Abstract
BACKGROUND
Developing newer strategies to improve outcomes in older patients with secondary AML is a critical unmet need. Establishing baseline metrics from which to evaluate newer approaches is important.
METHODS
Secondary AML was defined by one or more of the following: history of antecedent hematological disorder; the diagnosis of therapy related AML; AML with karyotype abnormalities characteristic of MDS. Newly diagnosed secondary AML (s-AML) patients aged 60–75 years were grouped into 5 treatment cohorts: 1) high/intermediate intensity chemotherapy (IC), 2) hypomethylating agent (HMA)/HMA combinations, 3) low dose Ara-c combinations (LDAC), 4) CPX-351, 5) investigational (INV). 931 patients met the criteria of age and s-AML.
RESULTS
Complete remission rates were statistically lower in the HMA (36%) when compared with IC (46%), CPX-351 (45%) and LDAC (43%). Patients receiving less intensive regimens [HMA and LDAC based, combined] had superior OS compared with IC-based regimens (6.9m vs. 5.4; P=0.048). Only 4.3% of IC patients proceeded to transplant as compared with 10.3% of patients on lower intensity regimens (p=0.001). There was no difference in median survival in patients treated with CPX-351 compared with conventional lower-intensity approaches (p=0.75). Age >70yrs, adverse karyotype, and prior AHD were associated with a decreased OS on multivariate analysis.
CONCLUSIONS
Lower-intensity approaches are associated with lower early mortality and improved OS when compared with intensive regimens. Overall survival is poor with currently available therapies, with average OS of 6 months (across regimens, 5.4–7.6 mo). Unsatisfactory outcomes using other investigational agents (INV) underscores the need for more effective therapies.
Keywords: acute myelogenous leukemia, secondary, outcomes, intensive, low dose cytarabine, epigenetic, hypomethylating, CPX-351
INTRODUCTION
Acute myeloid leukemia (AML) has been broadly categorized into de novo and secondary AML based on the absence or presence of a preleukemic hematologic disorder or therapy-related malignancies, respectively 1. Secondary AML is a heterogeneous and poorly defined category of disease entities that includes 1) AML secondary to prior MDS, myeloproliferative disorder (MPN), and aplastic anemia among others (s-AML) and 2) the development of AML secondary to a proven leukemogenic chemotherapeutic exposure (t-AML) 2. It has long been recognized that cytogenetic and molecular characteristics are major players in disease prognosis, and this has resulted in a gradual evolution of AML classification schemes from a morphologic based FAB system to a more prognostically informative classification incorporating cytogenetic and molecular abnormalities 3–5. In the 2008 revision, WHO refined their classification scheme by incorporating a new subcategory of AML defined by ‘AML with myelodysplasia-related changes’ (AML-MRC) that includes: AML from prior MDS, MDS/MPN; AML with a specific MDS-related cytogenetic abnormality, and AML with morphologic evidence of multilineage dysplasia 5. In the most recent 2016 update, ‘AML with myelodysplasia-related changes’ and ‘therapy related myeloid neoplasms’ have been retained as distinct categories, 6.
Age, cytogenetics, and antecedent myeloid malignancies are well established independent prognostic risk factors in defining AML outcomes 7, 8. Older patients (>60 yrs) experience significantly lower remission rates, and disease free survival compared with their younger counterparts 9, with a median time from treatment to death on conventional induction therapy estimated at 5–10 months (9, 10. Further refinements in prognostication have identified a relatively ‘favorable’ prognostic group among these patients - characterized by a younger age (55–65), de novo AML, favorable/intermediate cytogenetic profile, and lack of MDR expression 11, where a cytarabine based induction approach may be prioritized to improve survival. However, this likely excludes the older age group (>65 yrs) of patients who derive minimal OS benefit from conventional induction chemotherapy 12, 13. Palliation is often considered the only reasonable option in patients with advanced age (>80 years) or in elderly patients with a performance status of 3–4 9, 14, 15. Patients aged 60–75 years having high risk disease features but with minimal comorbidities and good performance status represent a subset of patients in whom there currently exists no recommended standard of care. In an effort to identify the most appropriate therapeutic strategy, we sought to review our own experience with various treatment approaches within these high risk patients. Establishing baseline metrics from which to evaluate and develop newer investigational strategies is important.
METHODS
MATERIALS AND METHODS
A retrospective chart review was done to evaluate older patients aged 60–75 years with newly diagnosed AML treated at our institution from 1990 to 2015. The patients were grouped into 5 study cohorts based on their treatment regimen: 1) High-dose or intermediate-dose Cytarabine based intensive chemotherapy (IC), 2) Hypomethylating agents (HMA)/HMA combinations, 3) Liposomal Ara-C: Daunorubicin (CPX-351), 4) Low-dose Cytarabine (Ara-C) combinations (LDAC) and 5) Other investigational agents (INV). Induction therapy in patients in group-1 consisted of either high dose cytarabine or 7+3 based regimen. Azacytidine and decitabine therapies, alone or in combination with other investigational therapies, constitute the second group (HMA). Patients who received non-conventional care and/or exclusively novel therapies were grouped under the INV category.
PATIENT POPULATION
Inclusion required a diagnosis of newly diagnosed AML, treatment at MDACC, and one or more of the following characteristics: 1) history of antecedent hematologic disorder, specifically: prior Myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), or aplastic anemia (AA), 2) the diagnosis of t-AML, or 3) AML with karyotype abnormalities characteristic of MDS as defined by the WHO 6. Patients with very poor performance status (≥ 3) were excluded. AML was defined by the presence of at least 20% myeloid blasts in the bone marrow. Patients’ karyotypes were divided into: adverse karyotype (complex: defined by > 2 chromosomal abnormalities, Del 5/5q, Del 7/7q, 11q, 3q abnormality), diploid karyotype, intermediate karyotype (karyotype other than adverse or diploid). We accorded a separate category to the diploid karyotype to establish a baseline comparison against which to compare other karyotypes. We did not include mutation status in our study analysis since we did not have molecular information in a large proportion of our patients, most of whom were treated in the ‘pre-mutational-profiling’ era of AML 16. Other study variables included in the analysis were: t-AML status (chemotherapy for prior malignancies), and therapy for prior antecedent hematologic disorder (AHD).
