Abstract
During development, cells of seemingly homogenous character sort themselves out into distinct compartments in order to generate cell types with specialized features that support tissue morphogenesis and function. This process is often driven by receptors at the cell membrane that probe the extracellular microenvironment for specific ligands and alter downstream signaling pathways impacting transcription, cytoskeletal organization, and cell adhesion to regulate cell sorting and subsequent boundary formation. This review will focus on two of these receptor families, Eph and Notch, both of which are intrinsically non-adhesive and are activated by a unique set of ligands that are asymmetrically distributed from their receptor on neighboring cells. Understanding the requirement of asymmetric ligand-receptor signaling at the membrane under homeostatic conditions gives insight into how misregulation of these pathways contributes to boundary disruption in diseases like cancer.
Keywords: Boundary, Eph receptor, ephrin, Notch, cancer, organogenesis
Introduction
Identifying how cells distinguish themselves from their neighbors allowing for segregation and boundary formation is essential to understanding embryogenesis and organ morphogenesis. These mechanisms are also important in adult tissues by maintaining tissue compartmentalization, which can breakdown in diseases like cancer.
The first mechanistic concepts of tissue separation and boundary formation emerged from observations that were made during sponge death. As a sponge dies, a subset of undifferentiated cells are spared and able to form aggregates that possess regenerative capacities and differentiate to produce an entire new sponge [1]. Similar cell aggregation and sorting processes have been seen throughout development, beginning as an early embryo transforms into a gastrula containing three germ layers. Compartmentalization is key throughout neurogenesis as the midbrain-hindbrain boundary (MHB) forms between the anterior and posterior segments of the neural tube. This is followed by the formation of seven or eight rhombomeres that are each separated by distinct boundaries [2]. The mechanisms governing boundary formation play a vital role in segmenting tissues and maintaining cellular compartments to support diverse organ functions [3].
During these stages of development, cells have the ability to communicate, recognize, and sort themselves out from their neighbors according to inherent differences in their adhesion properties [4, 5]. This can be caused by differences in cadherin expression, which are homophilic adhesion molecules. Differential expression of cadherins initiates cell sorting by generating compartments of like cells that segregate from neighboring cells with distinct cadherin subtypes [6].
As a boundary forms between two diverse populations of cells, mechanisms that help identify like and non-like cells in order to allow for clustering and segregation must also be activated [7]. An important factor found to play a role in this process is a biomechanical feature known as the differential adhesion hypothesis (DAH) [8]. The DAH proposes that cells have a liquid-like behavior that allows them to reorganize within a compartment and the major feature that governs their organizational pattern is mechanical force determined by the binding strength of the cell adhesion proteins expressed by the respective cell populations [9]. Consequently, increasing adhesive strength by changing the expression level of cadherins can directly impact cell aggregation and sorting. For example, mixing fibroblast cells that express different levels of N-cadherin results in aggregates with higher N-cadherin levels in the center and cells that have lower N-cadherin levels on the outer surface of colonies [10].
Since cadherins provide a link to the actin cytoskeleton, it has been suggested that adhesion strength works in combination with the cytoskeleton to generate changes in cell contractility that help compartmentalize tissues. This led to the differential interfacial tension hypothesis (DITH) that posits cells with similar surface tension will aggregate together [7, 11]. The DITH is supported by atomic force microscopy experiments quantifying differences in surface tension of zebrafish germ layers. These cells cluster according to their surface tension. Lower tension aggregates surround the higher tension aggregates, corresponding with the endoderm and mesoderm having a higher surface tension compared to ectoderm cells [12]. Interestingly, increasing the expression levels of cadherins in fibroblasts that lack endogenous cadherins directly increases cell surface tension, suggesting that adhesive strength and tension cooperate to direct cell segregation [10].
Tissue morphogenesis requires dynamic boundaries implying there must be a balance between pro-adhesive cadherins and repulsive signaling during this process. This equilibrium can be accomplished by integrating cadherin-mediated adhesion with signals from other membrane receptors, like erythropoietin-producing hepatoma (Eph) receptors, Notch, fibronectin and leucine-rich repeat proteins, and epithelial cell adhesion molecules [7, 13, 14]. These receptors help to form tissue boundaries by several non-mutually exclusive mechanisms including altering the cytoskeleton, activating transcriptional cell fate pathways, and directly modulating the adhesion strength of cadherins. In addition, there is often crosstalk between these receptor families to maintain cell segregation and tissue organization events.
