Abstract
Blastomycosis elicits a pyogranulomatous inflammatory response, which involves a prominent recruitment of neutrophils to the site of infection. While neutrophils are efficiently recruited to the site of infection, this event is paradoxically coupled with the host's inability to control infection by Blastomyces dermatitidis, the causative agent. The mechanisms underlying this characteristic pyogranulomatous response and inability of neutrophils to kill the yeast are poorly understood. We recently reported that the fungal protease DppIVA promotes B. dermatitidis virulence by cleaving a dipeptide from the N-terminus of C-C chemokines and GM-CSF, thereby inactivating them. Herein, we present evidence that DppIVA can also truncate the N-terminus of members of the ELR+ CXC chemokine family, which are known to modulate neutrophil function. We show that the DppIVA cleaved form of human (h) CXCL-2, e.g. hCXCL-2 (3-73), is a more potent neutrophil chemoattractant than its intact counterpart, but hCXCL-2 (3-73) is conversely impaired in its ability to prime the reactive oxygen species (ROS) response of neutrophils. Thus, DppIVA action on ELR+CXC chemokines may promote the pyogranulomatous response that is typical of blastomycosis, while also explaining the inability of neutrophils to control infection.
Introduction
Polymorphonuclear neutrophils (PMNs), or neutrophils, are central to the host immune response since conditions in which they are either reduced (neutropenia) or functionally impaired (e.g. chronic granulomatous disease) increase the risk of acquiring bacterial and fungal infections.(Babior, 1999; Geiszt et al., 2001; Zeidler et al., 2009) Neutrophils, once present at the site of infection, utilize a plethora of antimicrobial activities to kill invading microorganisms. These activities include phagocytosis of pathogens, production of toxic reactive oxygen species (ROS), initiation of the myeloperoxidase-hydrogen peroxide (H2O2) halide system, release of several types of granules that contain antimicrobial proteins and enzymes, and formation of neutrophil extracellular traps (NETs) that entangle and kill extracellular pathogens.(Brinkmann et al., 2004; Klebanoff, 1967, 1968; Kolaczkowska and Kubes, 2013; Laible and Germaine, 1985; Reeves et al., 2002) Therefore, in order for a pathogen to establish infection it must withstand or sabotage the neutrophil antimicrobial response.
Blastomyces dermatitidis is one such pathogen, as evidenced by the fact that neutrophils, in the absence of serum, inefficiently kill the yeast.(Brummer and Stevens, 1982, 1983; Drutz and Frey, 1985; Sixbey et al., 1979) Diminished killing in the absence of serum indicates a role for complement. This impairment also may be due to the large size of B. dermatitidis yeast since smaller, phagocytosable yeast such as Candida albicans demonstrate efficient fungal uptake and killing.(Kurita et al., 1991) A single B. dermatitidis yeast is about 8-10 μM, which rivals the size of human neutrophils.(Drutz and Frey, 1985) Neutrophils are unable to fully phagocytose this yeast, which spares the fungus from the concentrated acidic and degradative environment of phagolysosomes.(Flannagan et al., 2009) The activation of neutrophils may also be impaired via protease virulence factors of B. dermatitidis. The fungus releases an extracellular protease dipeptidyl peptidase-IVA (DppIVA) that cleaves and inactivates granulocyte/macrophage-colony stimulating factor (GM-CSF), which is a potent activator of neutrophils, thereby rendering cleaved GM-CSF an antagonist for its receptor.(Broxmeyer et al., 2012) This DppIVA action prevents neutrophil priming via GM-CSF and further reduces the fungicidal capacity of neutrophils.(Sterkel et al., 2016) Mammalian DppIV cleaves a large number of substrates including many chemokines and cytokines. (Ou et al., 2013) Blastomyces DppIVA may likewise act on its predicted cleavage site within additional substrates, including the immunologically active host targets of mammalian DppIV. DppIVA may thus thwart neutrophil function and promote disease pathogenesis in further ways that have not been studied, for example by altering the activity of ELR+CXC chemokines.
The CXC family of chemokines can be divided into two groups, ELR- chemokines and ELR+ chemokines. The ELR+ chemokines possess an ELR motif (glutamate, leucine, and arginine) N-terminally adjacent to the first cysteine of the CXC motif, and this group of chemokines has been shown to modulate neutrophil activity. The seven members of the human ELR+ CXC family (CXCL-1,-2,-3,-5,-6,-7,-8) differ in the extent to which they modulate neutrophil activity.(Ahuja and Murphy, 1996; Clark-Lewis et al., 1991; Geiser et al., 1993; Magazin et al., 1992; Proost et al., 1998; Wuyts et al., 1999) Once produced, these chemokines are subject to post-translational modifications, such as discrete N-terminal truncations that can enhance their activity relative to their intact counterparts.(Ahuja and Murphy, 1996; Clark-Lewis et al., 1991; Geiser et al., 1993; King et al., 2000; Proost et al., 1998; Wuyts et al., 1999) In general, the larger the N-terminal truncation, the more potent the chemokine becomes as long as the ELR-motif is undisturbed. For instance, human (h) CXCL-8 is secreted as a 77 amino acid protein, but naturally occurring forms where 5, 6, or 7, amino acids are removed from the N- terminus have been identified. These N-terminally truncated forms of hCXCL-8 promote neutrophil migration and elastase release to a greater extent than intact hCXCL-8.(Clark-Lewis et al., 1991) Similar trends have been observed for some N-terminally truncated forms of hCXCL-1, -2, -3, and -5, which induce greater calcium flux and are more chemotactic for neutrophils than their full-length forms.(King et al., 2000; Wuyts et al., 1999) For example, a 4 amino acid N-terminal truncated form of hCXCL-2 induces neutrophil ROS production to a greater extent than does the full-length protein.(King et al., 2000)
Although the enhancement of ELR+CXC chemokine function through N-terminal cleavage is the general trend, it is not the absolute rule. For instance, an N-terminally dipeptide truncated form of CXCL-6 fails to enhance neutrophil migration relative to the intact protein.(Proost et al., 1998) This finding justifies the need to experimentally validate the action of N-terminal truncation variants for each ELR+ CXC chemokine, especially for those forms that may function in a biologically relevant setting such as inflammation or infection. Because mammalian DppIV is predicted to cleave several ELR+ CXC chemokines, we tested which of these products are cleaved by B. dermatitidis DppIVA and assessed the effects of truncated chemokines on murine and human neutrophils. We hypothesized that the effects of DppIVA cleavage on ELR+CXC chemokines would alter neutrophil migration and priming, thereby modulating the host immune response.
Results
ELR+CXC chemokines are substrates of recombinant B. dermatitidis DppIVA (rDppIVA)
Mammalian DppIV most efficiently cleaves after, in descending order, proteins with an N-terminal penultimate proline, alanine, and serine.(Lambeir et al., 2003) Many human and murine ELR+ CXC chemokines possess such a residue in the penultimate N-terminal position, indicating that they may be substrates of fungal rDppIVA (Table 1).(Ou et al., 2013) We wondered whether B. dermatitidis rDppIVA can modulate neutrophil function by acting on ELR+CXC chemokines and, if so, whether its substrate specificity mirrors that of mammalian DppIV. We therefore investigated whether B. dermatitidis rDppIVA is capable of removing an N-terminal dipeptide from ELR+CXC chemokines with an N-terminal penultimate proline, as in murine CXCL-1 (mCXCL-1) and human CXCL-2 (hCXCL-2); an N-terminal penultimate alanine, as in human CXCL-8, (hCXCL-8); or an N-terminal penultimate serine, as in human CXCL-1 (hCXC-1).