STUDY ENDPOINTS
Study endpoints included complete response (CR) rate/CR with low platelets (CRp) according to the international working group criteria 17, overall survival (OS), and 8-week mortality rate. OS was calculated from time of AML diagnosis to date of death and censored at the time of last follow-up date if the patient was alive.
STATISTICAL ANALYSIS
Descriptive statistics were presented as medians and ranges for continuous data, and as numbers and percentages for dichotomous/categorical data. Chi-square and Mann Whitney U test were used to assess patient characteristics for the categorical and continuous data, respectively. Survival curves with Kaplan-Meier estimates were used to compare overall survival between groups, with comparison of survival curves by log rank test. Univariate and multivariate analyses were performed to examine the relationships between disease characteristics and disease outcomes (CR, OS). Only patients with a known karyotype were analyzed in the multivariate analysis. Logistic regression and cox proportional hazards regression models were used to investigate the effects of these variables on response rates and OS, respectively. Stepwise regression approach was used to study which variables were predictive for outcomes with an entry probability of < 0.10 and a stay probability of <0.05. Statistical significance was determined a p-value of 0.05.
RESULTS
We evaluated a total of 931 patients with newly diagnosed AML meeting these criteria of age and disease characteristics. The median age was 68 (range, 60–75) years. Karyotype information was available in 89% of the patients. Of the patients in whom we had karyotype information, 17 % had diploid karyotype, 57.5% belonged to adverse risk profile, and 31% belonged to intermediate risk profile (excluding diploid). All patients had a performance status ≤ 2. Molecular information was not available in majority of the patients, and hence, not included in the study analysis. Prognostically relevant inter-group differences were observed with respect to age (a larger percentage of younger patients in group-1) (Table 1a). Inter-group differences were also observed in other parameters such as LDH, creatinine, bone marrow blasts, and history of AHD. On further analyzing within in an AML population with high risk disease characteristics [i.e., WBC >50000K/μL, Creatinine >1.3 mg/dl, PS ≥ 2, and history of AHD], the CPX arm had a much higher proportion of patients with AHD compared to all other categories, except for INV group [Table 1b]. Additionally, only 1% (2/221) of AML patients treated with HMA-based therapies had a white blood cell count over 50000 K/μL, significantly lower than in other treatment groups, Patients within the INV group were significantly older when compared with other treatment category of patients (58% of the patients were above the age of 70 years) [Table 1b]. Induction therapies constituting each of the study groups are illustrated in Supplemental Figures 1–4. Treatment regimens, on which there were fewer than 10 study patients, were placed under ‘other’.
Table 1a.
Baseline Patient characteristics and outcomes
| Characteristics | Total | IC/HDAC combinations | HMA combinations | CPX | LDAC combinations | INV | P-Value | |
|---|---|---|---|---|---|---|---|---|
| N=931 | N=396 | N=220 | N=20 | N=186 | N=109 | |||
| Patient related characteristics: | ||||||||
| Age at diagnosis | 60–65 yrs (%) | 246 (26) | 148 (37) | 35 (16) | 5 (25) | 40 (22) | 18 (17) | <.001 |
| 65–70 yrs (%) | 325 (35) | 127 (32) | 84 (38) | 7 (35) | 80 (43) | 28 (26) | ||
| ≥70 yrs (%) | 360 (39) | 121 (30) | 101 (46) | 8 (40) | 66 (36) | 63 (58) | ||
| WBC (K/μL) | 3.4 (0.2–433) | 3.9 (0.3–1.9) | 2.7 (0.2–93) | 3.8 (0.7–61) | 3.2 (0.4–433) | 3.8 (0.6–77) | 0.02 | |
| PLT (K/μL) | 41 (1–936) | 41 (2–708) | 41 (1–454) | 33 (9–173) | 41 (5–445) | 47 (5–936) | 0.33 | |
| LDH (IU/L) | 668 (190–13688) | 723 (251–13688) | 603 (190–7585) | 630 (425–2493) | 626 (300–8887) | 670 (322–17486) | 0.01 | |
| Total Bilirubin (mg/dL) | 0.6 (0–2) | 0.6 (0.1–1.7) | 0.6 (0–1.9) | 0.7 (0.3–1) | 0.6 (0.1–1.7) | 0.5 (0.1–1.7) | 0.42 | |
| Creatinine (mg/dL) | 0.9 (0.4–1.9) | 1.0 (0.4–1.9) | 0.9 (0.4–1.9) | 0.8 (0.6–1.7) | 0.9 (0.4–1.9) | 1 (0.5–1.8) | 0.02 | |
| ECOG performance status (PS) | 0–1 | 649 (70) | 279 (70) | 149 (68) | 10 (50) | 148 (80) | 63 (58) | 0.9 |
| 2 | 147(16) | 79 (20) | 34 (15) | 4 (20) | 18 (10) | 12 (11) | ||
| undocumented | 135 (14) | 38 (10) | 37 (17) | 6 (30) | 20 (10) | 44 (31) | ||
| Disease related characteristics: | ||||||||
| Peripheral blood blasts (%) | 9 (0–98) | 91 (0–96) | 8 (0–95) | 5 (0–91) | 8 (0–98) | 10 (0–92) | 0.32 | |
| Bone marrow blasts (%) | 36 (0–96) | 40 (0–95) | 35 (3–89) | 36 (10–83) | 35 (4–96) | 28 (0–89) | 0.004 | |
| Prior therapy for antecedent disorder (%) | 244 (26) | 79 (20) | 35 (16) | 13 (65) | 63 (34) | 54 (49.5) | <.001 | |
| Cytogenetic category (per ELR): | ||||||||
| Adverse (%) | 537 (58) | 238 (60) | 125 (57) | 9 (45) | 116 (62) | 49 (45) | 0.44 | |
| Diploid (%) | 160 (17) | 62 (16) | 43 (20) | 5 (25) | 29 (16) | 20 (18) | ||
| Intermediate (%) | 132 (14) | 54 (14) | 38 (17) | 5 (25) | 23 (12) | 11 (10) | ||
| Unknown (%) | 102 (11) | 42 (11) | 14 (6) | 1 (5) | 16 (9) | 29 (27) | - | |
| Outcomes: | ||||||||
| Complete response [CR/CRp] (%) | 368 (39.5) | 183 (46) | 79 (36) | 9 (45) | 80 (43) | 17 (15.6) | <.001 | |
| Partial response [PR/HI] (%) | 43 (4.5) | 9 (2.2) | 26 (12) | 2 (10) | 6 (3.2) | 0 (0) | ||
| No response [NR] (%) | 335 (36) | 113 (28) | 88 (40) | 7 (35) | 64 (34.5) | 63 (57.8) | ||
| 8- (week mortality [LT-8W] | 185 (19.8) | 91 (22.9) | 27 (12.2) | 2 (10) | 36 (19.3) | 29 (26.6) | - | |
| Overall survival (median in months) | 6 | 5.4 | 6.7 | 7.6 | 7.1 | 4.6 | 0.002 | |
Table 1b.