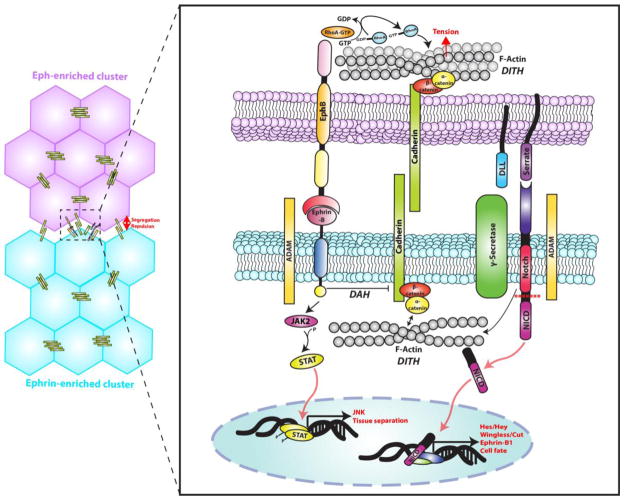
This review will go into the mechanisms driving boundary formation from two major cell-cell signaling networks involved during development, tissue maintenance and disease, namely Eph and Notch receptors. Both of these receptor families are distinguished by their non-adhesive character and asymmetrical distribution of ligand and receptor in neighboring cells that lends well for directing cell segregation and tissue formation (Figure). These receptors, along with cadherins, have points of convergence when tissue boundaries are formed [15]. The contribution of Eph and Notch pathways in the regulation of tissue morphogenesis may help us better understand scenarios where physical or functional boundaries are compromised in adult tissues and disease.
Figure. Eph/ephrin and Notch signaling drives boundary formation.
Eph and Notch receptors are asymmetrically distributed from their ligands at cell-cell contacts. Activation of both of these receptors can promote actin reorganization generating the tension needed to form separate clusters as suggested by the DITH. Also, ADAM metalloproteases can modulate these signaling pathways by cleaving ephrin and Notch extracellular domains. Reverse signaling through ephrin-Bs activate a JAK2/STAT/JNK pathway and inhibit cadherin-dependent adhesion strength resulting in tissue separation as proposed by the DAH. Initiation of Notch signaling causes cleavage of the intracellular domain by γ-secretase allowing for it to translocate to the nucleus and act as a transcription factor for cell fate pathways. Notch can directly affect Eph/ephrin signaling by modifying ephrin-B1 protein expression, initiating crosstalk between these receptor families in the regulation of cell segregation and boundary formation.
Signaling through Eph receptors and their ephrin ligands regulate tissue patterning and boundary formation
Eph receptors and their ephrin ligands are the largest family of receptor tyrosine kinases (RTK) in mammals and are asymmetrically expressed at cell-cell contacts. The Eph receptors are subdivided into A and B subfamilies; EphA receptors have a higher affinity for glycosylphosphatidylinositol-linked ephrin-A ligands, whereas EphB receptors preferentially bind ephrin-B ligands that contain a transmembrane domain with a cytoplasmic tail containing a PDZ-domain. The Eph family is comprised of nine EphA receptors, five EphB receptors, five ephrin-A ligands, and three ephrin-B ligands in humans [16]. Ephrin interaction with an Eph receptor on a neighboring cell can activate both forward and reverse signaling through the receptor and ligand, respectively. Although, there is promiscuity in Eph receptors binding to the alternative ephrin family, each receptor-ligand combination is formed by distinct binding affinities for one another and depending on the tissue there can be a variety of receptor-ligand combinations expressed at boundaries [17, 18]. Interestingly, this asymmetric expression pattern of receptor and ligand helps initiate and maintain cell segregation and boundary formation during development and once a tissue has reached homeostasis.
Upon activation by ephrins, Eph receptors alter their conformation resulting in receptor dimerization then oligomerization and can directly phosphorylate targets or act as a scaffold to alter downstream signaling for a variety of processes that enhance boundary formation [19–21]. Ephrin stability at the membrane can also modulate adhesive and transcriptional pathways that impact early stages of tissue morphogenesis. As early as gastrulation, the expression of XLerk, the Xenopus laevis ortholog of human ephrin-B1, increases and is important in the formation of mesoderm [22]. Initiation of ephrin-B1 signaling can activate RhoA and JAK2-induced STAT3 transcriptional activity, which can modulate the expression of genes involved in cell migration and invasion [23–25] (Figure). Concordantly, activation of STAT3 has been shown to be required for cell movement during gastrulation in zebrafish embryos [26]. These coordinated events initiated by ephrin reverse signaling result in early tissue separation events that lay the path for precise morphogenetic outcomes later in development.
As embryonic development progresses, there is differential expression of Ephs and ephrins at the ectoderm-mesoderm boundary [27]. The ectoderm has high expression of EphB3, EphB4, and ephrin-B3, whereas the mesoderm contains EphA4 and ephrin-B2. The separation between the mesoderm and ectoderm relies on the asymmetry of these receptor-ligand pairs in their distinctive compartments [28]. At this interface, there is a continuous cycle of cell repulsion, detachment, and attachment between receptor and ligand bearing cells. For example, forward signaling through EphB4 activates RhoA and Rac resulting in cell repulsion, but once this receptor signal decays at the membrane the boundary stabilizes allowing time to restore juxtamembrane presentation of receptor-ligand pairs that then set off another round of repulsive events [29].