Table 1. Human and murine ELR+CXC chemokines*.
| Chemokine | Human | Sequence | Mouse | Sequence |
|---|---|---|---|---|
| CXCL-1 | GRO-α |
|
KC |
|
| CXCL-2 | GRO-β |
|
MIP-2 |
|
| CXCL-3 | GRO-γ |
|
Gm1960 |
|
| CXCL-5 | ENA-78 (71AA) |
|
LIX |
|
| CXCL-6 | GCP-2 |
|
X | |
| CXCL-7 | NAP-2 |
|
X | |
| CXCL-8 | IL-8 (72 AA) |
|
X |
The family of ELR+CXC chemokines that modulate neutrophil function are listed along with the first five N-terminal amino acids of the human or murine biologically relevant form. (Bachelerie et al., 2014; Zlotnik and Yoshie, 2012) The two N-terminal residues of the ELR+CXC chemokines that are potential DppIV substrates are underlined and in bold; canonical penultimate proline or alanine residues are in red and non-canonical penultimate serine residues are in black. Human CXCL-1, CXCL-2, CXCL-3, and CXCL-6 share synteny with murine CXCL-3, CXCL-2, CXCL-1, and CXCL-5, respectively. (Bachelerie et al., 2014).
To monitor cleavage, we incubated each chemokine in the presence or absence of rDppIVA and analyzed the products by mass spectrometry. We found a reduction in mass of mCXCL-1 and hCXCL-2 after rDppIVA treatment that is equivalent to the loss of their respective N-terminal dipeptides (Fig. 1A and B). Thus, B. dermatitidis rDppIVA, like mammalian DppIV, cleaves proteins containing an N-terminal penultimate proline. However, there was no reduction in mass of hCXCL-8 or hCXCL-1 treated with fungal rDppIVA (Fig. 1C and D). Thus, despite predictions based on mammalian DppIV, fungal DppIVA did not cleave a dipeptide from substrates containing an N-terminal penultimate alanine (e.g. hCXCL-8) or serine (e.g. hCXCL-1). Mammalian DppIV and fungal DppIVA may therefore differ in their substrate specificities, with mammalian DppIV permitting a broader diversity of target residues in the N-terminal penultimate position compared to fungal DppIVA. In all, B. dermatitidis rDppIVA can cleave a dipeptide from the ELR+CXC chemokines hCXCL-2 and mCXCL-1 and could alter the function of these proteins and neutrophil response during pulmonary B. dermatitidis infection.
Figure 1. B. dermatitidis rDppIVA cleaves a dipeptide from human CXCL-2 and murine CXCL-1.

Recombinant (r) murine CXCL-1 [A] and human CXCL-2 [B], CXCL-8 [C] and CXCL-1 [D] were incubated alone [top panel] or with B. dermatitidis rDppIVA [bottom] in digestion buffer overnight at 37°C and then analyzed by mass spectrometry.
Cleaved mCXCL-1(2-72) and hCXCL-2(3-73) are more potent than intact mCXCL-1 and hCXCL-2 in chemoattraction of neutrophils
We previously characterized the effects of DppIVA sufficient and deficient B. dermatitidis (by RNA interference) in vivo, revealing that DppIVA plays and important role in virulence. (Sterkel et al., 2016) Neutrophils are recruited to tissues infected with B. dermatitidis (Littman et al., 1948), and many ELR+CXC chemokines are known to have enhanced chemotactic activity with small, discrete N-terminal truncations. (Clark-Lewis et al., 1991; Wuyts et al., 1999) Since murine CXCL-1 can rapidly recruit neutrophils to the lung when given intratracheally (i.t.) to mice with fungal infection (Jhingran et al. 2015), we delivered intact (1-72) or cleaved (3-72) mCXCL-1 i.t. to mice along with heat inactivated B. dermatitidis yeast. Five hours after i.t. administration, heat-killed yeast alone (vehicle) had not yet induced recruitment of neutrophils to the lung, as evidenced by a lack of neutrophil extravasation, however the addition of mCXCL-1 to yeast did induce recruitment and extravasation of neutrophils to the lung. mCXCL-1(3-72) induced significantly more recruitment of neutrophils to the lung than did intact mCXCL-1 (Fig 2A). Compared with intact mCXCL-1, cleaved mCXCL-1(3-72) increased the number of neutrophils that accumulated in the lung capillaries (Fig 2B,C). However, the proportion of neutrophils that had extravasated across the capillaries, as measured by staining with an intravenously injected antibody (Anderson et al., 2014), was not significantly increased by mCXCL-1(3-72) (Fig. 2D). Thus, the effect of DppIVA trimming on mCXCL-1 appears to be on neutrophil slowing and adhesion along the endothelium.
Figure 2. Cleaved murine CXCL-1(3-72) and human CXCL-2(3-73) induce greater neutrophil migration than uncleaved chemokine.

(A-D) Heat-killed B. dermatitidis yeast (2×104) were delivered i.t. into mice in PBS alone (vehicle) or with 100 ng of mCXCL-1 or mCXCL-1(3-72). Right before euthanizing mice, anti-CD45 was injected i.v. to label capillary leukocytes. The mean ± SEM of total neutrophils (A), extravasated neutrophils (B, negative for i.v. antibody), and capillary neutrophils (C, positive for i.v. antibody) across the lungs from a representative experiment (n=4-5 mice); ANOVA with Tukey test used to compare means of each group, *p<0.05, ***p<0.001. (D) Representative flow cytometry plots of gated lung neutrophils showing the percent (mean ± SEM) of neutrophils that had or had not extravasated from lung capillaries; both mCXCL-1 and mCXCL1 groups had a significantly greater proportion of CD45iv- neutrophils (p<0.01), but they were not significantly different from each other. (E-F) Human neutrophils: cells were fluorescently labeled with calcein AM and placed in transwells. Neutrophil migration in response to varying concentrations of hCXCL-2 and hCXCL-2(3-73) was measured after 45 minutes of incubation at 37°C in 5%CO2 in triplicate wells for each condition tested. (E) A representative migration experiment with neutrophils from donor A is shown from three separate experiments (F) The migration results over a range of chemokine concentrations were averaged for 3 to 6 different donors tested at a given condition, and the mean and SEM for each condition is displayed. A paired t-test was used to determine the statistical significance of differences at each chemokine concentration for neutrophil migration in response to hCXCL-2 vs. hCXCL-2(3-73). *p < 0.05; **** p < 0.0001.
The ELR+CXC family of chemokines is divergent across species. Murine CXCL-1 shares closest homology with human CXCL-3 that is not cleaved by rDppIVA (Fig. 1). (Nomiyama et al., 2010; Zlotnik and Yoshie, 2012) To understand how cleavage of ELR+CXC chemokines may impact the neutrophil response during blastomycosis in humans, we focused on human CXCL-2 because it is cleaved by rDppIVA. We postulated that rDppIVA cleavage of hCXCL-2 would enhance the chemokine's ability to induce human neutrophil migration, as we saw with mCXCL-1. We performed in vitro transwell assays where the migration of fluorescently labeled neutrophils collected from 6 healthy donors was measured in response to hCXCL-2 vs. hCXCL-2(3-73). Over a range of chemokine concentrations, significantly more neutrophils migrated in response to hCXCL-2(3-73) as compared to hCXCL-2 (Fig. 2E, F). Thus, both cleaved mCXCL-1(3-72) and hCXCL-2(3-73) displayed significantly greater neutrophil chemotactic activity than intact mCXCL-1 and hCXCL-2. This result suggests that rDppIVA cleavage of ELR+CXC chemokines during B. dermatitidis infection contributes to the exuberant neutrophil influx that is characteristic of this fungal infection.
