Abstract
Background
Individuals with psychotic disorders experience disruptions to both the sleep and circadian components of the sleep/wake cycle. Recent evidence has supported a role of sleep disturbances in emerging psychosis. However, less is known about how circadian rhythm disruptions may relate to psychosis symptoms and prognosis for adolescents with clinical high-risk (CHR) syndromes. The present study examines circadian rest/activity rhythms in CHR and healthy control (HC) youth to clarify the relationships among circadian rhythm disturbance, psychosis symptoms, psychosocial functioning, and the longitudinal course of illness.
Methods
Thirty-four CHR and 32 HC participants were administered a baseline evaluation, which included clinical interviews, 5 days of actigraphy, and a sleep/activity diary. CHR (n = 29) participants were re-administered clinical interviews at a 1-year follow-up assessment.
Results
Relative to HC, CHR youth exhibited more fragmented circadian rhythms and later onset of nocturnal rest. Circadian disturbances (fragmented rhythms, low daily activity) were associated with increased psychotic symptom severity among CHR participants at baseline. Circadian disruptions (lower daily activity, rhythms that were more fragmented and/or desynchronized with the light/dark cycle) also predicted severity of psychosis symptoms and psychosocial impairment at 1-year follow-up among CHR youth.
Conclusions
Circadian rhythm disturbances may represent a potential vulnerability marker for emergence of psychosis, and thus, rest/activity rhythm stabilization has promise to inform early-identification and prevention/intervention strategies for CHR youth. Future studies with longer study designs are necessary to further examine circadian rhythms in the prodromal period and rates of conversion to psychosis among CHR teens.
Keywords: Circadian Rhythm, Actigraphy, Prodromal, Clinical High-Risk, Psychosis, Schizophrenia
1. Introduction
Psychotic disorders are debilitating illnesses associated with marked distress and functional impairment, which often results in a significant socioeconomic burden (e.g., greater healthcare utilization, reduced occupational productivity; Jin and Mosweu, 2016). Thus, a critical programmatic priority has focused on elucidating risk factors for psychosis onset with the purpose of enhancing early identification and prevention/intervention approaches that reduce the rates of conversion (Cannon et al., 2008). Longitudinal investigations with adolescents with clinical-high risk (CHR) syndromes (e.g., subthreshold symptoms of psychosis, reduced psychosocial functioning) is a promising approach to identify etiological mechanisms implicated in disorder onset (Addington and Heinssen, 2012), as a high proportion (up to 36%) of CHR adolescents convert to psychosis within a two-year period (Fusar-Poli et al., 2012).
The sleep/wake cycle, which is comprised of two interacting processes (i.e., homeostatic sleep “Process S” and circadian pacemaker “Process C”; Borbely et al., 2016), is frequently disturbed in adults with psychotic disorders, including both medicated and medication-naïve individuals (Chan et al., 2016) and those with and without comorbid mood symptoms (Wulff et al., 2012). Specifically, disrupted sleep/wake cycles increase the severity of psychosis (Cohrs, 2008) and precede relapse of psychotic episodes (Benson, 2015), and interestingly, when addressed therapeutically, reduce psychotic symptom severity (Kantrowitz et al., 2010). While sleep/wake cycle disturbances have been documented and investigated since the earliest conceptions of psychosis (Kraepelin et al., 1919), related clinical features have also recently garnered interest as potential biomarkers for CHR individuals (Lunsford-Avery and Mittal, 2013; Zanini et al., 2013).
To date, investigations with at-risk adolescents have largely focused on the sleep component of the sleep/wake cycle with results showing increased self-reported, actigraphic-measured, and polysomnographic-measured (PSG) sleep disturbances among at-risk participants compared to healthy controls (HC) (Brietzke et al., 2015; Castro et al., 2015; Castro et al., 2012; Keshavan et al., 2004; Lunsford-Avery et al., 2015; Lunsford-Avery et al., 2013; Poe et al., In Press; Zanini et al., 2015). These disturbances in sleep are related to increased concurrent psychosis symptoms, poorer psychosocial functioning, cognitive deficits (i.e., impaired procedural learning), and sleep-related neural abnormalities (i.e., thalamus reductions) (Lunsford-Avery et al., Under Review; Lunsford-Avery et al., 2015; Lunsford-Avery et al., 2013; Poe et al., In Press). Perhaps most importantly, self-reported and actigraphic-measured sleep disturbances at clinical intake predict increased positive symptom severity over a 1-year period among CHR adolescents (Lunsford-Avery et al., 2015), suggesting potential risk markers for psychosis onset. Indeed, prior work has revealed self-reported sleep disturbances as an important factor in predicting transition to psychosis among CHR youth (Ruhrmann et al., 2010).