The complementary expression of Eph/ephrins is a common theme that drives tissue patterning and organization in the blastula, formation of stripes of the presumptive hindbrain in zebrafish embryos, and in patterning of the developing nervous system. For example, there is abundant expression of EphB4 in the presomitic mesoderm and ephrin-B2 in the notochord. EphB4 forward signaling results in activation of the RhoA/ROCK/MLCK signaling axis causing an accumulation of filamentous actin stress fibers within the mesoderm. This leads to the formation of contractile structures with high tension at the mesoderm-somite boundary interface [13] (Figure). Similarly, during neuroepithelial cell segregation, ephrin-B1 induces EphB2 forward signaling resulting in a ROCK-mediated increase in cortical actin within the EphB2 expressing cell population. This causes differential tension between the two cell populations based on their expression of receptor or ligand and resulting in cell segregation, providing a complementary molecular mechanism beyond cadherins that contributes to the DITH [30].
There is also a requirement of EphA4 and ephrin-B2a in opposing rhombomere-restricted domains allowing for the formation of asymmetric receptor-ligand pairs at these boundaries [31, 32]. Intermingling between EphA4 and ephrin-B2a expressing cells causes repulsion, however, contact among the EphA4 expressing cell cluster leads to increased cell-cell adhesion [32]. These differences in adhesion of cells with differential EphA4 and ephrin-B2a expression are critical in the segregation of rhombomeres. Similarly, blocking EphA4 activity or knocking down its ephrin-A1 ligand in the neuroectoderm causes defective gastrulation in Xenopus embryos and interferes with tissue separation between the involuting mesoderm and the non-involuting ectoderm [33]. This complementary expression of Eph receptors and their ephrin ligands thus guides tissue morphogenesis throughout embryonic development.
The topographic mapping of the visual system is largely dependent on complementary gradients of Eph/ephrins [34]. In the retina and superior colliculus, EphA receptors and ephrin-A ligands are expressed, respectively, along a gradient. Retinal neurons with the highest abundance of EphA receptors target regions in the superior colliculus that have the fewest ephrin-A ligands [35–38]. The high expression level of ephrin-A in the posterior colliculus repels retinal ganglion cell growth cones, particularly those from temporal retinal axons that have a high expression of EphA receptors [39]. Axon repulsion for retinotectal patterning is dependent on Eph RTK activity [40]. This mapping is partially controlled by the balance between cis and trans interactions between Eph receptors and their ligands. Cis interactions between Eph receptors and ephrin ligands on the surface of the same cell dampen RTK activity causing a loss of sensitivity to trans-induced ephrin activation from adjacent cells [41–43]. The relative degree of trans and cis interactions leads to either repulsion or attraction, respectively, thereby modulating downstream signaling pathways that control cell connectivity and ultimately neuronal responses.
Eph-induced neuronal repulsion responses are often dependent on receptor-ligand cleavage and alterations in cytoskeletal signaling pathways. For example, activation of ephrin-A2 with clustered EphA2-Fc results in the formation of an ephrin-A2-ADAM10 complex leading to ligand cleavage from the surface of neuroblastoma cells (Figure). This suggests a possible mechanism by which cell-cell contact between neurons in the optic tectum activate ganglion cells in the retina causing growth cone detachment, repulsion, and collapse [44]. Ephrin-A1 also induces retinal ganglion cell growth cone collapse through activation of EphA4. EphA4 then targets the RhoA guanine nucleotide exchange factor (GEF) ephexin leading to growth cone collapse [45]. As such, the complementary expression profile of Eph/ephrins and downstream signaling pathways lead to guidance cues for the trajectory of neurons and the formation of topographic maps in the visual system.
In addition to visual mapping, Eph/ephrins play a role in the maintenance of vasculature in the retina [46]. Specifically, reverse signaling through ephrin-B2 acts in endothelial cells to direct the formation of veins and arteries [47, 48]. Activation of ephrin-B2 also controls vessel pruning. In particular, regressing blood vessels have low levels of phosphorylated ephrin-B2 limiting its interaction with the phosphatase SHP2. This leads to activation of STAT1 and subsequently to JNK3-mediated induction of endothelial cell death-associated genes [49]. These opposing outcomes of ephrin signaling on angiogenesis are likely due to differences in the microenvironment of the vasculature in which they operate.
Counter gradients of Eph receptors and ephrin ligands in adult tissues also helps set functional boundaries within an organ system [21]. Many tissues including skin, intestine, and bone marrow contain a stem cell niche where the progenitor cell population is separated from their differentiated progeny, as well as cell types of different origins [50–52]. For instance, reciprocal expression pattern of ephrin-A1 in cardiomyocytes and EphA2 in cardiac stem cells helps create a segregated niche for the receptor bearing stem cell population. Upon injury, there is an increase in ephrin-A1 expression in cardiomyocytes, which drives the migration of EphA2 expressing progenitor cells into the infarcted area [53]. These differential Eph/ephrin expression patterns can be altered or misregulated under stress or in diseased states to disrupt cell-cell communication and tissue homeostasis [54–56].