Cleaved hCXCL-2(3-73) primes ROS production less efficiently than intact hCXCL-2
A central effector function of neutrophils is their production of ROS.(Klebanoff, 1967, 1968) This response is mediated by the NADPH oxidase protein complex, which is composed of several cytosolic proteins (p47phox, p67phox, p40phox, and Rac2) as well as the heterodimer of gp91phox and p22phox (flavocytochrome b558) present on the plasma membrane and the membrane of the specific granules.(Guichard et al., 2005; Sengelov et al., 1995) Binding of ELR+CXC chemokines to their receptors on neutrophils can induce a slight respiratory burst in neutrophils, as has been observed for hCXCL-8 and hCXCL-2.(Magazin et al., 1992; Yuo et al., 1991) Minor N-terminal truncations of ELR+CXC chemokines, such as the deletion of four amino acids from the NH2-terminus of hCXCL-2, can enhance its ability to induce the neutrophil oxidative burst.(King et al., 2000) In addition, ELR+CXC chemokines can “prime” the oxidative burst in neutrophils, so that, upon neutrophil recognition of a secondary stimulus, primed neutrophils produce a more robust ROS response than unprimed neutrophils. For example, hCXCL-8 primed neutrophils exposed to the secondary stimulus formylated-methionine-leucine-proline (fMLP) show much greater ROS production than unprimed neutrophils exposed to fMLP.(Guichard et al., 2005)
Since hCXCL-2(3-73) induced more neutrophil migration than hCXCL-2, we hypothesized that neutrophils primed with hCXCL-2(3-73) might show a more robust ROS response than those primed with intact hCXCL-2, upon stimulation with fMLP. To test this, we performed a cytochrome c reduction assay to indirectly monitor the production of superoxide anion. Neutrophils from 3 healthy donors first primed with hCXCL-2 or hCXCL-8 produced significantly more ROS in response to fMLP than did unprimed neutrophils (Fig. 3). Also, hCXCL-8 primed neutrophils produced the greatest ROS response, which was expected since hCXCL-8 is one of the most potent human ELR+CXC chemokine stimulators of neutrophil function.(Geiser et al., 1993; Magazin et al., 1992) However, to our surprise, hCXCL-2 primed neutrophils produced significantly more ROS in response to fMLP than did hCXCL-2(3-73) primed neutrophils (Fig. 3A). In fact, cleaved, hCXCL-2(3-73)-primed neutrophils produced as little ROS as did unprimed neutrophils in response to fMLP stimulation. This result contrasts with prior studies in which more extensive N-terminal truncations of hCXCL-2 showed greater activity upon cleavage.(King et al., 2000) To our knowledge, this is the first instance where an N-terminally truncated form of an ELR+CXC chemokine still possessing its ELR-motif is actually reduced in its capacity to prime neutrophil function relative to the intact chemokine.
Figure 3. hCXCL-2(3-73) does not prime ROS production of neutrophils to the same extent as intact hCXCL-2.

Neutrophils were primed with medium or 200 ng of hCXCL-2, hCXCL-2(3-73) or hCXCL-8 before stimulation with medium (control) or 100 nM fMLP. (A) Production of superoxide anion was measured using an SOD inhibitable cytochrome c reduction assay. (B) Production of ROS in quintuplicate wells was measured via isoluminol luminescence. The data represents the mean and SEM of results from 3 different donors tested in panel A and 6 different donors tested in panel B. **p<0.01, ***p<0.001, ****p<0.0001 for comparison of results between the groups indicated by brackets, including cleaved vs. un-cleaved CXCL-2.
While the results from the cytochrome c reduction assay are suggestive, this assay is relatively insensitive. To validate and refine our findings, we measured extracellular ROS production more accurately by its ability to cause isoluminol luminescence in a peroxidase-catalyzed manner. As above, neutrophils primed with hCXCL-8 produced the most ROS in response to fMLP (Fig. 3B). We again also saw that neutrophils primed with hCXCL-2 produced significantly more luminescence, e.g. ROS, in response to stimulation with fMLP, as compared to neutrophils primed with hCXCL-2(3-73). ROS production of neutrophils primed with hCXCL-2(3-73) did, however, exceed that of unprimed neutrophils in response to stimulation by fMLP. Thus, hCXCL-2(3-73) is able to prime the ROS response of neutrophils. Nonetheless, in contrast to other discrete, longer N-terminal truncations of hCXCL-2, the removal of only a dipeptide from the amino-terminus of hCXCL-2 actually reduces its ability to prime the ROS response of neutrophils. Thus, N-terminal truncations of varying lengths – even of the same ELR+CXC chemokine – can have disparate effects on neutrophil function. This finding, when combined with that of enhanced ability of hCXCL-2(3-73) to promote neutrophil migration, suggests a model in which fungal DppIVA cleavage of hCXCL-2 induces an influx of neutrophils that are relatively impaired in their antimicrobial functions during B. dermatitidis infection.
hCXCL-2 and hCXCL-2(3-73) induce degranulation to similar extents
Neutrophils are armed with membrane bound compartments chocked full of receptors, adhesins, antimicrobial proteins, and degradative enzymes.(Faurschou and Borregaard, 2003; Sengelov et al., 1995) Secretory vesicles and granules are released extracellularly in a specified order; vesicles are most readily mobilized, followed by gelatinase granules, specific granules, and azurophilic granules.(Sengelov et al., 1995) Flavocytochrome b558 is present in the membranes of specific granules and, upon their degranulation, is incorporated into the plasma membrane.(Borregaard et al., 1983; Jesaitis et al., 1990) Since hCXCL-2 (3-73) is impaired relative to hCXCL-2 in the ability to prime ROS production of neutrophils, we hypothesized that neutrophils primed with hCXCL-2(3-73) may likewise be impaired in degranulation.
Since secretory vesicles are the first to degranulate, we investigated whether neutrophils primed with hCXCL-2 (3-73) as opposed to intact hCXCL-2 were relatively impaired in the ability to release secretory vesicles in response to fMLP. We monitored neutrophil cell surface expression of CD35 (complement 1 receptor), which accumulates on the plasma membrane as secretory vesicles degranulate (Fig. 4A). Neutrophils stimulated with fMLP alone increased the expression of CD35 on their surface (Fig. 4B). Neutrophils primed with hCXCL-8 before fMLP stimulation showed the greatest increase in expression of CD35 e.g. release of secretory vesicles. Whereas priming neutrophils with either hCXCL-2 or hCXCL-2(3-73) before fMLP stimulation enhanced cell surface expression of CD35 over unprimed of fMLP stimulated neutrophils, there was no significant difference in expression level between the intact and cleaved forms of CXCL-2. Thus, hCXCL-2 (3-73) primed neutrophils were not impaired in their ability to prime degranulation of secretory vesicles relative to hCXCL-2 primed neutrophils.
Figure 4. hCXCL-2 and hCXCL-2(3-73) prime neutrophil degranulation of secretory vesicles and CD66b+ granules to similar extents.
