Abstract
Cell-cell adhesions are critical for the development and maintenance of tissues. Present at sites of cell-cell contact are the adherens junctions and tight junctions. The adherens junctions mediate cell-cell adhesion via the actions of nectins and cadherins. The tight junctions regulate passage of ions and small molecules between cells and establish cell polarity. Historically, the adherens and tight junctions have been thought of as discrete complexes. However, it is now clear that a high level of interdependency exists between the two junctional complexes. The adherens junctions and tight junctions are physically linked, by the zonula occludens proteins, and linked via signaling molecules including several polarity complexes and actin cytoskeletal modifiers. This review will first describe the individual components of both the adherens and tight junctions and then discuss the coupling of the two complexes with an emphasis on the signaling links and physical interactions between the two junctional complexes.
1. Introduction
The interactions between cells are important for the assembly and maintenance of three dimensional tissues. Ultrastructural studies reveal that cells are connected by multiple junctional complexes, including the tight junctions, adherens junctions, gap junctions, and desmosomes. These junctions are arranged with the tight junctions nearest the apical (lumen exposed) portion of the cell and the adherens junctions immediately underneath the tight junctions. Both the gap junctions and desmosomes are located more basally. Of these junctional components, the tight junctions and adherens junctions have been highly studied and are the subject of this review. It is appreciated that tight junctions are multiprotein complexes found in regions where membranes of two cells join together. The tight junctions have two functions: 1) a fence function, which prevents mixing of membrane lipids between the apical and basolateral membranes and 2) a gate function, which regulates the passage of molecules and ions between cells. In contrast, the adherens junctions contain two subcomplexes: the nectin-based adhesions, which form the first attachment of cells to their neighbors and the cadherin-based adhesions which mediate strong cell-cell adhesion.
While the adherens junctions and tight junctions have historically been studied as discrete complexes, evidence suggests a high level of interdependency. The formation of tight junctions is dependent on the cadherin- and nectin-based adhesions. Conversely, mutated tight junction proteins delay the maturation of adherens junctions [1]. Hence, these junctions are not discrete but highly interdependent. This review will first describe the main constituents of adherens junctions and tight junctions and then will focus on connections and interplay between the two junctions.
2. Adherens Junctions
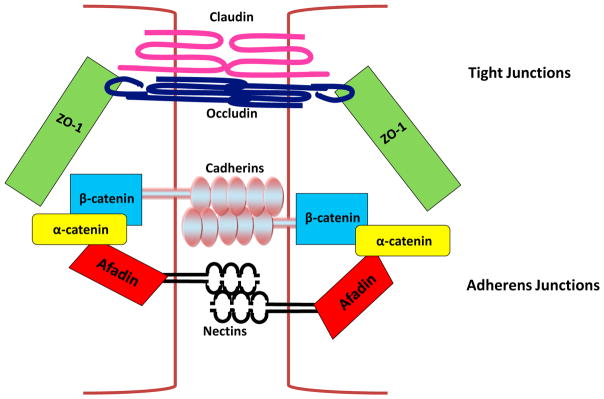
The adherens junctions are comprised of two families of transmembrane spanning, adhesive receptors: the cadherins and the nectins (Figure 1). The extracellular regions of these proteins mediate adhesion of cells to their neighbors while the intracellular regions interact with an array of proteins. These intracellular proteins control the assembly and dynamics of adherens junctions by modulating connections with the actin cytoskeleton and stimulating signaling pathways.
Fig. 1. Schematic of the adherens junctions and tight junctions.
The adherens junctions are composed of the nectin-based adhesions and the cadherin-based adhesions. The extracellular domains of nectins dimerize with nectins on neighboring cells and the cyotplasmic tail recruits afadin. Similarly, cadherins bind to cadherins on adjacent cells. The cadherin cytoplasmic tail recruits β-catenin which in turn binds α-catenin. More apical to the adherens junctions are the tight junctions. The main constituents of the tight junctions are two transmembrane spanning proteins (occludin and claudin). Occludin recruits ZO-1, an actin binding protein, that can during the formation of cell-cell junctions bind to the adherens junction protein, α-catenin. The brown lines indicate the plasma membranes of two adjacent cells.