The importance of ephrin signaling in tissue morphogenesis is also seen in diseases like craniofrontonasal syndrome (CFNS). CFNS is caused by mutations in the ephrin-B1 gene and leads to cleft palate [57] [58]. Ephrin-B1 expression is required in neural crest cells to form segregated ephrin-B1 and EphB compartments. At ephrin/ephrin interfaces, ephrin-B1 interacts with the gap junction protein Connexin-43 (Cx43) to promote osteogenic differentiation. However, at Eph/ephrin boundaries or when there is a loss of ephrin-B1 and its stabilizing influence on Cx43, bone differentiation is impaired [59]. In addition, decreased ephrin-B1 results in accumulation of EphB3 within these tissue compartments and a loss of MAPK-induced cell proliferation that is required for palatal shelf outgrowth [60]. Therefore, misregulation of Eph/ephrin boundaries leads to a spectrum of abnormal proliferation and differentiation programs that impact tissue organization and function.
Signaling pathways frequently merge during development and this type of crosstalk between Eph/ephrins and other cell surface receptors is important for orchestrating cellular events leading to boundary formation. For example, during gastrulation and mesoderm formation, the expression of XLerk increases under the guidance of fibroblast growth factor (FGF) signaling [22]. However, ectopic expression of XLerk causes cell dissociation in the blastula that can be normalized by overexpression of C-cadherin and FGF stimulation (Figure) [61]. Also, in retinal development, decreased FGF signaling causes a loss of retinal progenitor cells; a phenotype that can be rescued by activation of ephrin-B1. Ephrin-B1 thereby promotes repulsion of retinal progenitor cells that permits movement into and population of the eye field [62]. Alternatively, Notch1 increases the expression of ephrin-B1 while reducing the levels of EphB2 within the crypts of intestinal epithelium to maintain a boundary between progenitor cells and the more differentiated epithelium [63]. Ephrins can also directly modulate signaling of receptors beyond those in the Eph family as in the case of the PDZ domain of ephrin-B2 which interacts with the vascular endothelial growth factor receptors (VEGFRs) and promotes their internalization leading to filopodial extension and vessel sprouting [64]. In cancer, EphA2 expression is increased under the control of epidermal growth factor receptor (EGFR) signaling, which affects the motility of these cells and may lead to segregation of tumor cells from subpopulations that escape transformation by oncogenic Ras [65] [66]. Depending on the cell context, Eph/ephrin crosstalk with other cell surface receptors can therefore either modulate normal cell segregation and boundary formation or alter the organization of abnormal cell clusters in cancer.
Boundary regulation by Notch signaling
Similar to Eph/ephrins, Notch signaling is asymmetrically initiated when Notch receptors bind to either Delta-like (Dll) or Serrate/Jagged-like ligands on adjacent cells and its downstream signaling can affect tissue segregation and patterning [67]. Mammals possess four different Notch receptors, Notch1-4, and ligand binding results in unfolding of the Notch extracellular juxtamembrane domain allowing for cleavage by ADAM metalloproteases. The Notch fragment is recognized by a γ-secretase enzyme complex, which cleaves the Notch intracellular domain (NICD). The cleaved fragment can then translocate into the nucleus where it binds to transcription factors allowing for activation of Notch-target genes, like Hes and Hey [68] (Figure). Direct modulation of gene expression is a major difference between Notch and Eph/ephrin signaling pathways. Notch ligands can also undergo glycan modifications within specific EGF repeats that positively and negatively affect signaling depending on the ligand-receptor complex [67]. Similarly, ephrin-A1 glycosylation has been shown to play a role in binding and activating EphA2 signaling pathways in glioblastoma [69]. In addition to the similar juxtacrine mode of activation, Notch and Eph/ephrin signaling pathways share many converging downstream signaling pathways to regulate boundary formation and tissue development.
A major role for Notch signaling in boundary formation is found in the formation of the dorsoventral (DV) compartment boundary within the Drosophila wing imaginal disc during the third larval stage of development. At this stage of development, Notch is expressed in a set of cells that juxtapose both the dorsal and ventral compartment allowing for activation exclusively at this interface. Activated Notch can increase the expression of genes like Wingless and Cut, which are both required for wing development [70] [71]. Notch gain of function and loss of function studies show that this receptor is exclusively required at the DV interface for DV boundary formation and wing development [71] [70] [72].
There is a differential expression pattern of Notch ligands in distinctive DV regions of the wing disc. The Serrate (Ser) ligand is expressed in the dorsal compartment allowing for Notch activation in the ventral cells; whereas the Delta ligand is expressed in the ventral cells allowing for Notch activation in the dorsal cells [71] [70]. The dorsal-specific protein Apterous (Ap) induces the expression of Fringe, a glycosyltransferase that can modify several EGF-domains in the extracellular region of Notch [73] [74]. This modification causes Notch to increase its Delta-dependent signaling and suppress its Ser-dependent signaling [75]. Similarly, in the MHB neural tube the Notch ligands Ser1 and Dll1 are expressed in discrete regions of the midbrain and hindbrain, respectively, whereas Notch is ubiquitously expressed. Modifications by another Notch regulator, lunatic fringe, produces a narrow band of activated Notch at the MHB increasing the expression of the secreted proteins Wnt1 and Fgf8 that control neural cell fate [76]. These expression profiles of Notch and its ligands in the wing disc and at the MHB mirror what is seen with the complementary expression profiles of Ephs and ephrins throughout many different developmental processes, like embryogenesis and neurogenesis.
