Abstract
Background
We evaluated the efficacy, pattern of failure, and toxicity of stereotactic ablative radiotherapy (SABR) for medically inoperable clinical stage I non-small cell lung cancer (NSCLC) in a prospective clinical trial with 7 years follow-up.
Methods
Eligible patients with histologically confirmed NSCLC and PET clinically staged I disease were treated with SABR (50 Gy in 4 fractions). The primary endpoint was progression-free survival (PFS). Patients were followed with CT/PET-CT every 3 months for the first two years, then every 6 months for the next three years and then annually thereafter.
Results
Sixty-five patients were eligible for analysis. The median age was 71 years, and median follow-up was 7.2 years. A total of 18 (27.7%) patients had disease recurrence at a median of 14.5 months (range 4.3–71.5) after SABR. Estimated incidences of local, regional, and distant recurrence using competing risk analysis were 8.1%, 10.9%, and 11.0% at 5 years, respectively, and 8.1%, 13.6%, and 13.8% at 7 years. Second primary lung carcinoma (SPLC) developed in 12 (18.5%) patients at a median of 35 months (range 5–67) after SABR. Estimated 5- and 7-year PFS rates were 49.5% and 38.2%, respectively; corresponding overall survival rates were 55.7% and 47.5%. Three (4.6%) patients had grade 3 treatment-related adverse events. No patients had grade 4 or 5 events.
Conclusion
With long-term follow-up, our prospective study demonstrated outstanding local control and low toxicity after SABR in clinical stage I NSCLC. Regional recurrence and distant metastases were the dominant manifestations of failure. Surveillance for SPLC is recommended.
Keywords: stereotactic ablation radiotherapy (SABR), stereotactic body radiotherapy (SBRT), non-small cell lung cancer (NSCLC), stage I, pattern of failure
Introduction
Image-guided stereotactic ablative radiotherapy (SABR), also called stereotactic body radiation therapy (SBRT), as a non-invasive treatment, has become standard of care for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Population-based retrospective studies and a recent pooled analysis of two prospective randomized trials have indicated that SABR has effectiveness comparable to that of surgery for this population, with reported 3-year overall survival rates of 48–91% and local control rates of 85–96%. 1–4 Nonetheless, the efficacy, pattern of failure, and toxicity reported with SABR were mostly based upon relatively short-term follow-up. Currently, robust long-term outcomes data at follow-up times greater than 5 years are limited. As increasing numbers of early-stage lung cancers are being detected worldwide, and the effectiveness of SABR realized, SABR is gradually being applied to inoperable, borderline operable, or even operable early-stage NSCLC patients who have longer life expectancy. Thus, long-term data on the outcomes of SABR are urgently needed to strengthen confidence in its use.
We initiated a prospective clinical trial of SABR early in 2005. The aims of this study were to assess therapeutic effectiveness and toxicity of SABR for patients with medically inoperable stage I NSCLC. This is the first report of the study and the only one in the literature about the pattern of failure, prognosis, and toxicity after 7-year median follow-up. We also assess the literature on SABR vs surgery for early-stage NSCLC.
Methods
Patients and study design
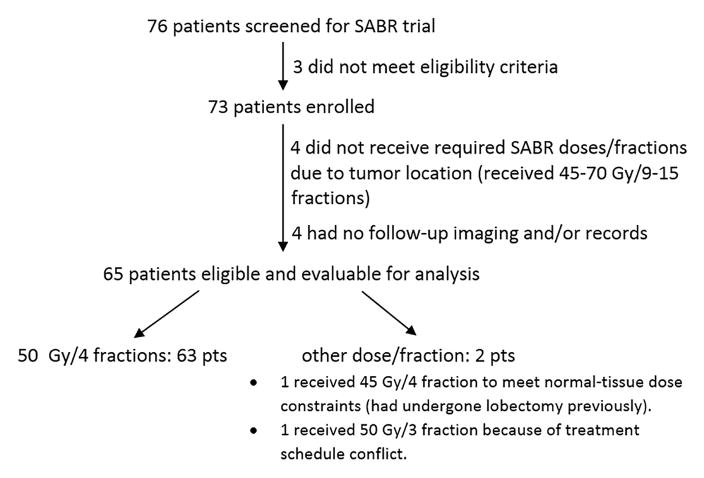
From November 2005 to March 2013, 73 patients with histologically confirmed NSCLC were prospectively enrolled in this trial (S1). Among 8 patients removed from final analysis, 4 patients received conventional radiotherapy due to normal tissue constraints and 4 patients didn’t come for any follow-up image or visit. This study was approved by MD Anderson’s Institutional Review Board, and all patients provided written informed consent to participate.
