Abstract
To prime adaptive immune responses from the female reproductive tract (FRT), particulate antigens must be transported to draining lymph nodes (dLNs) since there are no local organized lymphoid structures equivalent to those found in the respiratory or gastrointestinal tracts. However, little is known about how to safely and effectively navigate successive barriers to transport such as crossing the epithelium and gaining access to migratory cells and lymphatic drainage that provide entry into dLNs. Here, we demonstrate that intravaginal pre-treatment with chitosan significantly facilitates translocation of nanoparticles (NPs) across the multilayered vaginal epithelium to target dLNs. In addition, chitosan pre-treatment was found to enhance NP associations with immunogenic antigen presenting cells in the vaginal submucosa. These observations indicate that chitosan may have great potential as an adjuvant for both local and systemic protective immunity against viral infections in the FRT.
Keywords: mucosal delivery, chitosan, nanoparticle, reproductive tract, draining lymph node
Graphical Abstract
To induce robust immunity in the female reproductive tract, particulate antigens administered to the vaginal lumen must be transported to lymph nodes that drain the female reproductive tract. However, traversing the multilayered squamous epithelium to reach the draining lymph nodes is a formidable obstacle for particulate antigens. This study reports on the first evidence of using chitosan to facilitate nanoparticle trafficking to the draining lymph nodes. Observations indicate that it is feasible to safely overcome the physical barrier to nanoparticles, thereby enhancing efficacy of vaccines as well as drug delivery to the vaginal route.
Background
The large surface area of the vaginal epithelium provides the most substantial entry points in the female reproductive tract (FRT) for mucosal pathogens or vaccines. However, traversing this multilayered squamous epithelium to reach the iliac (ILLNs) and inguinal lymph nodes (IGLNs) that drain the lower FRT can be a formidable obstacle for large macromolecules and particulate antigens.1, 2 While ultrasmall nanoparticles (NPs) (≤40 nm) may appear in the dLNs following intravaginal (IVG) administration,3, 4 the robustness of NP delivery to the draining lymph nodes (dLNs) from the vaginal routes with respect to reproducibility and frequency of positive LNs in animal treatment groups has not been reported. As such, questions still remain about mechanisms of NP delivery across the vaginal epithelium to the dLNs. In addition, less is known about transport and accumulation to these same sites for larger NPs (≥100 nm), which may be more suitable as vaccine carriers.
Vaccine nanocarriers that mimic pathogen size (≤200 nm) and other attributes conducive for mucosal transport have been previously investigated for effective vaginal delivery.5 However, nanocarrier designs have focused mostly on surface modifications to overcome the mucus barrier, but stop short of describing subsequent barriers to transport encountered throughout the FRT. Some mucosal pathogens such as HIV have been shown to alter the integrity of intercellular junctions to facilitate paracellular translocation of cell-free virus across the epithelium.6 In addition, HIV-1 has recently been found to utilize transcellular trafficking through vagina epithelial cells.7 Alternatively, viruses can also hijack host cells to gain systemic entry. For example, vaginal Langerhans cells (LCs) capture and endocytose HIV virions from the epithelium and then, without being productively infected, migrate distally to transmit the virus to CD4+ T cells.8 Understanding the contribution of these different pathways in nanocarrier transport across the vaginal epithelium may present opportunities to improve their delivery in the FRT.
Strategies that can safely overcome the epithelial barrier without significantly compromising tissue integrity and inducing inflammation will be necessary to advance particulate drug and vaccine delivery via the transvaginal route. Chitosan is a cationic polysaccharide derived from the partial deacetylation of chitin, which is present in crustacean shells, insect exoskeleton, or the cell wall of fungi.9 Due to its non-toxic, biodegradable and biocompatible properties, chitosan has been extensively studied as a biomaterial for tissue engineering10–12 Chitosan has also been used as a pharmaceutical excipient electrostatically complexed with negatively charged hormones, proteins, nucleic acids, drugs, or NPs for mucosal delivery.13–15 When these complexes are delivered to the epithelium, studies have indicated that positively charged amino groups of chitosan interact directly with the negatively charged epithelial cell surface to induce a redistribution of cytoskeletal F-actin and tight junction proteins, which is suggested to subsequently increase paracellular permeability of the epithelium.13 The interaction of chitosan with the epithelial cell surface is also thought to destabilize the complexes formed with chitosan and release the complexed molecules or NPs for subsequent transport across the epithelium.16 However, it is unknown if other molecules or mechanisms are involved in this chitosan-induced cell modification and transportation.13 In addition, chitosan has an immunogenic capacity to stimulate intestinal epithelial cells17, 18 or murine immune cells19–21 to secrete a variety of pro-inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-1β. Of particular interest is the unique function of chitosan as a potent adjuvant for eliciting mucosal immune response that has been demonstrated in various animal models or human subjects.22–26
We hypothesized that chitosan could facilitate transport of NPs across the mouse vaginal epithelium to the dLNs in association with its capacity to open tight junctions of the epithelium and/or to elicit mucosal immune response as an adjuvant. The effect of chitosan on vaginal epithelial cell behavior and immune response of the vaginal mucosal tissue has not been previously investigated. To test our hypothesis, we examined integration and inflammation of the mouse vaginal epithelium following pre-treatment of the vaginal mucosal surface with chitosan, and then investigated NP translocation across the vaginal epithelium, NP accumulation in the dLNs, biodistribution of NPs, and interaction between NPs and immune cells. We observed that chitosan significantly enhanced NP transportation, in the absence of destruction or inflammation of the vaginal epithelium, not only across the vaginal epithelium but also to both ILLNs and IGLNs. Also, chitosan significantly enhanced NP association with immunogenic antigen presenting cells (APCs) in the vaginal submucosa. Since our study is not focused on mucus-penetration, we made no attempt to address effect of the mucus presence on our protocol and the mucus was removed before chitosan pre-treatment or NP administration. To the best of our knowledge, our study provides the first evidence of NP delivery, across the vaginal epithelium, to target LNs that drain the female mouse genital tract by taking advantage of multifunctional attributes of chitosan in the vaginal mucosal tissue.