Notch also controls DV boundary formation independent of transcriptional regulation. Along the DV boundary there is a Notch-dependent increase in mechanical tension at sites where adherens junctions are present. The enhanced tension causes cell re-arrangements to occur so that neighboring DV compartments remain separated from one another [77]. Also, Notch is required for increased F-actin that helps to create a barrier between the two regions at the DV boundary [72] (Figure). This non-canonical Notch transcription-independent signaling further suggests that Notch and Eph/ephrin pathways complement each other by promoting cytoskeletal reorganization at heterotypic cell-cell contacts.
Levels of Notch signaling are important for the formation of many other tissue boundaries. In the cochlea, for example, Notch signaling plays a role in the formation of the organ of Corti with the levels of expression needing to be tightly controlled for sensory cell fate determination [78]. Along with bone morphogenic protein signaling, proper localization of Notch signaling at the atrio-ventricular canal is necessary for cardiac valve morphogenesis. Changes in the distribution of Notch within this tissue compartment causes faulty valve development [79].
Similar to Eph/ephrins, Notch activation has been shown to play a variety of roles in angiogenesis. For example, loss of Dll4/Notch signaling leads to upregulation of the vasodilator genes, adrenomedullin and neurotensin, and downregulation of the vasoconstrictor gene, angiotensin II, independent of VEGFR [80]. Interestingly, inhibition of Notch signaling can downregulate the expression of proangiogenic genes ephrin-B1 and Sox17 at the transcriptional and post-transcriptional levels, respectively [81] [82] (Figure). Also, Notch activity has been shown to be crucial for transmitting differentiation, proliferation, survival, and angiogenesis signals to endothelial cells [83] [81] [84]. Thus, it is not surprising that homozygous mutations in Notch show embryonic lethality in mice in combination with vascular remodeling defects [85] [86].
Collectively, these examples highlight Notch as a major regulator of tissue morphogenesis. Reminiscent of Eph/ephrin signaling, asymmetric expression of Notch receptors and ligands help set a spatial distribution pattern that guides tissue morphogenesis via mechanisms directly or indirectly impacting transcriptional pathways and at key times involving Eph/ephrin signaling crosstalk [63].
Conclusions and future perspectives
Eph/ephrin and Notch signaling pathways provide a mechanism by which cells sense their cellular microenvironment to distinguish like from non-like cells that can then organize into discretely defined regions within a tissue or as distinct boundaries between tissue layers. Reliance on an asymmetric mode of activation makes these signaling molecules uniquely poised for directing morphogenetic processes at the level of cell-cell contact, and is enhanced by their diverse ligand/receptor combinations yielding distinct signaling outputs that further distinguish cells within a tissue. These same cell-cell communication pathways that govern developmental tissue formation and organization events by keeping cells in defined compartments also operate the boundary between tumors and the surrounding tissue making them attractive targets for restraining tumor growth and invasion in a variety of cancers [55] [87].
Epithelial-derived tumors need to breach through a basement membrane (BM) in order to invade into the surrounding tissue microenvironment. Interestingly, altered Eph and Notch signaling facilitates this escape from the epithelium by engaging proteolytic machinery mechanisms. For example, overexpression of EphA2 promotes invasion through the BM by increasing the expression of matrix-metalloprotease 9 (MMP), which subsequently cleaves type-IV collagen allowing for metastasis [88]. A number of studies have shown that delivery of ephrin ligand mimetics inhibit tumor invasion at least in part by limiting protease activity. This includes pancreatic adenocarcinoma cell lines treated with an ephrin-A1-Fc chimeric protein suggesting a possible path forward for limiting tumor cell invasion in cancer patients [89]. However, this strategy is likely to be specific for the Eph subtype being targeted as activation of EphB2 by ephrin-B2-Fc enhances the production of MMP1 and MMP13 [90]. Similarly, activation of Notch1 in human breast cancer cells can upregulate gene expression levels of MMP2, MMP9 and VEGF therefore promoting tumor cell migration and invasion [91]. Thus, careful consideration of tumor cell type and molecular characteristics will need to be taken when attempting to target Eph and Notch receptor signaling for mitigating metastatic cancer progression.