2.1 Cadherin-based adhesions
There are over 20 members of the cadherin superfamily. Epithelial cadherin, or E-cadherin, is found primarily in epithelial tissues and is the focus of this review. E-cadherin has five immunoglobulin-like extracellular cadherin repeat domains that mediate the adhesion of cells to adjacent cells [2]. To mediate adhesion, cadherins first form cis-dimers with cadherins on the same cells [3–5]. Contact with an adjacent cell is then initiated by the formation of trans-dimers with cadherins on neighboring cells across the paracellular space [3, 4]. Trans- and cis-cadherin dimers mediate strong adhesion, thereby preventing the separation of cells.
Like the N-terminal domains, the E-cadherin cytoplasmic tail is highly conserved and mediates interactions with the catenin proteins and other actin cytoskeletal binding proteins [6, 7]. Catenins recruited to the cadherin cytoplasmic domain include β-catenin, α-catenin, and p120-catenin (Figure 1). The interaction between E-cadherin and β-catenin retains β-catenin at sites of cell-cell contact. Under these conditions, E-cadherin prevents β-catenin from entering the nucleus, binding the transcription factor, Lef, and stimulating transcription [8]. In addition, β-catenin binds α-catenin, [7] which links E-cadherin to the actin cytoskeleton. α-Catenin is an actin bundling protein. The Nelson laboratory suggested α-catenin was unable to bind β-catenin and actin filaments simultaneously [9]. However, it is now widely appreciated that α-catenin binds both β-catenin and actin filaments, but this event is restricted to cells under force [10]. A third catenin, p120-catenin directly binds to the E-cadherin juxtamembrane domain [6, 11, 12]. This interaction stabilizes E-cadherin at the plasma membrane, promotes cadherin clustering, and stimulates the Rho family GTPases [13]. Subsequently, the Rho family GTPases bind effector proteins and modulate actin dynamics.
2.2 Nectins
The nectin family contains four members each with several splice variants [14]. The nectin extracellular domain contains three Ig-like loops, followed by a single pass transmembrane domain, and a cytoplasmic tail [15]. The Ig-like loops are necessary for the dimerization of nectins. Similar to the cadherins, nectin dimerization occurs through the formation of cis dimers followed by trans dimer formation across cell-cell junctions [15].
Like cadherins, nectins mediate cell-cell adhesion and facilitate the establishment of apical-basolateral polarity. This adhesion is fundamentally different from the cadherins in two ways. First, nectin dimers are unable to support strong cell-cell adhesion, thereby rendering cadherins as the major cell-cell adhesion receptor in adherens junctions. Second, unlike cadherins, nectins can form homophilic interactions with the same family member and heterophilic interactions with related family members [16].
The cytoplasmic tails of nectins are involved in protein-protein interactions (Figure 1). At the C-terminus of nectins, a PDZ binding motif binds Afadin [15, 17]. Afadin is an actin binding protein that anchors the nectins to the actin cytoskeleton. The nectin-afadin interaction is essential for adherens junction maturation, as loss of afadin delays cadherin localization to cell-cell junctions and weakens adherens junctions [18]. In addition to afadin, nectins interact with cell polarity proteins, such as partitioning-defective homolog 3 (Par3) [15, 19]. This interaction ensures the correct spatial and temporal localization of Par3 and the subsequent establishment of apico-basolateral polarity.
3. Tight Junctions
The most apical of all the junctional components are the tight junctions (Figure 1). The tight junctions have two functions. They have a barrier or gate function that regulates the passage of ions, water, and macromolecules through the regions between cells (i.e. paracellular space). Tight junctions also have a fence function that establishes and maintains cell polarity by restricting the distribution of lipids within the membrane.
Tight junctions are composed of greater than 40 proteins that are either transmembrane proteins or cytoplasmic actin-binding proteins. The former are responsible for establishing cell-cell contact in the intercellular space, while the latter serve as a link to the actin cytoskeleton. The primary constituents in the transmembrane group are the claudins, occludin, and junctional adhesion molecules (JAMs). The primary cytoplasmic actin binding proteins in the tight junctions are the zonula occludens (ZO) proteins.
3.1 Claudins
The claudins form the backbone of tight junctions and are the most important components of the tight junctions. They regulate the gate function of tight junctions by restricting the elements that pass by their size. The claudins have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm [20, 21]. These domains are arranged such that there are two large extracellular loops and a cytoplasmic tail. Extracellular loop 1 contains several charged amino acid residues that regulate paracellular charge selectivity [22]. The second extracellular loop mediates claudin dimerization between adjacent cells, helping to seal the junction and regulate paracellular transport [23]. The claudin cytoplasmic C-terminal tail has a PDZ motif through which it recruits PDZ scaffolding proteins [24–26].