Method Summary
All in vivo procedures were approved by the University of Washington Institutional Animal Care and Use Committee (Protocol #4260-01). The study was conducted to ensure humane care of animals as per the IACUC’s guidelines. In vivo administration of reagents and materials to mice is summarized in Figure 1a. Briefly, female C57BL/6J (8–10 weeks old) mice were synchronized with the menstrual cycle by medroxyprogesterone acetate injection, intravaginally administered reagents [ultra-pure chitosan, CpG-ODN, or nonoxynol-9 (N9)] and carboxylate-modified polystyrene (PS) NPs (20 and 200 nm diameter). At specified times, organs were isolated from euthanized mice, and processed for fluorescence biodistribution imaging, flow cytometry, histology, or cytokine secretion measurement. A complete version of the full methods is detailed in the supplement materials.
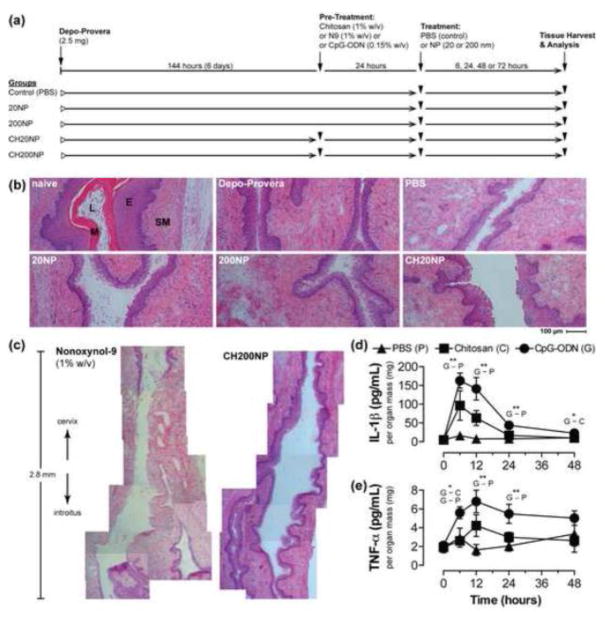
Figure 1. Chitosan does not alter tissue integrity or elicit pro-inflammatory cytokine secretion when applied topically to the vaginal mucosa.
(a) Schematic of treatment study design. Depo-Provera was administered to all C57Bl/6 mice prior to pre-treatment with chitosan followed by delivery of 20 nm or 200 nm NP (CH20 or CH200, respectively). Mice were also administered NPs without chitosan pre-treatment (20NP and 200NP). Control mice were only administered PBS. (b) Brightfield images (10× objective) of H&E-stained tissue sections of the mouse vaginal tract excised 24 hours after vaginal administration of PBS, 20NP, 200NP, and CH20NP. Tissue sections of naïve mice show effect of no treatment and Depo-Provera only treated mice show evidence of a thinned epithelium. SM: submucosal tissue, E: epithelium, L: lumen, M: mucus. (c) Image montage of a 2.8 mm length of vaginal tract comparing treatment of N9 and CH200NP. Images are collated from H&E-stained tissue sections collected from a single microtomed plane. (d–e) Dynamics of pro-inflammatory cytokine secretion following pre-treatment with chitosan or CpG-ODN without subsequent NP administration. G: CpG-ODN, C: chitosan, P: PBS control. Data show mean±SD, n>4 mice from independent experiments. *: p≤0.05, **: p≤0.01, ***: p≤0.001.
Results
Chitosan pre-treatment and prevention of oral ingestion of NPs facilitate NP retention in the RT and transport to dLNs
In small pilot studies (n=1 mouse per group), we optimized the mouse caging and dosing regimen to promote submucosal and dLN accumulation of NPs administered intravaginally. We found that when mice were abdominally taped and individually caged prior to and during NP administration to restrain them from oral ingestion of NPs via self- and inter-grooming, NPs were transported to dLNs but not to any other organs (Supplemental Figure 1). In addition, when NPs were administered following chitosan pre-treatment, NPs showed greater retention in the RTs and draining ILLNs and IGLNs compared to co-administration of chitosan with NPs (Supplemental Figure 2). Based on these observations, we used this optimized caging and dosing method for all mouse treatments in this study (see the supplement materials).
Chitosan pre-treatment and NP administration do not alter tissue integrity or elicit pro-inflammatory cytokine secretion
Tissue cytotoxicity and vaginal irritation were assessed to inform the safety of chitosan pre-treatment and NP administration in the RT (Figure 1). Based on the histology of RT tissue sections, we observed that all treatment groups maintained tissue integrity similar to our placebo controls but distinct from treatment with N9 (1% w/v). The Depo-Provera control mice showed a thinned epithelium compared to the naïve (untreated) mouse group (Figure 1b). However, all treatment arms except for N9 maintained an intact vaginal epithelium despite undergoing a vaginal lavage and swab procedure. Mice that were administered N9 intravaginally showed complete ablation of the epithelium to expose the vulnerable underlying submucosa, which extended the entire length of the lumen (Figure 1c). In contrast, we were unable to identify any damage to the vaginal epithelium anywhere along the lumen for mice subjected to chitosan pre-treatment and NP administration.