However, less is known about disruptions to the circadian process of the sleep/wake cycle (24-hour, time-dependent, light-dependent, oscillatory variation in sleep propensity; Borbely et al., 2016) among at-risk adolescents or how circadian disturbances may contribute to worsened clinical outcomes for affected youth. Although sleep and circadian processes are closely interrelated, they are also separate processes with distinct biological markers, neural underpinnings, and implications for treatment (Borbely et al., 2016). Thus, clarifying a role of circadian disruptions in addition to sleep problems in the development of psychosis has important implications for understanding the etiology of the illness as well as developing interventions. In a recent study, Castro and colleagues (2015) investigated circadian rest/activity rhythms in teens displaying At Risk Mental States (i.e., ARMS; risk of psychosis and/or bipolar disorder) and found rhythms to be more irregular, fragmented, and desynchronized with the light/dark cycle among at-risk youth compared to HC. In a follow-up case study of three youth from the ARMS project, there was evidence of irregular rest/activity rhythms in the period directly prior to psychosis conversion (Goncalves et al., 2016), suggesting that circadian disturbances may play a role in psychosis development. However, given the small sample size, this issue warrants further attention.
This study had three principal objectives. First, we aimed to replicate Castro and colleagues’ (2015) findings and hypothesized that CHR adolescents would display disrupted circadian rhythms compared to HC youth. Second, to extend this work, we tested whether circadian rhythm disturbances relate to concurrent psychosis symptom severity and psychosocial impairment among CHR youth. Finally, among CHR youth, we investigated whether circadian rhythm disturbances at intake predicted psychosis symptom severity and worsened psychosocial functioning at the 1-year follow-up assessment. Evidence linking circadian disturbances and worsened prognosis for CHR youth would support a possible role of rest/activity disruptions in psychosis pathophysiology as well as inform potentially beneficial prevention/intervention efforts for individuals at risk for psychosis.
2. Method
2.1 Procedure
Participants were recruited via internet and print advertisements and physician referrals as part of the Adolescent Development and Preventative Treatment (ADAPT) program’s ongoing longitudinal study of CHR youth. At the intake evaluation (time 1; T1), informed consent and assent were obtained and adolescents participated in clinical interviews to determine eligibility, and if deemed eligible, completed self-report forms as well as 5 days of actigraphy and a sleep/activity diary (described below). Participants returned for a 1-year follow-up (time 2; T2), at which time clinical interviews were repeated to assess for changes in symptom severity and psychosocial functioning during the follow-up period.
2.2 Participants
Participants (CHR = 45, HC = 42) included youth aged 12–21 years. Several adolescents in each group (CHR = 11, HC = 10) were excluded after the intake evaluation due to deficient data regarding rest/activity patterns (less than 5 days of registration or removing the actigraph for more than 3 hours), and only the final sample (CHR = 34, HC = 32) were included in T1 analyses. Participants who were excluded based on insufficient actigraphy data did not differ from included adolescents on any demographic or clinical variables. Inclusion criteria for the CHR group included: 1) attenuated positive psychosis symptoms (moderate severity) or 2) global functioning declines accompanied by family history of psychosis or schizotypal personality disorder (Miller et al., 1999). Histories of head injury/loss of consciousness, intellectual disability, and neurological disorder were exclusionary for both groups. Formal psychotic disorder was a further exclusionary criterion for CHR youth as was Axis I disorder and family history of psychosis for HC. Twenty-nine of 34 CHR participants had returned for the 1 year follow-up at the time T2 analyses were completed.
2.3 Clinical Assessments
The Structured Interview for Prodromal Symptoms (i.e., SIPS; McGlashan et al., 2001; Miller et al., 2003; Rosen et al., 2006), Structured Clinical Interview for DSM-IV (i.e., SCID, Research Version; First et al., 1995), and Global Assessment of Functioning (i.e., GAF; Jones et al., 1995) assessed CHR syndromes, Axis I disorders, and psychosocial functioning respectively at both time points (interrater reliability Kappa >.80). As depression is common in CHR youth (Rosen et al., 2006; Salokangas et al., 2012; Shioiri et al., 2007; Svirskis et al., 2005) and related to circadian rhythms among adolescents (Robillard et al., 2013), youth completed the 21-item Beck Depression Inventory-II at T1 (BDI-II; Beck et al., 1996) which served as a covariate in statistical analyses.
2.4 Actigraphic Measurement of Circadian Rhythms
Circadian rhythms were measured at T1 via an ActiSleep wrist monitor (approximate cost = $225; ActiGraph; Pensacola, FL), which was continuously worn by participants over a 5 day period. Participants concurrently completed a daily sleep/activity diary, which required participants to record bedtimes, wake times, and nap times, as well as information about activity participation, school attendance, and any medical concerns (Acebo and LeBourgeois, 2006; Ancoli-Israel et al., 2003). Participants were also asked to record any times they removed the actigraph (e.g., bathing), which was confirmed by ActiLife validation scoring (version 5.10.0).
Circadian rhythms variables were derived from the actigraphy data using the method described by Castro and colleagues (2015), including: autocorrelation function (Ac; slope of the time correlation line (log-transformed); indicative of rhythm fragmentation; lower values represent less fragmented rhythms), interdaily stability (IS; value for 1440 min provided by Sokolove–Bushell periodogram analysis; denotes synchronization of circadian rhythms with the light/dark cycle), intradaily variability (IV; mean of the first derivative of the actigraphy data normalized by the total variance; a measure of rest–activity rhythm fragmentation), M10 (mean activity level during the most active 10 hours of the day; higher values are indicative of a more active lifestyle), F10 (onset of M10), L5 (sum activity during the least active 5 hours of the day), F5 (onset of the least active 5 hour period/nocturnal activity), and relative amplitude (RA = (M10-L5)/(M10+L5); measures the relationship between diurnal amplitude and night amplitude, the maximum value of 1 occurs when there is no movement during the night) (Chiesa et al., 2010; Eke et al., 2002; Goncalves et al., 2015; Goncalves et al., 2014; Richman and Moorman, 2000; van Beek et al., 1989).