Baseline high risk disease characteristics by study regimen
| Characteristics | Type of regimen | ||||
|---|---|---|---|---|---|
| IC (n=396) | Epigenetic (n=221) | CPX-351 (n=20) | LDAC-combinations (n=189) | INV group (n=109) | |
| Age > 70 (%) | 121 (30) [p< 0.001] | 101 (46) [p=0.04] | 8 (40) [p=0.14] | 66 (36) [p< 0.001] | 63 (58) [ref] |
| Prior therapy for antecedent disorder (%) | 79 (20) [p< 0.001] | 35 (16) [p< 0.001] | 13 (65) [ref] | 63 (34) [p=0.005] | 54 (50) [p=0.2] |
| Creatinine ≥ 1.3 (%) | 74 (18.6) [ref] | 53 (23.9) [p=0.192] | 3 (15) [p= 0.686] | 21 (11.1) [p=0.023] | 13 (11.9) [p=0.09] |
| WBC ≥ 50 K/μL (%) | 23 (5.7) [p=0.003] | 2 (0.9) [ref] | 2 (10) [P = 0.002] | 8 (4.2) [p= 0.445] | 3 (2.8) [p= 0.19] |
| PS = 2 (%) | 79 (19.8) [ref] | 34 (15) [P = 0.164] | 4 (20) [P = 0.982] | 18 (9.5) [p=0.001] | 12 (11) [p=0.03] |
The overall CR/CRp rates for the entire group was 39.5%. Table 2 summarizes univariate analysis of CR/CRp by patient and disease characteristics. On univariate analysis, prior treatment for AHD, age, cytogenetics and type of treatment had an impact on response rates achieved. Patients who had prior treatment for AHD had inferior response rates (24.5% vs 45%; p <0.001). In addition, adverse and intermediate risk (non-diploid) karyotype (vs diploid, 39% vs 52%; p=0.001, 37% vs 52%; p=0.002) predicted inferior responses to therapy. Complete remission rates were lower in the HMA (36%) and INV (16%) groups compared to IC (46%) (p = 0.03, p=0.001 respectively). On the multivariate analyses, 5 factors correlated with response rates to induction therapy. Treatment with epigenetic (HMA-based) and investigational agents, the presence of intermediate (excluding diploid) and adverse karyotype, and a history of prior treatment for AHD negatively impacted response rates (Table 3). Eight week mortality rate for the entire group was 20%: IC (23%), HMA (12%), CPX (10%), LDAC (19%), and INV/other (27%).
Table 2.
Univariate analyses of response rates and overall survival by patient and disease characteristics (n=931)
| Variable | Univariate analysis (n=931) | ||||
|---|---|---|---|---|---|
| Response Rates | Overall Survival | ||||
| CR rates (%) | P value | Deaths (%) | Median survival (in months) | P value | |
| Overall | - | 856/931 (92) | 6 | - | |
| Rx Gp | |||||
| IC | 183 (46) | <0.001 | 377/396 (95) | 5.4 | 0.002 |
| HMA | 79 (36) | 187/220 (85) | 6.7 | ||
| CPX | 9 (45) | 14/20 (70) | 7.6 | ||
| LDAC | 80 (43) | 175/186 (94) | 7.1 | ||
| Inv | 17 (27) | 103/109 (94) | 4.6 | ||
| Cyto Gp | |||||
| Bad | 212 (39) | 0.011 | 501/537 (93) | 5.1 | <0.001 |
| Dip | 83 (52) | 142/160 (89) | 9.7 | ||
| Inter | 49 (37) | 120/132 (91) | 6.7 | ||
| Unk | 24 | 93/102 (91) | 5.1 | ||
| Age | |||||
| 60–64 | 111 (45) | 0.07 | 223/246 (91) | 6.6 | 0.05 |
| 65–69 | 128 (39) | 297/325 (91) | 6.2 | ||
| 70–75 | 129 (36) | 336/360 ( 93) | 5.4 | ||
| Rx-related | |||||
| No | 223 (38) | 0.22 | 533/589 (90) | 6.2 | 0.6 |
| Yes | 145 (42) | 323/342 (94) | 5.5 | ||
| Prior Rx for AHD | |||||
| No | 308 (45) | <0.001 | 635/687 (92) | 6.5 | 0.05 |
| Yes | 60 (25) | 221/244 (91) | 4.7 | ||
Table 3.