To understand if chitosan pre-treatment promoted inflammation, we also measured secretion of two pro-inflammatory cytokines (TNF-α and IL-1β) from homogenized tissues of the RTs. We used CpG-ODN (0.15% w/v) as a positive control since it is used commonly as an adjuvant for IVG vaccination in mouse models.27 For both CpG-ODN and chitosan treatment groups, we observed that secretion of TNF-α and IL-1β peaked at 12 hours and 6 hours post-administration, respectively, but then declined to basal levels by 48 hours (Figure 1d and 1e). CpG-ODN induced significant secretion of TNF-α and IL-1β that was at least 3- and 10-fold higher, respectively, than the PBS control at most time points. However, chitosan did not significantly elevate secretion of either cytokines compared to the PBS control despite being dosed over 6-fold (by weight) higher than CpG-ODN. As such, chitosan pre-treatment resulted in minimal pro-inflammatory cytokine secretion at levels that were similar to the PBS control.
Chitosan promotes accumulation of differently sized NPs from the RT to the distal IGLNs
Given the favorable safety profile of chitosan pre-treatment in the RT and the promising results from our pilot study, we performed larger-scale studies to further investigate the fate of differently sized NPs administered intravaginally. Here, we compared the biodistribution of 20 nm and 200 nm fluorescent NPs in both the presence and absence of chitosan pre-treatment. Similar to our pilot study, whole organ imaging showed that chitosan pre-treatment prior to NP administration resulted in fluorescence in the vaginal tract and uterine horns within 24 hours (Supplemental Figure 3a). Similar fluorescence distribution in these tissues was observed for NPs administered in the absence of chitosan, and no qualitative difference in RT fluorescence was observed between the 20NP and 200NP. Since an extensive vaginal lavage was performed prior to imaging, we expect that the observed fluorescence is indicative of NPs that are adherent to the mucosal epithelium or have penetrated into the tissue, which we also confirmed with tissue microscopy as discussed below.
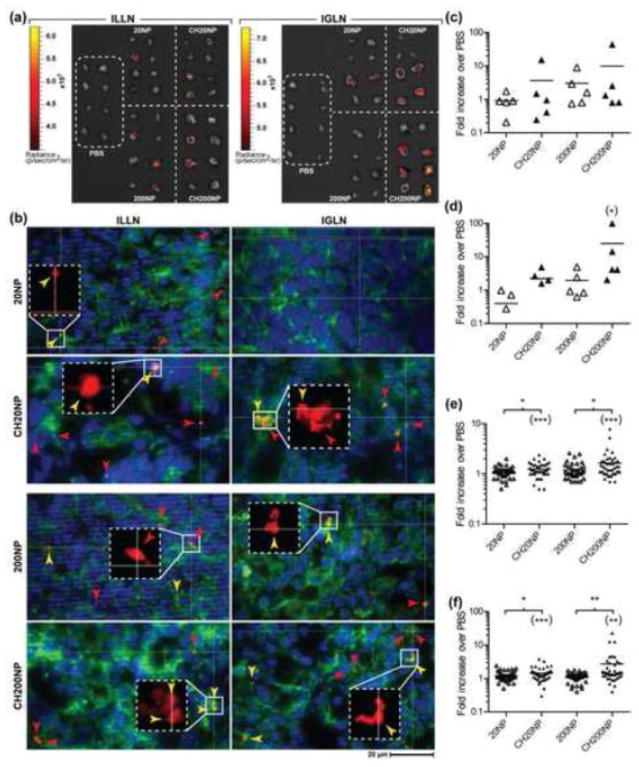
By 24 hours post-administration of 20NPs and 200NPs, fluorescence was detected in both the draining ILLNs and IGLNs and indicates that NPs are capable of trafficking into the dLN after vaginal delivery (Figure 2a). We calculated that chitosan pre-treatment for both sized NPs led to about 2-fold higher combined fraction of fluorescence-positive LNs compared to no pre-treatment (Table 1). In the absence of chitosan pre-treatment, we also observed that 20NP resulted in a similar fraction of positive ILLN and IGLN whereas 200NP showed a reduced trend of 4-fold for IGLN compared to ILLN. In contrast, chitosan pre-treatment led to a slight trend for 20NP in the ILLN but an opposite trend for 200NP. Overall, 20NP and 200NP showed a 2~8-fold higher fraction of positive IGLN when administered following chitosan pre-treatment. NP accumulation in the dLNs was also confirmed by fluorescent microscopy images of tissue sections taken from the ILLNs and IGLNs, where we observed both cell-free NPs and cell-associated NPs that are co-localized with cells (Figure 2b). Flow-cytometry analysis of total cells collected from dLNs also supported a trend for higher NP+ cells for the chitosan pre-treatment groups (Figure 2c and 2d). While these trends were not statistically significant, these data suggest that chitosan pre-treatment leads to differential transport of NP to the ILLN and IGLN depending on size.
Figure 2. Chitosan enhances NP accumulation in dLNs following IVG administration.
(A) Image overlay of whole organ tissue fluorescence and photograph of ILLN and IGLN excised 24 h following treatment with PBS, 20NP, 200NP, CH20NP and CH200NP. Fluorescent-positive lymph nodes are shown from pairs of dLNs collected from 4 mice per treatment. The images were obtained from a single trial out of 6 independent trials. (B) Fluorescent images (63× oil objective) of NPs (red) merged with IFHC-stained cell nucleus (DAPI, blue) and cytoskeleton (Phalloidin, green) from dLNs excised at 24 h post-treatment with 20NP, CH20NP, 200NP, and CH200NP. NP–cell association was confirmed by co-localization of fluorescence (yellow arrow head) from NPs (red arrow head) and cell cytoskeleton (green) detected in both x-y planes and z-axis using optical sectioning (images of z-axis not shown). Inset (additional 3×) confirms presence of NPs in co-localized fluorescence signal. (C-D) Percentage of NP+ cells from total LN cell population normalized to PBS control for (C) ILLN and (D) IGLN excised 24 h post-treatment. Data show mean ± SD from n = 5 independent trials (2-4 mice combined per treatment per trial). (E-F) Average tissue radiance normalized to PBS control measured from whole organ fluorescence imaging of (E) ILLN and (F) IGLN excised 24 h post-treatment. Data show mean ± SD of all LNs (40-44 ILLNs or IGLNs) collected from n = 6 independent trials (2-4 mice per treatment per trial). Asterisk marks in parentheses indicate significant difference compared to PBS control. *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001.