3.2 Occludin
Unlike the claudins, which are essential for the formation of tight junction, occludin is dispensable for tight junction assembly [27]. Rather it is important in maintaining the stability and barrier function of the tight junctions. Occludin spans the membrane four times forming two extracellular loops and cytoplasmic N- and C-termini [28, 29]. The first extracellular loop mediates interactions of occludin between adjacent cells, while the second extracellular loop is required for localization of occludin to the tight junctions [30]. The cytoplasmic tails are oriented in the cytoplasm where they are poised to mediate protein-protein interactions. The C-terminus is important for the barrier formation of tight junctions and binds several cytoplasmic proteins, including the ZO proteins [31].
3.3 ZO proteins
The zonula occludens (ZO) proteins, ZO-1, ZO-2, and ZO-3, are structurally similar with two domains important for mediating protein-protein interactions. These include a PDZ and a SH3 domain that are interspersed with conserved protein-binding domains known as unique (U) motifs [29]. Each domain mediates interactions with specific proteins known to localize to adherens junctions and/or tight junctions. For example, ZO-1 PDZ domains bind to tight junction proteins, such as claudins and JAMs, while the other ZO regions are thought to bind occludin [26, 32–34]. In addition to tight junction proteins, ZO-1 binds the adherens junction proteins afadin and α-catenin [35–37]. Given its interactions with adherens junction and tight junction components, ZO-1 is poised to link the two junctional complexes.
3.4 Junctional Adhesion Molecules
The final transmembrane components of the tight junctions are the junctional adhesion molecules (JAMs). JAMs are single membrane spanning proteins with an extracellular domain of two IgG-like folds, a transmembrane domain, and a cytoplasmic tail [38]. The extracellular domains mediate homophilic interactions between adjacent cells to stabilize cell-cell junctions. Like claudins and occludin, the cytoplasmic tail is involved in protein-protein interactions.
JAMs localize to tight junctions and support their formation. Experiments show that the appearance of JAMs at cell-cell junctions increases electrical resistance and reduces paracellular permeability [39]. In addition to maintaining the barrier function of tight junctions, JAMs recruit polarity complex proteins and scaffolding proteins, like ZO-1 to help establish the apical and basolateral polarity of cell-cell junctions [40].
4. Interplay between tight junctions and adherens junctions
4.1 Physical linkages between the adherens junctions and tight junctions: α-catenin, ZO-1 and afadin
The assembly of the tight junctions is coupled to the formation of the adherens junctions. To understand how this coupling occurs, we first must consider how cell-cell junctions are formed. Cell-cell junctions assemble via a highly concerted, multistep process. First, the membranes of neighboring cells extend actin-rich protrusions, placing two opposing cell membranes in close proximity. Second, nectins on one cell form homophilic interactions with nectins on adjacent cells. Following nectin dimerization, the main adhesion proteins of adherens junctions, cadherins, dimerize across the paracellular space and establish the first cell-cell adhesive contacts. Upon establishment of these primordial adherens junctions, the tight junctions begin to form.
The formation of the tight junctions is supported by the nascent adherens junctions. How the nascent adherens junctions are coupled to the assembly of tight junctions is an area of active investigation with clues provided from deletion or knockdown studies. Loss of the nectin binding protein, afadin, the cadherin binding protein, α-catenin, or the tight junction protein, ZO-1, results in cells that do not develop tight junctions, suggesting that these proteins physically link the two junctional types [36, 41]. Two of these proteins, afadin and α-catenin, localize exclusively to adherens junctions, while the other, ZO-1, is unique in that it associates with adherens junctions during assembly and later localizes exclusively to tight junctions [35–37, 42]. Mutational analyses identified the ZO-1 regions as critical for tight junction assembly [34, 43]. The U5 region binds α-catenin, and the SH3 domain binds afadin and both are essential for proper tight junction assembly [36]. Hence, both ZO-1 binding to afadin and ZO-1 binding α-catenin are critical for linking the assembly of tight junctions to adherens junctions.
To explain how the linkage between tight junctions and adherens junctions might occur mechanistically, Ooshio et al. proposed that afadin binds ZO-1 prior to the assembly of adherens junctions and provides the link to the tight junctions. In support of this idea, cells expressing a mutant version of afadin unable to bind ZO-1 assemble adherens junctions but not tight junctions [36]. However, this model is likely incomplete as other work suggests that cadherin-mediated adhesion is important for linking the two junctions and ZO-1 deletion delays but does not prevent tight junction assembly [41, 44]. Recently, our laboratory demonstrated that α-catenin transiently associates with ZO-1 in assembling cell-cell junctions and mutant versions of α-catenin unable to bind ZO-1 do not form functional tight junctions despite assembling robust adherens junctions [45]. These findings highlight a role for α-catenin binding to ZO-1 in coupling the tight junction assembly and adherens junction formation.
