Abstract
During tissue morphogenesis, cellular rearrangements give rise to a large variety of three-dimensional structures. Final tissue architecture varies greatly across organs, and many develop to include combinations of folds, tubes, and branched networks. To achieve these different tissue geometries, constituent cells must follow different programs that dictate changes in shape and/or migratory behavior. One essential component of these changes is the remodeling of cell-cell adhesions. Invasive migratory behavior and separation between tissues require localized breakdown of cadherin-mediated adhesions. Conversely, tissue folding and fusion require the formation and reinforcement of cell-cell adhesions. Cell-cell adhesion plays a critical role in tissue morphogenesis; its manipulation may therefore prove to be invaluable in generating complex topologies ex vivo. Recapitulating these shapes in engineered tissues would enable a better understanding of how these processes occur in vivo, and may lead to improved design of organs for clinical applications. In this review, we discuss work investigating the formation of folds, tubes, and branched networks with an emphasis on known or possible roles for cell-cell adhesion. We then examine recently developed tools that could be adapted to manipulate cell-cell adhesion in engineered tissues.
Keywords: morphodynamics, mechanobiology, mechanical force, branching morphogenesis
Introduction
During morphogenesis, cells and tissues rearrange themselves to generate complex three-dimensional (3D) architecture, such as folds, tubes, and branched networks [1]. Cell adhesions participate in these dynamic rearrangements and maintain tissue integrity throughout adult life. During collective cell movements that drive changes in tissue shape, cell-cell adhesions must be remodeled, broken down, or reinforced depending on the cellular behaviors required. For a given morphogenetic movement, the regulation of cadherin-based adhesions may be implicated in establishing cell polarity, mechanically coupling neighboring cells, and/or directing cell migration.
A large body of recent work has shed light on the physical mechanisms underlying organ development. Improved imaging capabilities have enabled us to study morphogenesis in vivo or using organ explants, and where this is not possible researchers have turned to organoid or cell culture models. However, replicating tissue shapes observed in vivo using cell culture is challenging. Nonetheless, if we can build organs in the lab, we can better understand how their development is misregulated and potentially generate organs for transplant into human patients. Using a variety of new technologies, manipulation of cell-cell adhesion in precise spatial or temporal ways could help to generate complex architecture in engineered tissues. Here, we review recent work highlighting the role of cell-cell adhesion in generating tissue folds, tubes, and branched networks. We then explore possible ways in which experimental control of cell-cell adhesion might be used to direct tissue morphogenesis in culture models.
Morphogenesis of 3D tissue architecture in vivo
Folds
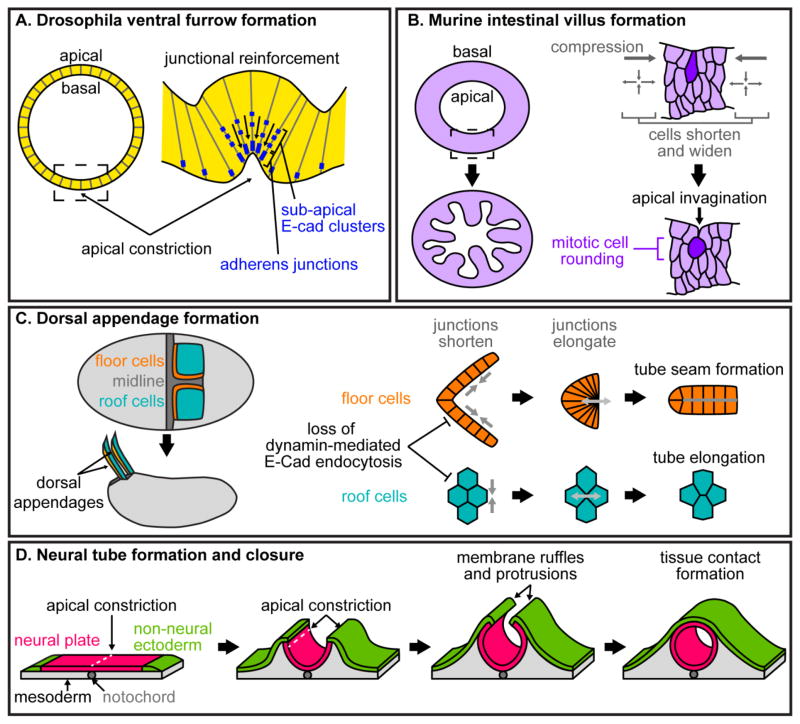
Many morphogenetic processes begin with a flat or curved sheet of cells that eventually gives rise to complex topologies such as folds. Folds can be generated by a monolayer of cells, by stratified cell sheets, or by multiple interacting tissues. Consequently, cell-cell adhesions must play different roles depending on the cellular behaviors required. A simple and well-studied example of tissue folding occurs during ventral furrow formation in the Drosophila embryo; in this case, folding is driven by pulsatile apical constriction of a row of cells within a monolayered epithelium (Figure 1A). Myosin-driven reduction of apical surface area causes the tissue to bend out of plane and fold into the center of the embryo [2–4]. Cell adhesion must be remodeled and reinforced to maintain tissue integrity in the presence of active, pulsatile contraction of actomyosin networks. Cycling of subapical clusters of E-cadherin is coupled to actomyosin pulses during gastrulation, allowing these clusters to join the apical junctions and reinforce intercellular adhesion [5].