2.5 Statistical Analyses
Independent t-tests and chi-square tests evaluated group differences in demographic variables. Group differences in circadian rest/activity variables at T1 were assessed using linear regressions. Within the CHR group, partial Pearson correlations assessed associations between rest/activity measures and SIPS symptoms (positive, negative) and psychosocial functioning at T1. Lastly, linear regressions covarying for T1 SIPS symptoms (positive, negative) or T1 psychosocial functioning evaluated the extent to which T1 circadian rest/activity disturbances predicted increases in psychosis symptom severity (positive, negative) and psychosocial impairment among CHR youth at T2. Age, sex, and depression were included as covariates in all analyses based on prior literature indicating the impact of these characteristics on circadian rhythms in adolescence (Mateo et al., 2012; Robillard et al., 2013).
3. Results
3.1 Sample Characteristics
Thirty-four CHR and 32 HC youth participated in the T1 assessment and actigraphy study. HC adolescents were slightly younger (trend level) and displayed significantly lower levels of T1 psychosis (positive, negative) and depressive symptoms and higher T1 psychosocial functioning compared to CHR. Groups did not differ by sex or parental education status. Anti-psychotic medication use was somewhat higher among CHR than HC adolescents at T1 (trend level); however, no HC and only 3 CHR (9%) adolescents were taking anti-psychotic medication. Four CHR participants (14%) were taking anti-psychotic medication at 1-year follow-up: 2 participants medicated at T1 continued antipsychotic use, 2 adolescents had initiated an anti-psychotic regimen during the follow-up period, and 1 participant previously medicated at T1 had suspended use. See Table 1.
Table 1.
Sample characteristics at T1
| Variable | CHR (n = 34) | HC (n = 32) | t value | p value |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age, years | 18.79 (1.93) | 17.75 (2.79) | 1.77 | 0.08 |
| Parent Education | 15.96 (2.22) | 15.97 (2.50) | −0.02 | 0.98 |
| SIPS-Positive | 12.35 (4.96) | 0.78 (1.52) | 12.65 | < 0.01 |
| SIPS-Negative | 9.56 (6.32) | 0.41 (0.84) | 8.13 | < 0.01 |
| GAF | 61.24 (13.08) | 85.75 (6.41) | −9.57 | < 0.01 |
| BDI | 17.50 (11.86) | 3.59 (5.13) | 6.11 | < 0.01 |
| Number (%) | Χ2value | |||
| Sex, male | 15 (44%) | 16 (50%) | 0.23 | 0.63 |
| Anti-Psychotic Use | 3 (9%) | 0 (0%) | 2.96 | 0.09 |
Abbreviations: CHR, Clinical High-Risk participants; HC, Healthy Control participants; SIPS, Structured Interview for Prodromal Symptoms (total positive and negative symptoms); GAF, Global Assessment of Functioning; BDI, Beck Depression Inventory
3.2 Differences in T1 Circadian Rhythms among CHR and HC Youth
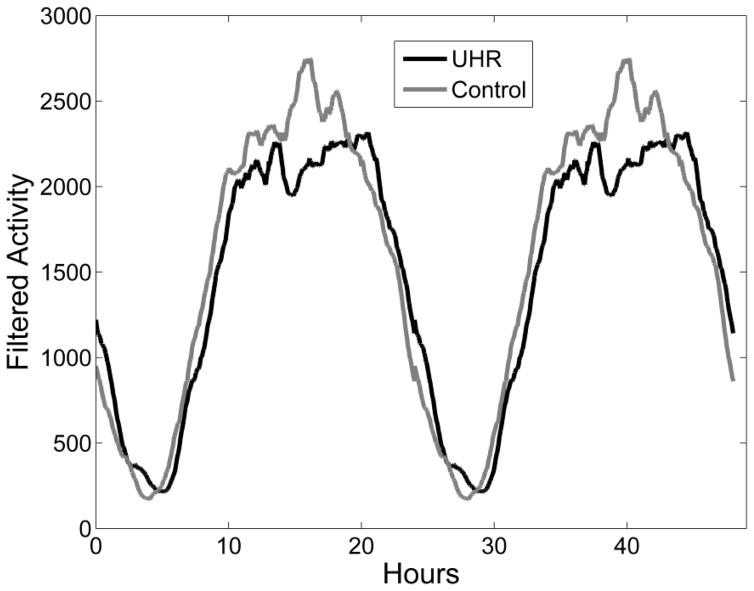
At T1, CHR adolescents displayed significantly lower autocorrelation (Ac) function and later onset of nocturnal rest (F5) compared to HC after accounting for age, sex, and depression. CHR and HC youth did not significantly differ on any other circadian measure (see Table 2 and Figure 1).
