Abstract
INTRODUCTION
Amyloid imaging is a tool that has recently become available to dementia specialists evaluating patients with possible Alzheimer’s disease. Studies have assessed the impact of amyloid imaging on diagnostic and treatment decisions, but patient and family perspectives have received less attention.
METHODS
To examine how amyloid imaging affects the diagnostic experience for patients and families, we interviewed members of 26 patient-caregiver dyads with whom a neurologist discussed the option of amyloid PET.
RESULTS
Most participants who chose to undergo amyloid imaging would choose to do so again. Regardless of the scan outcome, patients and caregivers commonly expressed relief upon learning the scan results. Some participants expressed expectations that were beyond scan capabilities.
DISCUSSION
Amyloid imaging may provide information that patients and their families find useful. Clinicians must set correct expectations and ensure that families understand the limitations of amyloid imaging.
Keywords: amyloid imaging, positron emission tomography, diagnosis, biomarker
1. Introduction
Clinicians diagnose Alzheimer’s disease (AD) by establishing core diagnostic features and systematically ruling out other causes of cognitive and functional impairment [1, 2]. Advances in understanding the pathobiology of AD and the development of technologies to demonstrate that pathobiology in vivo, have resulted in new biomarker tests that can support the clinical diagnosis of AD [3–5].
Three positron emission tomography (PET) imaging ligands specific for the fibrillar form of the amyloid beta (Aβ) protein, a neuropathological hallmark of AD, have achieved regulatory approval in the United States and other countries [6–9]. Amyloid imaging is indicated for use with a binary outcome. Positive scans indicate the presence of moderate to frequent amyloid plaques, while negative scans suggest no or sparse fibrillar amyloid–beta burden [6, 10]. An expert Amyloid Imaging Taskforce sponsored by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging developed Appropriate Use Criteria for amyloid PET, which outlined patients with persistent or progressive mild cognitive impairment (MCI), patients with possible AD due to atypical or mixed etiology presentation, and patients with young-onset dementia as appropriate for amyloid imaging [11]. Amyloid imaging may identify MCI patients at increased risk for progression to AD dementia [12, 13] and reduce ambiguity from a clinical diagnosis that is associated with substantial uncertainty [14–16]. It may help clinicians distinguish AD from other causes of dementia, especially in patients with atypical presentations [17, 18]. In patients with young-onset dementia, amyloid imaging may assist in important planning decisions related to employment and lifestyle and facilitate referral to clinical trials [11].
Recent studies suggest that amyloid imaging can increase diagnostic confidence [19, 20] and result in changed diagnosis or treatment decisions in some cases [18, 20–24]. Yet, amyloid imaging is not reimbursed for any patient group by the Center for Medicare and Medicaid Services or by most health insurers [25], except in the setting of specific approved clinical trials. Ongoing studies examine the healthcare costs associated with amyloid imaging and its impact on diagnosis, treatment, and health outcomes (http://www.ideas-study.org/; https://clinicaltrials.gov/ct2/show/NCT02317250). Collectively, these studies will provide valuable evidence to inform the debate on amyloid imaging coverage policies. What is missing from many of these studies is the impact that amyloid imaging has on the patient experience.
Receiving a diagnosis of AD alleviates anxiety for some patients and caregivers by providing an explanation for symptoms and advancing care from a diagnostic to a management phase [26]. Alternatively, applying the AD label to patients who do not meet the criteria for AD dementia could result in unnecessary concern [27, 28] or stigma [29, 30]. Amyloid imaging increases the level of information available to clinicians and may also aid in prognostication [31]. Few data are available to guide the clinical interaction around amyloid imaging, however, beyond the proposed appropriate use criteria [11, 32] and initial recommendations for communicating scan results [33, 34](Grill et al., submitted). To examine how amyloid imaging affects the diagnostic experience for patients and families, we performed a telephone interview study with patients and caregivers who received clinical care at an academic tertiary memory disorders clinic.