Figure 1. Folds and tubes.
(A). Apical constriction leads to tissue folding during ventral furrow formation in the Drosophila embryo. Subapical clusters of cadherin move apically to reinforce adherens junctions between apically constricting cells. (B) The internal (apical) surface of the murine intestine starts off smooth and gives rise to folded morphology and eventually villi. In the early stages of this process, epithelial cells shorten and widen, generating compressive forces on cells between future villi. Cells in these regions undergoing mitosis become rounded and generate apical invaginations, leading to folds in the intestinal epithelium. (C) Dorsal appendage formation in the Drosophila egg involves junctional remodeling and cell intercalation of roof cells (to extend the tube) and floor cells (to seal the tube). Rearrangements in both cell populations require dynamin-mediated cadherin endocytosis. (D) Neural tube formation begins with apical constriction along the length of the neural plate. A second round of constriction along both sides brings the neural plate and the non-neural ectoderm into apposition. Non-neural ectodermal cells extended protrusions towards their counterparts, leading to closure of the tube.
More complex folds exist on the interior surface of tubular tissues, including the intestine and the oviduct. In the chicken, intestinal epithelial morphogenesis occurs concomitantly with differentiation of the surrounding mesenchyme into layers of smooth muscle. Each topological change in the lumenal epithelium coincides with the formation of a new smooth muscle layer surrounding the intestine [6]. When the first layer of smooth muscle forms circumferentially, the inner surface of the tube buckles and forms longitudinal ridges. Subsequently, the formation of a second layer of smooth muscle longitudinally causes the epithelium to buckle perpendicular to these ridges and generates a zigzag pattern. Finally, the third layer of smooth muscle is assembled longitudinally between the epithelium and the circumferential layer, causing the development of villi [6]. The resulting topology generates an uneven pattern of morphogens, including sonic hedgehog (Shh), across the intestinal epithelium. Consequently, signals from the epithelium to the surrounding mesenchyme are concentrated in the tip of the emerging villus. Signals from the mesenchyme that suppress intestinal stem cell fate are thus enhanced at the villus tip, restricting intestinal stem cells to the crypt regions between villi [7].
Intestinal villus morphogenesis in the mouse occurs by different mechanisms than in the chicken; villi emerge fairly rapidly and without the intermediate ridges and zigzag patterns [7]. In the mouse intestine, regularly sized and spaced clusters of mesenchymal cells appear beneath future villi [8]. Formation of these clusters is achieved not by mechanical influences of the surrounding smooth muscle, but by a self-organizing Turing-like field of Shh and bone morphogenetic protein (BMP) signaling [8, 9]. The physical mechanisms underlying murine villus morphogenesis have recently been described by Freddo et al. After mesenchymal clusters have formed, epithelial cells directly above them shorten and widen, generating compressive forces felt by cells between clusters. Mitotic cells in these compressed regions undergo internalized cell rounding and generate apical invaginations that spread and deepen over the course of intestinal development (Figure 1B) [10].
E-cadherin is required for villus formation during mouse embryogenesis [11], but its specific role(s) remain unclear. Intercellular adhesion mechanically couples cellular cortices during cell rearrangements [12], and could therefore be involved in transmitting mechanical cues between epithelial cells above and between mesenchymal clusters. Alternatively, E-cadherin could play a role in establishing appropriate cell polarity for villus morphogenesis. For example, apical-basal polarity might be required to align mitotic cells between future villi in order to generate apical invaginations.
Cadherin-mediated planar cell polarity (PCP) has been implicated in similar morphogenetic contexts. Flamingo, a gene that encodes a seven-pass transmembrane cadherin receptor, has been shown to regulate PCP during development of the Drosophila wing [13]. Celsr1 is the mammalian homolog of Flamingo, and is required to establish PCP during folding in the mouse oviduct. The lumenal epithelium of the oviduct is arranged in ridges running along the length of the tube and is surrounded by a layer of smooth muscle. PCP is required to create a polarized network in which cells are elongated along the length of the tube; this allows for increased tissue-level tension along that axis, yielding well-aligned folds. Loss of Celsr1 abrogates cell elongation and reduces longitudinal tension, leading to misaligned folds [14, 15].
Tubes
In many different examples, tubulogenesis is initiated by tissue folding and is completed with the formation of new adhesions to seal the tube [16]. Tissue folding typically generates tubes of larger diameters requiring the concerted efforts of large groups of cells. For example, during respiratory appendage formation in the Drosophila egg, the initially flat appendage primordium bends out of plane from a focal point, and is then sealed and elongated by cell intercalation to generate a simple epithelial tube (Figure 1C) [17]. Using a two-dimensional (2D) model of this process, Osterfield et al showed that differences in apical tension in specific regions of the primordium are sufficient to generate the tissue bending and cell rearrangements observed in vivo [17]. Proper distribution and transmission of tension requires precisely controlled adhesion between cells; in line with this, disruption of dynamin-mediated endocytosis in the dorsal appendage epithelium results in abnormal E-cadherin localization and defects in tubulogenesis [18].