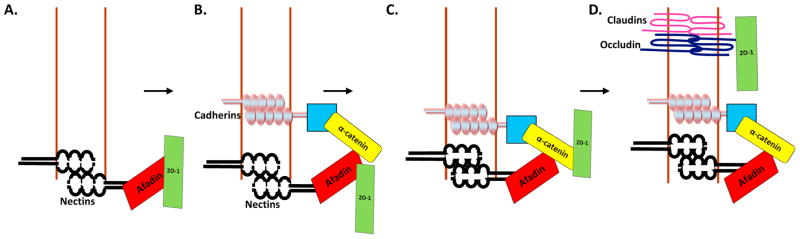
Based on this new information, we propose a new model to describe how tight junctions are linked to adherens junction assembly (Figure 2). Upon initiation of cell-cell contact, nectin-based adhesions form, recruiting afadin bound to ZO-1 [36, 46]. The nectin-based adhesions support the assembly of the cadherin adhesion complex, leading to the recruitment of α-catenin [15, 47, 48]. α-Catenin subsequently binds ZO-1. As adherens junctions mature, tight junction assembly begins with occludin and members of the claudin family being recruited. Occludin binds ZO-1 [49]. As the tight junctions continue to mature, the tight junction complex, including ZO-1, moves apically, leading to two distinct junctional complexes [42].
Fig. 2. Model for the coupling of tight junction assembly to adherens junctions.
A) During the formation of cell-cell contact, the membranes of adjacent cells protrude toward one another. Nectins on one cell bind nectins on neighboring cells and recruit afadin which in turn binds ZO-1. The nectins form a transient adhesion that brings two opposing membranes into close proximity. (B) The extracellular domains of cadherins dimerize with cadherins on adjacent cells and the cytoplasmic domain recruits β-catenin recruits α-catenin, an afadin binding protein. C) As the adherens junctions continue to mature, ZO-1 interacts transiently with α-catenin. D) The tight junctions begin to form, ZO-1 binds occludin, and then tight junctions migrate more apically. The brown lines indicate the plasma membranes of two adjacent.
This model (Figure 2) suggests the assembly of tight junctions and adherens junctions occurs in a sequential fashion. In support of this idea, previous studies show that cadherin-based adhesions are disrupted when nectin-based adhesions are lost, and tight junctions do not form in the absence of afadin [18, 36]. In further support of this notion, ZO-1 binding to α-catenin is an essential step in tight junction assembly, but loss of this interaction does not impair nectin-based adhesions. Thus, nectin-based adhesions are required for the assembly of cadherin-based adhesions, which in turn support tight junction formation.
This model does not address the nature of the complexes formed by cytoplasmic proteins during junctional assembly. It is tempting to propose that afadin hands off ZO-1 to α-catenin and α-catenin hands off ZO-1 to occludin. However, it is also possible that large protein complexes exist in which all the proteins are present. ZO-1, afadin, and α-catenin have been shown to bind one another in bipartite complexes but it is unknown whether a tripartite complex exists [34–36, 50]. Afadin and ZO-1 bind to distinct regions of α-catenin, and α-catenin and afadin do not compete for binding to ZO-1, suggesting that a tripartite complex is feasible [34, 50, 51]. In contrast, α-catenin and occludin compete for binding to ZO-1, with occludin having a stronger affinity for ZO-1 than α-catenin [34]. This competition is supported by cell-based assays that indicate ZO-1 association with adherens junctions and tight junctions is mutually exclusive [42]. Taken together, these observations suggest that α-catenin, afadin, and ZO-1 could potentially form a tripartite complex that then hands off ZO-1 to occludin.
In contrast to a role for ZO-1 binding to α-catenin, work from another group found that fragments of α-catenin lacking the ZO-1 binding site can support tight junction assembly. Twiss et al. identified α-catenin 1-402 is sufficient for proper localization of tight junction components and barrier formation in cells under force [52]. Hence while α-catenin binding to ZO-1 couples tight junctions to adherens junction, it is likely that other binding proteins may also play a role. One intriguing possibility is that cells under force have additional mechanisms for coupling the assembly of tight junctions to adherens junctions.