Inclusion criteria were a diagnosis of medically inoperable clinical stage IA ([T1N0M0]: tumor size < 3cm [T1], no regional lymph node metastasis [N0], no distant metastasis [M0]) or IB ([T2aN0M0]: tumor more than 3 cm but not more than 5 cm in greatest dimension [T2a]), histologically confirmed NSCLC. Operable patients who elected to have SABR were also eligible. For patients with prior lung cancer history, patients needed to be lung cancer free for more than 5 years for the same histology or more than 2 years for a different histology to reduce the impact of prior disease and treatment on the prognosis and toxicity of SABR. Patients were clinically staged by computed tomography (CT) and 18F-fluorodeoxyglucose (18F-FDG)-positron emission CT (PET/CT) within 3 months before SABR. Patients with hilar or mediastinal lymph nodes measuring ≤ 1 cm and no abnormal hilar or mediastinal uptake on PET were considered N0. Endobronchial ultrasound (EBUS) procedure were performed for patients with hilar or mediastinal lymph nodes measuring > 1 cm on CT or abnormal PET (including high avidity and suspicious but non-diagnostic uptake). Specifically, abnormal nodal SUVmax uptake higher than the SUVs of mediastinal blood pool was used as a reference to define abnormal uptake, but the final decision about EBUS was made based on treating physicians and radiologist’s PET clinical judgment and CT criteria as defined above.
SABR treatment
All patients underwent 4-dimensional CT-based simulation, and respiratory gating was considered if the tumor moved more than 1 cm. 15% of patients were treated with respiratory gate using video-feedback driven voluntary deep inspiration breath hold. Internal gross target volume (IGTV) was contoured according to maximal intensity projection and edited at different phases. Image-guided SABR for a total dose of 50 Gy in 4 fractions was prescribed to planning target volume (PTV). Unmodulated (3-dimensional conformal) or intensity modulated radiation therapy SABR plans were optimized by using 6–12 coplanar or non-coplanar 6-MV photon beams. The prescribed isodose line was required to cover more than 95% of the PTV and 100% of the IGTV. Detailed SABR simulation, planning, treatment delivery, and dose volume constraints were described previously. 5, 6
Follow-up evaluations
The primary endpoint was progression-free survival (PFS). Patients underwent chest CT scan every 3 months for the first 2 years, then every 6 months for the next 3 years, and then annually. PET/CT was required between 2–6 months after the completion of SABR; subsequent PET scan was indicated if there was any sign of recurrent disease. Local recurrence (LR) was defined as CT evidence of progressive soft tissue abnormalities in the same lobe that corresponded to avid (SUVmax>5) areas on PET/CT images >6 months after SABR. Biopsy was strongly recommended to confirm suspected recurrence. LR was classified as in-field recurrence (occurring in the area inside the PTV), involved lobe failure (out-field recurrence in the same lobe), and marginal failure (a recurrent lesion located within 1 cm in any direction around the PTV). Regional recurrence (RR) was defined as any intrathoracic lymph node relapse outside the PTV. Distant metastasis (DM) was defined as any recurrence in a different lobe or any failure outside the chest.
A second primary lung carcinoma (SPLC) after SABR was defined using modified criteria of Martini and Melamed: 7 A new tumor with different histological type/subtype or molecular genomic characteristics from the first tumor; a new tumor with the same histology in a different lobe or lung if (1) it occurred at tumor-free interval of >2 years and without any evidence of carcinoma in lymphatics and extrapulmonary metastases; (2) it was reviewed by a multidisciplinary tumor team (MDT) including treating physicians, pathologist, and radiologist for its pathological morphology, radiological features, tumor location, previous cancer history and imaging features and was verified by follow-up outcome with no tumor recurrence after definitive treatment for minimal 1 year of follow-up.
Statistical analysis
Progression-free survival (PFS) events, as the primary endpoints, including any recurrence (LR, RR and DM) and death, were calculated from the beginning date of SABR to the date of the first recurrence or death (for patients who were not known to have recurrence but died). The date of recurrence was the date of the first CT or PET/CT image that showed abnormalities. SPLC wasn’t counted in PFS event. Overall survival (OS) analysis was calculated from the beginning date of SABR to the date of death or last follow-up.
All statistical analyses were performed using SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA) and R statistical software 3.1.2 with packages cmprsk_v2.2-7 and survival_v2.38-1. Median follow-up was computed by the reverse Kaplan-Meier method. OS curve was performed using Kaplan-Meier method. In the presence of competing risks (death) when performing survival analyses for any recurrence, an alternative cumulative-incidence competing risk method was used to overcome the overestimated probabilities of recurrences. 8 All significance tests were two tailed with a significant P value defined as <0.05.
Results
Patient characteristics
A total of 65 patients were eligible for analysis (Figure 1, Table 1). Eighteen (27.7%) patients underwent EBUS staging, and 14 (21.5%) patients were potentially operable. EBUS was done in 6 patients with PET results of FDG-avid lymph nodes and in 6 patients with CT results showing a large short axis. A prior history of lung cancer was identified in 13 (20.0%) patients; 7 (10.8%) patients had had the same histology previously with more than 5 years tumor free, and 6 (9.2%) patients had had different histology previously with more than 2 years tumor free. Four of the 13 patients had received irradiation prior to SABR (1 had SABR, 3 had conventional thoracic radiation). A prior history of a malignancy other than lung cancer was identified in 12 (18.5%) patients.