Loss of signals between stem cells and differentiated cell populations within a tissue layer can also impact tissue homeostasis. For example, EphA2 and ephrin-A1 have complementary expression profiles in mammalian epidermis. EphA2 expression is increased in a basal to suprabasal gradient whereas ephrin-A1 is restricted to the basal layer. Deletion of EphA2 leads to increased basal cell proliferation and enhanced tumor susceptibility suggesting that this interface between progenitor and differentiated cells of the epidermis relies on Eph/ephrin signaling to maintain homeostasis [92]. While EphA2 appears to have tumor suppressive roles in skin, EphB2 positively regulates proliferation and vascularization of cutaneous squamous cell carcinomas (SCCs) in mouse xenograft models [90]. In breast tissue, ephrin-B2 and EphB4 are both expressed in mammary epithelial cells, but EphB4 is only expressed during proliferative phases of development. Overexpression of EphB4 causes hyperproliferation and invasion of mouse mammary tumors providing another example of the regulation of this pathway for maintaining tissue homeostasis [93]. Notch also plays a major role in the formation of skin tumors. Similar to EphA2 and ephrin-A1, Notch receptors are localized to the suprabasal layers of the epidermis whereas Dll1 is expressed in the basal cells [94]. Epidermal deletion of Notch-1 in mice results in increased susceptibility to basal cell carcinoma and SCC suggesting that Notch-1 functions as a tumor suppressor in the skin [95, 96]. In the mammary gland, Notch signaling is normally repressed in stem cells compared to luminal progenitor cells. Notch reactivation in stem cells promotes luminal cell differentiation at the expense of myoepithelial lineage commitment resulting in progenitor cell expansion and the development of tumors [97]. These studies emphasize how altered Eph/ephrin and Notch signaling within tissues can lead to a loss of homeostasis and ultimately tumor progression.
Given their important roles in cellular organization within tissues, it is not surprising that Eph and Notch pathways operate during the self-assembly of organoid cultures in vitro [98, 99]. As an example, inhibition of Eph/ephrin signaling causes mislocalization of Paneth stem cells in intestinal organoids mimicking its well-studied role in the process of the mouse gastrointestinal tract [100] [101]. Similarly, Notch signaling has been shown to be required for stem cell maintenance and differentiation in organoid cultures of the retina and fallopian tubes [102] [103]. Elucidating fundamental mechanisms utilized at the cell surface to modulate adhesive, cytoskeletal, and transcriptional changes in human organoid formation may provide important clues about how to normalize tissue form and function in diseased states on a personalized medicine level.
Boundary formation and tissue morphogenesis are largely dependent on differential adhesion and cytoskeletal tension. Eph/ephrin and Notch signaling are two major signaling pathways that have been shown to regulate these processes to induce cell segregation. Understanding how these receptors and ligands operate under homeostatic conditions can give insight into novel mechanisms that may normalize aberrant signaling in diseases like cancer, where there is a breakdown in boundary formation.
Highlights.
Cell segregation and boundary formation are required for tissue compartmentalization.
Eph/ephrin and Notch signaling are two pathways that regulate boundary formation.
Distinct expression patterns of Eph and Notch receptors initiate and maintain cell segregation.
Breakdown of boundaries can lead to developmental diseases and cancer.
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health [grant AR062110 to SG]; the Cellular and molecular basis of disease training fellowship [T32-GM08061 to RV]; and from the Cancer Smasher Fellowship [RV].
Abbreviation
- ADAM
A disintegrin and metalloproteinase
- Ap
Apterous
- BM
Basement membrane
- Cx43
Connexin-43
- CFNS
Craniofrontonasal syndrome
- Dll
Delta-like ligand
- DAH
Differential adhesion hypothesis
- DITH
Differential interfacial tension hypothesis
- DV
Dorsoventral
- EGFR
Epidermal growth factor receptor
- Eph
Erythropoietin-producing hepatoma
- FGF
Fibroblast growth factor
- GEF
Guanine nucleotide exchange factor
- MMP
Matrix-metalloprotease
- MHB
Midbrain-hindbrain boundary
- NICD
Notch intracellular domain
- RTK
Receptor tyrosine kinase
- Ser
Serrate
- SCC
Squamous cell carcinoma
- SHP2
Tyrosine-protein phosphatase non-receptor type 11
- VEGFR
Vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson HV. A New Method by Which Sponges May Be Artificially Reared. Science. 1907;25(649):912–5. doi: 10.1126/science.25.649.912. [DOI] [PubMed] [Google Scholar]
- 2.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6(7):553–64. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 3.Townes PL, JH Directed movements and selective adhesion of embryonic amphibian cells. The Journal of Experimental Zoology. 1955;128:53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 4.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86(3):287–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Townes PL, JH Directed movements and selective adhesion of embryonic amphibian cells. The Journal of Experimental Zoology. 1955;128(1):53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 6.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20(23):3199–214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 7.Fagotto F. The cellular basis of tissue separation. Development. 2014;141(17):3303–18. doi: 10.1242/dev.090332. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173(4):395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- 9.Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol. 2012;4(1):a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278(1):255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;124(2):188–97. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 12.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10(4):429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 13.Fagotto F, et al. A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev Cell. 2013;27(1):72–87. doi: 10.1016/j.devcel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Fagotto F. Regulation of cell adhesion and cell sorting at embryonic boundaries. Curr Top Dev Biol. 2015;112:19–64. doi: 10.1016/bs.ctdb.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Dahmann C, Oates AC, Brand M. Boundary formation and maintenance in tissue development. Nat Rev Genet. 2011;12(1):43–55. doi: 10.1038/nrg2902. [DOI] [PubMed] [Google Scholar]