2. Methods
2.1. Study design
In this exploratory study, one investigator (JDG) conducted telephone interviews with English-speaking members of patient-caregiver dyads with whom a neurologist discussed amyloid PET imaging. Interviews were performed between April 13, 2013 and October 21, 2014. At that time, only one FDA-approved ligand was available for clinical use, 18F-florbetapir (Amyvid®). Additionally, a voucher program that reduced the cost of the scan was available on a limited basis to referring clinicians. Five neurologists were invited to refer patients and/or family members with whom they discussed the option of amyloid imaging, including those who did and those who did not choose to undergo the scan. Information on the number of dyads who were potentially eligible for the current study was not available. Each referring neurologist was a fellowship-trained dementia specialist, exceeding the Amyloid Imaging Taskforce definition of ≥25% of patient contact time devoted to the evaluation and care of patients with cognitive impairment or dementia [32].
We interviewed patients and caregivers separately, using identical interview questions. Table 1 outlines the interview questions. All participants were asked an open-ended question about the rationale for their decision whether to have the scan. For participants from a dyad in which the patient underwent amyloid imaging, additional open-ended questions addressed expectations for the scan, the meaning of the scan results, how the neurologist communicated the scan results, the impact of learning the scan results, and the benefits and negatives of having the scan. All participants were asked two forced-choice questions related to the likelihood that they would repeat their decision whether to undergo the scan and whether they would recommend the scan to others in their position. A brief set of questions examined participant demographics, and participants gave permission for the study team to contact the neurologist to access minimal clinical information (i.e., reason for the scan, operationalized by the Appropriate Use Criteria [11]; outcome of the scan [if performed]; and the most recent Mini-Mental State Examination [MMSE [35]] score, relative to the scan date). Most interviews lasted 15–30 minutes.
Table 1.
Interview elements.
| • Did you choose to have the Amyvid PET scan? Can you tell me why you made this decision? |
| • What did you expect to learn by getting the Amyvid scan?* |
| • Were your expectations met? Why or why not?* |
| • What did the neurologist tell you when he or she shared the results of the scan?* |
| • And what did that (the Amyvid scan results) mean to you?* |
| • How did you feel after learning the scan results? Why did you feel that way?* |
| • Were there any benefits to having the Amyvid scan and learning the results? What were they? How important were they to you?* |
| • Were there any negatives to having the Amyvid scan and learning the results? What were they? How important were they to you?* |
| • If you could do it all over again, would you make the same decision whether to have the Amyvid scan and learn the results?# |
| • Would you recommend that others in your position choose to have the Amyvid scan and learn the results?# |
asked only of patients and caregivers from a dyad in which the patient underwent amyloid imaging;
multiple choice questions
2.2. Data analyses
Interviews were recorded and transcribed verbatim. Responses were considered equally for patients and caregivers, regardless of whether one or two members of the dyad completed the interview. Two investigators (JDG and CGC) independently reviewed each interview transcription qualitatively, to identify salient points related to the proposed categories of investigation. A simultaneous review and substantial discussion were performed to confirm or establish agreement about the number and definition of categories [36]. A template document was developed, which outlined the categories. The two reviewers completed an additional examination of each interview transcript, ensuring that all relevant responses, regardless of timing during the interview, were included in analyses. If participant responses fit multiple categories, this was noted in the study data. The frequency of comments for each category was assessed and exemplar comments were selected for each category. We present categories of responses that were endorsed by multiple patients and caregivers. Reported amyloid status is based on clinician-provided scan outcomes. Clinician and participant reports of amyloid scan results were concordant in all but one case.
2.3. Ethics
Two modes of informed consent were acceptable for this study. First, the referring neurologist had the option to perform informed consent and have the patient and/or caregiver sign a written consent document in person. Second, neurologists could refer patients and/or caregivers to the study and an investigator could secure telephonic consent. Participants or their legally authorized representative provided Health Information Portability and Accountability Act authorization prior to accessing patient medical records for the data elements listed above. The UCLA Institutional Review Board approved this study.
3. Results
3.1. Participants
Table 2 describes the patient population (n=26) with whom the neurologist discussed amyloid imaging, making them and their caregivers eligible for this study. Patients had a wide age range (52 to 88) and were mostly white and well educated. Seven patients had an atypical presentation of AD dementia, five had MCI, five presented with young-onset cognitive impairment, and one was both young-onset and atypical in the presentation. Three patients were typical in their presentation of AD and one was described as having memory complaints.
Table 2.