Figure 1.
Patient distribution for the prospective SABR trial.
Table 1.
Clinical characteristics and outcomes of the 65 enrolled patients with stage I non-small cell lung cancer
| Characteristic | Number of patients (%) |
|---|---|
| Age (years) | |
| Mean | 72.1 |
| Median (range) | 71.8 (54.7–91.8) |
| Sex | |
| Male | 32 (49.2) |
| Female | 33 (50.8) |
| ECOG performance status | |
| 0 | 7 (10.8) |
| 1 | 40 (61.5) |
| 2 | 18 (27.7) |
| Disease operability | |
| Operable | 14 (21.5) |
| Inoperable due to poor pulmonary function | 12 (18.5) |
| Inoperable due to comorbidity | 16 (24.6) |
| Inoperable due to both reasons | 23 (35.4) |
| Tumor stagea | |
| T1a | 38 (58.5) |
| T1b | 24 (36.9) |
| T2 (T2a: ≤5 cm, pleural invasion) | 3 (4.6) |
| Maximum tumor diameter (cm)b | |
| Mean | 2.0 |
| Median (range) | 1.9 (0.7–4.0) |
| Median iGTV volume (cm3, range) | 8.38 (0.70–37.03) |
| Median PTV volume (cm3, range) | 59.98 (6.72–169.31) |
| Tumor location 5 | |
| Peripheral | 57 (87.7) |
| Central | 8 (12.3) |
| Histology | |
| Adenocarcinoma | 30 (46.2) |
| Squamous | 26 (40.0) |
| NSCC NOS | 9 (13.8) |
| EBUS | |
| Performed | 18 (27.7) |
| Not performed | 47 (72.3) |
| FDG avidity of tumorc | |
| Mean | 8.0 |
| Median (range) | 5.3 (0.0–32.6) |
| Smoking status | |
| Past or current smoker | 57 (87.7) |
| Never smoker | 8 (12.3) |
| Median FEV 1, % predicted (range) | 60 (20–126) |
| Median DLCO, % predicted (range)c | 59.5 (18–112) |
| Treatment planning | |
| IMRT plan | 27 (41.5) |
| 3D-CRT plan | 38 (58.5) |
| Cumulative initial eventsd | 18 (27.7) |
| LR | 5 (7.7) |
| RR | 8 (12.3) |
| DM | 8 (12.3) |
| Median time to recurrence, m (range) | |
| Any recurrence | 14.5 (4.3–71.5) |
| Initial LR | 34.6 (7.8–48.1) |
| Initial RR | 15.6 (4.3–70.8) |
| Initial DM | 8.9 (4.5–71.5) |
| Death | 35 (53.8) |
| Due to lung cancer | 17 (48.6) |
| Due to other disease | 12 (34.3) |
| Unknown cause | 6 (17.1) |
| Median PFS time, m | 48.1 (21.0–75.2) |
| Median OS, m (95% CI) | 80.8 (36.9–124.8) |
| Median lung cancer-specific OS, m | Not reached |
Clinical staging according to the AJCC TNM staging seventh edition.
Tumor was measured on CT imaging within 3 months prior to SABR.
Specific FDG of tumor was unknown in 5 patients; DLCO was unknown or could not be performed in 9 patients.
Two patients had simultaneous failure (1 had RR and DM, 1 had all sites failure). Five patients had isolated RR, and 1 patient had more than 1 site of RR. Two patients had isolated DM, and 4 patients had more than 1 metastatic lesion.
Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV 1, forced expiratory volume in the first second of expiration; NSCC, non-small cell carcinoma; NOS, not otherwise specified; SUV, standardized uptake value; EBUS, endobronchial ultrasound; ECOG, Eastern Cooperative Oncology Group; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity modulated radiation therapy; LR, local recurrence; RR, regional recurrence; DM, distant metastasis; PFS, progression-free survival; m, month; CI, confidence interval.
Patterns of failure, salvage treatment and survival
At median follow-up time of 7.2 years (interquartile range 4.6–8.3), 18 (27.7%) patients had disease recurrence. The minimal and maximal follow up time for living patients were 3.1 years and 10.2 years, respectively. The initial recurrence manifested as LR in 5 (7.7%) patients, RR in 8 (12.3%), and DM in 8 (12.3%); 2 patients had simultaneous failure (Table 1). Of 5 patients who had LR as the first event, all patients had PET imaging with median SUVmax of 7.2 (range 5.0–12.2), and 4 patients had histological confirmation of recurrence. Furthermore, 3 patients’ recurrences were classified as in-field, 1 patient’s as out-field, and 1 patient’s as marginal.