- 16.Gale NW, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 17.Himanen JP, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7(5):501–9. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 18.Pasquale EB. Eph-ephrin promiscuity is now crystal clear. Nat Neurosci. 2004;7(5):417–8. doi: 10.1038/nn0504-417. [DOI] [PubMed] [Google Scholar]
- 19.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17(4):240–56. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Wang B, Getsios S. Eph/ephrin signaling in epidermal differentiation and disease. Semin Cell Dev Biol. 2012;23(1):92–101. doi: 10.1016/j.semcdb.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez White BE, Getsios S. Eph receptor and ephrin function in breast, gut, and skin epithelia. Cell Adh Migr. 2014;8(4):327–38. doi: 10.4161/19336918.2014.970012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones TL, et al. Identification of XLerk, an Eph family ligand regulated during mesoderm induction and neurogenesis in Xenopus laevis. Oncogene. 1997;14(18):2159–66. doi: 10.1038/sj.onc.1201082. [DOI] [PubMed] [Google Scholar]
- 23.Hwang YS, et al. The Smurf ubiquitin ligases regulate tissue separation via antagonistic interactions with ephrinB1. Genes Dev. 2013;27(5):491–503. doi: 10.1101/gad.208355.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bong YS, et al. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci U S A. 2007;104(44):17305–10. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel) 2014;6(2):897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita S, et al. Stat3 Controls Cell Movements during Zebrafish Gastrulation. Dev Cell. 2002;2(3):363–75. doi: 10.1016/s1534-5807(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 27.Fagotto F, Winklbauer R, Rohani N. Ephrin-Eph signaling in embryonic tissue separation. Cell Adh Migr. 2014;8(4):308–26. doi: 10.4161/19336918.2014.970028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohani N, et al. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 2014;12(9):e1001955. doi: 10.1371/journal.pbio.1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohani N, et al. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011;9(3):e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill AK, et al. Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. J Cell Biol. 2016;215(2):217–229. doi: 10.1083/jcb.201604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke J, et al. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development. 2001;128(4):571–80. doi: 10.1242/dev.128.4.571. [DOI] [PubMed] [Google Scholar]
- 32.Cooke JE, Kemp HA, Moens CB. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr Biol. 2005;15(6):536–42. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Park EC, et al. The involvement of Eph-Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Dev Biol. 2011;350(2):441–50. doi: 10.1016/j.ydbio.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 35.Reber M, Burrola P, Lemke G. A relative signalling model for the formation of a topographic neural map. Nature. 2004;431(7010):847–53. doi: 10.1038/nature02957. [DOI] [PubMed] [Google Scholar]
- 36.Yates PA, et al. Computational modeling of retinotopic map development to define contributions of EphA-ephrinA gradients, axon-axon interactions, and patterned activity. J Neurobiol. 2004;59(1):95–113. doi: 10.1002/neu.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higenell V, et al. Expression patterns of Ephs and ephrins throughout retinotectal development in Xenopus laevis. Dev Neurobiol. 2012;72(4):547–63. doi: 10.1002/dneu.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown A, et al. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102(1):77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 39.Drescher U, et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82(3):359–70. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 40.Feldheim DA, et al. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24(10):2542–50. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin Y, et al. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48(3):285–96. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho RF, et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9(3):322–30. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 43.Falivelli G, et al. Attenuation of eph receptor kinase activation in cancer cells by coexpressed ephrin ligands. PLoS One. 2013;8(11):e81445. doi: 10.1371/journal.pone.0081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289(5483):1360–5. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 45.Sahin M, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46(2):191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–6. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 47.Adams RH, et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104(1):57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 48.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129(6):1397–410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 49.Salvucci O, et al. EphrinB2 controls vessel pruning through STAT1-JNK3 signalling. Nat Commun. 2015;6:6576. doi: 10.1038/ncomms7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker MR, Stappenbeck TS. Deciphering the ‘black box’ of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol. 2008;24(2):115–20. doi: 10.1097/MOG.0b013e3282f4954f. [DOI] [PubMed] [Google Scholar]
- 51.Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13(2):103–14. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goichberg P, et al. The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction. Circ Res. 2011;108(9):1071–83. doi: 10.1161/CIRCRESAHA.110.239459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–80. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaught D, Brantley-Sieders DM, Chen J. Eph receptors in breast cancer: roles in tumor promotion and tumor suppression. Breast Cancer Res. 2008;10(6):217. doi: 10.1186/bcr2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wieland I, et al. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74(6):1209–15. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Twigg SR, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci U S A. 2004;101(23):8652–7. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4(10):e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes Dev. 2010;24(18):2068–80. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones TL, et al. Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proc Natl Acad Sci U S A. 1998;95(2):576–81. doi: 10.1073/pnas.95.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore KB, et al. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev Cell. 2004;6(1):55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 63.Koo BK, et al. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology. 2009;137(1):145–55. 155 e1–3. doi: 10.1053/j.gastro.