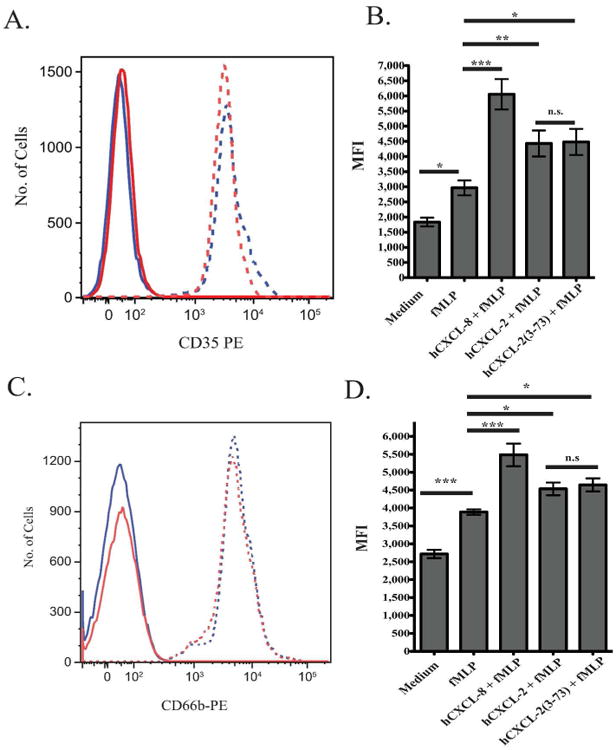
Neutrophils were primed with either medium alone or 200 ng of hCXCL-2, hCXCL-2(3-73), or hCXCL-8 and then stimulated with 1 nM fMLP or medium as indicated. Stimulated neutrophils were stained with either an IgG-PE isotype control, anti-CD35-PE (A, B) or anti-CD66b-PE (C, D) antibodies. Mean fluorescence intensity was measured by flow cytometry. The data represents the mean and SEM of results from 4 different donors. (A, C) Solid lines denote neutrophils labeled with isotype control, and dashed lines denote neutrophils labeled with anti-CD35 (A) or anti-CD66b (C). Blue lines denote hCXCL-2(3-73) primed neutrophils, and red lines denote hCXCL-2 primed neutrophils. (B, D) Quantification of mean fluorescence intensity of anti-CD35 (B) or anti-CD66 (D) on CD15+CD16+ staining of neutrophils. *** p <0.001, **p <0.01, *p <0.05.
We next investigated if hCXCL-2(3-73) is able to prime neutrophil degranulation of gelatinase and specific granules to the same extent as hCXCL-2 since these granules possess flavocytochrome b558 in their membranes. Degranulation was assayed by antibody staining with anti-CD66b, which is a marker in the membranes of both gelatinase and specific granules. Cell surface expression of CD66b was readily detected on primed neutrophils stimulated with fMLP (Fig. 4C) and increased compared to unstimulated neutrophils (Fig. 4D). CD66b expression was highest on hCXCL-8 primed neutrophils stimulated with fMLP. There was again no significant difference in CD66b surface expression between hCXCL-2 or hCXCL2 (3-73) primed neutrophils. This suggests that degranulation of gelatinase and specific granules and incorporation of flavocytochrome b558 occurred to a similar extent in both groups. Thus, the relative deficiency of hCXCL-2(3-73) in priming neutrophil ROS production is likely not the result of a lack of flavocytochrome b558 incorporation into the plasma membrane.
Although the sum of degranulation of specific and gelatinase granules was similar between hCXCL-2 and hCXCL-2(3-73) primed neutrophils, we could not discern if one was released to a greater extent than the other because CD66b is present on the surface of both granule types. Gelatinase granules contain a substantial amount of gelatinase B, or matrix metalloproteinase-9 (MMP-9), which can degrade several extracellular matrix components such as collagen. Degradation products of collagen induce neutrophil migration via the CXCR1 and CXCR2 receptors.(Weathington et al., 2006) Since we found above that hCXCL-2(3-73) enhances neutrophil migration better than hCXCL-2, we sought to determine if hCXCL-2(3-73) induces neutrophil release of MMP-9, which itself could augment cell influx.(Faurschou and Borregaard, 2003) To see if neutrophils primed with hCXCL-2 vs. hCXCL-2(3-73) differed in their ability to release MMP-9, we collected supernatants from unprimed neutrophils or primed neutrophils stimulated with fMLP. The supernatants were analyzed for extracellular MMP-9 by gel zymography. Based on gel inspection (Fig. 5A) and densitometry (Fig. 5B), both hCXCL-2-primed and hCXCL-2(3-73)-primed neutrophils induced release of MMP-9, and the levels were similar between the two stimuli. Thus, while the release of MMP-9 and resulting production of chemoattractants from digestion of collagen may foster recruitment of neutrophils during this infection, hCXCL-2(3-73) primes neutrophil degranulation of gelatinase granules to the same extent as hCXCL-2 indicating that this action would not contribute to differences in recruitment.
Figure 5. hCXCL-2 and hCXCL-2(3-73) prime release of MMP-9 from neutrophils to similar extents.
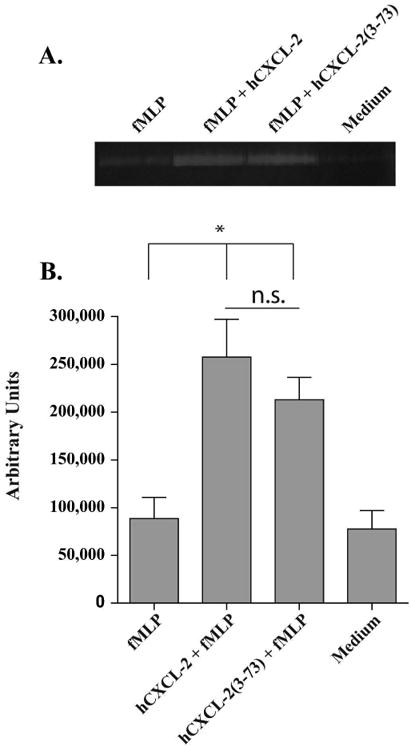
Neutrophils were primed with either medium alone or hCXCL-2, or hCXCL-2(3-73) and then stimulated with medium or 100 nMfMLP as indicated. Approximately 8 μL of diluted supernatant from each condition were run on triplicate 0.2% gelatin SDS-PAGE gels for 1 hour. Digestion of gelatin by MMP-9 was allowed to proceed at 37°C in zymography digestion buffer overnight. Gels were then stained with Coomaisse Blue and de-stained as needed. A representative gel is shown in (A). Densitometry of each MMP-9 band for each condition was quantified and subtracted from background in (B). Data represents the mean and SEM of results from 3 different donors. *p<0.05, n.s. denotes not significant.
Effect of rDppIVA cleavage of ELR+CXC chemokine on B. dermatitidis in vitro and in vivo
We previously reported that fungal DppIVA blunts antifungal immunity by targeting GM-CSF and CCR2 ligands (Sterkel et al. 2016). Cleavage of ELR+CXC chemokines may play an additional role in modulating neutrophil immunity. We investigated the effects of mCXCL-1 and mCXCL-1(3-72) on murine neutrophils exposed to B. dermatitidis. To assay the effect of intact and cleaved mCXCL-1 on neutrophil killing of B. dermatitidis, we incubated murine neutrophils in vitro with DppIVA deficient yeast (Sterkel et al. 2016) in the presence of mCXCL-1 or mCXCL-1(3-72). Murine CXCL-1 modestly enhanced neutrophil killing of B. dermatitidis yeast, but rDppIVA cleavage of mCXCL-1 did not further augment neutrophil killing (Fig. 6A).
Figure 6. DppIVA cleavage of murine CXCL-1 (3-72) has no effect neutrophil killing and ROS production.