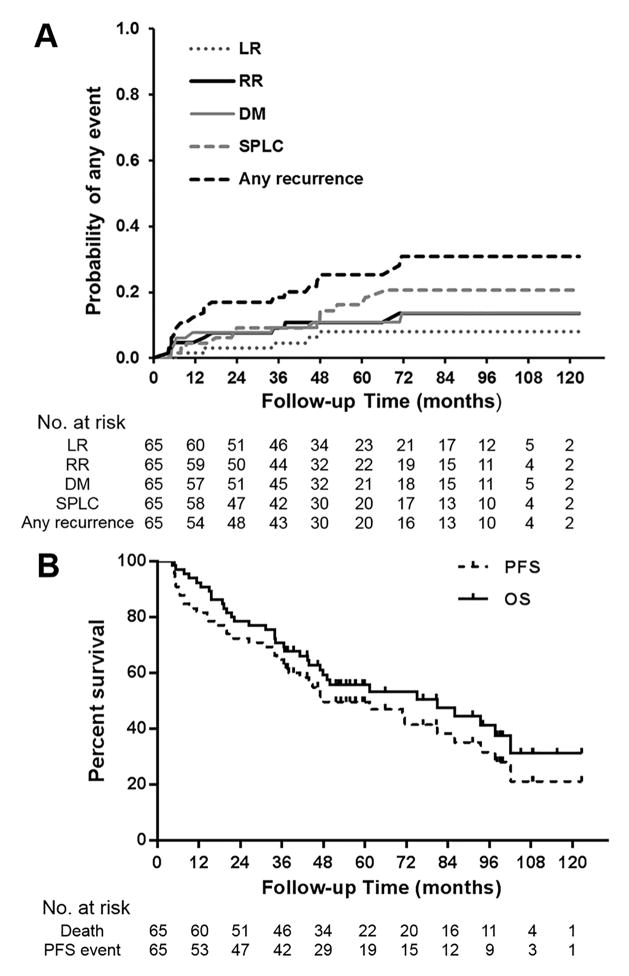
The median time to any initial recurrence was 14.5 months (range 4.3–71.5). Of note, 2 (25.0%) of 8 patients had very short times to RR, 4.3 and 5.2 months, and 4 (50.0%) of 8 patients had DM at a short interval of about 6 months after SABR. For the 8 patients who had initial DM, the most common site was pulmonary (50.0%); other sites were bone, liver, and distant nodal. Estimated cumulative 5-year rates of LR, RR, and DM using competing risk analysis were 8.1%, 10.9%, and 11.0%, respectively. Corresponding 7-year incidence rates increased to 8.1%, 13.6%, and 13.8%, respectively (Figure 2A, Table 2).
Figure 2.
Estimated cumulative incidence curves illustrating local recurrence (LR), regional recurrence (RR), distant metastasis (DM), second primary lung cancer (SPLC), and any recurrence over time by competing risk analysis (any cause of death as a competing risk event) (A); and overall survival (OS) and progression-free survival (PFS) by Kaplan-Meier analysis (B)
Table 2.
Pattern of failure, survival and second primary lung cancer over 7 years after stereotactic ablative radiotherapy
| Eventa | Actual incidence (%) | Estimated cumulative incidence, % (95% CI) | |||
|---|---|---|---|---|---|
|
| |||||
| 1-year | 3-year | 5-year | 7-year | ||
| Local recurrence | 7.7 | 1.5 (0.2–10.8) | 4.6 (1.5–13.9) | 8.1 (3.5–18.8) | 8.1 (3.5–18.8) |
| Regional recurrence | 12.3 | 4.6 (1.5–13.9) | 9.2 (4.3–19.8) | 10.9 (5.4–21.8) | 13.6 (7.0–26.5) |
| Loco-regional recurrence | 18.5 | 6.2 (2.4–15.9) | 12.3 (6.4–23.6) | 17.4 (10.1–29.8) | 20.0 (11.9–33.7) |
| Distant metastases | 12.3 | 7.7 (3.3–17.9) | 9.2 (4.3–17.8) | 11.0 (5.5–22.2) | 13.8 (7.1–26.8) |
| Any recurrence | 27.7 | 12.3 (6.4–23.6) | 18.5 (11.1–30.8) | 25.3 (16.6–38.8) | 30.9 (20.7–46.0) |
| Progression-free survivalb | - | 81.5 (76.7–86.3) | 64.6 (58.7–70.5) | 49.5 (43.2–55.8) | 38.2 (31.2–45.2) |
| Overall survivalb | - | 92.3 (89.0–95.6) | 70.8 (65.2–76.4) | 55.7 (49.4–62.0) | 47.5 (40.6–54.4) |
| Second primary lung cancer | 18.5 | 4.6 (1.5–13.9) | 9.2 (4.3–19.8) | 16.2 (9.2–28.6) | 20.7 (12.4–34.5) |
Any recurrence as the first event was calculated, and subsequent recurrence was not estimated here. The cumulative competing risk method was used to estimate the probabilities of any recurrence in the presence of competing risks (any cause of death).