Finally it is important to note cadherins are dispensable for the formation of tight junctions in some settings. For example, Yamada et al. observed cells lacking annexin II, a calcium-dependent phospholipid binding protein, could assembly tight junctions when cadherins were absent [53]. In these cells nectin-based adhesions alone supported tight junction formation. In other settings, tight junction assembly is independent of both the cadherins and nectins. For example, polarized human hepatocytes unable to form adherens junctions slowly form tight junctions [54]. Hence tight junctions can sometimes form in the absence of adherens junctions.
4.2 Physical linkages between the adherens junctions and tight junctions: ZO-1 binding to vinculin, VASP and cortactin
While ZO-1 binding to α-catenin is an established mechanism for linking the tight junctions to the adherens junctions, several other actin binding proteins present in cadherin containing cell-cell junctions bind ZO-1. Hence, ZO-1 may mediate additional linkages between the tight junctions and adherens junction. One such ZO-1 binding partner is vinculin. In cardiomyocytes, vinculin, an actin bundling protein, is localized to the intercalated disks, a structure that resembles adherens junctions [55, 56]. Furthermore, it directly binds ZO-1 and is necessary for ZO-1 localization to the intercalated disc [57]. Similarly, vasodilator activated phosphoprotein (VASP), an F-actin anti-capping protein, localizes to adherens junction and its loss disrupts the epithelial barrier, causing fatal vascular leakage [58–60]. VASP co-immunoprecipitates and co-localizes with ZO-1 [61]. Finally, cortactin, a weak activator of the actin nucleator Arp2/3 complex, localizes to adherens junctions [62]. Like vinculin and VASP, cortactin binds to ZO-1 but the functional consequence of this interaction has not been explored [63].
It is not fully understood how the interactions between ZO-1 and the various actin binding proteins coordinate the interplay between adherens junctions and tight junctions. Vinculin, VASP, and cortactin have different effects on the actin cytoskeleton. Furthermore, different types of actin structures are observed in epithelial cells [64]. Lomakin et al. noted a competition among branched and bundled F-actin networks during cell polarization [65]. Hence, it is possible that ZO-1 (by virtue of its ability to recruit different types of actin modifiers) orchestrates the assembly of different types of actin structures. Some of these structures could promote the assembly of the tight junction at the adherens junctions and others might remodel the actin structures to allow the tight junction to mature and discriminate from the adherens junction.
5. Conclusions
In summary, cell-cell contacts are critical for tissue establishment and homeostasis. The adherens junctions, composed of cadherins and nectins, mediate cell-cell adhesion. The tight junctions, including claudins, occludin, and JAMs, establish apical-basolateral polarity and regulate the paracellular transport of ions and solutes. Historically, the adherens and tight junctions were viewed as discrete complexes. However, new evidence has emerged highlighting their interdependency. From these studies, it is now appreciated that there are both physical and signaling linkages between the adherens junctions and tight junctions. ZO-1 physically links the two junctional complexes via its interactions with the actin binding proteins, α-catenin and afadin. These interactions promote the maturation of adherens junctions and the subsequent assembly of tight junctions. In addition to physical linkages, signaling couples the tight junctions to the adherens junctions. Polarity complexes, including Par, Crumbs, and Scribble, regulate the maturation of primordial adherens junctions, control the positioning of the tight junctions, and determine the apical and lateral surfaces. Finally, many events underlying the interplay between tight junction and adherens junctions are dependent upon the interaction of junctional proteins with the actin cytoskeleton and its regulation by myosin II.
6. Future Directions
Despite considerable progress in understanding how tight junction assembly is linked to the formation of adherens junctions, many questions remain. It is highly likely the full repertoire of proteins mediating coupling has not yet been determined. Also we still have a poor understanding for how the proteins involved in the coupling are regulated. This lack of information extends to understanding how the activities of these proteins are stimulated by polarity proteins to allow assembly and how these events are curtailed to allow for maturation and discrimination of the tight junction from the adherens junction. Finally we still need to establish how these linkages drive diverse cellular process. Understanding the latter events is likely to uncover novel mechanisms underlying the genesis and pathogenesis of disease.