Multivariate Analyses for response rate and overall survival
| Multivariate analyses for association of (CR/CRp) with clinic-pathological variables (n=829) | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | P value |
| IC, vs epigenetic therapy | 1.60 | (1.14–2.24) | 0.006 |
| IC, vs Investigational therapy | 3.70 | (2.04–6.74) | 0.001 |
| Intermediate karyotype, vs diploid | 2.07 | (1.27–3.38) | 0.003 |
| Adverse karytotype, vs diploid | 2.08 | (1.43–3.04) | 0.001 |
| Prior Rx for AHD, vs none | 2.35 | (1.58–3.48) | 0.001 |
| Multivariate cox regression for association of overall survival with patient and disease characteristics (n=829) | |||
| Variable | Hazards ratio | 95% CI | P value |
| Prior Rx | 1.21 | 1.01–1.46 | 0.035 |
| Karyotype, bad vs other | 1.55 | 1.33–1.81 | 0.001 |
| Age, >70 vs other | 1.29 | 1.11–1.49 | 0.001 |
The median OS for the entire group was 6 months. Overall survival by each treatment cohort is shown in Figure 1. There was a significant difference in OS between the 5 treatment groups (p<0.002). No statistically significant difference in median survival was identified when comparing individual lower intensity treatment groups against one another. However, despite a lower CR rate, there was a trend towards improved median survival in patients receiving HMA-based therapy over standard intensive care therapies (6.7 vs 5.2; p=0.058) and investigational agents (6.7 vs 4.6; p=0.06). Taken together, patients who received less intensive regimens [HMA- and LDAC- based] had a superior median OS compared to those receiving IC -based regimens (6.9 vs. 5.4; P=0.048, Figure 2). There was no difference in median survival in patients treated with CPX compared with conventional lower-intensity approaches (HMA + LDAC) (p=0.75). Of note, an overall survival analysis performed in patients with t-AML, by therapy regimen (i.e. IC vs Epi + LDAC), did not demonstrate a statistically significant difference in OS (6mo. vs 5.6mo, respectively; p=0.92) [data not shown].
Fig 1.
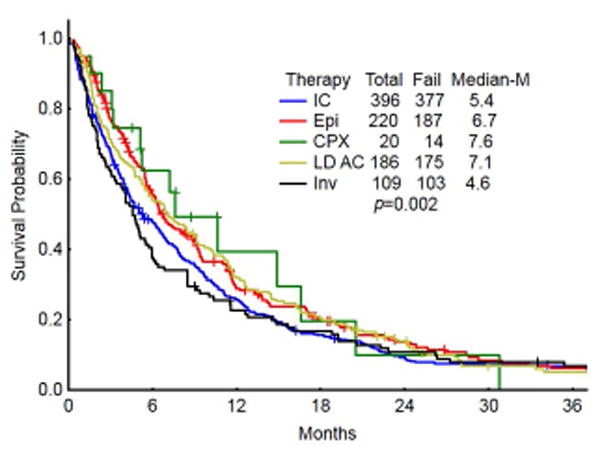
Kaplan-Meier Survival graph demonstrating OS in patients by the treatment regimen group. (IC- intensive chemotherapy; Epi – HMA or HMA combination therapies, CPX- CPX351, LDAC- low dose cytarabine/low dose cytarabine combinations, INV-Investigational therapies)
Fig 2.

OS comparison in patients who received IC vs less intensive (LDAC and HMA-based) therapies. (IC-Intensive chemotherapy; Epi+LDAC- HMA based or low dose cytarabine based therapies)
Patients who received transplant (7.1% of patients) had superior outcomes in terms of median survival (OS) compared with patients who did not proceed to transplant post chemotherapy (12.6 mo vs 4.5 mo, respectively; p<0.001) [Fig 3A]. 40% of the transplanted patients belonged to the age cohort of 60–65 years, as compared with 18.5% of patients who did not receive transplant [p<0.001]. Superior survival outcomes with transplant were observed across the groups, irrespective of being treated with intensive chemotherapy [OS in transplant vs non-transplant arms: 16.2 vs 4.8 mo, respectively; p <0.001 (figure 3B)] or with lower intensive approaches [OS in transplant vs non-transplant arms: 12.1 vs 5.8 mo, respectively; p<.001 (figure 3C)]. Patients who proceeded to transplant constituted only 4.3% of IC group (median age 64), while 10.3% of patients who received lower intensity regimens were able to proceed to transplant (median age 66) [p=0.001]. To assess the possible reasons for this paradoxical disparity in proportions, a survival analysis was performed comparing survival in lower-intensity complete responders versus IC complete responders. For this analysis, survival was calculated from the time of complete response to death or censored at the time of transplant or last follow up date if neither death nor transplant occurred (Figure 3d). The median survival in complete responders on low intensity approaches was 12.8 months vs high intensity approaches of 10.2 months [p=0.41]. On closer inspection, intersection of survival curves early after complete response created a lack of statistically significant survival difference.
Fig 3.
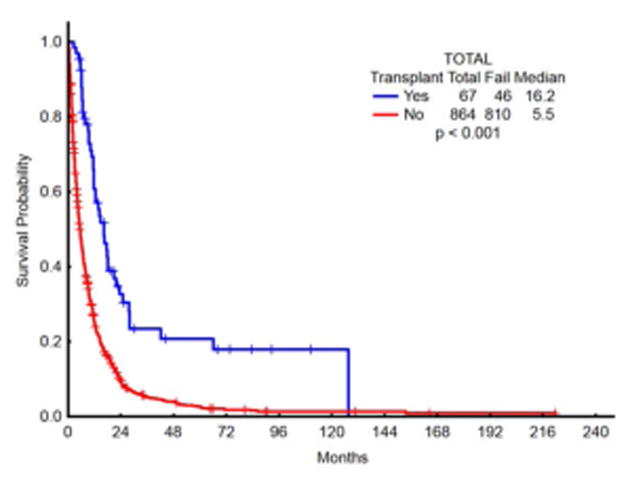
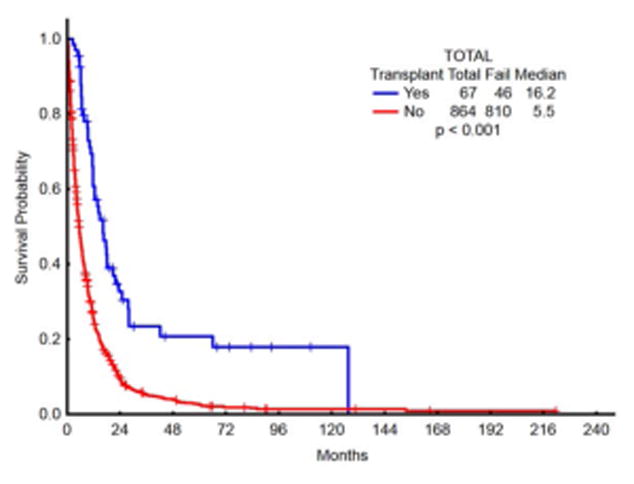


Fig 3a. OS comparison in patients who received chemo followed by transplant vs chemotherapy only
Fig 3b. OS comparison in IC patients who received chemo followed by transplant vs chemotherapy only
Fig 3c. OS comparison in less intensive (LDAC and HMA-based) patients who received chemo followed by transplant vs chemo only
Figure 3d. Overall survival after complete response [(LDAC + HMA) vs IC/HiDAC] while censoring for transplant.