Progression-free survival and overall survival were calculated using the conventional Kaplan-Meier method.
The estimated 5, 7-year rates were 49.5% and 38.2% for PFS, 55.7% and 47.5% for OS, respectively (Figure 2B, Table 2). Of the 10 (15.4%) patients with an initial local-regional recurrence without DM, 7 (70.0%) received salvage treatment: local modalities in 5 patients (2 received SABR, 1 received surgery, and 2 received conventional fractionated thoracic irradiation), concurrent chemoradiation in 1 patient, and chemotherapy in 1 patient. At last follow-up, 5 (50.0%) patients didn’t develop subsequent recurrence or death.
We reviewed individually 5 patients who had initial LR. They all had larger iGTV volume with a median of 20.76 cm3 (range 9.44–26.64), compared to a median of 8.38 cm3 in the entire group of patients. The 3 patients with primary site recurrence all had a suboptimal dose (minimum biologically effective dose (BED10) to 95% of PTV [PTVD95 BED10] < median value of 113.6 Gy; mean PTV BED dose [PTVmean BED10] < median value of 135 Gy), which might be because the dose was prescribed to an inappropriate isodose line. It should be noted that for 2 patients, a lesion located in the same lobe that had undergone SABR was found by biopsy to be a second lung malignancy with different histology. Because of the small number of events, we can’t draw conclusions about risk factors for any form of recurrence.
Second primary lung malignancy
SPLC developed in 12 (18.5%) patients at a median of 35 months (range 5–67) after SABR. Of the 12 patients, 10 underwent biopsy, and 2 of them had different histology in the same lobe of the prior SABR, indicating recurrence within the involved lobe should be carefully reviewed and ideally confirmed histologically. For the treatment of SPLC, 9 patients with stage I disease received SABR, 1 patient with stage I, centrally located lesion received hypofractionated radiation, 1 patient with stage II disease received conventional radiotherapy, and one 81-year-old patient with stage III disease received targeted therapy. Ten (83.3%) of the 12 patients did not have a subsequent recurrence event, and the median survival time from SPLC to last follow-up or death was 13.1 months (range 7.6–90.4).
Toxicity
The most common adverse effects were dermatitis, radiation pneumonitis, and chest wall pain (Table 3). In all, only 3 (4.6%) patients experienced grade 3 treatment-related adverse events (2 [3.1%] with dermatitis, 1 [1.5%] with chest wall pain and radiation pneumonitis). Most patients experienced asymptomatic changes in follow-up imaging, including grade 1 radiation pneumonitis (75.4%) and rib fracture (20.0%). No patient experienced grade 4 or 5 toxicity.
Table 3.
Adverse effects after stereotactic ablative radiotherapy, n (%)
| Adverse effect | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Acute adverse event | |||
| Dermatitis | 16 (24.6) | 3 (4.6) | 2 (3.1) |
| Radiation pneumonitis | 49 (75.4) | 7 (10.8) | 1 (1.5) |
| Dyspnea/shortness of breatha | 11 (16.9) | 8 (12.3) | 0 |
| Fatigue | 7 (10.8) | 2 (3.1) | 0 |
| Hemoptysis | 1 (1.5) | 0 | 0 |
| Late adverse event | |||
| Chest wall pain | 15 (23.1) | 7 (10.8) | 1 (1.5) |
| Rib fracture | 13 (20.0) | 3 (4.6) | 0 |
| Brachial plexopathy | 3 (4.6) | 2 (3.1) | 0 |
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Each symptom was scored separately. Late event was defined as >6 months after SABR.
Grade 2 event was also regarded as symptom that is related to radiation pneumonitis.
Discussion
In this prospective phase II study conducted over 10 years to investigate the efficacy of SABR for patients with clinical stage I NSCLC, with more than a 7-year median follow-up, we found an outstanding outcomes with low incidences of LR, RR, and DM at 5-years (8.1%, 10.9% and 11.0%, respectively). Interestingly, the 7-year estimated incidences of RR and DM slightly increased, indicating that a recurrence event could occur after 5 years. With respect to LR, it is only recently recognized that a smaller GTV and higher radiation dose delivered to the PTV are associated with better local control, which was supported by 5 LRs in the current study. 9 DM rate in our trial appears lower than reported results (Table 4). This is likely because all patients had a pathological diagnosis and rigorous PET staging pre-SABR, and most patients with high-risk features of lymph node involvement underwent an EBUS procedure before enrollment. 10–17 Further, most developed intrathoracic lesions were verified with biopsy, and some of them were found to be secondary lung malignancies. This routine biopsy of suspected recurrent lesions in the lung was missing in most other SABR studies. Therefore, it is helpful to have accurate staging pre-SABR and histological testing to differentiate a second primary lesion from recurrence. Additionally, SPLC developed in 18.5% patients during the 7-year surveillance. Given these data and published results, 18, 19 a long duration of follow-up (beyond 2 years) and routine post-SABR surveillance, perhaps even longer than currently believed is necessary, is important for detecting recurrence, including SPLC which are significant risks for all lung cancer patients.