Description of the patients with whom the neurologist discussed amyloid imaging.
| Patient Characteristic | Summary |
|---|---|
| N | 26 |
| Age, mean years (SD) [Range] | 73.1 (10.3) [52–88] |
| Sex, % female | 58 |
| Race, % white | 87 |
| Education, mean years (SD) | 17.2 (3.7) |
| MMSE, mean (SD) | 22.5 (5.2) |
| Reason for scan | |
| Possible AD, atypical present, n (%) | 7 (27) |
| Persistent MCI, n (%) | 5 (19) |
| Probable AD, young onset, n (%) | 5 (19) |
| Atypical AND Young onset | 1 (4) |
| Memory concerns, n (%) | 1 (4) |
| Typical AD, n (%) | 3 (11) |
| Missing, n (%) | 4 (15) |
The reason for the scan was missing for four cases. Among 20 patients who underwent amyloid imaging, 18 were amyloid positive and two were amyloid negative. The mean and standard deviation time between the amyloid PET scan and the study interview (available for 12 of 20 undergoing imaging) was 234 ± 176 days. Six patients did not undergo amyloid imaging.
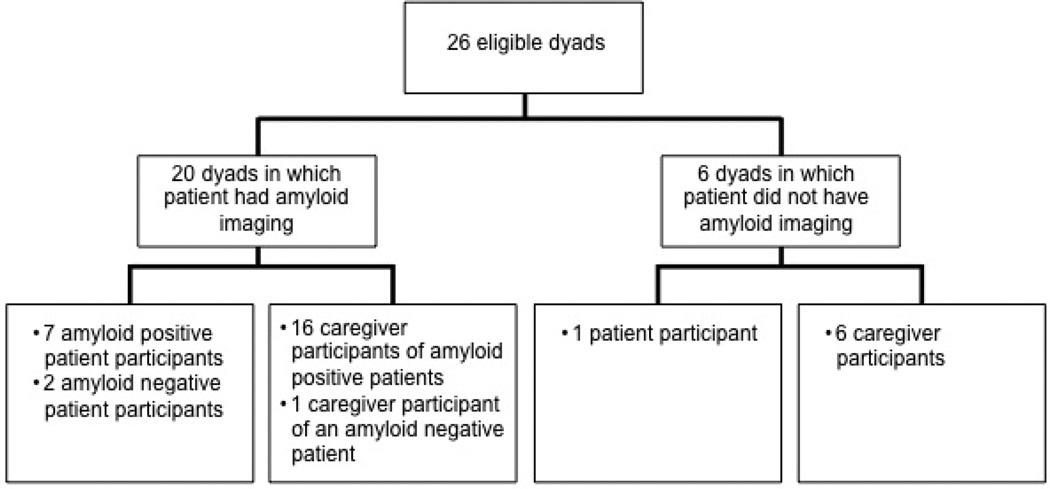
Figure 1 and Table 3 describe the 10 patients and 23 caregivers who completed the interview. For seven dyads, both the patient and the caregiver completed the interview. In the remaining 19 dyads, only one individual was interviewed (three patients and 16 caregivers).
Figure 1.
Schematic of study participants.
Table 3.
Description of the patients and caregivers interviewed.
| Characteristic | Summary |
|---|---|
| Patients | |
| N | 10 |
| Age, mean years (SD) [Range] | 70.0 (11.2) [52–83] |
| Female sex, n (%) | 4 (40) |
| White race, n (%) | 8 (80) |
| Latino ethnicity, n (%) | 2 (20) |
| Education, mean years (SD) | 18.0 (2.8) |
| MMSE, mean (SD) | 23.9 (4.7) |
| Scan details | |
| • Positive scan | 7 (70) |
| • Negative scan | 2 (20) |
| • No scan | 1 (10) |
| Caregivers | |
| N | 23 |
| Age, mean years (SD) [Range] | 64.3 (13.0) [38–89] |
| Female sex, n (%) | 14 (61) |
| White race, n (%) | 19 (82) |
| Latino ethnicity, n (%) | 3 (13) |
| Education, mean years (SD) | 17.8 (2.6) |
| Relation to the patient | |
| • Spouse, n (%) | 14 (61) |
| • Adult child, n (%) | 7 (30) |
| • Mother, n (%) | 1 (4) |
| • Missing, n (%) | 1 (4) |
| Scan details for the care recipient | |
| • Positive scan | 16 (70) |
| • Negative scan | 1 (4) |
| • No scan | 6 (26) |
Seven of nine patients who completed the interview and underwent amyloid imaging had a positive scan; two had negative scans. One patient who completed the interview chose not to have amyloid imaging. Six of 23 caregivers who completed the interview were members of a dyad who chose not to have amyloid imaging. Sixteen were caregivers of a patient that had a positive scan and one was a caregiver of a patient that had a negative scan. Fourteen caregivers who completed the interview were spouses, seven were adult children, and one was the mother of a young-onset patient. The relationship to the patient was missing for one caregiver who completed the interview.