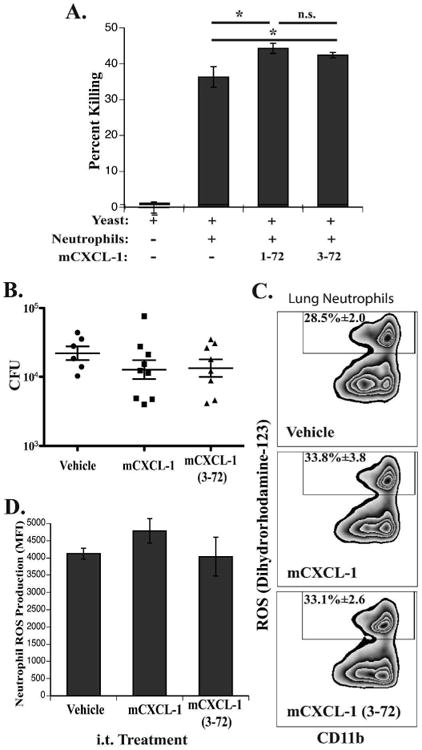
(A) Murine bone marrow neutrophils were incubated with DppIVA RNAi (silenced) B. dermatitidis yeast with mCXCL-1, mCXCL-1 (3-72), or without chemokine; shown are the combined means of three independent experiments; *p<0.05, n.s. denotes not significant. (B-D) Mice were challenged i.t. with 2 × 104 DppIVA RNAi yeast and two days later given PBS (vehicle), mCXCL-1, or mCXCL-1 (3-27) i.t. Lung homogenates were plated for CFU (B) and lung leukocytes were incubated ex vivo with dihyrorhodamine-123 followed by surface antibody staining for flow cytometry (C-D). (B) Fungal burdens of mice receiving each i.t. treatment; each point is a single mouse. (C) Representative flow cytometry plots showing the percent of ROS+ neutrophils (only showing gated neutrophils). (D) ROS production from total neutrophil populations determined by mean fluorescence intensity (MFI) of rhodamine fluorescence. N=5 mice/group for C & D.
We further investigated the effects of mCXCL-1 and mCXCL-1(3-72) on neutrophil functions in vivo during early murine pulmonary blastomycosis. Two days after infection with DppIVA-deficient B. dermatitidis, we delivered mCXCL-1 or mCXCL-1(3-72) i.t. and investigated the effects on fungal burden and neutrophil ROS production. In vivo, the effect of mCXCL-1 mirrored neutrophil killing of yeast in vitro, where mCXCL-1 treatment reduced the fungal burden, albeit insignificantly, and treatment with mCXCL-1(3-72) did not differ compared to intact mCXCL-1 (Fig. 6B). We used ex vivo accumulation of dihydrorhodamine-123 to track ROS production in lung neutrophils (Balaguer et al. 2015; Sterkel et al. 2016). Treatment with mCXCL-1 during infection had a small but insignificant effect on ROS production by neutrophils (p=0.2), inducing a slight increase in the proportion of ROS-producing neutrophils in the lungs. DppIVA cleavage of mCXCL-1 similarly affected the percent of ROS+ neutrophils (Fig 6C). The total ROS production indicated by mean DHR-123 fluorescence was not significantly affected by mCXCL-1 or mCXCL-1(3-72) (Fig. 6D). Thus, cleaved mCXCL-1 enhances neutrophil migration, but these cells fail to show enhanced ROS production or killing.
Discussion
Overall, we demonstrate here that the ELR+CXC chemokines, mCXCL-1 and hCXCL-2, are substrates of the B. dermatitidis virulence factor DppIVA. DppIVA cleaves a dipeptide from the N-terminus of mCXCL-1 and hCXCL-2 to form mCXCL-1 (3-72) and hCXCL-2(3-73). This cleavage event is biologically significant since mCXCL-1 (3-72) and hCXCL-2(3-73) modulate neutrophil function differently than do the intact chemokines. Like several other N-terminally truncated forms of ELR+CXC chemokines, mCXCL-1 (3-72) and hCXCL-2(3-73) are more potent neutrophil chemoattractants than mCXCL-1 and hCXCL-2, respectively. Both mCXCL-1 and hCXCL-2 are important chemokine signals for recruiting neutrophils to sites of infection. In pulmonary aspergillosis, mCXCL-1 recruits neutrophils into the lung to limit fungal burden (Jingran et al. 2015). However, unlike pulmonary aspergillosis, blastomycosis is characterized by an overwhelming infiltrate of neutrophils with inefficient clearance of fungal burden. DppIVA produced by B. dermatitidis may have a role in augmenting neutrophil recruitment to lung by enhancing the chemotactic actions of ELR+CXC chemokines.
While DppIVA cleavage of both mCXCL-1 and hCXCL-2 enhanced neutrophil migration, we found that only hCXCL-2(3-73) had a diminished effect on neutrophil priming. This difference is probably due to the alternate effects of the chemokines on the CXCR2 receptor. Human CXCL-2 had a profound effect on neutrophil priming while murine CXCL-1 caused little-to-no enhancement of neutrophil ROS production or fungal killing, so a decrease in neutrophil priming by mCXCL-1 might not be observed. The differences in the relative priming of hCXCL-2 and mCXCL-1 are probably the result of the fact that mice and humans have evolved differently vis-à-vis ELR+CXC chemokines, and these two chemokines are not perfect homologues of each other. (Zlotnik and Yoshie, 2012) The manner in which we investigated the effect of ELR+CXC chemokines on human and murine neutrophils also differed. We interrogated mCXCL-1 in vivo looking at the effects of the chemokine over the course of hours. Human neutrophils enriched from peripheral blood expire quickly; thus, our in vitro studies with hCXCL-2 lasted only minutes. If ELR+CXC chemokines transiently prime neutrophils, we would only expect to see these effects in the brief assays of human neutrophils. Another caveat to our work is that we studied E. coli produced recombinant chemokines, which would not be glycosylated. However, relatively little is known about how such modifications impact the function of these chemokines. (Mortier et al., 2011)
The most surprising result of this study was that DppIVA cleavage of hCXCL-2 reduced the priming by this chemokine. When neutrophils are stimulated with hCXCL-2(5-73) alone, where a tetrapeptide has been removed from the N-terminus of hCXCL-2,(King et al., 2000) neutrophils produce more ROS than neutrophils stimulated with intact hCXCL-2. The deficiency in priming neutrophil ROS production by hCXCL-2(3-73) is likely not due to a reduction in flavocytochrome b558 incorporation into the plasma membrane. This is because neutrophils primed with hCXCL-2 or hCXCL-2(3-73) and then stimulated with fMLP degranulate specific and gelatinase granules to the same extent, as measured by surface expression of CD66b. The release of gelatinase granules also appears to be similar between the two groups of primed neutrophils, since they release similar amounts of MMP-9 based on gel zymography. Whereas granule release of MMP-9 may enhance degradation of collagen and production of neutrophil chemoattractants that act on CXCR1 or CXCR2 receptors (Weathington et al., 2006), our findings imply that hCXCL-2(3-73) and intact hCXCL-2 would act similarly in this regard.
It is remarkable that hCXCL-2(3-73) has opposing effects on neutrophil function, enhancing neutrophil migration while hampering priming of ROS production. N-terminal truncations of other ELR+CXC chemokines typically induce neutrophil function to a similar or greater extent than their intact counterparts, although few of these studies have investigated priming of ROS production.(Clark-Lewis et al., 1991; King et al., 2000; Proost et al., 1998) One potential explanation for the difference in neutrophil priming by hCXCL-2 and hCXCL-2(3-73) may lie in their relative ability to signal through the ELR+CXC chemokine receptors on human neutrophils, CXCR2 and CXCR1. hCXCL-1, -2, -3, -5, -6 and -7, preferentially bind CXCR2 rather than CXCR1. Conversely, hCXCL-8 preferentially binds CXCR1 although it can also bind CXCR2.(Ahuja and Murphy, 1996; Baggiolini et al., 1997; Geiser et al., 1993) Nonetheless, stimulating neutrophils with either hCXCL-2 or hCXCL-8 alone can induce a similar suite of functions: an increase in intracellular calcium, neutrophil migration, and ROS production. (Magazin et al., 1992) Since hCXCL-2 likely signals through CXCR2 and hCXCL-8 mainly signals through CXCR1, this suggests that there may be functional overlap between the two receptors. This point notwithstanding, Jones et. al. (Jones et al., 1996) reported that mAb against CXCR1 but not CXCR2 blocked ROS production in neutrophils induced by hCXCL-8. This finding would suggest that CXCR1 mediates neutrophil ROS production when ELR+CXC chemokines are the sole stimulus. While the design of our study is different, e.g. the ROS response is primed by ELR+CXC chemokines and stimulated by fMLP, the fact that ROS production may be mediated more efficiently by CXCR1 rather than CXCR2 could explain why hCXCL-2(3-73) does not prime neutrophil ROS production as well as intact hCXCL-2. That is, perhaps the two forms have varied affinity for CXCR1 and CXCR2.