Table 4.
Patterns of failure and outcomes of stereotactic ablative radiotherapy vs surgery for the treatment of clinical early-stage NSCLC reported in the current and other studies
| Publication | Total no. of pts | Median follow-up (m) | Prescribed dose/fractions | LR (%) | RR (%) | DM (%) | PFS (%) | OS (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 3y | 5y | 3y | 5y | 3y | 5y | 3y | 5y | 3y | 5y | ||||
| SABR studies | |||||||||||||
| Baumann et al. (prospective, 2009) 10 | 57 | 35 | 45 Gy/3f | 8 | - | 5c | 16c | 52 | - | 60 | - | ||
| Timmerman et al. (prospective, 2010) a 11 | 59 | 34.4 | 54 Gy/3f | 9.4 | - | 12.8b | - | 22.1 | - | 48.3 | - | 55.8 | - |
| Onishi et al. (retrospective, 2011) 12 | 87 | 55 | 45–72.5 Gy/3–10f | - | 13.3 | - | 14.7 | - | 24.9 | - | - | - | 69.5 |
| Senthi et al. (retrospective, 2012) 13 | 676 | 32.9 | 54–60 Gy/3f; 55–60 Gy/5f; 60 Gy/8f |
- | 10.5 | - | 12.7 | - | 19.9 | - | - | - | ~32 |
| Lagerwaard et al. (prospective database, 2012) 14 | 177 | 31.5 | 60 Gy/3,5,8f | 7 | - | 9.7 | - | 9.7 | - | 81 | - | 84.7 | - |
| Zheng et al. (retrospective, 2014)d15 | Meta-analysis | 28 | BED10 ≥100 Gy | 12.2b | 16.1b | - | - | - | - | 65.8 | 65.8 | 56.6 | 41.2 |
| Ricardi et al. (retrospective, 2014) 17 | 196 | 30 | 48–60 Gy/3–8f | 10.3 | - | 14.5 | - | 24.1 | - | 65.5 | - | 68 | - |
| Nagata et al. (prospective, 2015) 16 | 100 (in) | 47 | 48 Gy/4f | 12.7 | - | 8c | 23c | 48.9 | - | 59.9 | 42.8 | ||
| 64 (op) | 67 | 14.6 | - | 25c | 32.8c | 54.5 | - | 76.5 | 54 | ||||
| Spratt et al. (retrospective, 2015) 18 | 366 | 23 | 45–50 Gy/5f; 48 Gy/4f; 54–60 Gy/3f |
14.2 | - | 17.4 | - | 19.5 | - | - | - | ~53 | - |
| Chang et al. (prospective, 2015) 1 | 31 | 40.2 | 54 Gy/3f; 50 Gy/4f; 60 Gy/5f | 4 | - | 10 | - | 3 | - | 86 | - | 95 | - |
| 27 | 35.4 | Lobectomy | 0 | - | 4 | - | 9 | - | 80 | - | 79 | - | |
| Current study | 65 | 86.2 | 50 Gy/4f | 4.6 | 8.1 | 9.2 | 10.9 | 9.2 | 11.0 | 64.6 | 49.5 | 70.8 | 55.7 |
| Surgery studies for clinical stage I NSCLC | |||||||||||||
| Okada et al. (prospective nonrandomized, 2006) 31 | 262 | >60 | Lobectomy | 6.9b,c | 10.3c | - | 83.4 | - | 89.1 | ||||
| 305 | >60 | SLR | 4.9b,c | 9.2c | - | 85.9 | - | 89.6 | |||||
| Whitson et al. (retrospective, 2007) 30 | 88 | - | Thoracotomy | - | - | - | - | - | - | - | - | 77 | 64 |
| 59 | - | VATS | 78 | - | |||||||||
| Crabtree et al. (PSM, 2010) 2 | 462 | 31 | Surgery | 6b | - | - | - | - | - | - | - | 68 | 55 |
| 76 | 19 | SABR | 11 | - | 3.9c | - | - | - | - | 32 | - | ||
| Verstegen et al. (PSM, 2013) 3 | 64 | 16 | VATS | 17.4b | - | - | - | 34.5 | - | 63.2 | - | 76.9 | - |
| 64 | 30 | SABR | 6.7b | - | - | - | 14.8 | - | 79.3 | - | 79.6 | - | |
| Port et al. (PSM, 2014) 4 | 76 | 35 | Wedge±brachy | 4b,c | 5c | 88 | - | 87 | - | ||||
| 23 | 35 | SABR | 13b,c | 17c | 72 | - | 75 | - | |||||
| Landreneau et al. (PSM, 2014) 29 | 312 | 64.8 | Segmentectomy | 5.5b,c | 14.8c | - | 70 | - | 54 | ||||
| 312 | 64.8 | Lobectomy | 5.1b,c | 11.6c | - | 71 | - | 60 | |||||
– indicates the specific number was not provided.
