Bayer, DalCor Pharma UK, Genzyme, Lilly, Medicines Company, MedImmune, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Salix, Sanderling, Sanofi, Takeda, Teva, Thrasos and Vericel. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of MI with Novartis. Dr Pfeffer is a coinventor. His share of the licensing agreement is irrevocably transferred to charity. The other authors report no conflicts.
References
- 1.Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996;53:163–170. doi: 10.1016/0167-5273(95)02502-2. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 3.Gorcsan J, Sogaard P, Bax JJ, Singh JP, Abraham WT, Borer JS, Dickstein K, Gras D, Krum H, Brugada J, Robertson M, Ford I, Holzmeister J, Ruschitzka F. Association of persistent or worsened echocardiographic dyssynchrony with unfavourable clinical outcomes in heart failure patients with narrow QRS width: a subgroup analysis of the EchoCRT trial. Eur Heart J. 2016;37:49–59. doi: 10.1093/eurheartj/ehv418. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 6.Joseph J, Claggett BC, Anand IS, Fleg JL, Huynh T, Desai AS, Solomon SD, O’Meara E, Mckinlay S, Pitt B, Pfeffer MA, Lewis EF. QRS Duration Is a Predictor of Adverse Outcomes in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:477–486. doi: 10.1016/j.jchf.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Santos ABS, Kraigher-Krainer E, Bello N, Claggett B, Zile MR, Pieske B, Voors AA, McMurray JJV, Packer M, Bransford T, Lefkowitz M, Shah AM, Solomon SD. Left ventricular dyssynchrony in patients with heart failure and preserved ejection fraction. Eur Heart J. 2014;35:42–47. doi: 10.1093/eurheartj/eht427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menet A, Greffe L, Ennezat P-V, Delelis F, Guyomar Y, Castel AL, Guiot A, Graux P, Tribouilloy C, Marechaux S. Is mechanical dyssynchrony a therapeutic target in heart failure with preserved ejection fraction? Am Heart J. 2014;168:909–916. e1. doi: 10.1016/j.ahj.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim H-Y, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O’Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 12.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJV, Solomon SD PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl. 1986;1:307–310. [PubMed] [Google Scholar]
- 16.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 17.Pouleur A-C, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, McNitt S, Hall WJ, Zareba W, Goldenberg I, Moss AJ, Pfeffer MA, Solomon SD MADIT-CRT Investigators. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J. 2011;32:1720–1729. doi: 10.1093/eurheartj/ehr185. [DOI] [PubMed] [Google Scholar]
- 18.Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J, Gras D, Krum H, Sogaard P, Holzmeister J EchoCRT Study Group. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 19.Tan YT, Wenzelburger FW, Sanderson JE, Leyva F. Exercise-induced torsional dyssynchrony relates to impaired functional capacity in patients with heart failure and normal ejection fraction. Heart Br Card Soc. 2013;99:259–266. doi: 10.1136/heartjnl-2012-302489. [DOI] [PubMed] [Google Scholar]
- 20.Baltabaeva A, Marciniak M, Bijnens B, Moggridge J, He FJ, Antonios TF, MacGregor GA, Sutherland GR. Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. 2008;9:501–508. doi: 10.1016/j.euje.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Bauer M, Cheng S, Unno K, Lin F-C, Liao R. Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress. PloS One. 2013;8:e59915. doi: 10.1371/journal.pone.0059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJV Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 23.Knappe D, Pouleur A-C, Shah AM, Cheng S, Uno H, Hall WJ, Bourgoun M, Foster E, Zareba W, Goldenberg I, McNitt S, Pfeffer MA, Moss AJ, Solomon SD Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy Investigators. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 24.Kutyifa V, Pouleur A-C, Knappe D, Al-Ahmad A, Gibinski M, Wang PJ, McNitt S, Merkely B, Goldenberg I, Solomon SD, Moss AJ, Zareba W. Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6:432–444. doi: 10.1016/j.jcmg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Mollema SA, Liem SS, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Gorcsan J, Bax JJ. Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol. 2007;50:1532–1540. doi: 10.1016/j.jacc.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Donal E, Lund L, Linde C, Daubert J-C KaRen investigators. Is cardiac resynchronization therapy an option in heart failure patients with preserved ejection fraction? Justification for the ongoing KaRen project. Arch Cardiovasc Dis. 2010;103:404–410. doi: 10.1016/j.acvd.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Donal E, Lund LH, Oger E, Hage C, Persson H, Reynaud A, Ennezat P-V, Bauer F, Drouet E, Linde C, Daubert C KaRen investigators. New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: a subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur J Heart Fail. 2015;17:680–688. doi: 10.1002/ejhf.291. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ MADIT-CRT Investigators. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122:985–992. doi: 10.1161/CIRCULATIONAHA.110.955039. [DOI] [PubMed] [Google Scholar]
- 29.Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004;44:1883–1888. doi: 10.1016/j.jacc.2004.06.074. [DOI] [PubMed] [Google Scholar]
- 30.Yu C-M, Chan JY-S, Zhang Q, Omar R, Yip GW-K, Hussin A, Fang F, Lam KH, Chan HC-K, Fung JW-H. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123–2134. doi: 10.1056/NEJMoa0907555. [DOI] [PubMed] [Google Scholar]
- 31.Santos ABS, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016:9. doi: 10.1161/CIRCHEARTFAILURE.115.002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Risum N, Williams ES, Khouri MG, Jackson KP, Olsen NT, Jons C, Storm KS, Velazquez EJ, Kisslo J, Bruun NE, Sogaard P. Mechanical dyssynchrony evaluated by tissue Doppler cross-correlation analysis is associated with long-term survival in patients after cardiac resynchronization therapy. Eur Heart J. 2013;34:48–56. doi: 10.1093/eurheartj/ehs035. [DOI] [PubMed] [Google Scholar]
- 35.Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, Lauridsen TK, Saba S, Kisslo J, Gorcsan J, Sogaard P. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. J Am Coll Cardiol. 2015;66:631–641. doi: 10.1016/j.jacc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, Figulla HR, Poerner TC, Edvardsen T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2012;25:667–673. doi: 10.1016/j.echo.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, Florian A, Sjøli B, Brunvand H, Køber L, Voigt J-U, Desmet W, Smiseth OA, Edvardsen T. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging. 2013;6:841–850. doi: 10.1016/j.jcmg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Biering-Sørensen T, Olsen FJ, Storm K, Fritz-Hansen T, Olsen NT, Jøns C, Vinther M, Søgaard P, Risum N. Prognostic value of tissue Doppler imaging for predicting ventricular arrhythmias and cardiovascular mortality in ischaemic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:722–731. doi: 10.1093/ehjci/jew066. [DOI] [PubMed] [Google Scholar]
- 39.Negishi K, Negishi T, Zardkoohi O, Ching EA, Basu N, Wilkoff BL, Popović ZB, Marwick TH. Left atrial booster pump function is an independent predictor of subsequent life-threatening ventricular arrhythmias in non-ischaemic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:1153–1160. doi: 10.1093/ehjci/jev333. [DOI] [PubMed] [Google Scholar]
- 40.Biering-Sørensen T, Knappe D, Pouleur A-C, Claggett B, Wang PJ, Moss AJ, Solomon SD, Kutyifa V. Regional Longitudinal Deformation Improves Prediction of Ventricular Tachyarrhythmias in Patients With Heart Failure With Reduced Ejection Fraction: A MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) Circ Cardiovasc Imaging. 2017;10:e005096. doi: 10.1161/CIRCIMAGING.116.005096. [DOI] [PubMed] [Google Scholar]
- 41.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