3.2. Categories of responses
We classified participants’ responses to the open-ended questions in the following categories: reasons for the decision whether to undergo amyloid imaging; responses to the scan; and interactions with the neurologist.
3.2.1. Reasons for the decision whether to undergo amyloid imaging
The desire to receive a definitive diagnosis, to learn if AD was the cause of the patient’s cognitive impairment, and the general desire for more information about the patient’s condition were the most frequent reasons patients and caregivers who completed the interview endorsed for proceeding with amyloid imaging (Table 4).
“We wanted to know whether I had Alzheimer’s or not. That was pretty much what I was pulling for, that they could determine whether I did.” 72-year-old amyloid negative patient.
Table 4.
Factors* related to participants’ decision whether to undergo amyloid imaging.
| Category | N |
|---|---|
| Reasons to undergo amyloid imaging | |
| To receive a definitive diagnosis | 13 |
| To learn if AD | 10 |
| To gain information/learn about condition | 9 |
| Physician recommendation | 7 |
| To learn if amyloid present | 5 |
| To learn disease severity/plaque coverage | 3 |
| To instruct treatment decisions | 2 |
| To inform planning for the future | 2 |
| Preference over lumbar puncture | 2 |
| To instruct research/trial participation | 2 |
| To help others | 2 |
| To aid the physician | 2 |
| To provide physical evidence | 2 |
| Reasons to not undergo amyloid imaging | |
| Cost/lack of insurance coverage | 9 |
| Lack of direct benefit | 3 |
| No impact on treatment/care | 3 |
Participants were able to present more than one reason to undergo or not undergo the scan. Those who chose to undergo the scan and those who chose not to could provide responses in both categories. We present responses provided by multiple individuals interviewed.
Several individuals stated that a recommendation by the physician was important to their decision to have the scan, with some noting only this as a reason they chose to proceed. One patient and one caregiver stated that they underwent the scan to provide additional information for the neurologist to include in the patient’s workup.
“We made the decision on the advice of our doctor, who felt that this would be an important diagnostic tool in determining if there was the onset or preliminary indications of cognitive impairment for my mother.” 52-year-old caregiver of an amyloid positive patient.
One patient and four caregivers specifically articulated that they underwent the scan to learn if amyloid plaques were present in the brain, and three caregivers stated that they expected the scan to provide information about the level of plaque deposition and/or the severity of the disease.
“[I expected to learn] if there was plaque present on my mom’s brain and how much was there. If it was there, how advanced it was.” 58-year-old caregiver of an amyloid positive patient.
Among all patients and caregivers interviewed, including those who did and those who did not proceed with the scan, the most frequently endorsed reason not to undergo the scan was cost or the lack of coverage by insurance (Table 4). Additional reasons to not have the scan included a lack of direct benefit to the patient and that the results would have no bearing on treatment or management of the disease.
“It wouldn’t have changed the management. It didn’t really matter if it was Alzheimer’s or something else. The medications prescribed and the prognosis wouldn’t have changed. It didn’t matter if you put a name on it.” 38-year-old caregiver of a patient who did not have amyloid imaging.
3.2.2. Responses to the scan
When patients and caregivers were asked what the scan results meant to them, they most often cited that they now needed to engage in planning (n=8; 4 patients and 4 caregivers).
“But from a standpoint of managing her care and figuring out how best to take care of her with her symptoms, I feel like the scan was really positive in that it let me know she probably couldn’t go home and live by herself again and that I would really need to take her care in a direction that none of us had anticipated or could have predicted.” 69-year-old caregiver of an amyloid positive patient.
Six caregivers and one patient reported that they now had a definitive diagnosis, while four caregivers and one patient stated that the scan provided physical evidence that indicated the presence of amyloid. Four caregivers and one patient stated that the scan confirmed what they had suspected, that AD was the cause of cognitive problems. Three caregivers from an amyloid positive dyad responded that the scan meant the patient had AD and two patients and one caregiver from amyloid negative dyads responded that the scan meant the patient did not have AD.