Neural tube formation in vertebrates can also be driven by tissue folding (Figure 1D). In contrast with tube formation in the dorsal appendage in which bending occurs at a specific point, neurulation begins with apical constriction and folding along the length of the tissue, parallel to the dorsal midline. As the neural plate bends out of plane, it folds once more on each side to bring the lateral sides into apposition. The neural plate is initially located between two separate sheets of non-neural ectoderm; during folding, these sheets are also brought together, and new cell-cell adhesions form between them to seal the tube [19, 20]. Fusion between these two portions of the non-neural ectoderm is achieved either by a zippering mechanism, where the tube closes from one end of the tissue to the other, or by a “button-up” mechanism, where protrusions from the non-neural epithelium extend and join to generate multiple closure sites along the length of the tube [20, 21]. These protrusions are regulated by Cdc42 and Rac1, and are required for successful neural tube closure [21]. When protrusions from cells in culture meet, they initiate the formation of new cell-cell contacts [22]. It is likely that protrusions from the non-neural ectoderm are required for initially establishing cell-cell adhesions during neural tube closure.
Alternatively, tubes can form by cavitation of a cylindrical mass of cells [19]. The process of generating a lumen from a multi-layered epithelium has been observed in a variety of other contexts, including the mammary gland. During embryonic development, the mammary epithelium is a simple, bi-layered tube. At the onset of puberty, the epithelium becomes stratified and branching morphogenesis is achieved by migration of the highly dynamic, multi-layered terminal end bud (TEB). Cells of the TEB remain epithelial, as evidenced by the presence of E-cadherin-based adhesions, but they are incompletely polarized. The entire epithelial layer maintains apical polarity at the lumenal side and basal polarity at the surface in contact with the basement membrane, but individual cells lack clearly established apical and basal domains. As ductal elongation ends, the epithelium returns to a simple, bi-layered state and apical-basal polarity is re-established [23, 24].
In its bi-layered state, the mammary gland is composed of a layer of lumenal epithelial cells surrounded by a layer of myoepithelial cells. Lumenal epithelial cells adhere to each other via E-cadherin, while myoepithelial cells adhere via placental (P)-cadherin [25]. It is possible that E- and P-cadherin provide differential adhesion leading to cell sorting, which may play a role in achieving normal ductal architecture during development. Myoepithelial cells appear to have some control over the location of branch initiation – new branches typically form in regions with less myoepithelial cell coverage in mammary gland organoids [24]. However, the role of myoepithelial cells in lumen formation and whether they are implicated in the transition from a stratified to simple epithelium is still unknown.
Tubes can also be formed on a much smaller scale, either between two cells or through a single cell. Examples of these include secretory lumena in the liver [26], the tracheal lumen in Drosophila embryos [27], and lumena of blood vessels. In blood vessel tubulogenesis, cell-cell adhesions participate in establishing polarity and may also act as mechanosensors linking blood flow to vascular remodeling. Vascular endothelial (VE)-cadherin and apical-basal polarity are required for endothelial cells to form lumena in cell culture and in the embryo [28, 29]. Polarity is established through the interactions between VE-cadherin and cell polarity complex proteins Par3 and Pals1 [29]. Without proper localization of cell-cell junctions and establishment of cell polarity, lumen formation and therefore blood flow are prevented.
Blood flow is major determinant of vascular morphogenesis, including sprouting and lumen elongation [30, 31]. Endothelial cells that experience higher levels of shear stress, such as those in arteries, are more strongly axially polarized against the direction of flow, while cells in regions of low shear stress, such as capillaries, exhibit less axial polarization [32]. Pressure or shear stress must be sensed by endothelial cells to cause changes in their behavior. These mechanical signals may be transduced via VE-cadherin, which leads to cytoskeletal and junctional remodeling across endothelial cells [33]. Recent work has shed light on possible mechanisms by which endothelial cells transduce mechanical signals from blood flow. Non-canonical Wnt ligands (Wnt5a and Wnt11) secreted by endothelial cells prevent vessel regression and are thus implicated in stabilizing vascular networks [34]. Furthermore, depletion of these ligands in capillaries, where endothelial cells experience lower shear forces, causes increased sensitivity to flow as evidenced by stronger axial polarization [34]. While these results help to bridge the connection between cell behavior and blood flow, the exact mechanisms through with endothelial cells sense mechanical forces remains unclear.
Branches
Many tubular organs form branched networks, and can achieve their shape using vastly different physical mechanisms [35]. These various branching modes all require some level of regulation of cell-cell adhesion. For invasive branching, cells may need to break down intercellular contacts and become more mesenchymal or migratory. Branching by epithelial folding involves cells remaining as a coherent sheet, and may therefore require more persistent cell-cell adhesions. Alternatively, branched networks can be shaped by the surrounding extracellular matrix (ECM) or mesenchyme and differential growth of the epithelium.