55 patients were evaluable. The primary tumor failure rate was 2.4% at 3 years. Three additional patients had recurrence within the involved lobe.
Local-regional failure rate.
Actual recurrence rate instead of estimated cumulative 3-, 5-year recurrence rates.
A meta-analysis of 40 SABR studies (4850 patients) published after 2005. Local-regional control data were from 30 studies.
Abbreviations: LR, local recurrence; RR, regional recurrence; DM, distant metastasis; PFS, progression-free survival; OS, overall survival; m, month; y, year; f, fraction; SLR, sublobar resection; VATS, lobectomy by video-assisted thoracoscopic surgery; PSM, propensity-matched analysis; in, inoperable; op, operable.
Notably, one third of patients in this study with RR and/or DM had the recurrence within 6 months after SABR. The short interval suggests the existence of occult tumor before SABR. Subclinical disease is difficult to detect by current image modalities, so RR and DM are still the predominant pattern of failure. A more accurate method than PET-CT to detect occult tumor before SABR should be explored. More importantly, treatments that are effective in eradicating occult metastases would alter the disease status and improve prognosis. A combination of an immunotherapeutic approach and SABR (termed ISABR) has recently been proposed, as there is evidence of synergy between immune therapy and SABR, as well as evidence that immune therapy works in the metastatic setting and may be best, in fact, at eliminating micrometastatic rather than bulky disease. 20–22 Thus, ISABR, a promising and currently investigated approach, may address both visible and occult disease, and further improve outcomes of SABR for early-stage NSCLC.
In the present study, the actual incidence of developing second lung malignancy appears relatively high. The historical estimated risk of SPLC ranges from 1% to 6% per person-year after resection, and can slightly increasing over time. 23, 24 The crude rate is reported to be 3.2–15.3% with short follow-up, 1, 13, 18, 25 and its actuarial risk reaches 11.7–20% at 5–8 years after initial surgery for NSCLC. 19, 26, 27 It is noteworthy that although SPLC is an undesirable occurrence, it is often highly curable, with few recurrences occurring after definitive treatment. In addition, half of patients with only LR or RR did not develop a subsequent recurrence after salvage treatment. Thus, second lung malignancy is a concern for long-term follow-up, and close surveillance will help to identify a new lesion/recurrence at an early stage when it can be considered for curative local therapy to obtain a better prognosis.
To date, the role of SABR in operable stage I NSCLC has been debated. 28 Table 4 outlines studies reporting outcomes after SABR or surgery for early-stage NSCLC. A pooled analysis of two randomized, controlled trials indicated that SABR is comparable to lobectomy in terms of PFS with improved OS at 3 years and a lower toxicity profile in operable patients. 1 The limitations of this non-planned pooled analysis include short follow-up and a small sample size of the two early closed trials due to poor accrual. However, when comparing the two modalities using nonrandomized studies, it should be noted that there are several pitfalls and potential biases that affect interpretation of results. First, there are discrepancies about the definitions of LR, RR, and DM between surgery and SABR studies, and even among different SABR studies. For example, in many surgery studies, failure in the ipsilateral hilum and mediastinum is defined as RR, and failure in a contralateral lymph node is defined as DM, 4, 13 while in most SABR studies recurrence in an intrathoracic lymph node is regarded as RR. Thus, RR may appear lower in surgical studies simply by virtue of definition. A second pitfall is that all SABR cases were clinically rather than pathologically staged, whereas most surgical patients underwent surgical lymph node sampling/dissection that result in upstaging in 7.3~35% of patients who were considered preoperatively to have early-stage disease. 2, 4, 29–31 Many node-positive and upstaged patients receive postoperative chemotherapy and/or radiotherapy, which improves outcomes, and/or these upstaged patients are typically excluded from outcome analysis that could be biased toward favoring surgery. Third, even in some propensity-matched analyses, about half of SABR patients were considered medically inoperable but were still matched with patients received surgery despite the fact that some important baseline characteristics, such as ECOG, pulmonary function, and pretreatment reliable lymph node evaluation, were rarely matched. 3, 4 A fourth pitfall is that many surgical studies that reported local-regional recurrence were actual percentages instead of estimated cumulative incidences with time point that will be higher than actual rate over time.
One of the criticisms for SABR is not having mediastinal lymph node dissection, which raises the concern of lower loco-regional control. However, it is not quite clear whether lymph node dissection is therapeutic or diagnostic. In the current study, we estimated the probabilities of recurrence using a competing risk model with 7-year follow-up, the longest among all published literature to date. The results showed that although local-regional recurrence rate after SABR was on the high end (12.3% and 17.4% at 3- and 5-year, respectively) it still fell within the range of that reported with surgery (Table 4). However, the results reported here for DM, PFS, and even OS are not only within the range, but also at the average rate of that observed with surgery (except for data from Japan, Table 4), even though most of our patients were older and medically inoperable. When we included patients who received SABR and death dates are available but didn’t come back for images and follow up visit, the 5-, 7-year OS rates were 54.3% and 46.4%, respectively. However, we would like to point out that the tumor size in the current study was relatively small ( ≤ 4cm) and majority (95%) of the patients had T1 tumors (≤ 3cm), which could contribute to the better outcomes as demonstrated in other study. 17 Again, larger prospective randomized trials comparing the two modalities are needed and ongoing.