“It was kind of expected I think. I was hopeful that it wouldn’t be the case, but I remember our family thinking ‘at least now we know’ and that was helpful. It identified it for us, I think.” 44-year-old caregiver of an amyloid positive patient.
The most frequent participant emotional response to learning the scan results was relief (Table 5). The two patients and one caregiver who were members of a dyad in which the patient had a negative scan each endorsed feeling relief as a result of having the scan. For one, this relief was tempered by knowledge that a negative scan does not rule out other potential causes of cognitive problems.
“I was greatly relieved. That’s what it meant to me. But it didn’t mean, I know there are other issues, there are different things that can cause dementia, so of course it didn’t mean that I’m 100% free of everything. But I felt comfortable ruling out Alzheimer’s at this time.” 73-year-old amyloid negative patient.
Table 5.
Participant responses* related to how learning the scan results made them feel.
| Category | n |
|---|---|
| Relieved | 10 |
| Upset/hopeless/depressed | 8 |
| Satisfied | 4 |
| Concerned/anxious | 3 |
| Validated | 3 |
| Shocked | 2 |
| No change in feelings | 2 |
Only participants who chose to undergo the scan responded. Participants were able to present more than one response. We present responses provided by multiple individuals interviewed.
One patient and six caregivers for whom amyloid imaging was positive also expressed relief.
“Well, it was, me personally, it was what I expected. I didn’t really think it was anything other than Alzheimer’s. I think my wife really did really believe that it was psychological--she’s a psychologist--she thought the whole thing was stress related or something like that. She was upset with the diagnosis. But I had already decided a long time earlier that that was what I had and it was just a relief to hear it and to know that this is what we are dealing with and now we can attack it.” 52-year-old amyloid positive patient.
“I think they put to rest any doubt about it being Alzheimer’s and so in that sense…Certainly on a cognitive level it was a relief in a sense to know that yes, this is it and this is what it is. Although we really figured that that’s what it was, it was still there was kind of a finality to it and that’s a relief.” 66-year-old caregiver of an amyloid positive patient.
Eight individuals, all of whom were caregivers from dyads in which the patient had a positive scan, reported that learning the scan results caused them to feel sadness or despair.
“Terrible. Horrible. It sucks. All of the hope that my wife didn’t have Alzheimer’s or had something that was less, I’m not even sure what the word is, but was less freighted with anxiety and hopelessness.” 62-year-old caregiver of an amyloid positive patient.
When asked about the benefits of amyloid imaging, the most frequent responses were the information gained through the procedure (n=8; 3 patients and 5 caregivers), the usefulness of that information in making a plan (n=6; 1 patient and 5 caregivers), the validation of memory concerns (n=5; 2 patients and 3 caregivers), that it informed care decisions (n=4; 1 patient and 3 caregivers), and that the participant now had a diagnosis (n=2 caregivers).
“Well I think the importance again is that there’s kind of the physicality to it that we can see and that the doctor can see and be able to tell us, you know, this looks like it could be signs that my mom has, you know, early onset Alzheimer’s. And that was important for us to know as a family. The confirmation was a bit of a relief in an odd way. We would have preferred that there had not been a confirmation, but the fact that we did allowed us to make a plan after that.” 44-year-old caregiver of an amyloid positive patient.
Fourteen of the 26 individuals interviewed who were members of a dyad in which the patient underwent amyloid imaging (including the two patients and one caregiver from dyads with a negative scan and 11 of 23 patients and caregivers from dyads with a positive scan), when asked, stated that they felt there were no negative aspects to having the scan. Among those who did acknowledge negative aspects, patients and caregivers cited cost (n=3 caregivers), time and inconvenience (n=3; 2 patients and 1 caregiver), fear and anxiety (n=2 patients), and burden on the patient (n=2 caregivers).
3.2.3. Interaction with the neurologist
Patients (n=4) and caregivers (n=9) most frequently reported that during the clinical interaction, neurologists informed them that the amyloid PET scan was supportive of or confirmed a diagnosis of AD. The caregivers of one amyloid positive and one amyloid negative patient reported that the neurologist changed the preliminary diagnosis (n=2). Similarly, two caregivers, one of a positive and one of a negative patient, reported that the neurologist changed the treatment plan (n=2). One caregiver of an amyloid negative patient and one amyloid negative patient responded that the neurologist indicated the patient did not have AD (n=2) based on the scan results. Six caregivers, all from dyads with a positive scan, stated that the neurologist shared the scan images with them and felt that this was helpful.