Table 1.
Fractiona of fluorescence-positive lymph nodes resulting from vaginal dosing of NPs in the presence and absence of chitosan pre-treatment.
| PBS (control) | 20NP | CH20NP | 200NP | CH200NP | |
|---|---|---|---|---|---|
|
|
|||||
| ILLN | 0 | 0.3 (0.5) | 0.6 (0.3) | 0.4 (0.3) | 0.3 (0.3) |
| IGLN | 0 | 0.3 (0.5) | 0.5 (0.6) | 0.1 (0.3) | 0.8 (0.5) |
|
| |||||
| Combined dLNs | 0 | 0.3 (0.3) | 0.6 (0.2) | 0.3 (0.2) | 0.5 (0.4) |
Values are the mean (±SD) frequency for every pair of lymph nodes collected from n=4 mice per treatment group shown in Figure 2a. Scoring criteria for positive lymph node are described in the Methods.
In addition to the frequency calculations, we also measured relative tissue fluorescence collected by imaging to further inform NP accumulation to the dLNs from the RT. We found that chitosan pre-treatment resulted in up to a 2.4-fold enhancement of average radiance in both the ILLN and IGLN for 20NP and 200NP (Figure 2e and 2f). We attribute the enhanced radiance in the chitosan pre-treatment groups to accumulation of more NPs in these organs. Finally, we attempted to understand if higher NP fluorescence in the RT would also predict higher NP accumulation in the dLNs. When we plotted the measured NP fluorescence in the dLNs (ILLN and IGLN) against the fluorescence measured in RT tissues (vaginal tract and uterine horns), we observed a small positive correlation indicating that more NPs retained in RTs led to more NP accumulation in dLNs irrespective of their size (Supplemental Figure 3b).
Chitosan pre-treatment promotes translocation of NP across the epithelium to associate with migratory immune cells of the RT
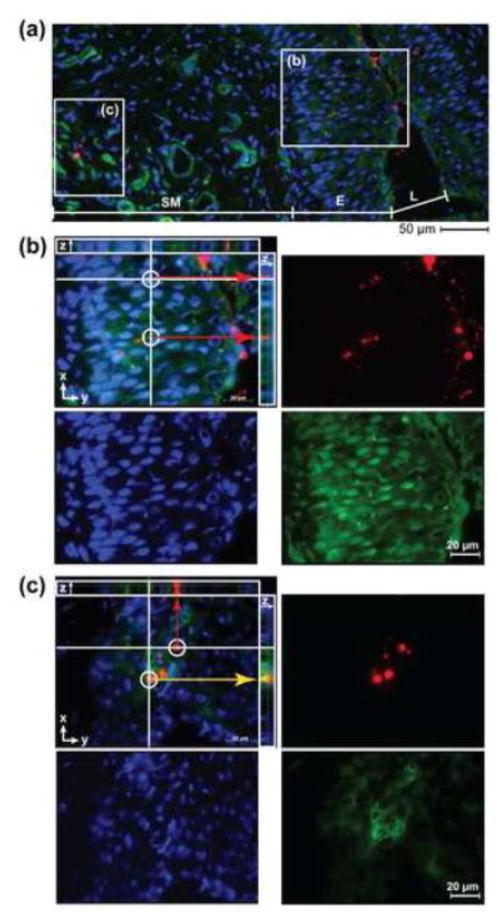
We looked at the site of NP administration to understand how events that occur locally may facilitate NP transport distally. In particular, we investigated if cells in the RT interact with NPs and contribute to their transport to the dLNs. We first used immunofluorescent histochemical (IFHC) staining of tissue sections from the RT to visualize that NPs were transported across the vaginal epithelium into the submucosa for all treatment groups (Figure 3a). Representative IFHC microscopy data from CH200NP show co-localization of NPs with the cell cytoskeleton proximally within the vaginal epithelium (Figure 3b) and distally within the submucosa (Figure3c). Optical sectioning along the tissue depth (z-axis) indicates that NPs are present in the extracellular spaces, which may be evidence for a paracellular route of transport across the vaginal epithelium (Figure 3b, merge). In addition, we observed that NPs are associated with cells of the submucosa (Figure 3c, merge).
Figure 3. Chitosan promotes translocation of NPs across the vaginal epithelium.
Fluorescent images of NPs (red) merged with IFHC-stained cell nucleus (DAPI, blue) and cytoskeleton (Phalloidin, green) from vaginal tissue sections excised at 24 h following treatment with CH200NP. (A) Image (20× objective) of FRT tissue section demarcating a region of the vaginal epithelium and submucosa. (B) Inset (b) of vaginal epithelium (63× oil objective) from (A) showing an optical section where NPs (red) have penetrated beneath the epithelium. (C) Inset (c) of submucosa (63× oil objective) from (A) showing an optical section where NPs (red) have reached the lamina propria and co-localize (yellow arrows) with cells (green). Images are representative of n = 3 mice.