While hCXCL-2 has a low affinity for CXCR1, it can signal through this receptor.(Ahuja and Murphy, 1996; Geiser et al., 1993; Magazin et al., 1992) We speculate that hCXCL-2(3-73) may have a higher affinity than hCXCL-2 for the CXCR2 receptor, and therefore more hCXCL- 2(3-73) binds CXCR2, reducing the amount of hCXCL-2(3-73) available to bind to CXCR1 and prime neutrophil ROS production. To test this premise, one could perform CXCR1 and CXCR2 receptor binding studies with hCXCL-2 and hCXCL-2(3-73) to determine the affinities of each ligand for each receptor. Such studies could also determine whether CXCR1 is primarily responsible for ELR+CXC chemokine priming of neutrophil ROS production in response to a secondary stimulus such as fMLP.
In addition to producing DppIV A, B. dermatitidis also evades neutrophil killing by blunting production of tumor necrosis factor-α (TNF-α). This action is likely to further undermine priming of neutrophils and killing of B. dermatitidis since neutrophils kill more yeast after stimulation with TNF-α.(Finkel-Jimenez et al., 2001; Finkel-Jimenez et al., 2002) B. dermatitidis impairs TNF-α production by elaborating the virulence factor BAD-1 (Blastomyces adhesin-1), which blocks phagocyte TNF-α production through transforming growth factor-β (TGF-β)-dependent and -independent mechanisms.(Finkel-Jimenez et al., 2001; Finkel-Jimenez et al., 2002) The ability of B. dermatitidis yeast to undermine neutrophil activation likely retards the activity of the myeloperoxidase-H2O2 halide system, which in turn promotes fungal survival since B. dermatitidis yeast are sensitive to H2O2.(Sugar et al., 1983)
In conclusion, B. dermatitidis DppIVA cleaves the ELR+CXC chemokines, murine CXCL-1 and human CXCL-2, increasing their chemotactic effect on neutrophils. Blastomycosis is characterized by the exuberant recruitment of neutrophils to sites of infection (Littman et al., 1948), and DppIVA may play a role in promoting pyogranulomatous inflammation and host tissue damage in part through this mechanism. While DppIVA cleavage of mCXCL-1 did not impair other actions of murine neutrophils, cleavage of hCXCL-2 blunted induction of human neutrophil ROS production. Thus, fungal DppIVA cleavage of ELR+CXC chemokines, while recruiting more neutrophils to tissue, does not enhance and may retard antifungal immunity. Aside from action on ELR+CXC chemokines, neutrophils are impaired by fungal DppIVA directly through inactivation of GM-CSF, and indirectly through blunted recruitment of inflammatory monocytes upon inactivation of CC chemokine ligands of CCR2. (Sterkel et al. 2016) As a virulence factor, fungal DppIVA thus acts to recruit many neutrophils incapable of efficient fungal clearance, leading to immunopathology with limited pathogen clearance.
Material and Methods
DppIV digestion of recombinant chemokines
Recombinant (r) DppIVA was cloned from B. dermatitidis and expressed in Pichia pastoris as described (Sterkel et al., 2016). Recombinant (r) hCXCL-1 (Catalog #300-11), -2 (#300-39), and -8 (#200-08M), and murine (m) CXCL-1 (#250-11) were purchased from Peprotech (Rocky Hill, NJ). Chemokines were re-suspended in digestion buffer (40 mM NaCl, 20 mM HEPES, pH 7.4) at a concentration of 40μg /ml. For rDppIVA digestion of chemokines, 1.5 μg of chemokine was incubated with 300 ng of rDppIVA in a total of 50 μl of buffer. Control reaction lacked rDppIVA to obtain uncleaved chemokine. Reactions were incubated at 37°C overnight and submitted for mass spectrometry analysis the next day. Cuvettes containing 1.5 μg of chemokine without rDppIVA were also incubated at 37°C overnight and submitted for mass spectrometry analysis. To purify cleaved and intact chemokines for in vitro and in vivo assays, samples were loaded onto a 30K centricon filter (Millepore) and spun at 13,000 × g for 10 minutes, and the flow-through containing chemokine was collected. To determine the concentration of filtered hCXCL-2 and hCXCL-2(3-73), a Pierce BCA Protein Assay (Thermo Scientific-23227) was performed following the manufacturer's instructions for a microplate procedure.
Mass spectrometry
Protein samples were cleaned/concentrated using solid phase extraction C18 cartridges (ZipTip-C18, EMD Millipore, Billerica, MA) according to the manufacturer's protocol, but eluted with 1μl solution of 60%Acetonitrile/39%H2O/1%TFA. Half of the protein sample (0.5μl volume) was directly deposited onto the Opti- TOFTM 384 well plate (Applied Biosystems, Foster City, CA) and re-crystalized with 0.4μl of matrix [5mg/ml of α- Cyano-4-Hydroxycinnamic acid in acetonitrile/H2O/TFA (70/30/0.1)]. Mass spectrum was acquired on a 4800 Matrix-Assisted Laser Desorption/Ionization-Time of Flight-Time of Flight (MALDI TOF-TOF) mass spectrometer (Applied Biosystems) scanning 5,000-40,000 Da mass range using 1000 shots acquired from 20 randomized regions of the sample spot at 3,800 intensity and 0.88V Detector Voltage Multiplier of OptiBeamTM on-axis Nd:YAG laser with 200Hz firing rate and 3 to 7ns pulse width in Positive Linear Mid Mass mode. External calibration with a Cytochrome C protein standard was performed to validate mass accuracy.
Mice
Wild-type C57BL/6 mice were purchased from Charles River. Mice used in experiments were 8-12 week old males. Intratracheal (i.t.) and intravenous (i.v.) treatments were given under anesthesia. All mouse work was in compliance with a protocol approved by the University of Wisconsin IACUC.
In vivo recruitment by mCXCL-1
To track the effects of cleaved or intact mCXCL-1 (KC), 2 × 104 heat-inactivated B. dermatitidis yeast (as described by Sterkel et al., 2016) were delivered i.t. with 100 ng of murine CXCL-1 that had been incubated in digest buffer with or without DppIV as described above. Control mice received PBS vehicle containing heat-inactivated yeast. Five hours after i.t. delivery mice were euthanized and lungs were harvested and processed for flow cytometry as previously described (Sterkel et al., 2016). To track the location of neutrophils in the lung, prior to euthanizing mice 2 μg of anti-CD45 (clone 30-F11; BioLegend, San Diego, CA) was injected i.v. to label all leukocytes in the blood (Anderson et al. 2014).