“When we were able to look at what the doctor represented as a normal scan and then we looked at my wife’s, it was very apparent that there was a difference.” 58-year-old caregiver of an amyloid positive patient.
3.3. Likelihood of repeating the decision whether to undergo amyloid imaging and recommending the scan to others
Ninety-three percent of individuals interviewed (n=31) stated that they would probably or definitely make the same decision whether to undergo the scan. There was no difference in the frequency of responses between those who underwent the scan vs. those who did not, or between those who were members of a dyad in which the patient had positive vs. negative scan results (data not shown).
The two patients and one caregiver who were members of dyads with a negative scan reported that they would probably or definitely recommend others in their position undergo the scan. Twenty of 23 members of dyads with a positive scan reported that they would probably or definitely recommend others in their position undergo the scan. Among those who chose not to undergo the scan, responses were more mixed. One caregiver reported that they definitely would not recommend the scan to others in their position; one caregiver reported that they would probably not recommend the scan to others; one patient and one caregiver reported that they were unsure; and one caregiver reported that they would probably recommend the scan to others in their position. Two participants refused to respond.
4. Discussion
These are among the first data documenting the patient and caregiver experience related to clinical amyloid imaging. Like one study of caregivers of atypical AD patients [24], our data suggest that amyloid imaging can provide information desired by patients with cognitive disorders and their families and enhance the diagnostic experience in many cases. Nearly all participants in our study who underwent amyloid imaging stated that they would repeat the decision to do so. In fact, a common reaction to learning scan results, regardless of amyloid status, was relief. Those with positive results frequently endorsed that the scan added a reality or “physicality” to the working diagnosis, validated concerns, satisfied curiosity, and spurred patients and families to take actions such as advance care management planning, increasing physical exercise, or participating in clinical trials. Although most participants acknowledged no negative aspects of undergoing amyloid imaging, several caregivers of amyloid positive participants experienced sadness as a result of learning the scan results.
Cost and lack of coverage by insurance were viewed as the largest deterrents to undergoing amyloid imaging, suggesting that changes in coverage policies might increase utilization of this biomarker test. Among dyads who chose not to have the scan, participants endorsed the lack of direct benefit for the patient and the lack of implications to the management plan. These participants were referred by multiple neurologists in the study, suggesting that this observation was not due to a single physician’s attitudes or description of the value of amyloid imaging.
Participant responses suggested that there is a substantial opportunity and need for education during the clinical encounter. Several participants used the term definitive diagnosis when discussing the scan. The definitive diagnosis of AD requires information related to both of two pathological disease hallmarks, amyloid plaques and neurofibrillary tangles [37]. Additionally, some patients with other dementias may scan positive [38] and some (albeit few) patients with AD may scan negative [39]. Therefore, clinicians must be careful to appropriately set expectations for the degree of diagnostic confidence they will have when incorporating amyloid PET information into their workup. Some participants stated that they valued learning the extent of plaque coverage or information related to disease severity. The Amyloid Imaging Taskforce specifically indicated determination of disease severity as an inappropriate use [11]. Thus, these comments raise concern about patient and family understanding of the capacity of the current indicated uses of amyloid imaging. Clinicians in this study also referred typical AD patients and one patient with memory concerns. These referrals conflict with the established Appropriate Use Criteria [11].
Our data are limited by a small sample size and by possible sample biases. A disproportionate number of caregivers participated in the interview, limiting the representation of the patient perspective on amyloid imaging. Participants were highly educated and were most commonly from a dyad in which the patient underwent amyloid imaging and had positive scan results. While this risks an overemphasis of the current data on the implications of having the scan, it facilitates informing clinicians about the experiences of undergoing amyloid imaging and learning (especially positive) results, and how those results affect care. In particular, understanding reactions to negative scans will require further study since, even in the few participants here, we observed a potential dichotomy in dyads’ reactions between the “good news” of being amyloid negative and the reality of a clinical uncertainty. The disproportionate number of amyloid positive dyads referred to the study suggests a possible physician bias in referral in favor of those who had positive scans. Another limitation of our study is that we were not able to make comparisons among diagnostic groups or specific categories of patients as defined in the Appropriate Use Criteria. Data were collected at one academic medical center tertiary care clinic with only a few referring neurologists. Interviews were performed during a period in which a voucher program enabled scans at reduced cost, potentially resulting in some participants choosing to undergo imaging who otherwise might not have. The time that occurred between the scan and the study interviews introduces the possibility of bias in participant recollection of the events and reactions related to undergoing amyloid imaging.