Univariably, type of treatment approach, age, cytogenetics, and prior treatment for AHD were associated with differences in OS (Table 2). On multivariate cox regression analysis, age >70yrs, adverse karyotype, and prior treatment for AHD were associated with a significantly decreased OS (table 3).
DISCUSSION
Unlike in the younger AML population, older AML patients have witnessed little improvement in prognosis over the past few decades 18. Improvement in outcomes in the younger AML population is partly related to more effective post remission therapies 19. In contrast to the younger AML patients, post-remission therapy has shown to be of limited value in older patients 20. Although low-risk older AML patients may achieve up to 50% remission rates on standard 7+3 based approach, risk of relapse is as high as 85% at 3 years 21. These response rates drop to less than 30% in patients with high risk cytogenetics 8, 22. High-dose cytarabine based therapies may result in improved response duration in patients who respond but this comes at the cost of increased early treatment-related mortality. This is of major consequence in older AML patients, the majority of whom do not ‘tolerate’ standard induction doses. Randomized control trials comparing standard induction and less intensive chemotherapies in older AML patients are scarce and there is currently no recommended standard of care in the management of this subset of patients, with the National Comprehensive Care Network (NCCN) and European Leukemia Net (ELN) divided in opinion on the optimal mode of therapeutic strategy. One of the major challenges in conducting comparison trials between intensive versus non-intensive therapies in older AML patients are concerns over issues related to ‘tolerability’, comorbidity, and performance status, among others. In this context, conducting trials in AML patients with high risk prognostic features calls for further genomic annotation to define who may benefit from cytarabine-based therapy. The most recently updated ELN guidelines recommend standard induction cytarabine-anthracycline based regimen for patients aged 60–74 years with a good performance status and favorable cytogenetics. They suggest an investigational treatment approach for patients with adverse-risk cytogenetics recognizing the significant impact that cytogenetics have on response to standard induction therapy 23, 24. NCCN guidelines recommend either participation in a clinical trial, treatment with standard therapy, or lower intensity approaches including HMAs (azacytidine, decitabine), and low dose cytarabine 25.
Azacytidine and decitabine are the two most commonly used low intensity therapies, along with LDAC in the management of AML. It is important to note that responses in patients receiving hypomethylating agents may not be evident until after a few cycles of therapy. As an example, in older (≥65 years) patients with newly diagnosed AML treated with decitabine, the median time to response for patients achieving CR/CRp was 4.3 months 26. In our study, only 36% of patients on HMA-based regimens had shown a complete response, far lower than the CR rates achieved on IC (45%). In fact, the multivariate analysis in our study showed that use of HMAs was a predictor for decreased CR rate. However, we observed that this did not reflect in the overall survival rates, as noted by the trend towards superior median overall survival in patients who received HMA based therapy compared with those who had IC 27. In this regard, OS of patients receiving HMA therapy may not be predicted by the type of response as much by the evidence of any degree of improvement (either CR, PR or HI), including stable disease 28. Furthermore, retrospective data have suggested possible superiority of hypomethylating agents over traditional cytotoxic agents in patients with adverse risk cytogenetics 29.
Achieving complete remission may be of lesser consequence in an older AML patient whose primary goal of therapy is to improve survival while maintaining quality of life and is less to achieve cure by proceeding for a transplant 30, 31. That being said, reduced intensity nonmyeloablative chemotherapy- transplantation may still be an option if applicable in select patients. Interestingly, we noted that a fewer percentage of AML patients in the IC arm were able to proceed to transplant compared with patients who received low intensive therapy (4.3% vs 10.3%, respectively; p<0.001). This, despite higher complete response rates in the IC arm. Although there was no statistically significant survival difference between the two groups, separation of survival curves later in the time-course suggest that complete responders on lower intensity approaches seem to fare better when compared with complete responders on high intensive chemotherapy approaches [Figure 3d]. One might surmise that the lower proportion of patients proceeding to transplant in IC arm is likely related in part to the relatively higher rates of morbidity (which are not systematically captured) and mortality in this group precluding the option of transplant. Also, patients on the lower intensity approaches [such as (clofarabine/cladribine +LDAC) or HMA] are typically able to continue receiving further cycles, thus maintaining their response while awaiting transplant, and averting relapse-related mortality. Nevertheless, there may have been other factors involved including the availability of suitable donor, patient/physician choice of therapy, and fitness for transplant. A study assessing the feasibility of reduced intensity-allo transplant (RIC) in first CR in older AML patients demonstrated improved event free and OS. However, the median age of the SCT patients was 57 with only 6 patients above the age of 60, and only 2 patients with adverse karyotype 32. A prospective study in older patients (>60 years) is required to address the feasibility of non-myeloablative transplant in higher risk settings.