Flow cytometry
Enriched neutrophils or lung digest samples were stained and acquired on a LSRII cytometer (BD Biosciences). Data was analyzed using Flowjo version 9 or 10 software (Tree Star, Ashland, OR). Antibodies were purchased either from BD Biosciences or BioLegend. Clones for mouse targets were M1/70 (CD11b), N418 (CD11c), 30-F11 (CD45), HK1.4 (Ly6C), AL-21 (Ly6C), 1A8 (Ly6G), and E50-2440 (Siglec F). Clones for human targets were HI98 (CD15), 3G8 (CD16), E11 (CD35), G10F5 (CD66b). Dead cells were gated out of analysis using Live/Dead Yellow or Near Infrared stains (ThermoFisher Scientific). Murine neutrophils were defined as CD11c-, Siglec F-, CD11b+, Ly6Cint, Ly6G+. Human neutrophils were defined as CD15+CD16+ (Pillay et al., 2013). Mean fluorescence intensities (MFI) were determined by the geometric mean of the population.
Human neutrophil (hPMN) collection and purification
Blood was obtained from volunteer donors with written informed consent through a protocol approved by the University of Wisconsin Internal Review Board (IRB). Blood was collected in 50 ml heparin treated conical tubes. Human neutrophils were purified using the Miltenyi Biotec MACxpress® Neutrophil Isolation Kit, human (130-104-434) following the manufacturer's instructions. Erythrocytes were removed from the purified neutrophils following the instructions of the Miltenyi Biotec MACSxpress Erythrocyte Depletion Kit, human (130-098-196).
Priming of human neutrophils
For the ROS degranulation assays, and MMP-9 zymography, 1.5-2 × 106 purified hPMNs were primed by the addition of modified HBSS (mHBSS) (HBSS, 20 mM HEPES, 0.1% human serum albumin), 200 ng of hCXCL-8, 200 ng of hCXCL-2, or 200 ng hCXCL-2(3-73) for 20 minutes at 37°C with 5% CO2.
Transwell migration assays
Purified hPMNs were re-suspended in mHBSS. Calcein AM dye re-suspended in DMSO (Sigma-Aldrich D2650) was added to the neutrophils at a final concentration of 3 μM, and cells were incubated in the dark for 20 minutes to fluorescently label the neutrophils. Labeled hPMNs were pelleted by spinning at 1500 RPM at 25°C for 5 minutes without accelerator and brake. The supernatant was removed and neutrophils were re-suspended in mHBSS. hPMNs were again pelleted without the accelerator and brake. After this, the supernatant was removed and cells were re-suspended at 106 hPMNs/ml. 200 μl of the appropriate chemotactic stimulus was added to the flat-bottom, black-walled, 96-well plates (Corning 3583) in triplicate. Fluorescently labeled neutrophils were then loaded into a 96-well transwell plate with 3.0 μM pore size (Corning 3385); 105 hPMNs were loaded per well. The transwell plate was placed in the black-bottom plate and incubated at 37°C with 5% CO2 for 45 minutes to allow migration. After migration, 50 μl of 0.5 M EDTA was placed in each of the lower wells and 10 μl of 0.5 M EDTA was placed in the upper wells. The plates were incubated at 4°C for 15 minutes after which the transwells were removed and the amount of fluorescence in the lower wells was quantified using a Molecular Devices Filter Max F5 Multi-Mode Microplate Reader.
Neutrophil superoxide anion cytochrome c reduction assay
Once primed, neutrophils from each condition were added to two separate reactions in 1.5 mL cuvettes. The first “experimental” reaction contained 1.5 mg of Cytochrome C (Sigma-Aldrich C2037), about 950,000 hPMNs, and 100 nM fMLP or an equivalent volume of mHBSS for controls (Sigma-Aldrich F3506) in a final total volume of 1 ml. The second “reference” reaction was the same as the first except with the addition of 200 Units of superoxide dismutase (Sigma-Aldrich S7571). The reduction of cytochrome c was determined by measuring absorbance at 550 nM wavelength for which both reactions (with and without superoxide dismutase) for each “primed” condition were measured simultaneously in a Cary Bio400 UV/Vis Spectrophotometer (Varian) at the University of Wisconsin-Madison Biophysics Instrumentation Facility. The nanomoles of superoxide anion/106 neutrophils was determined.
Neutrophil isoluminol reactive oxygen species assay
Approximately 1.9 × 106 purified hPMNs were primed with mHBSS (control) or chemokines as described above. Reactions were performed in a 96-well black walled, clear bottom plates in quintuplicate. Each 200 μl well reaction contained a final concentration of 5 mM isoluminol (Sigma-Aldrich A8264), a final concentration of 100 nM fMLP (except for the medium control), 0.8 units of horse radish peroxidase (Sigma-Aldrich), and 190,000 primed hPMNs. Luminescence was measured by using a 4 × 4 area scan of each well in a Molecular Devices Filter Max F5 Multi-Mode Microplate Reader.
Cell surface marker degranulation assays
Approximately 1.9 × 106 purified human PMNs were primed with mHBSS (control) or chemokine as described above. hPMNs from each primed condition were then aliquoted into hextuplicate tubes and stimulated with 0.75nM fMLP (for CD35 experiments) or 1 nM fMLP (CD66b experiments) and incubated at 37°C for 30 minutes with 5% CO2. For the medium control, hPMNs were aliquoted to triplicate tubes to be labeled with anti-CD35 or -CD66b antibody, and were not treated with fMLP, but were incubated at 37°C for 30 minutes at 5% CO2.
MMP-9 zymography
Approximately 1.9 × 106 purified hPMNs were primed for each condition tested. hPMNs were then treated with mHBSS or 1 nM fMLP for 30 minutes at 37°C with 5% CO2. Treated hPMNs were pelleted at 1500 RPM for 5 minutes without the accelerator and brake. Supernatants were collected and stored at -80°. 10% PAGE gels containing 0.2% gelatin were prepared and 1:8 dilutions of supernatant from each condition were made, mixed with an equal volume of 2× sample buffer (62.5 mM Tris-HCL pH 6.8, 25% glycerol, 4% SDS, 0.01% Bromophenol Blue), and run for 60 minutes at 200 Volts. Gels were incubated in a 2.5% Triton X-100 Buffer for 30 minutes while shaking at room temperature, at which time the buffer was changed and incubated for another 30 minutes shaking at room temperature. Gels were next submerged in zymography digestion buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, and 5 mM CaCl2) and incubated at 37°C overnight. The gels were then incubated with 0.1% Coomassie Blue and imaged on a BioRad Versadoc 5000 (Hercules, CA).
In vitro neutrophil killing assay
Bone marrow neutrophils were obtained from mice as previously described through Percoll-gradient enrichment (Sterkel et al., 2016). Neutrophils were pre-incubated alone or with 50 ng (final concentration of 250 ng/ml) of mCXCL-1 or mCXCL-1 (3-72) for 30 minutes at 37° C in a 96-well plate. After the priming incubation, DppIVa RNAi yeast (Sterkel et al., 2016) were added to neutrophils to reach an effector-to-target ratio of 50:1. After six hours of incubation at 37° C, neutrophils were lysed with water and yeast were spread on brain heart infusion (BHI) agar plates to innumerate CFU. Percent killing was calculated based on the reduction of CFU compared to control wells lacking neutrophils:
Effect of mCXCL-1 (3-72) and mCXCL-1 on neutrophil function in vivo
Mice were challenged i.t. with 2 × 104 DppIVA RNAi yeast to induce a natural neutrophil response. Two days later, mice received i.t. 100 ng of mCXCL-1 (3-72), mCXCL-1, or PBS alone (vehicle). To quantify fungal burden, four hours after chemokine delivery, lungs were harvested and homogenates were spread on BHI agar plates to quantify CFU. To analyze ROS production of in vivo neutrophils, lungs were harvested just two hours after chemokine delivery to limit the effects of additional neutrophil recruitment. Lungs were harvested and processed as noted above. Leukocytes were incubated at 37° C for four hours unstimulated with fluorescent ROS probe dihydrorhodamine-123 (DHR-123, Chemodex) at a final concentration of 10 μg/ml in RPMI-1640 with 10% heat-inactivated FBS (Balaguer et al. 2015; Sterkel et al. 2016). After DHR-123 incubation, leukocytes were washed and stained for flow cytometry as described above.