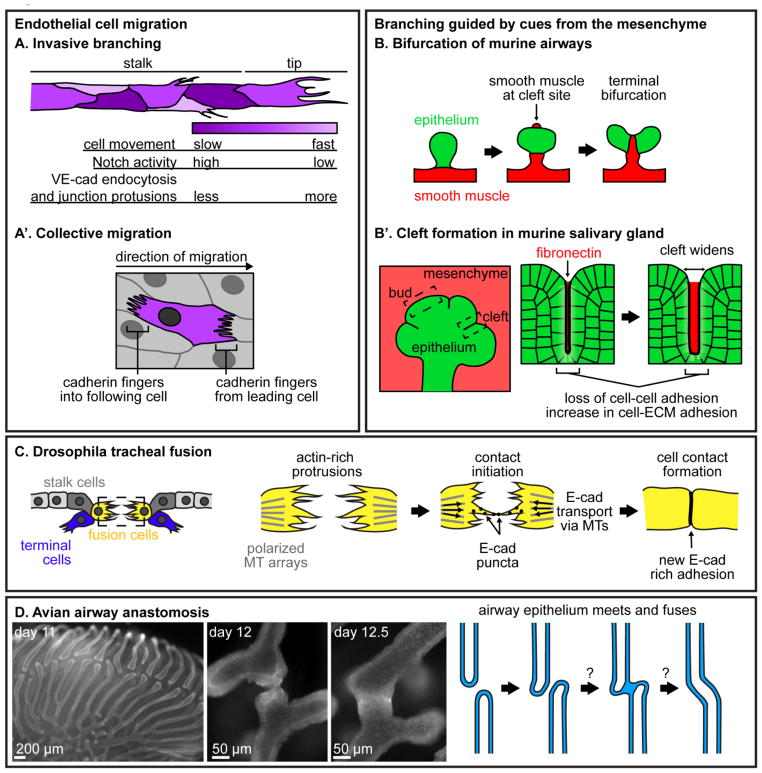
Invasive branching of endothelial cells during vascular development or remodeling involves tip or leader cells that guide migration of the ensemble and extend protrusions into the surrounding microenvironment (Figure 2A) [36]. Live-imaging analysis in culture and in vivo has shown that cells near the migrating front move both forwards and backwards, effectively taking turns at the leading edge of the vessel [37]. Tip-cell selection involves Notch-dependent signaling through vascular endothelial growth factor receptor (VEGFR), which can specify tip versus stalk cell identity [36, 38]. As cells rearrange and encounter new neighbors, relative levels of VEGFR are re-evaluated, resulting in constant competition for tip-cell status [38]. Recent work has shown that the VEGF-Notch signaling module directly affects VE-cadherin stability, providing a mechanism for Notch-dependent junctional remodeling [39]. Cells with low levels of Notch activity extend junctional protrusions into neighboring cells, increase VE-cadherin endocytosis, and move faster. Recent work examining collective migration of vascular endothelial cells in culture has revealed that leader cells extend protrusions called “cadherin fingers” into trailing cells (Figure 2A′) [40]. These cadherin fingers may provide signaling or guidance cues required for collective migration. However, it is not yet known whether these structures form in vivo, or how they might be remodeled or adapted to allow for tip-cell shuffling.
Figure 2. Branching and tube fusion.
(A-A′) Migration of endothelial cells in different contexts. (A) During invasive branching, endothelial cells at the tip of a blood vessel exhibit different levels of Notch signaling, endocytose VE-cadherin at different rates, and shuffle either more quickly or more slowly. As a result of this dynamic behavior, tip-cell identity switches between neighbors as the vessel migrates. (A′) Endothelial cells migrating collectively engulf cadherin fingers from cells ahead of them and extend cadherin fingers into the cells following them. (B-B′) Examples of branching guided by cues from the surrounding mesenchyme. (B) Bifurcation of the murine airway epithelium is guided by smooth muscle differentiation. As the parent branch grows, smooth muscle appears at the future cleft site. Smooth muscle wraps around the epithelium in a specific pattern, dividing it in two and leading to bifurcation. (B′) During branching morphogenesis of the murine salivary gland, fibronectin accumulates in clefts, leading to an increase in cell-ECM adhesion and a breakdown of cell-cell adhesion to allow clefts to widen. (C) During tracheal fusion in the Drosophila embryo, fusion cells extend filopodia towards each other. When filopodia meet, E-cadherin puncta move along filopodia to initiate a new adhesion, and E-cadherin is trafficked along microtubules (MTs) towards the new cell-cell junction. (D) Anastomosis of parabronchi during avian airway morphogenesis has not yet been extensively studied. Multicellular parabronchi extend towards each other and fuse by unknown mechanisms to generate loops required for unidirectional air flow. Images of E-cadherin immunofluorescence in lungs from embryonic days 11, 12 and 12.5 show the different stages of airway anastomosis.