Acknowledgments
This publication was supported by The National Institutes of General Medicine (Award Number R01GM112805 to K.A.D). The authors are also thanks Jennifer Bays and Christy Heidema for their comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikenouchi J, et al. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176(6):779–86. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20(23):3199–214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 3.Pertz O, et al. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18(7):1738–47. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, et al. Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc Natl Acad Sci U S A. 2010;107(41):17592–7. doi: 10.1073/pnas.1011247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1(3):a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141(3):779–89. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle H, et al. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–63. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 8.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drees F, et al. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley CD, et al. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346(6209):1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkubo T, Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem. 1999;274(30):21409–15. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- 12.Jou TS, et al. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A. 1995;92(11):5067–71. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noren NK, et al. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150(3):567–80. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takai Y, et al. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94(8):655–67. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145(3):539–49. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong X, et al. Crystal structure of the V domain of human Nectin-like molecule-1/Syncam3/Tsll1/Igsf4b, a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule. J Biol Chem. 2006;281(15):10610–7. doi: 10.1074/jbc.M513459200. [DOI] [PubMed] [Google Scholar]
- 17.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116(Pt 1):17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, et al. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281(8):5288–99. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- 19.Takekuni K, et al. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278(8):5497–500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- 20.Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149(1):13–6. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 22.Colegio OR, et al. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283(1):C142–7. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 23.Piontek J, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22(1):146–58. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 24.Hamazaki Y, et al. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277(1):455–61. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 25.Roh MH, et al. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem. 2002;277(30):27501–9. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, et al. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulzke JD, et al. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669(1):34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 30.Medina R, et al. Occludin localization at the tight junction requires the second extracellular loop. J Membr Biol. 2000;178(3):235–47. doi: 10.1007/s002320010031. [DOI] [PubMed] [Google Scholar]
- 31.Balda MS, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134(4):1031–49. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebnet K, et al. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275(36):27979–88. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 33.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16(13):1835–7. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 34.Muller SL, et al. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280(5):3747–56. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran AK, et al. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132(3):451–63. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooshio T, et al. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 2010;285(7):5003–12. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh M, et al. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138(1):181–92. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazzoni G, et al. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275(40):30970–6. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–74. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 40.Ebnet K, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116(Pt 19):3879–91. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 41.Capaldo CT, I, Macara G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18(1):189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ando-Akatsuka Y, et al. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1999;179(2):115–25. doi: 10.1002/(SICI)1097-4652(199905)179:2<115::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 43.Rodgers LS, et al. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci. 2013;126(Pt 7):1565–75. doi: 10.1242/jcs.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17(4):1922–32. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiers JL, et al. ZO-1 recruitment to alpha-catenin--a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci. 2013;126(Pt 17):3904–15. doi: 10.1242/jcs.126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama S, et al. alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol Biol Cell. 2001;12(6):1595–609. doi: 10.1091/mbc.12.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikeda W, et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146(5):1117–32. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asakura T, et al. Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4(10):573–81. doi: 10.1046/j.1365-2443.1999.00283.x. [DOI] [PubMed] [Google Scholar]
- 49.Furuse M, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127(6 Pt 1):1617–26. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pokutta S, et al. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277(21):18868–74. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachibana K, et al. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150(5):1161–76. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Twiss F, et al. Vinculin-dependent Cadherin mechanosensing regulates efficient epithelial barrier formation. Biol Open. 2012;1(11):1128–40. doi: 10.1242/bio.20122428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada A, et al. Requirement of nectin, but not cadherin, for formation of claudin-based tight junctions in annexin II-knockdown MDCK cells. Oncogene. 2006;25(37):5085–102. doi: 10.1038/sj.onc.1209525. [DOI] [PubMed] [Google Scholar]
- 54.Theard D, et al. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol Biol Cell. 2007;18(6):2313–21. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koteliansky VE, Gneushev GN. Vinculin localization in cardiac muscle. FEBS Lett. 1983;159(1–2):158–60. doi: 10.1016/0014-5793(83)80437-2. [DOI] [PubMed] [Google Scholar]
- 56.Noorman M, et al. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47(1):23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Zemljic-Harpf AE, et al. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J Cell Sci. 2014;127(Pt 5):1104–16. doi: 10.1242/jcs.143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott JA, et al. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17(3):1085–95. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasioukhin V, et al. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 60.Furman C, et al. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179(4):761–75. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol. 2002;282(6):C1235–45. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 62.Helwani FM, et al. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164(6):899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katsube T, et al. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273(45):29672–7. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 64.Revenu C, et al. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol. 2004;5(8):635–46. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- 65.Lomakin AJ, et al. Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nat Cell Biol. 2015;17(11):1435–45. doi: 10.1038/ncb3246. [DOI] [PMC free article] [PubMed] [Google Scholar]