Table 2.
Differences in T1 circadian rhythms among CHR and HC youth
| CHR mean (SD) | HC mean (SD) | R2 | df | F | p | |
|---|---|---|---|---|---|---|
| Ac | −0.29 (0.11) | −0.26 (0.07) | .05 | 4, 61 | 3.18 | 0.04 |
| IV | 0.70 (0.16) | 0.65 (0.14) | .02 | 4, 61 | 0.93 | 0.17 |
| IS | 0.47 (0.13) | 0.46 (0.12) | .00 | 4, 61 | 0.03 | 0.44 |
| RA | 0.96 (0.02) | 0.97 (0.02) | .01 | 4, 61 | 0.77 | 0.19 |
| M10 | 1599775.71 (449886.43) | 1697687.00 (547432.99) | .02 | 4, 61 | 1.09 | 0.15 |
| F5 | 1587.35 (108.44) | 1542.95 (115.06) | .05 | 4, 61 | 3.03 | 0.04 |
| F10 | 679.82 (138.28) | 654.97 (129.58) | .01 | 4, 61 | 0.40 | 0.27 |
| L5 | 27732.93 (13892.24) | 26016.27 (9157.06) | .00 | 4, 61 | 0.20 | 0.33 |
Abbreviations: CHR, Clinical High-Risk participants; HC, Healthy Control participants; Ac, Autocorrelation function (log-transformed); IV, Intradaily Variability; IS, Interdaily Stability; RA, Relative Amplitude
Figure 1.
Graphic depiction of group differences in T1 circadian rest/activity rhythms over 48 hour period
3.3 T1 Associations between Circadian Rhythm Disruptions and Psychosis Symptoms, Psychosocial Functioning among CHR Youth
Lower autocorrelation function (Ac), lower diurnal activity (M10), and increased intradaily variability (IV) were significantly associated with T1 positive symptom severity (all p values <.05) after accounting for age, sex, and depression. Lower relative amplitude (RA) and later onset of nocturnal rest (F5) were related to increased T1 positive symptoms at a trend level (p < .10). Decreased activity during the most and least active periods of the day (M10 and L5) was correlated with increased T1 negative symptoms over and above age, sex and depression (p < .05). Decreased activity during the most active period of the day was associated with reduced T1 psychosocial functioning at the trend level (p < .10). No additional circadian variables were associated with negative symptoms or psychosocial functioning at T1 among CHR youth (See Table 3).
Table 3.
Associations of T1 circadian rhythms, psychosis symptom severity, and psychosocial impairment in the CHR group
| Circadian Variable | T1 Positive Symptoms | T1 Negative Symptoms | T1 GAF |
|---|---|---|---|
| Ac | − 0.34* | −0.03 | 0.05 |
| IV | 0.36* | 0.07 | −0.05 |
| IS | − 0.13 | −0.04 | 0.16 |
| RA | − 0.29+ | 0.19 | 0.03 |
| M10 | − 0.32* | −0.35* | 0.24+ |
| F5 | 0.25+ | 0.06 | −0.14 |
| F10 | − 0.04 | −0.09 | 0.03 |
| L5 | 0.08 | −0.34* | 0.14 |
Values represent partial Pearson correlations. Significance:
indicates p < .05,
indicates p <.10
Abbreviations: Ac, Autocorrelation function; IV, Intradaily Variability; IS, Interdaily Stability; RA, Relative Amplitude; GAF, Global Assessment of Functioning
3.4 Disrupted T1 Circadian Rhythms Predict Increased Psychosis Symptom Severity and Psychosocial Impairment at 1-Year Follow-up
Reduced diurnal activity (M10), relative amplitude (RA), interdaily stability (IS), and autocorrelation function (Ac) and increased intradaily variability (IV) were associated with greater positive symptom severity at the 1-year follow-up after covarying for age, sex, depression, and T1 positive symptom severity. Decreased T1 diurnal activity (M10) also was predictive of greater negative symptom severity at 1-year follow-up after accounting for age, sex, depression, and T1 negative symptom severity. Finally, reduced diurnal activity (M10), relative amplitude (RA), and autocorrelation function (Ac), greater intradaily variability (IV), and later onset of the most active period of the day (F10) at T1 were related to poorer psychosocial functioning at T2 above and beyond age, sex, depression, and T1 psychosocial functioning. See Table 4.
Table 4.