Branched networks in the kidney, mammary gland, and lung are generated by different mechanisms than those observed during angiogenesis. In these contexts, individual cells do not exhibit protrusive or migratory behavior – instead, the entire epithelial collective bends or grows into its surrounding microenvironment. In this context, cell-cell adhesions must be regulated in a different manner than during invasive branching. Kidney branching morphogenesis proceeds via outgrowth and bifurcation of the ureteric bud. Epithelial cells of the kidney tubules adhere to each other via E-cadherin; in the tip, E-cadherin is enriched apically, while in the stalk or medullary regions it is evenly distributed along the entire lateral membrane [41]. Bifurcation of the ureteric bud requires signaling through mitogen-activated protein kinase (MAPK); when MAPK activity is perturbed, E-cadherin is enriched in tip cells and localizes more basally than in the native kidney [41]. Loss of MAPK activity results in decreased phosphorylation of Paxillin but also redistribution of pPaxillin to and accumulation of vinculin at cell-cell adhesions, which could theoretically stabilize E-cadherin-mediated adhesion [41]. Abnormal E-cadherin levels and distribution likely prevent the cellular rearrangements required for tip bifurcation, leading to defective branching morphogenesis when MAPK activity is reduced.
MAPK has also been implicated in regulating cell behavior during mammary branching morphogenesis and ductal elongation [42]. Mammary epithelial cells migrate collectively to drive tube elongation [24]. Cells at the tip of the TEB that exhibit higher levels of MAPK activity are more migratory, and blocking MAPK signaling prevents branching morphogenesis [42]. Given the role of MAPK in determining E-cadherin localization during branching of the ureteric bud, it is possible that MAPK affects remodeling of cell-cell adhesions and thus controls cell motility in the mammary gland. However, this has not yet been directly tested.
Collective tissue movements also drive branching morphogenesis of the airways in the lung, and interestingly, this may occur by very different mechanisms in the chicken and in the mouse. In avian lung development, the first branches form laterally off the primary bronchus. Initiation of these lateral branches is driven by apical constriction, which drives tissue folding out of the circumference of the tube and into the surrounding mesenchyme [43]. While there are many differences between the avian lung and the early Drosophila embryo, both generate folds via apical constriction of an epithelial tissue. Remodeling of cell-cell adhesions, like that observed during ventral furrow formation, is likely required to reinforce intercellular junctions during apical constriction in the chicken lung as well [5].
The physical mechanisms of branching in the mouse lung are less clear. Cell shape changes like those expected during apical constriction and tissue folding have been observed in emerging lateral branches; furthermore, inhibiting cytoskeletal regulators Rho-associated protein kinase (ROCK) or phosphorylated myosin light-chain kinase (pMLC) prevents these cell shape changes and causes defective branching [44]. Apical constriction may therefore play a role in branching morphogenesis of the mouse lung. Alternatively, localized cell proliferation could drive the formation of new branches; in line with this, cell divisions occur far more frequently in branching versus non-branching regions of the epithelium [45]. Increased proliferation has also been observed during bifurcation events; however, the levels within the parent branch are homogeneous and these patterns of proliferation alone cannot explain how bifurcation occurs [45]. The orientation of cell division varies throughout the branch, with divisions parallel to the plane of the tissue more predominant in the future cleft region, and divisions perpendicular to the plane slightly elevated in the rest of the branch [45]. This oriented growth could potentially help to direct branching, yet it is unclear how growth is spatially controlled. Signals from the surrounding mesenchyme are likely candidates for guiding branching of the airway epithelium. Kim et al. showed that smooth muscle differentiation in the cleft region is required for bifurcation, demonstrating an active role for smooth muscle wrapping in shaping the developing airways (Figure 2B) [46]. Whether similar events occur during the formation of lateral branches is still unknown.
Mechanical cues from the mesenchyme are also critical in directing branching of the salivary gland. For clefts to form in this tissue, epithelial cells between emerging buds must locally detach from one another (Figure 2B′). During branching morphogenesis, fibronectin accumulates in clefts and triggers the expression of BTB/POZ domain-containing protein 7 (Btbd7), which leads to a local increase in cell-ECM adhesion and decrease of E-cadherin-mediated cell-cell adhesion. Subsequently, the epithelial tissue becomes disorganized and the cleft deepens [47, 48]. Differences in cell-cell adhesion throughout the developing salivary gland are also associated with specific cell morphologies and motility. Outer cells, along the surface of the bud, are columnar and exhibit integrin-dependent motility, frequently coming in and out of contact with the basement membrane. Cells further from the edge, called inner cells, are more rounded and undergo E-cadherin-dependent movement, and show overall less mobility than outer cells [49]. These differences in cell shape and movement may be achieved through differential regulation of cadherin-mediated adhesion; however, the mechanisms by which cell-cell adhesions could be remodeled to allow for salivary gland branching are unknown. Signaling from the integrin-matrix adhesions could potentially modulate the stability of intercellular adhesions, leading to differences in cell shape and motility.