Future research is needed to better understand the implications of performing amyloid imaging. Studies should incorporate multisite recruitment from a variety of clinics in which the option of amyloid imaging is presented as part of the diagnostic workup of patients with cognitive disorders. Systematic invitations to participate would enhance inclusion of those who choose not to undergo amyloid imaging and those who scan negative, two particularly important groups in clinical care who are underrepresented in the current study. Clinician referrers to our study did not adhere to a specific protocol. Thus, the type and extent of information and education related to amyloid imaging that was provided is likely to have been variable. While this may add ecological validity to our results, it also introduces the potential confound that we cannot distinguish between differences in patient interpretation of the clinical interaction and differences in the clinical delivery of information. Studies to better understand referring physicians’ perspectives and attitudes toward amyloid imaging, the clinical encounter, and patient and caregiver reactions to amyloid imaging are needed.
Conclusions
Amyloid imaging is a relatively new tool for dementia specialists. There remains much debate over the value of amyloid imaging in the clinical evaluation of AD, though health economic research studies are collecting additional evidence to address this controversy. It will be important to consider the value of amyloid imaging in the lives of patients and families, beyond diagnostic changes, prescriptions, and cost. The data presented here suggest that, although it can cause sadness and despair, amyloid imaging may remove ambiguity and result in relief, confidence, and satisfaction for some patients and families, even when learning that AD is the most likely cause of cognitive problems. This information may allow patients and families to move toward important disease and lifestyle management strategies. Physicians may need to provide increased education to ensure that families understand the capabilities of the scan and reduce misunderstanding.
Research in context.
Systematic review
The authors reviewed the literature using traditional sources (i.e. PubMed). Few data are available related to the clinical use of amyloid imaging and fewer still examine how amyloid imaging affects the diagnostic experience for patients and families.
Interpretation
Our findings lead us to conclude that, though not all patients, caregivers, and family members see value in amyloid imaging, many do. The information provided by amyloid imaging may validate concerns, spur action, and provide relief from ambiguity and anxiety related to uncertain diagnoses and prognoses. Some participants in our study, however, held misconceptions about the capabilities of amyloid imaging and this will require careful attention by practicing clinicians using this new technology.
Future directions
These preliminary results will require validation in larger clinical studies.
Acknowledgments
We thank the participants for making this study possible. Drs. Grill, Kremen, Teng, Mendez, Shapira, Ringman, and Apostolova were supported by NIA AG016570. Dr. Grill and Ms. Cox are supported by NIA AG016573. Joshua Grill designed and oversaw the study, performed the interviews and data analyses, drafted the article, and approved the final draft. Chelsea Cox participated in the analyses, edited the article for content, and approved the final draft. Sarah Kremen, Mario Mendez, Edmond Teng, Jill Shapira, John Ringman, and Liana Apostolova referred participants, edited the article for content, and approved the final draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL, Cole G. Alzheimer disease. Jama. 2002;287(18):2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 3.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Rieves D, Ganley C. Brain amyloid imaging--FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367(10):885–887. doi: 10.1056/NEJMp1208061. [DOI] [PubMed] [Google Scholar]
- 7.Sabri O, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015;11(8):964–974. doi: 10.1016/j.jalz.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Curtis C, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72(3):287–294. doi: 10.1001/jamaneurol.2014.4144. [DOI] [PubMed] [Google Scholar]
- 9.Clark CM, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 11.Johnson KA, et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caroli A, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): Prediction of progression. Neurology. 2015;84(5):508–515. doi: 10.1212/WNL.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos SJ, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(Pt 5):1327–1338. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beard RL, Neary TM. Making sense of nonsense: experiences of mild cognitive impairment. Sociol Health Illn. 2013;35(1):130–146. doi: 10.1111/j.1467-9566.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence V, et al. Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2014;29(1):22–31. doi: 10.1002/gps.3958. [DOI] [PubMed] [Google Scholar]