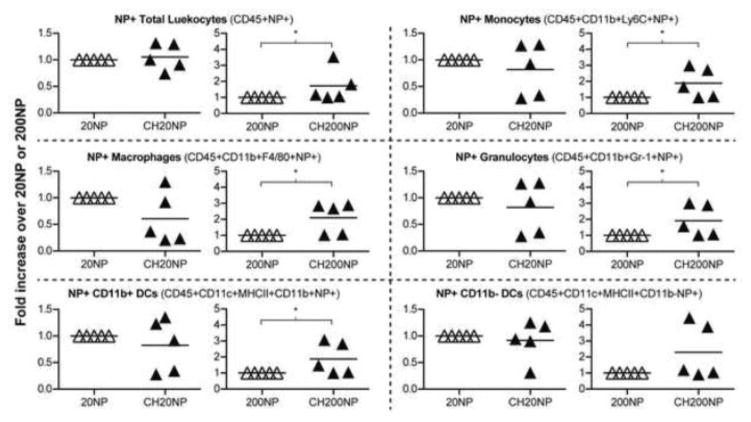
Flow cytometry was used to measure recruitment of immune cells to the RT and interaction of NPs with specific migratory immune cells. We tracked NP association with CD45+ leukocytes stained for total mucosal dendritic cells (DCs) (CD11c+MHCII+), macrophages (CD11b+F4/80+), monocytes (CD11b+Ly6C+) and granulocytes (CD11b+Gr-1+). We observed that the frequencies of these cell populations remained the same after IVG administration of chitosan and NP alone and in combination (data not shown), which indicates no immune cells were recruited to the RT and supports our previous findings on the mucosal safety of these reagents. We found that NPs associated with 0.1~0.3% of CD45+ leukocytes and 5~15% of CD45-epithelial cells irrespective of chitosan treatment (Supplemental Figure 4a and 4b). We also measured the mean fluorescence intensity (MFI) of NP+ cell populations as an effect of chitosan treatment and NP size (Supplemental Figure 4a). These MFIs of NPs per immune cell population for 20NP and CH20NP were statistically similar across all immune cell types. However, CH200NP showed a significantly higher MFI compared to 200NP for all immune cell types except for CD11b-DCs (Figure 4).
Figure 4. NPs associate with various immune cells in mouse RTs.
Fold-increase in mean fluorescence intensity (MFI) of NP+ cells resulting from chitosan pre-treatment relative to no pre-treatment. Reproductive tracts were excised 24 h post-administration of NPs and isolated cells were stained with fluorescently labeled antibodies to identify specific immune cells under flow cytometry. Data show mean ± SD from n = 5 independent trials (2-4 mice combined per treatment per trial). *: P ≤ 0.05.
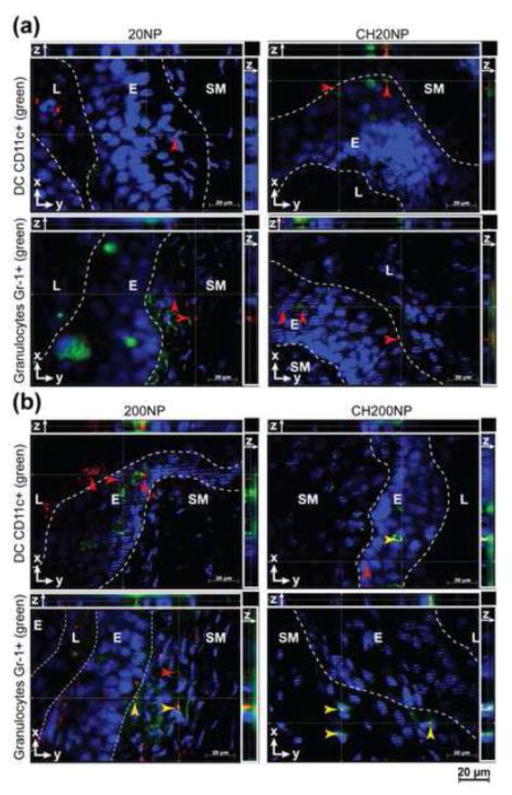
IFHC staining of RT tissue sections was performed to visualize the interactions of NP with DCs (CD11c +) and granulocytes (Gr-1+), which are both phagocytic cells known to migrate to the dLNs upon antigen uptake.28 Although we had observed NP fluorescence in the uterine horns irrespective of their size or chitosan pre-treatment (Supplemental Figure 3a), IFHC tissue sections did not show NPs in the uterine submucosa or associated with DCs or granulocytes for any treatment groups (Supplemental Figure 4c). As expected, DCs were found in both the vaginal epithelium and submucosa whereas granulocytes were detected mainly in the submucosal tissue (Figure 5). We did not detect co-localization of the smaller sized 20NP or CH20NP treatment groups with either cell type from the vaginal epithelium. In contrast, DCs and granulocytes both showed co-localization with 200 nm NP and the interaction appeared to be enhanced with chitosan pre-exposure. We also found that 200NP appeared to associate with CD11c+ DCs in the vaginal epithelium following chitosan pre-exposure, which may be evidence for a transcellular migration route across the epithelium. In summary, we found that chitosan facilitates NP transportation across the vaginal but not uterine epithelia via paracellular or transcellular routes, and enhances association of larger NPs with various immune cells except CD11b-DCs in the vaginal submucosa.
Figure 5. NPs associate with CD11c+ and Gr-1+ immune cells in mouse vaginal tract.
Fluorescent images (63× oil objective) of NPs (red) merged with IFHC-stained cell nucleus (DAPI, blue) and CD11c+ or Gr-1+ (green) markers from vaginal tissue sections excised at 24 hours following treatments with (a) 20NP and CH20NP and (b) 200NP and CH200NP. NP-cell associations were confirmed by co-localization of fluorescence (yellow arrow head) from NPs (red arrow head) and cell marker (green) detected in both x-y planes and z-axis using optical sectioning, and only select NPs or NP-cell associations are shown with arrow heads. Images are representative of n=3 mice.