In certain biological systems, tubes elongate and then merge with other tubes in the network in a process known as anastomosis or fusion. Anastomosis requires the formation of new adhesions between cells at the ends of adjacent tubes. In culture, cells extend actin-rich protrusions to initiate contact between the two populations and form new cell-cell adhesions [22, 50]. Tube fusion in the Drosophila tracheal system involves similar processes (Figure 2C) [27, 51]. The cell at the dorsal-most tip of the branch, known as the fusion cell, extends filopodia towards its counterpart across the dorsal side of the embryo. The two fusion cells elongate until they make contact, at which point they pack in towards each other. The stalk cells, which are immediately behind the fusion cell, elongate concomitantly with this packing. Finally, as fusion cell bodies come into closer contact, the lumen of each branch connects [27].
As fusion cells approach each other, E-cadherin puncta localize to the filopodia extending from the leading edge. Junctional cadherin remains stable at contacts between fusion and stalk cells, but becomes more dynamic and increases in intensity at the newly formed contact between fusion cells. Live imaging revealed that E-cadherin flows towards to the fusion cell contact, and genetically depleting E-cadherin in tracheal cells impaired the formation of new contacts without disrupting cell morphology or migration, suggesting that newly synthesized E-cadherin is preferentially directed to the newly formed adhesion site. Fusion cells also exhibit polarized arrays of microtubules, and when these arrays are genetically disrupted, E-cadherin fails to enrich at the leading edge, preventing the formation of contacts between fusion cells [51]. Microtubule-mediated transport of E-cadherin to the newly formed adhesion is thus a critical component of tube fusion.
Another example of anastomosis occurs during angiogenesis. In the embryo and the adult, vascular networks are remodeled to meet changing demands for oxygen and nutrients of each organ. New vessels sprout from existing ones, migrate, and then fuse with the existing network. Vascular anastomosis has been extensively characterized in the context of zebrafish development, and bears some resemblance to the process of Drosophila tracheal fusion. Tip cells of migrating vessels extend filopodia towards each other and form single VE-cadherin-mediated adhesion sites upon contact [52]. These adhesions then expand to form a ring, bringing the apical domains of each tip cell into apposition. VE-cadherin is essential for initial contact formation; loss of VE-cadherin results in persistent tip cell migratory behavior and the formation of multiple new contact sites along the length of each cell [52]. Surprisingly, VE-cadherin is not required to establish of the apical domain in contacting tip cells, suggesting a role for other adhesion or polarity proteins in this process. Subsequent fusion of vessel lumens occurs either via cord hollowing to form a multicellular tube, or by membrane invagination to generate a unicellular tube [53]. Interestingly, the mode of lumen fusion used by zebrafish vasculature seems to depend upon blood flow; apical invagination dominates under higher blood pressure, while cell rearrangement and cord hollowing is used under low pressure [52].
Anastomosis also occurs in the avian lung. During avian airway morphogenesis, tertiary bronchi, also known as parabronchi, form off secondary bronchi and subsequently anastomose to generate air capillaries (Figure 2D) [54]. The resulting looped architecture is a characteristic feature of the parabronchial lung that allows for unidirectional air flow [54]. Fusion of these tubes is an integral part of avian lung development, but the exact mechanisms of anastomosis in this context have not yet been studied. Compared to the Drosophila trachea or zebrafish vasculature, where single cells establish new contacts to join adjacent lumens, the mechanisms used by the much larger avian lung may be more complex. Instead of just joining simple tubes of fewer cells, closed, multicellular tubes that make up the airway must meet and fuse.
Future directions: Replicating tissue shape to engineer organs
Generating complex 3D tissue architecture in culture, while extremely challenging, will likely prove to be a fruitful line of research. Replicating cell and tissue rearrangements using culture models could be a powerful tool for investigating the mechanisms that drive morphogenesis in vivo. Further, building tissues in the lab could lead to construction of organs for regenerative medicine. Given the importance of cadherin-mediated adhesion in generating complex shapes during tissue morphogenesis, one possible strategy to approach this problem is to experimentally manipulate intercellular adhesion.
Scaffolds, microfluidic devices, and synthetic matrices have been used successfully to grow cells, tissues, and organoids. For example, engineered microenvironments have been used to study vascular sprouting and anastomosis using 3D matrices [55, 56] or microfluidic devices [57, 58]. Scaffolds and matrices have been designed to mimic tissue shape for studying intestinal differentiation [59] or to promote intestinal organoid growth [60]. Each of these structural or ECM-mediated constraints will directly affect cell shape and cell-ECM adhesion, and indirectly affect cell-cell adhesion. Cross-talk between cadherin- and integrin-mediated adhesions has been observed in several different contexts both in vivo and in culture [61, 62]. Therefore, signals from a synthetic microenvironment may be communicated via cell-ECM adhesions and result in changes in cell-cell adhesion.
