PATIENT DESCRIPTION
A 24 month-old girl was referred to our clinic for evaluation of hypotonia. She was born at 39 weeks gestation to a G7P4T4AB3 with non-consanguineous parents. Polyhydramnios was noted at 28 weeks on prenatal ultrasounds and she was delivered via normal spontaneous vaginal delivery. At birth in an outside hospital, meconium stained fluid was noted and she required non-invasive ventilation at delivery; she was given surfactant once and her respiratory status improved (Apgars 3, 7, 8). Birth weight was 2815 grams. After delivery, she was noted to have congenital abnormalities including a midline U shaped cleft palate with intact lip and maxillary ridge, micrognathia, adducted thumbs and club feet. A brain MRI at the outside hospital was unremarkable, but ultrasound of her diaphragm showed right-sided elevation suggesting hemi-diaphragmatic paralysis. Hip ultrasound noted right hip dysplasia. Placental pathology was unremarkable. She was transferred to our facility due to suspected Pierre-Robin Sequence and for further work-up.
Upon arrival, she required oxygen supplementation and was noted to have a weak cry, weak suck and minimal spontaneous movements. She also had feeding difficulties and a gastric tube was placed to provide adequate fluids and nutrition. To help delineate the cause of hypoxia, a sleep study was done suggesting mild obstructive sleep apnea. She was discharged at two months of age from our NICU on 0.25 L oxygen and 100% tube feeds with close follow up. Thereafter, she had three admissions for respiratory distress and probable aspiration pneumonia requiring non-invasive ventilation. She continued to have difficulty with emesis after bolus feeds and was found to have severe gastroesophageal reflux. She had a fourth admission for respiratory failure requiring intubation due to adenovirus and was eventually weaned from the ventilator but placed on non-invasive ventilation overnight to keep her more stable. She continued to have notable muscle weakness and had developed scoliosis. At 6 months of age, she required significant support to sit and continued to have poor head control. She was discharged home on nocturnal non-invasive ventilation and 0.5L oxygen awaiting surgery for a Nissen fundoplication.
She was admitted for her Nissen fundoplication and evaluation by ENT under sedation. She tolerated the beginning of the procedure but the Nissen was not completed due to high fever, hypercarbia and muscular rigidity of her abdomen. She was resuscitated in the OR and recovered. She was evaluated by the neuromuscular team and found to have diminished spontaneous movements, with moderate head lag and marked hypotonia, but had anti-gravity strength in shoulder abduction, elbow flexion and hip flexion. She recovered and was able to have the Nissen and muscle biopsy performed without difficulty in the OR when precautions for malignant hyperthermia were taken.
After the fundoplication, she improved from a respiratory standpoint with fewer episodes of pneumonia. She was weaned to oxygen only overnight when asleep. She continued to have poor tone and weakness but did gain skills over the first two years of her life.
DIFFERENTIAL DIAGNOSIS
Here we report a patient with dysmorphic features, hypotonia and poor respiratory effort at birth. In cases of hypotonia with poor respiratory effort at birth, one must consider both central and peripheral etiologies. Central considerations include a hypoxic ischemic event, brainstem or cerebellar malformations, Prader-Willi syndrome, substance exposures and other metabolic abnormalities. However, our patient’s brain MRI, laboratory and toxicology studies were all normal. Prader-Willi methylation testing was not pursued due to additional clinical findings (as discussed below) in our patient which are not typically seen in Prader-Willi syndrome.
Peripheral causes include disorders of the motor unit, the most common of which is spinal muscular atrophy, due to mutations in the SMN gene, and which commonly presents with hypotonia, poor feeding and respiratory distress. Spinal muscular atrophy with respiratory distress (SMARD1) presents with more prominent distal involvement and frequently with hemi-diaphragmatic weakness, as in our patient and mutations in the IGHMBP2 gene are causative. Other causes include disorders of the peripheral nerves, such as the congenital hypomyelinating neuropathies. Disorders of the neuromuscular junction include neonatal myasthenia gravis (MG) due to maternal antibodies (typically in the setting of known maternal MG) and congenital myasthenic syndromes. Disorders of muscle include congenital myopathies and metabolic myopathies such as acid maltase deficiency (Pompe disease). Congenital myotonic dystrophy, which is relatively common, results in symptoms from both central nervous system and muscle dysfunction.
