Abstract
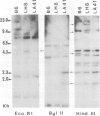
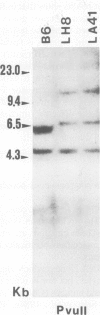
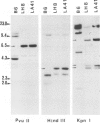
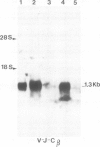
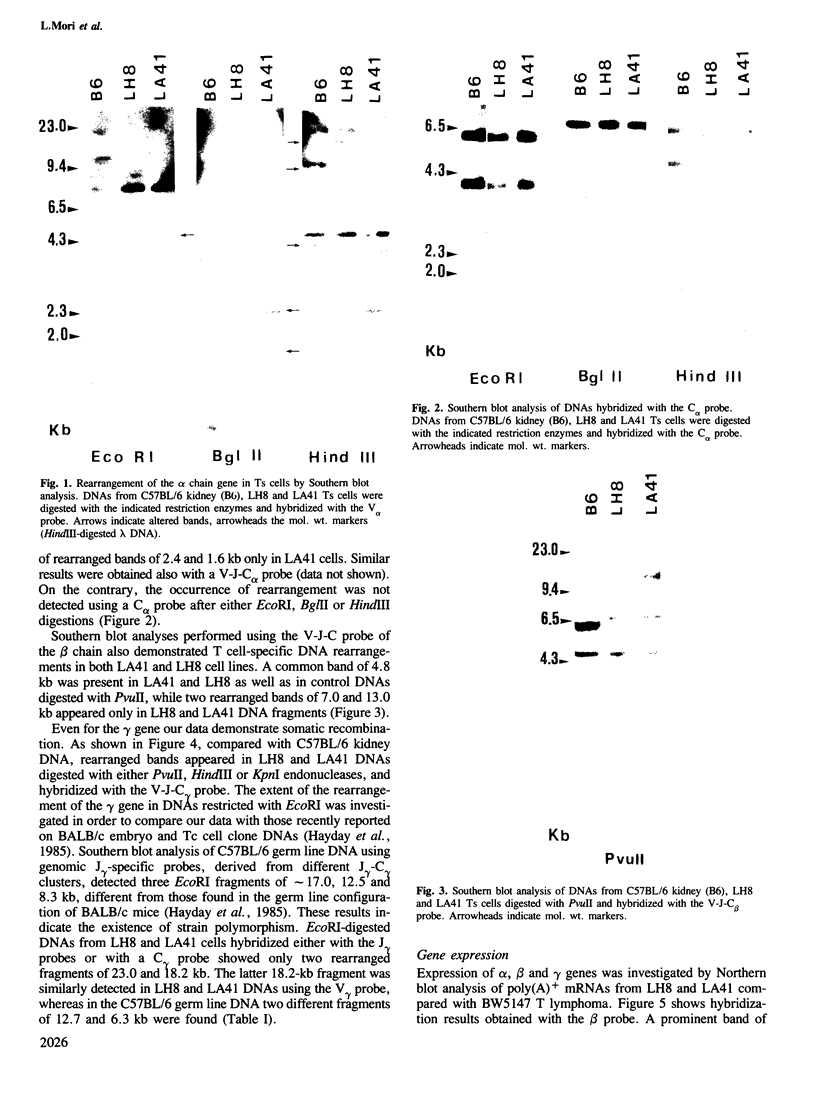
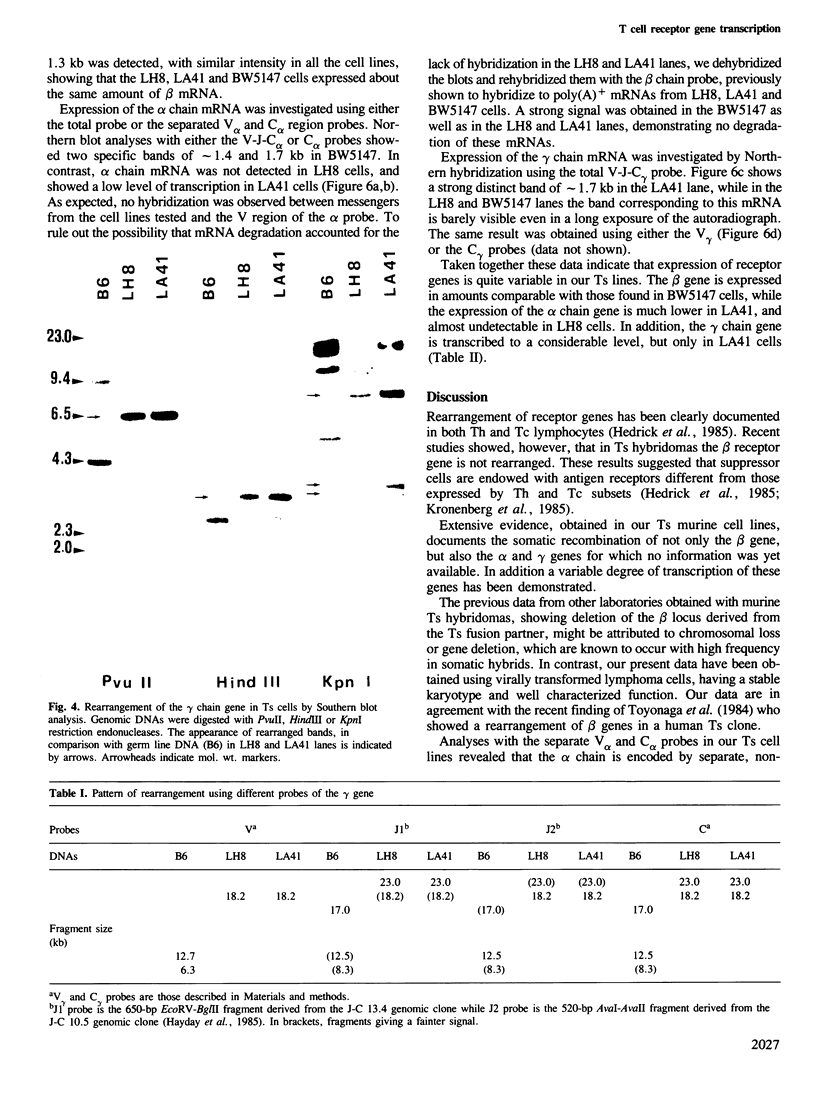
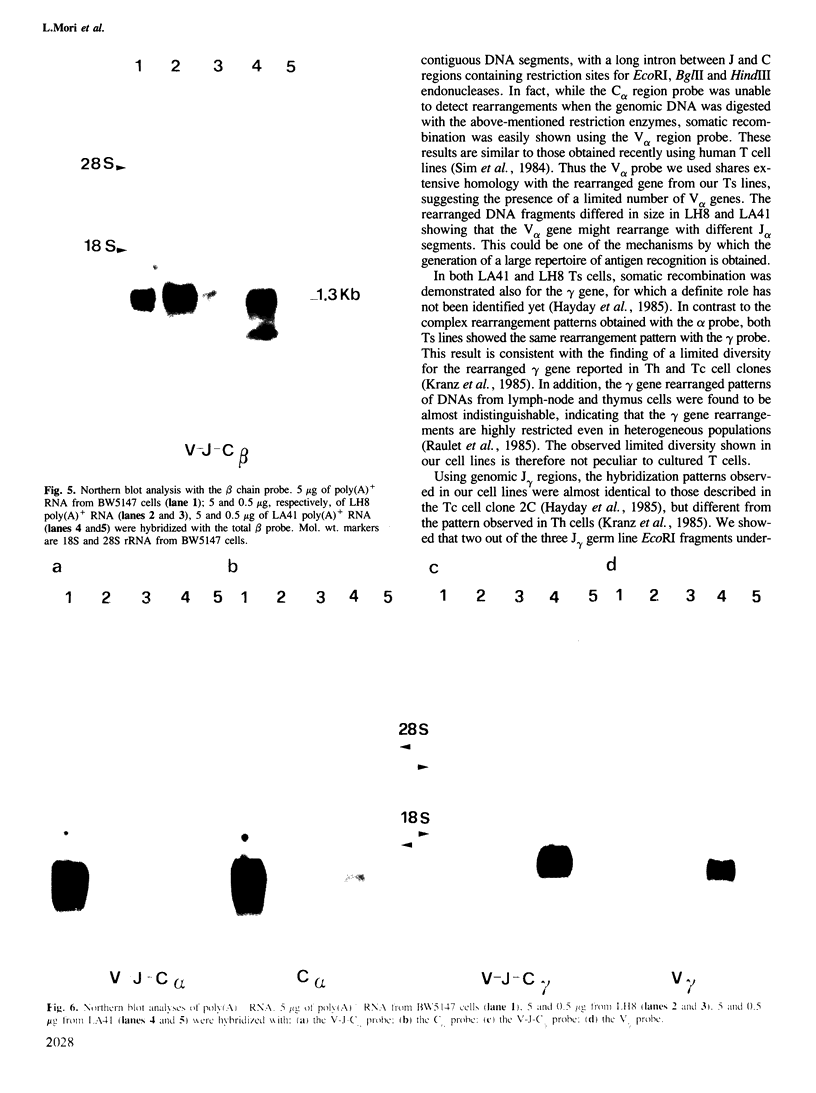
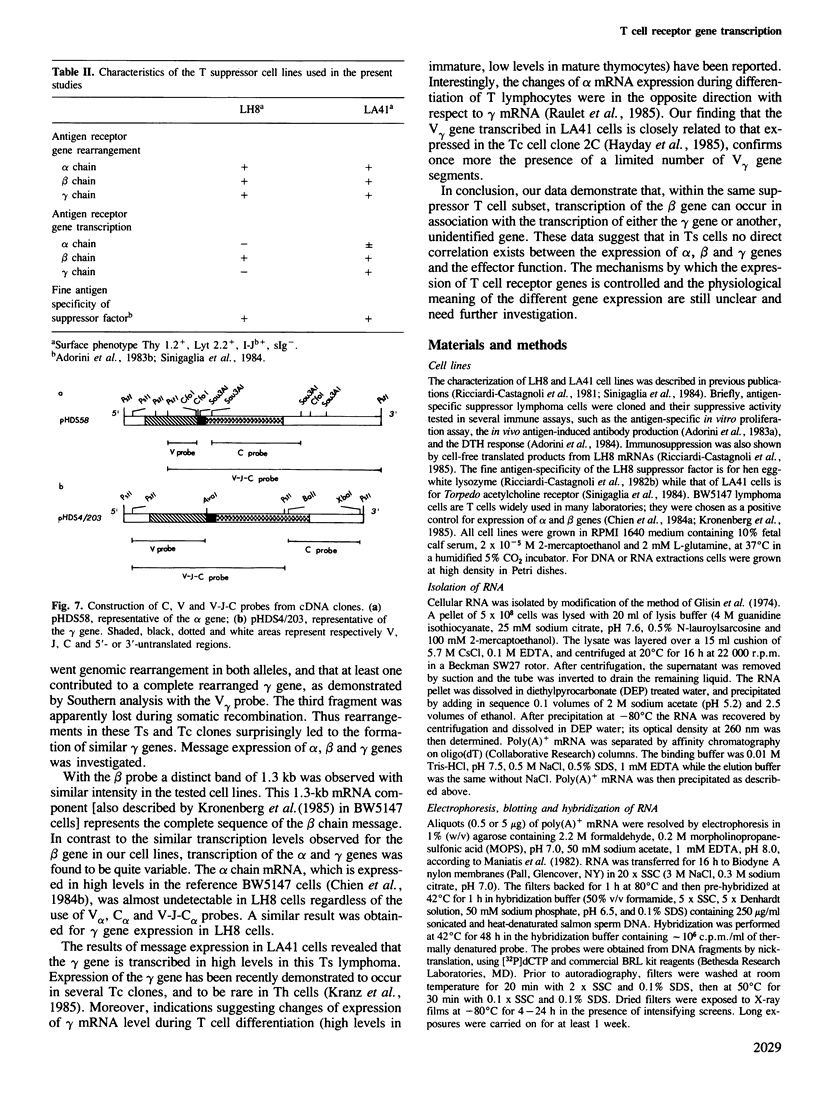
The rearrangement and transcription of the antigen receptor alpha, beta and gamma genes were investigated in murine antigen-specific suppressor T cell lines, to establish whether the suppressor T cell subset expresses the same antigen receptor transcripts previously found in helper and cytotoxic T lymphocytes. The genomic organization of the alpha, beta and gamma chain loci was investigated using probes representative of the entire gene or fragments from variable, joining and constant regions. The present results show that in functional suppressor T cells the three antigen receptor genes are all rearranged. The beta gene is expressed in all the tested cell lines, while