Recently, many new tools have been developed that could be used to specifically target cell-cell adhesion. Spatial and temporal optogenetic control of motor proteins including kinesin, dynein, and myosin has been used to direct movement of organelles and vesicles [63]. Activating light-sensitive dynein or kinesin to remove or recruit endosomes to axon growth cones effectively prevented or enhanced growth, respectively. Theoretically, similar tools could be used to regulate cadherin endo/exocytosis to stabilize or weaken cell-cell adhesions. This could be applied to encourage disassembly of junctions required for cell motility and rearrangements by enhancing endocytosis, or to encourage trafficking of cadherin to the membrane to promote formation of new junctions for tissue fusion or reinforcement of junctions for tissue folding.
Optogenetic tools could also potentially be used to more directly affect cadherin-mediated adhesion. Light-sensitive Cry2 molecules oligomerize in the presence of light; engineering Cry2 molecules with binding domains for specific transmembrane proteins therefore induces clustering of these proteins of interest [64]. This technique has successfully been used to reversibly cluster receptor tyrosine kinases and integrins [64]. The design of appropriate binding domains would enable the spatiotemporal control of cadherin clustering and could be used to drive adhesion assembly or to reinforce existing adhesions.
Molecular engineering has recently been applied to achieve reversible, light-controlled assembly of cell adhesions without directly targeting cadherin [65]. In this technique, β-cyclodextrin is incorporated into plasma membranes allowing cells to bind to azobenzene, a photo-switchable compound whose trans but not cis isomer binds β-cyclodextrin. Using engineered molecules including two azobenzene components connected by a polyethylene glycol chain, nearby cells displaying β-cyclodextrin can be induced to bind each azobenzene in the presence of visual light [65]. This binding forces cells into close apposition, which may encourage the formation of cadherin-based adhesions. Further, this interaction can be broken using UV light. This technique could be used to force groups of cells to build adhesions in specific spatiotemporal patterns. None of these tools have been applied directly to cadherin-mediated adhesion or to the formation of complex tissue architecture. Nonetheless, adapting them to control intercellular adhesion in space and time will greatly enhance our ability to recapitulate tissue geometries observed in vivo.
Highlights.
Cell shape changes and rearrangements give rise to highly varied tissue topology.
Generating 3D structures requires differential remodeling of cell-cell adhesions.
Cell-cell adhesion could be experimentally manipulated to engineer tissues.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- BMP
bone morphogenetic protein
- Btbd7
BTB/POZ domain containing 7
- Cdc42
cell division control protein 42
- ECM
extracellular matrix
- MAPK
mitogen-activated protein kinase
- PCP
planar cell polarity
- pMLC
phospho-myosin light-chain kinase
- ROCK
Rho-associated protein kinase
- Shh
sonic hedgehog
- TEB
terminal end bud
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Misra M, et al. Shape Transformations of Epithelial Shells. Biophys J. 2016;110(7):1670–8. doi: 10.1016/j.bpj.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason FM, Tworoger M, Martin AC. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat Cell Biol. 2013;15(8):926–36. doi: 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457(7228):495–9. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin AC, et al. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188(5):735–49. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng M, Wieschaus E. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J Cell Biol. 2016;212(2):219–29. doi: 10.1083/jcb.201508056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyer AE, et al. Villification: how the gut gets its villi. Science. 2013;342(6155):212–8. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyer AE, et al. Bending gradients: how the intestinal stem cell gets its home. Cell. 2015;161(3):569–80. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walton KD, et al. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci U S A. 2012;109(39):15817–22. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton KD, et al. Villification in the mouse: Bmp signals control intestinal villus patterning. Development. 2016;143(3):427–36. doi: 10.1242/dev.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freddo AM, et al. Coordination of signaling and tissue mechanics during morphogenesis of murine intestinal villi: a role for mitotic cell rounding. Integr Biol (Camb) 2016;8(9):918–28. doi: 10.1039/c6ib00046k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondow BJ, et al. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol. 2012;371(1):1–12. doi: 10.1016/j.ydbio.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maitre JL, et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338(6104):253–6. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- 13.Usui T, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98(5):585–95. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 14.Shi D, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141(23):4558–68. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- 15.Koyama H, et al. Mechanical Regulation of Three-Dimensional Epithelial Fold Pattern Formation in the Mouse Oviduct. Biophys J. 2016;111(3):650–65. doi: 10.1016/j.bpj.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iruela-Arispe ML, Beitel GJ. Tubulogenesis. Development. 2013;140(14):2851–5. doi: 10.1242/dev.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterfield M, et al. Three-dimensional epithelial morphogenesis in the developing Drosophila egg. Dev Cell. 2013;24(4):400–10. doi: 10.1016/j.devcel.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters NC, Berg CA. Dynamin-mediated endocytosis is required for tube closure, cell intercalation, and biased apical expansion during epithelial tubulogenesis in the Drosophila ovary. Dev Biol. 2016;409(1):39–54. doi: 10.1016/j.ydbio.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayraghavan DS, Davidson LA. Mechanics of neurulation: From classical to current perspectives on the physical mechanics that shape, fold, and form the neural tube. Birth Defects Res A Clin Mol Teratol. 2016 doi: 10.1002/bdra.23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyrgaki C, et al. Dynamic imaging of mammalian neural tube closure. Dev Biol. 2010;344(2):941–7. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolo A, et al. Regulation of cell protrusions by small GTPases during fusion of the neural folds. Elife. 2016;5:e13273. doi: 10.7554/eLife.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasioukhin V, et al. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 23.Ewald AJ, et al. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci. 2012;125(Pt 11):2638–54. doi: 10.1242/jcs.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewald AJ, et al. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14(4):570–81. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel CW, Strickland P, Friedmann Y. Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol. 1995;169(2):511–9. doi: 10.1006/dbio.1995.1165. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, et al. Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension. Nat Cell Biol. 2016;18(3):311–8. doi: 10.1038/ncb3310. [DOI] [PubMed] [Google Scholar]