- 16.Lingler JH, et al. Making sense of mild cognitive impairment: a qualitative exploration of the patient’s experience. Gerontologist. 2006;46(6):791–800. doi: 10.1093/geront/46.6.791. [DOI] [PubMed] [Google Scholar]
- 17.Mendez MF, Sabodash V. Clinical Amyloid Imaging in Logopenic Progressive Aphasia. Alzheimer Dis Assoc Disord. 2013 doi: 10.1097/WAD.0b013e3182a683de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Juan P, et al. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology. 2014;82(3):230–238. doi: 10.1212/WNL.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schipke CG, et al. Impact of beta-amyloid-specific florbetaben PET imaging on confidence in early diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(6):416–422. doi: 10.1159/000339367. [DOI] [PubMed] [Google Scholar]
- 20.Grundman M, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27(1):4–15. doi: 10.1097/WAD.0b013e318279d02a. [DOI] [PubMed] [Google Scholar]
- 21.Ossenkoppele R, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9(4):414–421. doi: 10.1016/j.jalz.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Zannas AS, et al. Impact of (1)(8)F-florbetapir PET imaging of beta-amyloid neuritic plaque density on clinical decision-making. Neurocase. 2014;20(4):466–473. doi: 10.1080/13554794.2013.791867. [DOI] [PubMed] [Google Scholar]
- 23.Mitsis EM, et al. A consecutive case series experience with [18 F] florbetapir PET imaging in an urban dementia center: impact on quality of life, decision making, and disposition. Mol Neurodegener. 2014;9:10. doi: 10.1186/1750-1326-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensaidane MR, et al. Clinical Utility of Amyloid PET Imaging in the Differential Diagnosis of Atypical Dementias Its Impact on Caregivers. J Alzheimers Dis. 2016 doi: 10.3233/JAD-151180. [DOI] [PubMed] [Google Scholar]
- 25.Williams SC. Alzheimer’s imaging agents struggle to find a market outside trials. Nat Med. 2013;19(12):1551. doi: 10.1038/nm1213-1551. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter BD, et al. Reaction to a dementia diagnosis in individuals with Alzheimer’s disease and mild cognitive impairment. J Am Geriatr Soc. 2008;56(3):405–412. doi: 10.1111/j.1532-5415.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 27.Shulman MB, et al. Using AD biomarker research results for clinical care: a survey of ADNI investigators. Neurology. 2013;81(13):1114–1121. doi: 10.1212/WNL.0b013e3182a55f4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JS, et al. Mild cognitive impairment in clinical care: a survey of American Academy of Neurology members. Neurology. 2010;75(5):425–431. doi: 10.1212/WNL.0b013e3181eb5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauthier S, et al. Diagnosis and management of Alzheimer’s disease: past, present and future ethical issues. Prog Neurobiol. 2013;110:102–113. doi: 10.1016/j.pneurobio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Arias JJ, Karlawish J. Confidentiality in preclinical Alzheimer disease studies: when research and medical records meet. Neurology. 2014;82(8):725–729. doi: 10.1212/WNL.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holloway RG, Gramling R, Kelly AG. Estimating and communicating prognosis in advanced neurologic disease. Neurology. 2013;80(8):764–772. doi: 10.1212/WNL.0b013e318282509c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson KA, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106–e109. doi: 10.1016/j.jalz.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Witte MM, et al. Clinical use of amyloid-positron emission tomography neuroimaging: Practical and bioethical considerations. Alzheimer’s & Dementia: Diagnosis, Assessment, & Disease Monitoring. 2015;1:10. doi: 10.1016/j.dadm.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinovici GD, et al. Testing and disclosures related to amyloid imaging and Alzheimer’s disease: Common questions and fact sheet summary. Alzheimers Dement. 2016;12(4):510–515. doi: 10.1016/j.jalz.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Ryan GW, Bernard HR. Techniques to identify themes. Field Methods. 2003;15(1):85–109. [Google Scholar]
- 37.Montine TJ, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ossenkoppele R, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landau SM, et al. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86(15):1377–1385. doi: 10.1212/WNL.0000000000002576. [DOI] [PMC free article] [PubMed] [Google Scholar]