After limited success with the addition of various agents to the cytarabine- daunorubicin backbone, attempts were made to optimize efficacy by locking both agents in a fixed ratiometric dosing within a liposomal drug carrier 33. Pre-clinical leukemic models showed markedly improved efficacy with CPX-351 against leukemic blasts 33, 34. A muticenter, randomized phase II trial data analysis reported improved response rate (57.6% vs 31.6%, P = .06), and increased OS (12.1 vs 6.1 months, HR = 0.46, P = .01) in older s-AML patients assigned to CPX-351 over conventional 7+3 regimen 35. Importantly, responses were not affected even if patients had received HMAs for prior MDS. The superior efficacy was later confirmed in a phase III trial comparing CPX 351 with standard 7+3 induction chemotherapy (OS 9.56 months vs 5.95 months, p=0.005, CR+CRi of 47.7% versus 33.3%; p=0.016) 36. Our data on outcomes in patients who received CPX-351 compared less favorably, in terms of response rates and median survival, to data from both of these trials 35, 36. The reason for the lower median survival observed in our CPX-351 study group may relate to differences in dosing of CPX-351 and a higher percentage of poor risk disease (45% adverse karyotype, 75% aged >65) 31,37. Further, we did not observe a statistical difference in median overall survival between the CPX-351 and IC groups. This may again be due to the lower median survival, as compared to historical data, in the CPX-351 group secondary to factors mentioned above and the nature of the comparator IC arm which was constituted by regimens such as IA (idarubicin, HiDAC), FIA (fludarabine, idarubicin, cytarabine) (both containing higher dose cytarabine), among others. Finally, patients who received other conventional low intensity approaches (HMA and LDAC therapies) had similar overall survival when compared with patients who received CPX-351 (p=0.75). This is especially important to note in the context of CPX-351 as a potential first line option in high risk AML patients.
Cytarabine may be administered at a reduced dose (LDAC) in patients who may not tolerate standard dosing induction chemotherapy. LDAC is an alternative in older infirm AML patients who would otherwise not tolerate standard induction 38. However, LDAC alone is not considered curative and is not favored among physicians due to its minimal efficacy. Complete response rates can vary from 0% to above 30% depending on whether cytogenetics are adverse or favorable, respectively 13, 39–41. Efforts to improve outcomes have resulted in a bevy of clinical trials, of novel agents in combination with LDAC. Purine nucleoside analogs in particular have proven to be effective in improving response rates and median survival, when used in combination with low dose cytarabine 42, 43. A propensity-score matched comparison of patients given clofarabine and LDAC over standard induction chemotherapy in older AML patients (>60) found the former to be non-inferior with no difference in response rates or overall survival. As was expected, the lower intensity therapy patient cohort also experienced few toxicities 44. A number of other novel therapeutics have entered into trials either as single agents or in combination with other low intensity agents. Trials employing these agents in combination regimens with LDAC have shown improvements in response rates and overall survival when compared with LDAC alone. Of them, Volsertib (a Polo-like kinase 1 inhibitor) 45, vosaroxin (a quinolone derivative which is a topoisomerase II inhibitor) 46, guadecitbine (a dinucleotide of decitabine and deoxyguanosine) 47, gemtuzumab ozagomicin 48, venetoclax 49 are of notable mention. Our data on the LDAC group compares favorably with historical data on LDAC alone, and this is likely due to the fact almost all our patients belonging to the LDAC group were on combination regimens (supplemental figure 2). Of consequence is the fact that this group did not perform inferiorly to other groups, in terms of either response rates or median survival despite the majority having adverse cytogenetics (a major factor determining response to LDAC). This points to the potential effect of combining agents (such as clofarabine or cladribine) to LDAC to circumvent the problem of poor responsiveness to low dose cytarabine.
Most prior studies assigned low-intensity therapy approaches to patients considered ‘unfit’ for intensive chemotherapy. One of the important strengths of our study is that patients in each group were fairly well balanced in prognostically-relevant disease characteristics, and had minimal comorbidities and a reasonable performance status. This allowed us to comment and make comparisons of various treatment approaches in these high risk patients considered to be in ‘the zone of therapeutic uncertainty.’ Our study may be criticized on several grounds. Our methods involved a retrospective study design which suffers from its inherent limitations including a potential for treatment selection biases. We did not have data and hence were not able to include molecular status, an emerging prognostic factor in AML outcomes going forward. Recent studies have shown that specific molecular mutation patterns have an impact on response durations and survival outcomes 50, 51. Molecular characterization will facilitate further prognostic risk stratification of this high risk AML population. Efforts are underway to prospectively molecularly characterize all new AML patients at our institution. The CPX-cohort consisted of a small group of patients, and this may have contributed to the statistically insignificance despite high CR rates and a median survival of 7.6 months. However, results from this study should encourage future prospective studies comparing CPX-351 against other lower intensity approaches. Patient assignment in the two of the five groups was based on the intensity of the cytarabine backbone, irrespective of what the other combination agents involved were (i.e, either standard induction or LDAC-based). The resulting within-group heterogeneity caused by pooling intervention regimens of varying efficacy would undermine external validity comparisons. That being said, the primary intention of this study was to examine relationships between disease characteristics and outcomes, and to assess response rates and overall survival on different types of therapies.
Acknowledging these limitations, a few generalizations can be made. Outcomes of older patients with newly diagnosed high risk AML are poor and reflective of their advanced age and poor-risk karyotype. Patients on standard induction and high dose cytarabine regimens have marginally higher complete response rates but also experience a higher 8 week treatment mortality. In comparison, patients on lower-intensity approaches involving HMA and LDAC-based regimens experience lower early mortality with a trend towards improved overall survival. No single treatment group is predictive of improved survival but lower intensity regimens promise longer survival and lower early mortality when compared with high intensity regimens. Our study data on the INV group is disappointing in light of the need for new treatments but we refrain from commenting further due to many different novel agent combinations constituting this group. Also, patients in the INV group were older and had other high risk features. The less than optimal outcomes, in this group, are likely related in part to selection biases associated with the retrospective study analysis.
In conclusion, the results of our study reiterate the fact that standard induction or high intensity regimens would not be the ideal option in older AML patients who harbor poor-risk disease characteristics. With improvements in supportive care and of tools predicting treatment-related mortality with reasonable accuracy, the decision of which therapy to give in this subset of patients is a challenge that is still open to debate and requires intensive study 20. Results from our study should help reflect on the paradigm shift towards favoring lower intensity combination approaches even in patients who are expected to ‘tolerate’ high intensity chemotherapy. As was discussed above, patient with high risk disease characteristics are typically resistant to conventional care high intensity approaches and are most appropriate for investigational-low intensity combination therapies. Rational combination of newer agents in novel combinations with low intensity cytarabine, epigenetic therapies and liposomal agents like CPX-351 may be the best step forward toward improving outcomes in these patients. Choosing appropriate therapy must be made after due consideration to the applicability of RIC in the few select group of patients 52. Unfortunately, unfavorable cytogenetic risk status predicts increased relapse after non-myeloablative SCT 53. It remains to be seen if the deleterious effects of an unfavorable cytogenetic and mutational profile can be circumvented by incorporating novel pre- and post- SCT regimens into future investigational trials 31, 54.