Associations between T1 circadian rhythms and 1-year follow-up psychosis symptoms and psychosocial functioning among CHR adolescents
| T1 Predictor Variable | T2 Positive Symptoms | T2 Negative Symptoms | T2 GAF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | R2 | F | p | R2 | F | p | R2 | F | p | |
| Ac | 5, 23 | .20 | 5.95 | 0.01* | .06 | 1.44 | 0.12 | .42 | 17.15 | <0.001** |
| IV | 5, 23 | .17 | 4.84 | 0.02* | .04 | 0.98 | 0.17 | .37 | 13.23 | <0.001** |
| IS | 5, 23 | .13 | 3.69 | 0.03* | .06 | 1.46 | 0.12 | .03 | 0.61 | 0.22 |
| RA | 5, 23 | .17 | 4.88 | 0.02* | .04 | 1.02 | 0.16 | .21 | 6.16 | 0.01* |
| M10 | 5, 23 | .13 | 3.35 | 0.04* | .18 | 5.20 | 0.02* | .33 | 11.11 | 0.002** |
| F5 | 5, 23 | .02 | 0.61 | 0.22 | .06 | 1.44 | 0.12 | .08 | 1.94 | .09 |
| F10 | 5, 23 | .00 | 0.12 | 0.36 | .00 | 0.05 | 0.41 | .13 | 3.43 | .04* |
| L5 | 5, 23 | .04 | 0.92 | 0.17 | .00 | 0.00 | 0.48 | .03 | 0.71 | .20 |
Significance:
indicates p < .01,
indicates p <.05
Abbreviations: Global Assessment of Functioning, GAF; Ac, Autocorrelation function; IV, Intradaily Variability; IS, Interdaily Stability; RA, Relative Amplitude
4. Discussion
The current study tested whether circadian rhythms are disturbed in CHR adolescents in comparison to healthy peers. In addition, we examined how disturbed rest/activity rhythms may relate to concurrent psychosis symptoms and psychosocial functioning as well as prognosis over a 1-year period. First, consistent with prior research (Castro et al., 2015), CHR individuals displayed more fragmented circadian rhythms and later onset of nocturnal rest relative to HC youth. Notably, later onset of nocturnal rest may suggest increased alertness and reduced readiness to sleep at bedtime (Gradisar and Crowley, 2013), which may contribute to the longer sleep latencies (and REM-latencies) observed in prior CHR studies as measured by PSG (Zanini et al., 2015) and self-report (Lunsford-Avery et al., 2013). Second, among CHR youth, more fragmented circadian rhythms and lower daily activity were cross-sectionally associated with increased positive symptom severity, and lower daily activity was related to negative symptoms at T1. Last, after controlling for T1 symptoms, greater fragmentation of rhythms, desynchronization of circadian rhythms with the light/dark cycle, and decreased daily activity at T1 were predictive of increased positive symptoms one year later, while lower T1 daily activity levels were associated increased negative symptoms at follow-up among CHR youth. Lower activity level and more fragmented/less regular circadian rhythms at intake were further associated with poorer functioning at follow-up for CHR adolescents.
In previous research, we proposed a neurodevelopmental diathesis-stress model of psychosis wherein sleep/wake disturbances are hypothesized to reflect both an underlying biological vulnerability as well as a stressor in adolescence (through its impact on neuromaturational, endocrine, biological, and psychological factors) that interact to drive psychosis onset (Lunsford-Avery and Mittal, 2013). The current findings that rest/activity disturbances are predictive of worsened prognosis suggest that in addition to disturbances to the sleep component of the sleep/wake cycle (Lunsford-Avery et al., 2015), circadian pacemaker disruptions may also contribute to psychosis development. This is an important addition to the model, as it suggests that the distinct but interactive biological processes underlying nighttime sleep and daytime rest/activity rhythms may each contribute to mechanisms underlying psychosis etiology as well as inform interventions with this population.
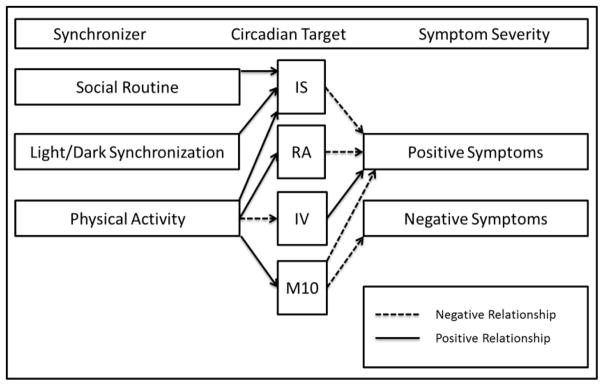
For example, while it is not possible to disentangle the direction of causality in the current study (circadian rhythm disruptions may contribute to or result from emerging psychosis), results suggest a potential mechanism by which circadian disruptions may result in psychosis onset. Specifically, among vulnerable individuals, disturbances in daily rhythm synchronizers (e.g., exposure to light, food intake, amount and timing of social interaction and exercise; Golombek and Rosenstein, 2010) may result in disruptions to the circadian clock, which in turn, may interact with other genetic (e.g., genes supporting brain development) and environmental (e.g., social/academic/occupational stress) vulnerabilities to drive psychosis emergence (see Figure 2).
Figure 2.
Hypothesized mechanisms by which circadian synchronizers may impact psychosis symptoms in CHR youth
Future longitudinal research utilizing longer study designs and investigating a range of potential mechanisms through which sleep/wake disturbances may result in illness onset (e.g., through changes in cortisol response, neurodevelopment, or cognition) is essential for informing our understanding of the possible contribution of sleep/wake abnormalities to psychosis etiology. Experimental designs using PSG sleep/wake assessment, cortisol sampling, and next-day measures of neurocognitive functioning (e.g., memory impairment; Keshavan et al., 2010; Li et al., 2016) may also be useful for clarifying risk factors and mediators of relationships between specific sleep/wake disturbances and clinical and functional outcomes in CHR youth. In addition, research has suggested an influence of circadian phase on cortical excitability (i.e., neural reactivity), which in turn impacts cognitive performance (Ly et al., 2016). Cortical excitability, as measured by scalp EEG responses to transcranial magnetic stimulation, may represent another potential mediator of sleep/wake-cognition relationships for future study with CHR adolescents.