Discussion
This is the first in vivo study showing that chitosan, a naturally derived biomaterial, enhances NP targeting of the draining ILLN and IGLN after delivery to the mouse vaginal tract. In addition, chitosan administration in the vaginal mucosa modulated immune cell association with NPs but did not promote local tissue inflammation. In the present study, IVG washing, swabbing, or chitosan pre-treatment did not induce damage of the mouse vaginal epithelium. We also confirmed that NPs were transported to the dLNs following NP transport across the vaginal but not uterine epithelium, which was enhanced by chitosan pre-treatment.
Only a few studies have investigated NP trafficking from the mouse vaginal mucosa to the dLNs of the FRT. However, questions remain about the frequency and reproducibility of NP accumulation in the dLNs following vaginal dosing, particularly for larger sized NPs that may be more useful as vaccine carriers. Moreover, the routes in which NP can be transported (cellular and non-cellular pathways) to the dLNs have not been fully described and may present untapped opportunities for local immunization and drug delivery. For example, quantum dots (≤20 nm) dosed vaginally after pre-treatment with the surfactant N9 were transported to the ILLNs but not the distal IGLNs.3 While quantum dot accumulation was quantified in the ILLN, it is unclear if the positive nodes were from the same or separate animals given that only five individual nodes were scored from an expected total of 10 ILLNs collected. The mice used in this study were also not collared or separately caged, which may confound data if quantum dots were ingested orally. In a separate study by Howe et al., PS NPs (≤40 nm) were imaged in the ILLN by microscopy after vaginal dosing but the robustness of the delivery was not quantified or discussed.4 Here, we quantify for the first time that only a low frequency of about 1 in 4 nodes (25%) accumulates 20 nm- or 200 nm-sized NP that are dosed vaginally. In this case, we also observed only limited NP-cell interactions in the vaginal tissue and the dLNs, which may support evidence of NP transport by the paracellular route. As such, our findings suggest that NPs dosed vaginally without chitosan pre-treatment are only infrequently trafficked to the dLNs of the FRT where the epithelium presents a formidable barrier to particulate carriers.
In contrast, chitosan pre-treatment of the vaginal epithelium enhanced NP accumulation to both dLNs and increased NP-cell interactions detected locally in the vaginal tissue as well as distally in the dLNs. Most importantly, we found that chitosan did not alter the integrity of the vaginal epithelium while still facilitating transepithelial migration of NPs to the dLNs, as well as association between larger NPs and various immune cells including immunogenic APCs in the RT. NPs dosed vaginally after chitosan pre-treatment resulted in a doubling of the frequency of positive dLNs. Notably, we saw that chitosan improved transport of larger 200 nm NP to the IGLN from a frequency of ~10% to ~75%. NP-cell interactions detected in the vaginal epithelium between CD11c+ cells and 200 nm NP may be indicative of transcellular routes of migration across the epithelial barrier. Likewise, we saw evidence of paracellular transport of both smaller and larger NPs across the multilayered vaginal epithelium. Since this is the first study investigating effect of chitosan pre-treatment on transport of intravaginally administered NPs, we limited our examination of NP biodistribution to the ILLN and IGLN. However, we do not exclude the possibility of farther systemic transport beyond the IGLNs of larger NPs past 24 hours. Future studies that investigate the impact of NP size on biodistribution may inform protocol development for systemic delivery of NPs from female genital mucosa.
Chitosan molecules have positively charged primary amine groups that are well recognized for their role in opening tight junctions of the epithelium.29, 30 These amine groups have been described to reversibly destabilize cell plasma membrane,31 which enhances uptake of cargo co-administered with chitosan.32 In addition, when orally administered to mice, chitosan is transported primarily associated with DCs or macrophages to LNs that drain the gastrointestinal mucosa.18 In the present study, it is conceivable that chitosan opens tight junctions of the vaginal epithelium to facilitate NP transepithelial delivery while also promoting greater association between NPs and cells locally in the RT to cause accumulation distally in the dLNs. Pro-inflammatory cytokines, TNF-α and IL-1β, are autocrine cytokines produced from immune cells as well as epithelial cells and promote recruitment of inflammatory cells.33, 34 These cytokines have recently been found to also play a role in modulating tight junctions and thereby contribute to viral translocation across the genital epithelium.6 As a natural adjuvant for intranasal or intestinal mucosal vaccines,17, 18, 26, 35–37 chitosan is known to induce various cells to secrete pro-inflammatory cytokines including TNF-α or IL-1β.17, 19–21, 38–42 Interestingly, for both CpG-ODN and chitosan treatment groups, secretion of TNF-α and IL-1β was found to peak in the mouse RT at 12 and 6 hours post-administration, respectively. These levels declined to basal levels by 48 hours possibly due to continuous turnover of the mucus layer that may expel chitosan as well as CpG-ODN out of the vaginal lumen.43–45 Unexpectedly, a single dose of chitosan (200 μg) per mouse was insufficient to induce significant secretion of either TNF-α or IL-1β nor result in immune cell recruitment to the RT compared to our PBS control. However, we found that chitosan pre-exposure still enhanced NP transport across the vaginal epithelium and further transit to both ILLN and IGLN irrespective of NP size.