the expression of the alpha and gamma genes is variable. In one cell line (LH8) alpha and gamma genes are not efficiently transcribed; in the other cell line (LA41) the gamma mRNA is found in amounts similar to beta mRNA, whereas the alpha gene is expressed at low levels. These data suggest that in suppressor T cells no direct correlation exists between the expression of alpha, beta and gamma antigen receptor genes and the effector function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Colizzi V., Doria G., Ricciardi-Castagnoli P. Immunoregulation of lysozyme-specific suppression. II. Hen egg-white lysozyme-specific monoclonal suppressor T cell factor suppresses the afferent phase of delayed-type hypersensitivity and induces second-order suppressor T cells. Eur J Immunol. 1984 Sep;14(9):826–830. doi: 10.1002/eji.1830140911. [DOI] [PubMed] [Google Scholar]
- Adorini L., Doria G., Ricciardi-Castagnoli P. Fine antigenic specificity and genetic restriction of lysozyme-specific suppressor T cell factor produced by radiation leukemia virus-transformed suppressor T cells. Eur J Immunol. 1982 Sep;12(9):719–724. doi: 10.1002/eji.1830120905. [DOI] [PubMed] [Google Scholar]
- Adorini L., Pini C., De Santis R., Robbiati F., Doria G., Ricciardi-Castagnoli P. Monoclonal suppressor T-cell factor displaying V H restriction and fine antigenic specificity. Nature. 1983 Jun 23;303(5919):704–706. doi: 10.1038/303704a0. [DOI] [PubMed] [Google Scholar]
- Allison J. P., McIntyre B. W., Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982 Nov;129(5):2293–2300. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Germain R. N., Bevan M. J., Dorf M., Engel I., Fink P., Gascoigne N., Heber-Katz E., Kapp J., Kaufmann Y. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985 Jan;82(2):531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi-Castagnoli P., Doria G., Adorini L. Production of antigen-specific suppressive T cell factor by radiation leukemia virus-transformed suppressor T cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3804–3808. doi: 10.1073/pnas.78.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi-Castagnoli P., Robbiati F., Barbanti E., Doria G., Adorini L. Establishment of functional, antigen-specific T cell lines by radLV-induced transformation of murine T lymphocytes. Curr Top Microbiol Immunol. 1982;100:89–96. doi: 10.1007/978-3-642-68586-6_10. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Gotti C., Castagnoli R., Clementi F. Acetylcholine receptor-specific suppressive T-cell factor from a retrovirally transformed T-cell line. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7569–7573. doi: 10.1073/pnas.81.23.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga B., Yanagi Y., Suciu-Foca N., Minden M., Mak T. W. Rearrangements of T-cell receptor gene YT35 in human DNA from thymic leukaemia T-cell lines and functional T-cell clones. 1984 Sep 27-Oct 3Nature. 311(5984):385–387. doi: 10.1038/311385a0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]