The patient we describe had additional clinical features of cleft palate, micrognathia, low set ears, adducted thumbs, hip dysplasia and club feet. The presence of a cleft palate and micrognathia were consistent with the Pierre-Robin sequence. Seen in isolation (and typically with glossoptosis), the Pierre-Robin sequence is categorized as non-syndromic, but it can also be associated syndromically with Stickler syndrome (OMIM #108300 and #604841), which is characterized by midface hypoplasia, cleft palate, micrognathia, myopia and skeletal abnormalities1; congenital disorder of glycosylation type 1 (CDG1T; OMIM #614921), which is characterized by myopathy, cleft palate, micrognathia, cardiomyopathy and short stature2; and possibly Camera-Marugo-Cohen syndrome (OMIM #604257), which is characterized by hypotonia, cleft palate, micrognathia, joint contractures, and cognitive impairment/developmental delay though its genetic etiology is currently unknown3.
During her initial hospitalization, it was suspected that her symptoms of poor feeding and impaired respiratory function were all related to her Pierre Robin sequence, so suspicion was highest for Stickler syndrome. Karotyping, SNP microarray and specific gene testing for the most common Stickler genes (COL2A1 & COL11A1) were performed; all were normal and she was discharged home from the NICU without a specific diagnosis. However, due to her hypotonia, poor feeding and inability to breathe unassisted, further work up was pursued during one of her frequent hospitalizations. Examination by a neuromuscular consultant revealed significant hypotonia and weakness, without the tongue fasciculations, flaccid tone and symmetric proximal greater than distal weakness suggestive of SMA type 1; SMN genetic analysis was nevertheless performed and found to be negative for causative mutations. Testing for expansions in the DMPK gene (the cause of congenital myotonic dystrophy) was similarly negative.
Her serum CK was 26 U/L. An EMG revealed normal sensory nerve conductions, variable mild reduction of motor nerve amplitudes and fibrillation potentials on EMG which was interpreted as consistent with denervation due to a motor neuron cause. Given her hemi-diaphragmatic elevation on chest radiograph, the leading diagnosis was SMARD1, although congenital myopathies remained under consideration. Lymphocyte derived DNA was sent for analysis by the Expanded Neuromuscular Disorders Gene Panel (Emory; MM360), which encompasses 78 genes, including IGHMBP2; no causative mutation was found in the entire panel. A myopathic process remained high on the differential, particularly after she developed an episode of suspected malignant hyperthermia with the first attempt at Nissen fundoplication; a muscle biopsy was therefore performed with the subsequent successful Nissen fundoplication.
MUSCLE BIOPSY PATHOLOGY
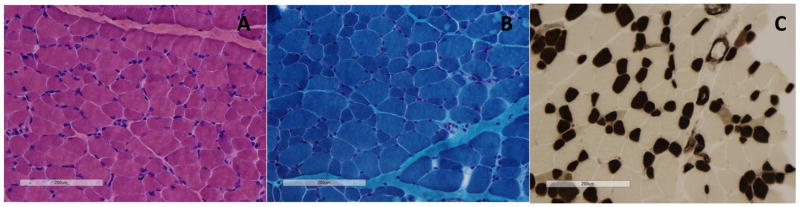
Muscle biopsy was obtained from her left quadriceps and stained with standard H&E, Gomori Trichrome, SDH, NADH, COX, ATPase at pHs 4.2, 4.6 and 9.4 (Figure 2). H&E staining revealed abnormally increased fiber size variability but no increased internal nuclei and no signs of inflammation, necrosis or fibrosis. Gomori Trichrome did not reveal any abnormal inclusions, structural abnormalities, vacuoles or ragged red fibers. NADH staining did not reveal any abnormality of internal architecture. Mitochondrial stains (COX/SDH) were normal. ATPase stains revealed mild type 1 fiber predominance, type 1 fiber hypotrophy (~25+% smaller than type 2 fibers overall) and a relative paucity of type 2B fibers, without any obvious fiber type grouping. In addition to the above mentioned panel of stains, tissue was fixed and embedded for possible electron microscopy but the semi-thin sections showed no evidence of any architectural abnormalities or structural inclusions such as rods, caps or core-like areas, thus complete electron microscopy evaluation was deferred.
Figure 2. Muscle biopsy of the left quadriceps. Images shown were photographed at 200X magnification.
A: Hematoxylin and Eosin stain shows increased fiber size variability with scattered rounded smaller than expected fibers for patient age (<10 μm in diameter) alternating with normal-sized to larger than expected fibers for age (up to ~40μm in diameter); the normal fiber size range for patient age is 13 to 19 μm in diameter.
B: Gomori Trichrome stain shows no suggestion of any abnormal inclusions, architectural disarray or abnormal structures (rods, cores, caps, or cytoplasmic bodies) and no evidence of any possible increase in subsarcolemmal mitochondria.
C: ATPase pH 4.2 stain shows the darker staining type 1 fibers (55% of fibers in this image as quantified using ImageJ software are significantly smaller (hypotrophic appearing) overall, compared to the more normal to hypertrophic type 2 fibers which appear generally up to about twice as large. Notably, the small fibers have retained a polygonal shape and are not angular, consistent with hypoplasia rather than atrophy.