Our flow cytometry and fluorescence microscopy data detect NPs in the dLNs and confirm the Xenogen IVIS results showing NP transport to the dLNs. However, flow cytometry was unable to measure statistically significant enhancement of NP transport to the dLNs upon chitosan pre-treatment. Previous studies reported that NPs are easily lost during repeated washing steps46 and flow cytometry can underestimate cell number by 70-fold compared to microscopy.47 Therefore, it is possible in our study that preparing samples for flow cytometry analysis led to a significant loss of NPs or target cells, which reduced sensitivity compared to our imaging methods. We found that chitosan pre-treatment significantly enhanced association between larger NPs and immunogenic APCs in the vaginal mucosa (Figure 4). In previous studies, drainage via lymphatics from intradermal or intracutaneous injection sites in mice has shown that immune cell-associated transport of NPs depends on the carrier size.48, 49 Our flow cytometry and microscopy results showing NP-cell association locally in the RT indicates that larger NPs may indeed be transported to the dLNs by increased association with immune cells. We also observed that other immune cells such as granulocytes associate with NPs in the vaginal mucosa.49 More comprehensive studies are needed wherein various immune cell-associated mechanism of NP transport in both lymphatics and dLNs are further investigated.
Parenteral, oral, or intranasal immunizations have been examined for the ability to elicit protective immunity in the genital mucosa against sexually transmitted infections (STIs). Recently, a NP-based oral vaccine was shown to protect animals challenged intravaginally with vaccinia virus.50 However, oral vaccination in this case still induced lower IgG and IgA antigen-specific antibody responses at the local mucosa (colorectal tissue or vaginal wash) compared to intracolorectal vaccination. Eliciting protective mucosal immunity in the genital mucosa from systemic rather than local immunization is challenging due to the unique anatomy and organization of immune function of the FRT compartment. For example, it has been reported that only CD11b+ DCs resident in vaginal submucosa induce protective immunity against virus infection whereas CD11b-DCs resident in vaginal epithelium promote immunosuppression.51, 52 Furthermore, the FRT has a highly compartmentalized immune system wherein entry of circulating memory lymphocytes into the vaginal mucosa is strictly limited. For this reason, IVG immunization is considered to elicit greater local cellular and humoral (IgG and IgA) immunity than parenteral, oral, or intranasal immunization.53–57 In the present study, we found that chitosan enhanced association between larger NPs and CD11b+ DCs in the vaginal submucosa as well as NP transport across the vaginal epithelium and to the dLNs. These interactions and the trafficking of NPs may be conducive for the development of a systemic immunity. Therefore, our findings suggest that chitosan could have great potential for delivering NPs loaded with vaccine antigen/adjuvant formulations targeted to CD11b+ submucosal DCs in the genital mucosa and promote protective immunity against viral infections.
In this study, we removed the mucus in the vaginal lumen before chitosan pre-treatment or NP administration to induce direct contact between chitosan or NPs and the vaginal epithelium. In the presence of mucus, hydrophobic/charged interactions may lead to preferential interaction of conventional NPs with the mucus layer rather than with the epithelium.58 This interaction could reduce the probability for NP transport across the vaginal epithelium. However, we expect if mucus-penetrating NPs59, 60 are employed, transport of such NPs across the vaginal epithelium can still be enhanced by chitosan despite the presence of mucus.
We have shown that NPs are transported across the multilayered vaginal epithelium via paracellular or transcellular pathways depending on NP size or chitosan pre-treatment, and further transported to both dLNs (ILLNs and IGLNs) irrespective of NP sizes. We also found that chitosan pre-treatment significantly enhanced the NP transport across the vaginal epithelium to the dLNs as well as association between larger NPs and immunogenic APCs in the vaginal submucosa. These results indicate that it is feasible to safely overcome the physical barrier of thick vaginal epithelium of the FRT and increase NP transport and association with specific immune cells. Thus, we expect that our chitosan-based protocol may be useful for immunization by the vaginal route to target immunogenic APCs (via cell-associated mechanism) in the vaginal submucosa, thereby inducing both local and systemic immunity against viral infections. In addition, our chitosan-based protocol may enhance efficacy of drug delivery to the vaginal route by increasing NP transport across the vaginal epithelium.
Supplementary Material
Acknowledgments
This work is supported by NIH grant AI094412 and HD075703 to K.A.W.
The authors thank Prof. Dr. J. Bryers’ research group (supported by NIH grant 2R56AI074661-06) for technical assistance with the fluorescence microscopy.
Footnotes
The authors declare no conflicts of interest that would be perceived to bias the research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert opinion on drug delivery. 2010;7:37–48. doi: 10.1517/17425240903338055. [DOI] [PubMed] [Google Scholar]
- 3.Ballou B, Andreko SK, Osuna-Highley E, McRaven M, Catalone T, Bruchez MP, et al. Nanoparticle transport from mouse vagina to adjacent lymph nodes. Plos One. 2012;7:e51995. doi: 10.1371/journal.pone.0051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe SE, Konjufca VH. Protein-coated nanoparticles are internalized by the epithelial cells of the female reproductive tract and induce systemic and mucosal immune responses. Plos One. 2014;9:e114601. doi: 10.1371/journal.pone.0114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical reviews. 2015;115:11109–46. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS pathogens. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinlock BL, Wang Y, Turner TM, Wang C, Liu B. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. Plos One. 2014;9:e96760. doi: 10.1371/journal.pone.0096760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballweber L, Robinson B, Kreger A, Fialkow M, Lentz G, McElrath MJ, et al. Vaginal langerhans cells nonproductively transporting HIV-1 mediate infection of T cells. Journal of virology. 2011;85:13443–7. doi: 10.1128/JVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueter CL, Specht CA, Levitz SM. Innate sensing of chitin and chitosan. PLoS pathogens. 2013;9:e1003080. doi: 10.1371/journal.ppat.1003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–42. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 11.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 12.Seol YJ, Lee JY, Park YJ, Lee YM, Young K, Rhyu IC, et al. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnology letters. 2004;26:1037–41. doi: 10.1023/B:BILE.0000032962.79531.fd. [DOI] [PubMed] [Google Scholar]