These results also open an opportunity to intervene with this population by modifying daily rhythm synchronizers. For example, increasing social routine regularity (e.g., bedtime, wake time, meals) and improving light/dark synchronization (e.g., increasing daytime light exposure, decreasing nighttime light exposure from electronic devices such as television, smartphones, and computers) may improve interdaily stability and reduce positive symptoms in CHR youth. Additionally, increasing daytime physical activity may enhance several circadian stability and activity measures (IS, RA, M10) and reduce intradaily variability, which may attenuate both positive and negative symptoms and improve functioning.
Recent evidence suggests that treatments such as Interpersonal and Social Rhythm Therapy (i.e., IPSRT), which targets circadian rhythm stabilization, result in improved clinical outcomes for teens diagnosed with or at risk for severe mental illnesses such as bipolar disorder (Goldstein et al., 2014; Inder et al., 2015). In addition, treatments focused on increasing physical activity through exercise are associated with clinical, functional, and neurocognitive improvements among individuals with schizophrenia (Firth et al., 2015). Such interventions may also reduce current CHR symptom severity, enhance functioning, and prevent or delay psychosis onset, and future studies should examine this possibility. Technological innovations (e.g., smart phone applications that assess circadian/activity patterns and provide real-time feedback to patients) could be considered an additional tool of interest to augment and increase access to circadian/activity interventions for CHR youth.
Noninvasive brain stimulation (NIBS) techniques during sleep, such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS), have been successfully employed to improve sleep/wake functioning in individuals with severe mental illnesses, such as bipolar disorder (Minichino et al., 2014), and appear to have a positive impact on neurocognitive functioning in individuals with psychosis (e.g., memory; Goder et al., 2013). In addition, given our prior finding of relationships between sleep disturbances and bilateral thalamic volume reductions in CHR youth (Lunsford-Avery et al., 2013), it is important to consider that supplements that enhance GABAB, such as gamma hydroxybutyric acid (GHB), have been shown to improve sleep in adults with schizophrenia (Kantrowitz et al., 2010) and may be useful for targeting sleep and related impairments in CHR teens. However, potential complications and issues associated with use of NIBS and GHB with youth are currently unclear (Davis, 2014; Kamal et al., 2016). Future studies may assess the utility, tolerability, and feasibility of incorporating these treatments into preventative and intervention work with CHR youth.
This study is subject to several limitations. First, our current analysis only includes two time points. Only a portion of CHR youth (~36%) develop psychosis and identifying those most likely to convert is vital to the development of targeted prevention strategies (Cannon et al., 2008). Given the relatively few conversions in our sample during the follow up period (N = 2 at T2), our investigation was limited to relationships between circadian disruptions and symptom and functional prognosis. Second, our sample was relatively small and may have been underpowered to detect the full range of circadian disturbances experienced by CHR youth. Third, our study was only 5 days in duration. Although this duration is consistent with prior studies of circadian rhythms in youth with severe mental illness (e.g., Faedda et al., 2016), longer studies would optimize consistency of sampling across participants (e.g., equal proportion of weekend/weekdays assessed). Future studies using larger samples, multiple time points (i.e., past the typical window for conversion), and longer circadian rhythm assessments (7+ days) will further clarify the sleep/wake disturbances experienced by CHR youth and their role in the emergence of psychosis. Finally, several CHR youth (9%) were medicated during the actigraphy assessment; however, the direction and magnitude of effects did not differ when medicated individuals were removed from analyses (data not shown).
In conclusion, results suggest that CHR youth display circadian rhythm disturbances that are associated with clinical presentation and prognosis. Paired with prior research investigating sleep disturbances in at-risk samples, findings support a potential role of both sleep and circadian processes of the sleep/wake cycle in psychosis etiology and suggest that interventions targeting these phenomena may have utility in improving the clinical course of illness for adolescents at-risk for severe psychopathology.
Acknowledgments
Role of Funding Source
Funding for this work was provided by National Institutes of Health grants RO1-MH-094650 and R21/R33-MH-103231 to Dr. Mittal and K23-MH-108704 to Dr. Lunsford-Avery. We express our gratitude to Raeana Newberry for her assistance with data management.
Footnotes
Conflict of Interest
Dr. Mittal serves as a consultant to Takeda Pharmaceuticals. There are no other conflicts of interest to report.
Contributors
Authors Lunsford-Avery, Gonçalves, and Mittal conceptualized and designed the study. Authors Lunsford-Avery and Gonçalves performed the statistical analyses, and Dr. Lunsford-Avery wrote the initial draft of the paper. Authors Mittal, Brietzke, Bressan, Gadelha, and Auerbach contributed to results interpretation and drafting of the manuscript. The submitted manuscript reflects the contributions of all of the authors, who unanimously approved the final draft for submission.