Graphical and statistical analysis
Graphs were produced using Graphpad Prism 5 or Flowjo software. A student's t-test was used to statistically analyze the migration of neutrophils; an ANOVA with Tukey test was used to analyze flow and CFU data; an ANOVA using a Newman-Keuls Multiple Comparison Test was performed to statistically analyze ROS production, CD35 and CD66b mean fluorescence intensity, and zymography densitometry data.
Acknowledgments
We thank Chad Johnson and Jonathan Cabezas-Olcoz for their assistance with the collection and preparation of neutrophils. This work was supported by grants AI035681 and AI040996 (to BSK) and AI108727 (JN) from NIH, and BWF 1012299 (JN) from the Burroughs Wellcome Fund, and American Heart Association Postdoctoral Fellowship (17POST32790004 to JSF).
References
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. The Journal of biological chemistry. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual review of immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. The Journal of cell biology. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Hoggatt J, O'Leary HA, Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag S, Rohrabaugh SL, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nature medicine. 2012;18:1786–1796. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E, Stevens DA. Opposite effects of human monocytes, macrophages, and polymorphonuclear neutrophils on replication of Blastomyces dermatitidis in vitro. Infection and immunity. 1982;36:297–303. doi: 10.1128/iai.36.1.297-303.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E, Stevens DA. Enhancing effect of murine polymorphonuclear neutrophils (PMN) on the multiplication of Blastomyces dermatitidis in vitro and in vivo. Clinical and experimental immunology. 1983;54:587–594. [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. The Journal of biological chemistry. 1991;266:23128–23134. [PubMed] [Google Scholar]
- Drutz DJ, Frey CL. Intracellular and extracellular defenses of human phagocytes against Blastomyces dermatitidis conidia and yeasts. The Journal of laboratory and clinical medicine. 1985;105:737–750. [PubMed] [Google Scholar]
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes and infection / Institut Pasteur. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Finkel-Jimenez B, Wuthrich M, Brandhorst T, Klein BS. The WI-1 adhesin blocks phagocyte TNF-alpha production, imparting pathogenicity on Blastomyces dermatitidis. J Immunol. 2001;166:2665–2673. doi: 10.4049/jimmunol.166.4.2665. [DOI] [PubMed] [Google Scholar]
- Finkel-Jimenez B, Wuthrich M, Klein BS. BAD1, an essential virulence factor of Blastomyces dermatitidis, suppresses host TNF-alpha production through TGF-beta-dependent and -independent mechanisms. J Immunol. 2002;168:5746–5755. doi: 10.4049/jimmunol.168.11.5746. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nature reviews Microbiology. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. The Journal of biological chemistry. 1993;268:15419–15424. [PubMed] [Google Scholar]
- Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? Journal of leukocyte biology. 2001;69:191–196. [PubMed] [Google Scholar]
- Guichard C, Pedruzzi E, Dewas C, Fay M, Pouzet C, Bens M, Vandewalle A, Ogier-Denis E, Gougerot-Pocidalo MA, Elbim C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. The Journal of biological chemistry. 2005;280:37021–37032. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- Jesaitis AJ, Buescher ES, Harrison D, Quinn MT, Parkos CA, Livesey S, Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. The Journal of clinical investigation. 1990;85:821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AG, Johanson K, Frey CL, DeMarsh PL, White JR, McDevitt P, McNulty D, Balcarek J, Jonak ZL, Bhatnagar PK, Pelus LM. Identification of unique truncated KC/GRO beta chemokines with potent hematopoietic and anti-infective activities. J Immunol. 2000;164:3774–3782. doi: 10.4049/jimmunol.164.7.3774. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. The Journal of experimental medicine. 1967;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. Journal of bacteriology. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kurita N, Terao K, Brummer E, Ito E, Nishimura K, Miyaji M. Resistance of Histoplasma capsulatum to killing by human neutrophils. Evasion of oxidative burst and lysosomal-fusion products. Mycopathologia. 1991;115:207–213. doi: 10.1007/BF00462229. [DOI] [PubMed] [Google Scholar]
- Laible NJ, Germaine GR. Bactericidal activity of human lysozyme, muramidaseinactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infection and immunity. 1985;48:720–728. doi: 10.1128/iai.48.3.720-728.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Critical reviews in clinical laboratory sciences. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- Littman ML, Wicker EH, Warren AS. Systemic North American blastomycosis; report of a case with cultural studies of the etiologic agent and observations on the effect of streptomycin and penicillin in vitro. The American journal of pathology. 1948;24:339–365. [PMC free article] [PubMed] [Google Scholar]
- Magazin M, Vita N, Cavrois E, Lefort S, Guillemot JC, Ferrara P. The biological activities of gro beta and IL-8 on human neutrophils are overlapping but not identical. European cytokine network. 1992;3:461–467. [PubMed] [Google Scholar]
- Mortier A, Gouwy M, Van Damme J, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp Cell Res. 2011;317:642–654. doi: 10.1016/j.yexcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine & growth factor reviews. 2010;21:253–262. doi: 10.1016/j.cytogfr.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ou X, O'Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood. 2013;122:161–169. doi: 10.1182/blood-2013-02-487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and molecular life sciences: CMLS. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost P, De Meester I, Schols D, Struyf S, Lambeir AM, Wuyts A, Opdenakker G, De Clercq E, Scharpe S, Van Damme J. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. The Journal of biological chemistry. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- Sixbey JW, Fields BT, Sun CN, Clark RA, Nolan CM. Interactions between human granulocytes and Blastomyces dermatitidis. Infection and immunity. 1979;23:41–44. doi: 10.1128/iai.23.1.41-44.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkel AK, Lorenzini JL, Fites JS, Subramanian Vignesh K, Sullivan TD, Wuthrich M, Brandhorst T, Hernandez-Santos N, Deepe GS, Jr, Klein BS. Fungal Mimicry of a Mammalian Aminopeptidase Disables Innate Immunity and Promotes Pathogenicity. Cell host & microbe. 2016;19:361–374. doi: 10.1016/j.chom.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar AM, Chahal RS, Brummer E, Stevens DA. Susceptibility of Blastomyces dermatitidis strains to products of oxidative metabolism. Infection and immunity. 1983;41:908–912. doi: 10.1128/iai.41.3.908-912.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature medicine. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Wuyts A, Govaerts C, Struyf S, Lenaerts JP, Put W, Conings R, Proost P, Van Damme J. Isolation of the CXC chemokines ENA-78, GRO alpha and GRO gamma from tumor cells and leukocytes reveals NH2-terminal heterogeneity. Functional comparison of different natural isoforms. European journal of biochemistry / FEBS. 1999;260:421–429. doi: 10.1046/j.1432-1327.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- Yuo A, Kitagawa S, Kasahara T, Matsushima K, Saito M, Takaku F. Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood. 1991;78:2708–2714. [PubMed] [Google Scholar]
- Zeidler C, Germeshausen M, Klein C, Welte K. Clinical implications of ELA2-, HAX1-, and G-CSF-receptor (CSF3R) mutations in severe congenital neutropenia. British journal of haematology. 2009;144:459–467. doi: 10.1111/j.1365-2141.2008.07425.x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