- 13.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced drug delivery reviews. 2010;62:59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Cosco D, Cilurzo F, Maiuolo J, Federico C, Di Martino MT, Cristiano MC, et al. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Scientific reports. 2015;5:17579. doi: 10.1038/srep17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14:585–92. doi: 10.1208/s12249-013-9943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YH, Mi FL, Chen CT, Chang WC, Peng SF, Liang HF, et al. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8:146–52. doi: 10.1021/bm0607776. [DOI] [PubMed] [Google Scholar]
- 17.Canali MM, Porporatto C, Aoki MP, Bianco ID, Correa SG. Signals elicited at the intestinal epithelium upon chitosan feeding contribute to immunomodulatory activity and biocompatibility of the polysaccharide. Vaccine. 2010;28:5718–24. doi: 10.1016/j.vaccine.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Porporatto C, Bianco ID, Correa SG. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. Journal of leukocyte biology. 2005;78:62–9. doi: 10.1189/jlb.0904541. [DOI] [PubMed] [Google Scholar]
- 19.Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, et al. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem. 2011;286:35447–55. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo Y, Burdick JA, Highley CB, Marini R, Langer R, Kohane DS. Peritoneal application of chitosan and UV-cross-linkable chitosan. J Biomed Mater Res A. 2006;78:668–75. doi: 10.1002/jbm.a.30740. [DOI] [PubMed] [Google Scholar]
- 21.Bajaj G, Van Alstine WG, Yeo Y. Zwitterionic chitosan derivative, a new biocompatible pharmaceutical excipient, prevents endotoxin-mediated cytokine release. Plos One. 2012;7:e30899. doi: 10.1371/journal.pone.0030899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbal-Gill I, Fisher AN, Rappuoli R, Davis SS, Illum L. Stimulation of mucosal and systemic antibody responses against Bordetella pertussis filamentous haemagglutinin and recombinant pertussis toxin after nasal administration with chitosan in mice. Vaccine. 1998;16:2039–46. doi: 10.1016/s0264-410x(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 23.van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Coos Verhoef J, Junginger HE. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine. 2003;21:1400–8. doi: 10.1016/s0264-410x(02)00686-2. [DOI] [PubMed] [Google Scholar]
- 24.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007;25:8673–86. doi: 10.1016/j.vaccine.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read RC, Naylor SC, Potter CW, Bond J, Jabbal-Gill I, Fisher A, et al. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23:4367–74. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. Journal of virology. 2006;80:5283–91. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 29.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Advanced drug delivery reviews. 2001;52:117–26. doi: 10.1016/s0169-409x(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 30.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Advanced drug delivery reviews. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 31.Bowman K, Leong KW. Chitosan nanoparticles for oral drug and gene delivery. International journal of nanomedicine. 2006;1:117–28. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porporatto C, Bianco ID, Cabanillas AM, Correa SG. Early events associated to the oral co-administration of type II collagen and chitosan: induction of anti-inflammatory cytokines. Int Immunol. 2004;16:433–41. doi: 10.1093/intimm/dxh051. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 34.Khan MM. Immunopharmacology. Springer; 2008. Role of Cytokines; pp. 33–59. [Google Scholar]
- 35.Svindland SC, Pedersen GK, Pathirana RD, Bredholt G, Nostbakken JK, Jul-Larsen A, et al. A study of Chitosan and c-di-GMP as mucosal adjuvants for intranasal influenza H5N1 vaccine. Influenza and other respiratory viruses. 2013;7:1181–93. doi: 10.1111/irv.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buffa V, Klein K, Fischetti L, Shattock RJ. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. Plos One. 2012;7:e50529. doi: 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert review of vaccines. 2009;8:937–53. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- 38.Peluso G, Petillo O, Ranieri M, Santin M, Ambrosio L, Calabro D, et al. Chitosan-Mediated Stimulation of Macrophage Function. Biomaterials. 1994;15:1215–20. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura K, Ishihara C, Ukei S, Tokura S, Azuma I. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine. 1986;4:151–6. doi: 10.1016/0264-410x(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 40.Mori T, Irie Y, Nishimura SI, Tokura S, Matsuura M, Okumura M, et al. Endothelial cell responses to chitin and its derivatives. Journal of biomedical materials research. 1998;43:469–72. doi: 10.1002/(sici)1097-4636(199824)43:4<469::aid-jbm15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Mori T, Okumura M, Matsuura M, Ueno K, Tokura S, Okamoto Y, et al. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 1997;18:947–51. doi: 10.1016/s0142-9612(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. European cells & materials. 2012;24:136–52. doi: 10.22203/ecm.v024a10. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 43.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced drug delivery reviews. 2009;61:158–71. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kieweg SL, Geonnotti AR, Katz DF. Gravity-induced coating flows of vaginal gel formulations: in vitro experimental analysis. Journal of pharmaceutical sciences. 2004;93:2941–52. doi: 10.1002/jps.20194. [DOI] [PubMed] [Google Scholar]
- 45.Kieweg SL, Katz DF. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. Journal of biomechanical engineering. 2006;128:540–53. doi: 10.1115/1.2206198. [DOI] [PubMed] [Google Scholar]
- 46.McCall RL, Sirianni RW. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. Journal of visualized experiments : JoVE. 2013:51015. doi: 10.3791/51015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–49. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature biotechnology. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 49.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nature medicine. 2012;18:1291–6. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–62. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwasaki A. Mucosal dendritic cells. Annual review of immunology. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 53.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infection and immunity. 1997;65:1387–94. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Mucosal vaccination strategies for women. The Journal of infectious diseases. 1999;179(Suppl 3):S493–8. doi: 10.1086/314810. [DOI] [PubMed] [Google Scholar]
- 55.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008;180:2504–13. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- 57.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical journal. 2001;81:1930–7. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Science translational medicine. 2012;4:138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J Control Release. 2011;156:258–64. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.