References
- Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Addington J, Heinssen R. Prediction and Prevention of Psychosis in Youth at Clinical High Risk. Annu Rev Clin Psycho. 2012;8:269. doi: 10.1146/annurev-clinpsy-032511-143146. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benson KL. Sleep in Schizophrenia: Pathology and Treatment. Sleep Med Clin. 2015;10(1):49–55. doi: 10.1016/j.jsmc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25(2):131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- Brietzke E, Zanini MA, Castro JV, da Cunha GR, Asevedo E, Pan P, Bittencourt LRA, Morgadinho F, Tufik S, Gadelha A, Bressan RA. Abnormalities in Sleep Patterns in Individuals At-Risk for Psychosis and Bipolar Disorder. Biol Psychiat. 2015;77(9) doi: 10.1016/j.schres.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk. Arch Gen Psychiat. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Zanini M, da Goncalves BS, Coelho FM, Bressan R, Bittencourt L, Gadelha A, Brietzke E, Tufik S. Circadian rest-activity rhythm in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;168(1–2):50–55. doi: 10.1016/j.schres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Castro JP, Brietzke E, Bittencourt LR, Zanini M, Bressan RA, Tufik S. Changes in sleep patterns in indivuduals in ultra-high risk for psychosis. Early Interv Psychia. 2012;6:123–123. [Google Scholar]
- Chan MS, Chung KF, Yung KP, Yeung WF. Sleep in schizophrenia: A systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Chiesa JJ, Cambras T, Carpentieri AR, Diez-Noguera A. Arrhythmic rats after SCN lesions and constant light differ in short time scale regulation of locomotor activity. J Biol Rhythms. 2010;25(1):37–46. doi: 10.1177/0748730409352843. [DOI] [PubMed] [Google Scholar]
- Cohrs S. Sleep Disturbances in Patients with Schizophrenia Impact and Effect of Antipsychotics. Cns Drugs. 2008;22(11):939–962. doi: 10.2165/00023210-200822110-00004. [DOI] [PubMed] [Google Scholar]
- Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci. 2014;8:600. doi: 10.3389/fnhum.2014.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke A, Herman P, Kocsis L, Kozak LR. Fractal characterization of complexity in temporal physiological signals. Physiol Meas. 2002;23(1):R1–38. doi: 10.1088/0967-3334/23/1/201. [DOI] [PubMed] [Google Scholar]
- Faedda GL, Ohashi K, Hernandez M, McGreenery CE, Grant MC, Baroni A, Polcari A, Teicher MH. Actigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls. J Child Psychol Psychiatry. 2016;57(6):706–716. doi: 10.1111/jcpp.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I), Patient Edition. American Psychiatric Press; Washington, D.C: 1995. [Google Scholar]
- Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45(7):1343–1361. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting Psychosis Meta-analysis of Transition Outcomes in Individuals at High Clinical Risk. Arch Gen Psychiat. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, Marshall L. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144(1–3):153–154. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Fersch-Podrat R, Axelson DA, Gilbert A, Hlastala SA, Birmaher B, Frank E. Early intervention for adolescents at high risk for the development of bipolar disorder: pilot study of Interpersonal and Social Rhythm Therapy (IPSRT) Psychotherapy (Chic) 2014;51(1):180–189. doi: 10.1037/a0034396. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Goncalves B, Castro J, Zanini MA, Bittencourt L, Gadelha A, Cunha GR, Coelho FM, Tufik S, Bressan RA, Brietzke E. Circadian rhythm disturbances and conversion to psychosis in ultra high-risk youth. Rev Bras Psiquiatr. 2016;38(2):178–179. doi: 10.1590/1516-4446-2015-1859. [DOI] [PubMed] [Google Scholar]
- Goncalves BS, Adamowicz T, Louzada FM, Moreno CR, Araujo JF. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev. 2015;20:84–91. doi: 10.1016/j.smrv.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Goncalves BS, Cavalcanti PR, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: An update. Sleep Sci. 2014;7(3):158–164. doi: 10.1016/j.slsci.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Crowley SJ. Delayed sleep phase disorder in youth. Curr Opin Psychiatry. 2013;26(6):580–585. doi: 10.1097/YCO.0b013e328365a1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder ML, Crowe MT, Luty SE, Carter JD, Moor S, Frampton CM, Joyce PR. Randomized, controlled trial of Interpersonal and Social Rhythm Therapy for young people with bipolar disorder. Bipolar Disord. 2015;17(2):128–138. doi: 10.1111/bdi.12273. [DOI] [PubMed] [Google Scholar]
- Jin H, Mosweu I. The Societal Cost of Schizophrenia: A Systematic Review. Pharmacoeconomics. 2016 doi: 10.1007/s40273-016-0444-6. [DOI] [PubMed] [Google Scholar]
- Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166(5):654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- Kamal RM, van Noorden MS, Franzek E, Dijkstra BA, Loonen AJ, De Jong CA. The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review. Neuropsychobiology. 2016;73(2):65–80. doi: 10.1159/000443173. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Oakman E, Bickel S, Citrome L, Spielman A, Silipo G, Battaglia J, Javitt DC. The importance of a good night's sleep: an open-label trial of the sodium salt of gamma-hydroxybutyric acid in insomnia associated with schizophrenia. Schizophr Res. 2010;120(1–3):225–226. doi: 10.1016/j.schres.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3(3):163–168. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, Seidman LJ, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E, Barclay RM, Robertson GM. Dementia præcox and paraphrenia. E. & S. Livingstone; Edinburgh: 1919. [Google Scholar]