In summary, this prospective study represents the first literature investigating SABR for early-stage NSCLC with longest follow-up of 7 years. The results demonstrate outstanding OS with rates of local/regional and distant control for SABR comparable to those of surgery but with lower toxicity. LR needs to be reviewed carefully to rule out second primary malignancy. RR and DM remain the dominant failures, indicating that pre-SABR staging is important, and the combination of SABR with systemic therapy such as immunotherapy should be explored to improve the outcome. Second malignancy remains one of the most common issues with longer follow-up, again consistent with surgical data. Therefore, closely post-therapeutic surveillance scan and aggressive salvage treatment are crucial as many cases of recurrent disease and second primary malignancy are highly curable.
Acknowledgments
FUNDING SUPPORT: This research was supported in part by the National Cancer Institute at the National Institutes of Health through Cancer Center Core Support Grant (Grant No. P30CA016672, used the Clinical Trials Support Resource) and through Clinical and Translational Science Award (Grant No. UL1 RR024148) to MD Anderson Cancer Center.
We thank the Thoracic Radiation Oncology section in the Division of Radiation Oncology and Thoracic Center of MD Anderson Cancer center for their help/support and the Department of Scientific Publications (Ms. Sunita Patterson) for editorial assistance. The manuscript and the database were critically reviewed by an independent thoracic surgeon and a thoracic medical oncologist.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors had no disclosures.
ClinicalTrials.gov: NCT00489008; https://clinicaltrials.gov/ct2/show/NCT00489008.
AUTHOR CONTRIBUTIONS: Bing Sun: Formal analysis, investigation, data curation, writing–original draft, writing–review and editing, and visualization. Eric Brooks: Formal analysis, resources, writing–original draft, writing–review and editing. Ritsuko Komaki: Conceptualization, investigation, resources, writing–review and editing, and supervision. Zhongxing Liao, Melenda Jeter, Mary McAleer, James D. Welsh, Michael O’Reilly, and Daniel Gomez: Methodology, investigation, data curation, writing–review and editing, and visualization. Pamela Allen: Formal analysis, investigation and data curation. Peter A. Balter: Validation, investigation, and writing–review and editing. Stephen. Hahn, Jack Roth, Reza Mehran, John Heymach: Investigation, resources, writing–review and editing, and supervision. Joe Chang: Conceptualization, methodology, formal analysis, validation, investigation, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration and funding acquisition.
References
- 1.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24:1543–1548. doi: 10.1093/annonc/mdt026. [DOI] [PubMed] [Google Scholar]
- 4.Port JL, Parashar B, Osakwe N, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg. 2014;98:1152–1159. doi: 10.1016/j.athoracsur.2014.04.128. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Bezjak A, Mornex F. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. 2015;10:577–585. doi: 10.1097/JTO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 6.Chang JY, Liu H, Balter P, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol. 2012;7:152. doi: 10.1186/1748-717X-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 8.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J AM STAT ASSOC. 1999;94:496–509. [Google Scholar]
- 9.Zhao L, Zhou S, Balter P, et al. Planning target volume D95 and mean dose should be considered for optimal local control for stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1226–1235. doi: 10.1016/j.ijrobp.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011;81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 13.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 14.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90:603–611. doi: 10.1016/j.ijrobp.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 17.Ricardi U, Frezza G, Filippi AR, et al. Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer. 2014;84:248–253. doi: 10.1016/j.lungcan.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clin Lung Cancer. 2015 doi: 10.1016/j.cllc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verstegen NE, Lagerwaard FJ, Hashemi SM, Dahele M, Slotman BJ, Senan S. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J Thorac Oncol. 2015;10:1195–1200. doi: 10.1097/JTO.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 24.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–82. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Lamont JP, Kakuda JT, Smith D, Wagman LD, Grannis FW., Jr Systematic postoperative radiologic follow-up in patients with non-small cell lung cancer for detecting second primary lung cancer in stage IA. Arch Surg. 2002;137:935–940. doi: 10.1001/archsurg.137.8.935. [DOI] [PubMed] [Google Scholar]
- 26.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 27.Pastorino U, Infante M, Maioli M, et al. Adjuvant treatment of stage I lung cancer with high-dose vitamin A. J Clin Oncol. 1993;11:1216–1222. doi: 10.1200/JCO.1993.11.7.1216. [DOI] [PubMed] [Google Scholar]
- 28.Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5:183–189. doi: 10.21037/tlcr.2016.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol. 2014;32:2449–2455. doi: 10.1200/JCO.2013.50.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–1970. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 31.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]