DISCUSSION
These findings are consistent with congenital fiber type (size) disproportion (CFTD). CFTD is histopathologically defined by the presence of type 1 fibers of small cross sectional area (CSA) and typically found in predominance in comparison to type 2 fibers; because such features may be seen in a variety of congenital (structural) myopathies, this diagnosis is reserved for biopsies in which there are no other histopathologic features suggesting another specific diagnosis, such as nemaline myopathy or central core myopathy4. The definition of what constitutes disproportion is inexact; initially, classification as CFTD was based on type 1 fibers >12% smaller than type 2 fibers but this was later found to be relatively non-specific and the cutoff was raised to 25%, with an argument that the cutoff should be raised to >35–40% to heighten specificity5. Mutations causing CFTD have been discovered in multiple genes, including TMP2, TMP3, RYR1, ACTA1, MYH7, and SEPN1, in both autosomal dominant and recessive inheritance patterns5. In addition, CFTD has been seen in posterior fossa malformations of the brainstem and cerebellum that lead to abnormal suprasegmental influences on the developing motor unit.6 Despite this genetic heterogeneity in peripheral etiologies, common clinical features include a long face, high arched palate, hypotonia, reduced reflexes and contractures7.
Nearly all genes associated with CFTD had been excluded as containing causative mutations by expanded neuromuscular gene panel testing. We therefore proceeded to whole exome sequencing (WES) and identified a homozygous pathogenic variant in STAC3 (c.851G>C; p.W280S) inherited in trans from each parent. This variant has been described as the causative mutation responsible for the autosomal recessive Native American Myopathy (NAM), first described in 1987 in a 3 month old of Lumbee American Indian descent with Pierre Robin sequence, talipes equinus and arthrogryposis8,9. The variant p.W280S was originally described as c.1046G>C9, but is c.851G>C based upon the reference sequence ENST00000332782.6. Since the original description, several more cases have been reported with typical clinical features of short stature, cleft palate, myopathic facies, hypotonia, scoliosis and congenital joint contractures10,11, all of which are seen in our patient (Figure 1). In NAM there is a susceptibility to malignant hyperthermia, which is characterized by hyperthermia, muscle hyperactivity, rigidity, tachycardia/tachypnea, and metabolic acidosis in response to inhalation anesthetics and depolarizing muscle relaxants which also appeared to be present in our patient. Malignant hyperthermia is typically seen in core myopathies in association with RYR1 gene mutations. However, it can be seen in several other gene mutations associated with congenital myopathies, including STAC312. Notably, upon direct questioning the parents subsequently described remote Cherokee (mother) and Shawnee and Cherokee (father) heritage, but denied Lumbee heritage.
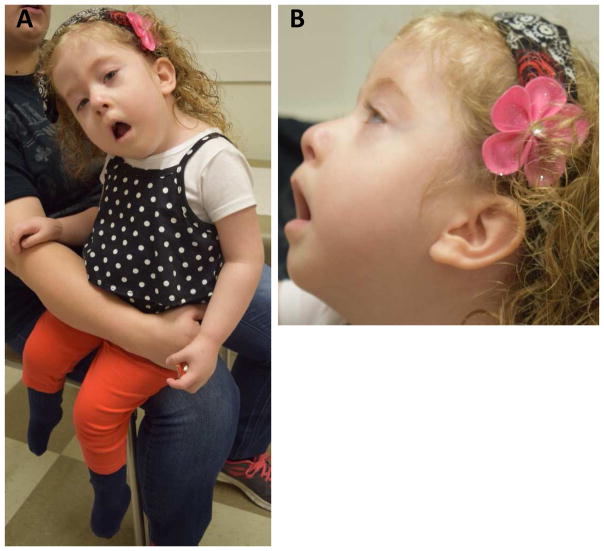
Figure 1. Clinical Features.
Photographs were taken at 33 months of age. A: note the myopathic facies (ptosis, tented mouth), hypotonia and club feet; B: note the low set ears and micrognathia
STAC3 encodes the SH3 and cysteine-rich domain 3 (STAC3) protein, which is almost exclusively expressed in skeletal muscle. It is an important component in excitation-contraction coupling because of direct involvement in conformational coupling between slow activating calcium channels and the type 1 ryanodine receptor13. STAC3 knockout mice die at birth, and mice with STAC3 knocked out at age 4 weeks have reduced muscle mass and muscle strength14. Although the specific mechanism resulting in the histopathologic finding is not clear, CFTD has been previously described in muscle biopsies from NAM patients.