Supplementary Material
Supp. Figure 1: Treatment regimens constituting the HiDAC/IC arm. IDA+HDAC- Idarubicin+high dose cytarabine; IA-based- Idarubicin+standard dose Arac; FIA-Fludarabine+IA; BIDFA-Twice daily Fludarabine and cytarabine; GO- Gemtuzumab ozogamicin; FLAG-Fludarabine+high dose Ara-C+G-CSF; CAT-Cyclophosphamide+Ara-C+Topotecan; VNP- Laromustine; Lipo DNR+HDAC- Liposomal Daunorubicin+ high dose Ara-C.
Supp. Figure 2: Treatment regimens constituting hypomethylating treatment group
Supp. Figure 3: Treatment regimens constituting the low dose cytarabine combination group arm .LDAC- low dose cytarabine; CLAD- cladribine; CLOF- clofarabine)
Supp. Figure 4: Treatment regimens constituting the investigational group arm
Key points.
Lower-intensity approaches (LDAC-based and hypomethyating agent-based) are associated with lower early mortality and improved OS when compared with intensive regimens in secondary AML.
CPX-351 is not statistically associated with a superior median survival compared with lower intensity therapy approaches
Acknowledgments
FUNDING SUPPORT
This study was supported by University of Texas MD AQ7 Anderson Cancer Center Support Grant (P30 CA16672 principal investigator, Dr. Ronald DePinho) and award P01 CA049639 (PI, Dr. Richard Champlin) from the National Cancer Institute (NCI). Jorge Cortes is recipient of a grant from the NCI (P01 CA049639).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Tapan Kadia, Jorge Cortes consulting Jazz Pharmaceuticals
AUTHOR CONTRIBUTIONS
TMK, PCB provided conception and design of the study, collection, analysis, and interpretation of data, provided patient data for study, writing and revising the article, and providing final review and approval. SP provided collection and analysis of data. HK, FR, CDD provided patient data for study, revising the article, and providing final review and approval. GGM, SV, EJJ, KT, KB, MK, MO, NP, NJ, WGW, and JEC provided data for study, final review and approval
References
- 1.Bennett JM. Secondary acute myeloid leukemia. Leuk Res. 1995;19:231–232. doi: 10.1016/0145-2126(95)00049-t. [DOI] [PubMed] [Google Scholar]
- 2.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20:29–37. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Arber DA. Realistic pathologic classification of acute myeloid leukemias. Am J Clin Pathol. 2001;115:552–560. doi: 10.1309/K2PH-L2U7-722B-B1YR. [DOI] [PubMed] [Google Scholar]
- 4.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 5.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 9.Baudard M, Marie JP, Cadiou M, Viguie F, Zittoun R. Acute myelogenous leukaemia in the elderly: retrospective study of 235 consecutive patients. Br J Haematol. 1994;86:82–91. doi: 10.1111/j.1365-2141.1994.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 10.Stone RM, Berg DT, George SL, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 11.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 12.Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 13.Tilly H, Castaigne S, Bordessoule D, et al. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1990;8:272–279. doi: 10.1200/JCO.1990.8.2.272. [DOI] [PubMed] [Google Scholar]
- 14.DeLima M, Ghaddar H, Pierce S, Estey E. Treatment of newly-diagnosed acute myelogenous leukaemia in patients aged 80 years and above. Br J Haematol. 1996;93:89–95. doi: 10.1046/j.1365-2141.1996.4771012.x. [DOI] [PubMed] [Google Scholar]
- 15.Estey EH. How I treat older patients with AML. Blood. 2000;96:1670–1673. [PubMed] [Google Scholar]
- 16.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotlib J, Pardanani A, Akin C, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121:2393–2401. doi: 10.1182/blood-2012-09-458521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alibhai SM, Leach M, Minden MD, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115:2903–2911. doi: 10.1002/cncr.24373. [DOI] [PubMed] [Google Scholar]
- 19.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 20.Walter RB, Estey EH. Management of older or unfit patients with acute myeloid leukemia. Leukemia. 2015;29:770–775. doi: 10.1038/leu.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 22.Malfuson JV, Etienne A, Turlure P, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93:1806–1813. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 23.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 24.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 25.Appelbaum FR, Baer MR, Carabasi MH, et al. NCCN Practice Guidelines for Acute Myelogenous Leukemia. Oncology (Williston Park) 2000;14:53–61. [PubMed] [Google Scholar]
- 26.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120:4840–4845. doi: 10.1182/blood-2012-06-436055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore S, PF, Santini V, Bennett JM, Silverman LR, Seymour JF, Hellstrom-Lindberg E, Swern AS, Beach CL, List AF. Time-dependent decision analysis: Stable disease in azacitidine (AZA)-treated patients (pts) with higher-risk MDS. J Clin Oncol. 2010;28(15s) (suppl; abstr 6503). 2010 ASCO Annual Meeting. [Google Scholar]
- 29.Ravandi F, Issa JP, Garcia-Manero G, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer. 2009;115:5746–5751. doi: 10.1002/cncr.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estey E. Recent developments in management of older patients with acute myeloid leukemia. Ther Adv Hematol. 2012;3:89–96. doi: 10.1177/2040620711434319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 33.Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36:1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Lim WS, Tardi PG, Xie X, et al. Schedule- and dose-dependency of CPX-351, a synergistic fixed ratio cytarabine:daunorubicin formulation, in consolidation treatment against human leukemia xenografts. Leuk Lymphoma. 2010;51:1536–1542. doi: 10.3109/10428194.2010.490312. [DOI] [PubMed] [Google Scholar]