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 64.Sawamiphak S, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465(7297):487–91. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 65.Larsen AB, et al. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5(3):283–93. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- 66.Porazinski S, et al. EphA2 Drives the Segregation of Ras-Transformed Epithelial Cells from Normal Neighbors. Curr Biol. 2016;26(23):3220–3229. doi: 10.1016/j.cub.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 67.D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascia F, et al. The black box illuminated: signals and signaling. J Invest Dermatol. 2012;132(3 Pt 2):811–9. doi: 10.1038/jid.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferluga S, et al. Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase. J Biol Chem. 2013;288(25):18448–57. doi: 10.1074/jbc.M113.464008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Micchelli CA, Blair SS. Dorsoventral lineage restriction in wing imaginal discs requires Notch. Nature. 1999;401(6752):473–6. doi: 10.1038/46779. [DOI] [PubMed] [Google Scholar]
- 71.de Celis JF, Garcia-Bellido A, Bray SJ. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development. 1996;122(1):359–69. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- 72.Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132(17):3823–33. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- 73.Blair SS, et al. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development. 1994;120(7):1805–15. doi: 10.1242/dev.120.7.1805. [DOI] [PubMed] [Google Scholar]
- 74.Delanoue R, et al. Interaction between apterous and early expression of vestigial in formation of the dorso-ventral compartments in the Drosophila wing disc. Genes Cells. 2002;7(12):1255–66. doi: 10.1046/j.1365-2443.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 75.Bruckner K, et al. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406(6794):411–5. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 76.Tossell K, et al. Notch signalling stabilises boundary formation at the midbrain-hindbrain organiser. Development. 2011;138(17):3745–57. doi: 10.1242/dev.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michel M, et al. The Selector Gene apterous and Notch Are Required to Locally Increase Mechanical Cell Bond Tension at the Drosophila Dorsoventral Compartment Boundary. PLoS One. 2016;11(8):e0161668. doi: 10.1371/journal.pone.0161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basch ML, et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. Elife. 2016;5 doi: 10.7554/eLife.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarmah S, Muralidharan P, Marrs JA. Embryonic Ethanol Exposure Dysregulates BMP and Notch Signaling, Leading to Persistent Atrio-Ventricular Valve Defects in Zebrafish. PLoS One. 2016;11(8):e0161205. doi: 10.1371/journal.pone.0161205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lobov IB, et al. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117(24):6728–37. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 81.Dimova I, et al. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16(4):921–37. doi: 10.1007/s10456-013-9366-5. [DOI] [PubMed] [Google Scholar]
- 82.Lee SH, et al. Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res. 2014;115(2):215–26. doi: 10.1161/CIRCRESAHA.115.303142. [DOI] [PubMed] [Google Scholar]
- 83.Arboleda-Velasquez JF, et al. Notch signaling functions in retinal pericyte survival. Invest Ophthalmol Vis Sci. 2014;55(8):5191–9. doi: 10.1167/iovs.14-14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tattersall IW, et al. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis. 2016;19(2):201–15. doi: 10.1007/s10456-016-9501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCright B, et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128(4):491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 86.Krebs LT, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14(11):1343–52. [PMC free article] [PubMed] [Google Scholar]
- 87.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11(5):338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 88.Yuan W, et al. Silencing of EphA2 inhibits invasion of human gastric cancer SGC-7901 cells in vitro and in vivo. Neoplasma. 2012;59(1):105–13. doi: 10.4149/neo_2012_014. [DOI] [PubMed] [Google Scholar]
- 89.Duxbury MS, et al. Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochem Biophys Res Commun. 2004;320(4):1096–102. doi: 10.1016/j.bbrc.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 90.Farshchian M, et al. EphB2 Promotes Progression of Cutaneous Squamous Cell Carcinoma. J Invest Dermatol. 2015;135(7):1882–92. doi: 10.1038/jid.2015.104. [DOI] [PubMed] [Google Scholar]
- 91.Li L, et al. Notch-1 signaling promotes the malignant features of human breast cancer through NF-kappaB activation. PLoS One. 2014;9(4):e95912. doi: 10.1371/journal.pone.0095912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Guo H, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66(14):7050–8. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 93.Munarini N, et al. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002;115(Pt 1):25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- 94.Nowell C, Radtke F. Cutaneous Notch signaling in health and disease. Cold Spring Harb Perspect Med. 2013;3(12):a017772. doi: 10.1101/cshperspect.a017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nicolas M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33(3):416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 96.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16(1):55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouras T, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3(4):429–41. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12(2):79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 99.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–54. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 100.Langlands AJ, et al. Paneth Cell-Rich Regions Separated by a Cluster of Lgr5+ Cells Initiate Crypt Fission in the Intestinal Stem Cell Niche. PLoS Biol. 2016;14(6):e1002491. doi: 10.1371/journal.pbio.1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herath NI, Boyd AW. The role of Eph receptors and ephrin ligands in colorectal cancer. Int J Cancer. 2010;126(9):2003–11. doi: 10.1002/ijc.25147. [DOI] [PubMed] [Google Scholar]
- 102.Kessler M, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989. doi: 10.1038/ncomms9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Volkner M, et al. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports. 2016;6(4):525–38. doi: 10.1016/j.stemcr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]