PATIENT OUTCOME
At almost 3 years of age, our patient requires 0.5L of oxygen by nasal cannula at night and is now eating teaspoons of soft thickened foods by mouth; otherwise, she is predominantly fed through her feeding tube. Motor development is delayed but she is now sitting unsupported, crawling and just started pulling herself to stand. Cognitively she appears normal; she can understand and follow complex commands and appears to be a good problem solver. Her speech development is delayed, as she is babbling and just beginning to say words, but this is likely limited by her yet to be repaired cleft palate.
FINAL DIAGNOSIS
Native American Myopathy due to the p.W280S missense mutation, exhibiting classic muscle biopsy findings of congenital fiber type disproportion.
CONCLUSIONS
This case highlights the importance of considering a neuromuscular etiology in all patients with hypotonia and significant respiratory issues at birth. Here, there was an assumption that her symptoms were all related to a non-syndromic Pierre Robin sequence; although she had significant hypotonia and weakness that was not well characterized until she was several months of age. This case also highlights the changing landscape of rare neuromuscular disease diagnosis with the emergence of clinically-available WES to facilitate molecular diagnosis. As use of WES becomes more widespread, it is likely that there will be an increasing number of patients diagnosed with rarely reported syndromes. When common diagnostic strategies such as targeted gene panel mutation analysis have been unrevealing, WES may be an appropriate next step to help clinicians in rare disease diagnosis.
Acknowledgments
We would like to thank Dr. Z. Sahenk, MD, PhD and Dr. C. R. Pierson, MD, PhD for their help during the interpretation of the muscle biopsy. We would like to thank Matthew Pastore, MS, LGC for his help in obtaining the genetic diagnosis.
FUNDING
This work was supported by the National Institutes of Health T32 grant # 5T32NS077984-04.
Footnotes
CONFLICTS OF INTEREST
MAW, DRB, ES: none
KMF: Formal Advisor for Audentes Therapeutics and Sarepta Therapeutics and Consultant for PTC Therapeutics
RS: Consultant for AveXis Inc
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gomez-Ospina N, Bernstein JA. Clinical, cytogenetic, and molecular outcomes in a series of 66 patients with Pierre Robin sequence and literature review: 22q11.2 deletion is less common than other chromosomal anomalies. American journal of medical genetics. Part A. 2016;170A(4):870–880. doi: 10.1002/ajmg.a.37538. [DOI] [PubMed] [Google Scholar]
- 2.Tegtmeyer LC, Rust S, van Scherpenzeel M, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. The New England journal of medicine. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert DM, Watters G, Andermann F, Der Kaloustian VM. The Camera-Marugo-Cohen syndrome: report of two new patients. American journal of medical genetics. 1999;86(3):208–214. doi: 10.1002/(sici)1096-8628(19990917)86:3<208::aid-ajmg3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Jungbluth H, Ochala J, Treves S, Gautel M. Current and future therapeutic approaches to the congenital myopathies. Seminars in cell & developmental biology. 2016 doi: 10.1016/j.semcdb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Clarke NF. Congenital fiber-type disproportion. Seminars in pediatric neurology. 2011;18(4):264–271. doi: 10.1016/j.spen.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Sarnat HB. Cerebral dysgeneses and their influence on fetal muscle development. Brain & development. 1986;8(5):495–499. doi: 10.1016/s0387-7604(86)80093-6. [DOI] [PubMed] [Google Scholar]
- 7.Clarke NF, North KN. Congenital fiber type disproportion--30 years on. Journal of neuropathology and experimental neurology. 2003;62(10):977–989. doi: 10.1093/jnen/62.10.977. [DOI] [PubMed] [Google Scholar]
- 8.Bailey AG, Bloch EC. Malignant hyperthermia in a three-month-old American Indian infant. Anesthesia and analgesia. 1987;66(10):1043–1045. [PubMed] [Google Scholar]
- 9.Horstick EJ, Linsley JW, Dowling JJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nature communications. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamm DS, Aylsworth AS, Stajich JM, et al. Native American myopathy: congenital myopathy with cleft palate, skeletal anomalies, and susceptibility to malignant hyperthermia. American journal of medical genetics. Part A. 2008;146A(14):1832–1841. doi: 10.1002/ajmg.a.32370. [DOI] [PubMed] [Google Scholar]
- 11.Stamm DS, Powell CM, Stajich JM, et al. Novel congenital myopathy locus identified in Native American Indians at 12q13.13-14.1. Neurology. 2008;71(22):1764–1769. doi: 10.1212/01.wnl.0000325060.16532.40. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet journal of rare diseases. 2015;10:93. doi: 10.1186/s13023-015-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polster A, Nelson BR, Olson EN, Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(39):10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cong X, Doering J, Grange RW, Jiang H. Defective excitation-contraction coupling is partially responsible for impaired contractility in hindlimb muscles of Stac3 knockout mice. Scientific reports. 2016;6:26194. doi: 10.1038/srep26194. [DOI] [PMC free article] [PubMed] [Google Scholar]