- 27.Gervais L, Lebreton G, Casanova J. The making of a fusion branch in the Drosophila trachea. Dev Biol. 2012;362(2):187–93. doi: 10.1016/j.ydbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Lampugnani MG, et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123(Pt 7):1073–80. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 29.Brinkmann BF, et al. VE-cadherin interacts with cell polarity protein Pals1 to regulate vascular lumen formation. Mol Biol Cell. 2016;27(18):2811–21. doi: 10.1091/mbc.E16-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebala V, et al. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat Cell Biol. 2016;18(4):443–50. doi: 10.1038/ncb3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galie PA, et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014;111(22):7968–73. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco CA, et al. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol. 2015;13(4):e1002125. doi: 10.1371/journal.pbio.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barry AK, Wang N, Leckband DE. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J Cell Sci. 2015;128(7):1341–51. doi: 10.1242/jcs.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco CA, et al. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. Elife. 2016;5:e07727. doi: 10.7554/eLife.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varner VD, Nelson CM. Cellular and physical mechanisms of branching morphogenesis. Development. 2014;141(14):2750–9. doi: 10.1242/dev.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentley K, et al. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol. 2009;5(10):e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arima S, et al. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 2011;138(21):4763–76. doi: 10.1242/dev.068023. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–53. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 39.Bentley K, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol. 2014;16(4):309–21. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 40.Hayer A, et al. Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol. 2016;18(12):1311–1323. doi: 10.1038/ncb3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ihermann-Hella A, et al. Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion. PLoS Genet. 2014;10(3):e1004193. doi: 10.1371/journal.pgen.1004193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huebner RJ, Neumann NM, Ewald AJ. Mammary epithelial tubes elongate through MAPK-dependent coordination of cell migration. Development. 2016;143(6):983–93. doi: 10.1242/dev.127944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140(15):3146–55. doi: 10.1242/dev.093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadzik RS, et al. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A. 2014;111(34):12444–9. doi: 10.1073/pnas.1406639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnatwinkel C, Niswander L. Multiparametric image analysis of lung-branching morphogenesis. Dev Dyn. 2013;242(6):622–37. doi: 10.1002/dvdy.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HY, et al. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Dev Cell. 2015;34(6):719–26. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 48.Onodera T, et al. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010;329(5991):562–5. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu JC, et al. Region-specific epithelial cell dynamics during branching morphogenesis. Dev Dyn. 2013;242(9):1066–77. doi: 10.1002/dvdy.24000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNeill H, et al. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J Cell Biol. 1993;120(5):1217–26. doi: 10.1083/jcb.120.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato K, et al. Microtubule-dependent balanced cell contraction and luminal-matrix modification accelerate epithelial tube fusion. Nat Commun. 2016;7:11141. doi: 10.1038/ncomms11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenard A, et al. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell. 2013;25(5):492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Herwig L, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21(22):1942–8. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Maina JN. The Lung-Air Sac System of Birds. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 55.Diaz-Santana A, Shan M, Stroock AD. Endothelial cell dynamics during anastomosis in vitro. Integr Biol (Camb) 2015;7(4):454–66. doi: 10.1039/c5ib00052a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen DH, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110(17):6712–7. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, et al. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip. 2016;16(2):282–90. doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song JW, Bazou D, Munn LL. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr Biol (Camb) 2012;4(8):857–62. doi: 10.1039/c2ib20061a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costello CM, et al. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng. 2014;111(6):1222–32. doi: 10.1002/bit.25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 61.Maruthamuthu V, et al. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108(12):4708–13. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Julich D, et al. Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology. Dev Cell. 2015;34(1):33–44. doi: 10.1016/j.devcel.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Bergeijk P, et al. Optogenetic control of organelle transport and positioning. Nature. 2015;518(7537):111–4. doi: 10.1038/nature14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bugaj LJ, et al. Regulation of endogenous transmembrane receptors through optogenetic Cry2 clustering. Nat Commun. 2015;6:6898. doi: 10.1038/ncomms7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi P, et al. Spatiotemporal control of cell-cell reversible interactions using molecular engineering. Nat Commun. 2016;7:13088. doi: 10.1038/ncomms13088. [DOI] [PMC free article] [PubMed] [Google Scholar]