- Li X, Thermenos HW, Wu Z, Momura Y, Wu K, Keshavan M, Seidman L, DeLisi LE. Abnormal interactions of verbal- and spatial-memory networks in young people at familial high-risk for schizophrenia. Schizophr Res. 2016;176(2–3):100–105. doi: 10.1016/j.schres.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Dean DJ, Mittal VA. Self-Reported Sleep Disturbances Associated with Procedural Learning Impairment in Adolescents at Ultra-High Risk for Psychosis. Schizophrenia Research. doi: 10.1016/j.schres.2017.03.025. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, LeBourgeois MK, Gupta T, Mittal VA. Actigraphic-measured sleep disturbance predicts increased positive symptoms in adolescents at ultra high-risk for psychosis: A longitudinal study. Schizophr Res. 2015;164(1–3):15–20. doi: 10.1016/j.schres.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Mittal VA. Sleep Dysfunction Prior to the Onset of Schizophrenia: A Review and Neurodevelopmental Diathesis-Stress Conceptualization. Clin Psychol-Sci Pr. 2013;20(3):291–320. [Google Scholar]
- Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, Smith Watts AK, Bernard J, Millman ZB, Mittal VA. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151(1–3):148–153. doi: 10.1016/j.schres.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JQ, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, Archer SN, Phillips C, Dijk DJ, Maquet P, Massimini M, Vandewalle G. Circadian regulation of human cortical excitability. Nat Commun. 2016;7:11828. doi: 10.1038/ncomms11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo MJC, Diaz-Morales JF, Barreno CE, Prieto PD, Randler C. Morningness-eveningness and sleep habits among adolescents: Age and gender differences. Psicothema. 2012;24(3):410–415. [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27(4):563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Minichino A, Bersani FS, Spagnoli F, Corrado A, De Michele F, Calo WK, Primavera M, Yang B, Bernabei L, Macri F, Vergnani L, Biondi M, Delle Chiaie R. Prefronto-cerebellar transcranial direct current stimulation improves sleep quality in euthymic bipolar patients: a brief report. Behav Neurol. 2014;2014:876521. doi: 10.1155/2014/876521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe SL, Brucato G, Bruno N, Arndt LY, Ben-David S, Gill KE, Colibazzi T, Kantrowitz JT, Corcoran CM, Girgis RR. Sleep Disturbances in Individuals at Clinical High Risk for Psychosis. Psychiat Res. doi: 10.1016/j.psychres.2016.12.029. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, Hickie IB. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28(7):412–416. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Rosen JL, Miller TJ, D'Andrea JT, McGlashan TH, Woods SW. Comorbid diagnoses in patients meeting criteria for the schizophrenia prodrome. Schizophr Res. 2006;85(1–3):124–131. doi: 10.1016/j.schres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkotter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Ruhrmann S, von Reventlow HG, Heinimaa M, Svirskis T, From T, Luutonen S, Juckel G, Linszen D, Dingemans P, Birchwood M, Patterson P, Schultze-Lutter F, Klosterkotter J group E. Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr Res. 2012;138(2–3):192–197. doi: 10.1016/j.schres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Shinada K, Kuwabara H, Someya T. Early prodromal symptoms and diagnoses before first psychotic episode in 219 inpatients with schizophrenia. Psychiatry Clin Neurosci. 2007;61(4):348–354. doi: 10.1111/j.1440-1819.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- Svirskis T, Korkeila J, Heinimaa M, Huttunen J, Ilonen T, Ristkari T, McGlashan T, Salokangas RK. Axis-I disorders and vulnerability to psychosis. Schizophr Res. 2005;75(2–3):439–446. doi: 10.1016/j.schres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- van Beek JH, Bassingthwaighte JB, Roger SA. Fractal networks explain regional myocardial flow heterogeneity. Adv Exp Med Biol. 1989;248:249–257. doi: 10.1007/978-1-4684-5643-1_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200(4):308–316. doi: 10.1192/bjp.bp.111.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini M, Castro J, Coelho FM, Bittencourt L, Bressan RA, Tufik S, Brietzke E. Do sleep abnormalities and misaligned sleep/circadian rhythm patterns represent early clinical characteristics for developing psychosis in high risk populations? Neurosci Biobehav R. 2013;37(10):2631–2637. doi: 10.1016/j.neubiorev.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Zanini MA, Castro J, Cunha GR, Asevedo E, Pan PM, Bittencourt L, Coelho FM, Tufik S, Gadelha A, Bressan RA, Brietzke E. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schizophrenia Research. 2015;169(1–3):262–267. doi: 10.1016/j.schres.2015.08.023. [DOI] [PubMed] [Google Scholar]