- 35.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lancet effrey E, JEC, Newell Laura F, Lin Tara L, Ritchie Ellen K, Stuart Robert K, et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol. 2016:34. (suppl; abstr 7000) [Google Scholar]
- 37.Feldman Eric Jay, JEL, Kolitz Jonathan E, Hogge Donna, Tallman Martin S, Goldberg Stuart L, Kovacsovics Tibor, Louie Arthur Chin, Cortes Jorge E. Multivariate analysis of factors affecting overall survival, event free survival, and 60-day mortality among AML patients treated with CPX-351 or intensive chemotherapy. J Clin Oncol. 2013;31 (suppl; abstr 7100). 2013 ASCO Annual Meeting. [Google Scholar]
- 38.Housset M, Daniel MT, Degos L. Small doses of ARA-C in the treatment of acute myeloid leukaemia: differentiation of myeloid leukaemia cells? Br J Haematol. 1982;51:125–129. doi: 10.1111/j.1365-2141.1982.tb07297.x. [DOI] [PubMed] [Google Scholar]
- 39.Bashir Y, Geelani S, Bashir N, et al. Role of low dose cytarabine in elderly patients with acute myeloid leukemia: An experience. South Asian J Cancer. 2015;4:4–6. doi: 10.4103/2278-330X.149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castaigne S, Tilly H, Sigaux F, Daniel MT, Degos L. Treatment of leukemia with low dose Ara-C: a study of 159 cases. Haematol Blood Transfus. 1985;29:56–59. doi: 10.1007/978-3-642-70385-0_13. [DOI] [PubMed] [Google Scholar]
- 41.Deschler B, de Witte T, Mertelsmann R, Lubbert M. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: problems and approaches. Haematologica. 2006;91:1513–1522. [PubMed] [Google Scholar]
- 42.Kadia TM, Faderl S, Ravandi F, et al. Final results of a phase 2 trial of clofarabine and low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Cancer. 2015;121:2375–2382. doi: 10.1002/cncr.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadia T, Borthakur G, Ferrajoli A, et al. Phase II Trial Of Cladribine and Low-Dose AraC (LDAC) Alternating With Decitabine In Older Patients With Acute Myeloid Leukemia (AML) Blood. 2013;122:5011–5011. [Google Scholar]
- 44.Takahashi K, Kantarjian H, Garcia-Manero G, et al. Clofarabine Plus Low-Dose Cytarabine Is as Effective as and Less Toxic Than Intensive Chemotherapy in Elderly AML Patients. Clin Lymphoma Myeloma Leuk. 2016;16:163–168. e161–162. doi: 10.1016/j.clml.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dohner H, Lubbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennis M, Russell N, Hills RK, et al. Vosaroxin and vosaroxin plus low-dose Ara-C (LDAC) vs low-dose Ara-C alone in older patients with acute myeloid leukemia. Blood. 2015;125:2923–2932. doi: 10.1182/blood-2014-10-608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kropf Patricia, EJ, Yee Karen, O’Connell Casey, Tibes Raoul, Roboz Gail J, Walsh Katherine, Podoltsev Nikola A, Savona Michael, Issa Jean-Pierre, Hao Yong, Naim Sue, Azab Mohammad, Kantarjian Hagop. Late responses and overall survival (os) from long term follow up of a randomized phase 2 study of sgi-110 (guadecitabine) 5-day regimen in elderly aml who are not eligible for intensive chemotherapy. EHA Abstract. 2015:P571. [Google Scholar]
- 48.Piccaluga PP, Martinelli G, Rondoni M, et al. First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leuk Res. 2004;28:987–990. doi: 10.1016/j.leukres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Wei Andrew, SAS, Roboz Gail J, Hou Jing-Zhou, Fiedler Walter, Lin Tara L, Martinelli Giovanni, Walter Roland B, Enjeti Anoop, Fakouhi Kaffa, Darden David E, Dunbar Martin, Zhu Ming, Agarwal Suresh, Salem Ahmed H, Mabry Mack, Hayslip John. Safety and Efficacy of Venetoclax Plus Low-Dose Cytarabine in Treatment-Naive Patients Aged ≥65 Years with Acute Myeloid Leukemia. ASH session: 616. Acute Myeloid Leukemia: Novel Therapy, excluding Transplantation: Novel Agents in AML Therapy. 2016 [Google Scholar]
- 50.Ohgami RS, Ma L, Merker JD, et al. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Mod Pathol. 2015;28:706–714. doi: 10.1038/modpathol.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devillier R, Gelsi-Boyer V, Brecqueville M, et al. Acute myeloid leukemia with myelodysplasia-related changes are characterized by a specific molecular pattern with high frequency of ASXL1 mutations. Am J Hematol. 2012;87:659–662. doi: 10.1002/ajh.23211. [DOI] [PubMed] [Google Scholar]
- 52.Farag S, Maharry K, Perez WS, et al. Allogeneic Hematopoietic Stem Cell Transplantation (HCT) Compared to Chemotherapy Only in Acute Myeloid Leukemia (AML) Patients 60 Years and Older: A Center for International Blood and Marrow Transplantation Research (CIBMTR)/Cancer and Leukemia Group B (CALGB) Study. Blood. 2009;114:657–657. [Google Scholar]
- 53.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure 1: Treatment regimens constituting the HiDAC/IC arm. IDA+HDAC- Idarubicin+high dose cytarabine; IA-based- Idarubicin+standard dose Arac; FIA-Fludarabine+IA; BIDFA-Twice daily Fludarabine and cytarabine; GO- Gemtuzumab ozogamicin; FLAG-Fludarabine+high dose Ara-C+G-CSF; CAT-Cyclophosphamide+Ara-C+Topotecan; VNP- Laromustine; Lipo DNR+HDAC- Liposomal Daunorubicin+ high dose Ara-C.
Supp. Figure 2: Treatment regimens constituting hypomethylating treatment group
Supp. Figure 3: Treatment regimens constituting the low dose cytarabine combination group arm .LDAC- low dose cytarabine; CLAD- cladribine; CLOF- clofarabine)
Supp. Figure 4: Treatment regimens constituting the investigational group arm


