Abstract
Two wild-type (WT) Aspergillus strains, A. flavus HAk1 and A. oryzae HAk2, were selected for kojic acid (KA) biosynthesis. Malt extract sucrose culture medium (MES) was the best culture medium for maximum production of KA. The maximum production of KA has been estimated at pH 4 after 7 days of incubation at 30 °C. Overproduction of KA was attained by mutagenesis of both A. flavus HAk1 and A. oryzae HAk2 through their exposer to different doses of gamma irradiation. The mutant strains (MT) A. flavus HAk1-M2 and A. oryzae HAk2-M26 were the most stable mutants for maximum production of KA through four generations. Yield of KA by A. oryzae HAk2-M26 and A. flavus HAk1-M2 has been 2.03-fold and 1.9-fold, respectively, higher than their wild-type strains. All WT and MT strains were used for KA production from different agricultural raw materials. Apple peel was the best waste for KA production by WT strains of A. flavus and A. oryzae, while orange peel and rice stalk are best material for KA production by MT strains, A. flavus HAk1-M2 and A. oryzae HAk2-M26, respectively. All experimental strains have the ability to produce considerable amounts of KA from sugarcane molasse (SCM) and sugar-beet molasse (SBM). SBM was better than SCM for KA production by all strains. The antioxidant activity of biosynthesizing KA was strongly affected with production conditions, where the highest antioxidant activity of all strains was recorded at the optimum environmental and nutritional conditions for KA production.
Keywords: Aspergillus, Kojic acid, Mutagenesis, Raw materials, Antioxidant
Introduction
Kojic acid (KA) is a chelation agent produced by several species of fungi during aerobic fermentation of various substrates. KA has the Japanese common name koji which was derived from “Koji”, the inoculum of fungus starter used in food fermentations for many centuries. Its chemical structure was previously investigated and defined as 5-hydroxy-2-hydroxymethyl-4-pyrone (Nandan and Polasa 1985; Kahn et al. 1995). The attention for KA is increasing extremely because of its commercial application in industry. KA has various applications in several fields such as cosmetic industry, medicine, food industry, agriculture, and chemical industry. Nowadays, KA plays a crucial role in cosmetics (Rosfarizan et al. 2010), especially skin care products which prevent exposure to UV radiation. It has been used in the production of skin whitening creams, skin protective lotions, whitening soaps, and tooth care products, and it acts as ultraviolet protector. KA suppresses hyperpigmentation in human skins by restraining the formation of melanin through the inhibition of tyrosinase formation, the enzyme that is responsible for skin pigmentation (Ohyama and Mishima 1990; Noh et al. 2009). KA plays an extensive role in prevention of browning formation (speck) during processing and storage of uncooked noodles. In addition, it has also an inhibitory effect on polyphenol oxidase in different fruits and vegetables including apples, potatoes, and crustaceans (Chen et al. 1991). KA interferes with the oxygen required for enzymatic browning leading to the reduction of o-quinones to diphenols and prevents the formation of melanin pigment (Mohamad et al. 2010). Moreover, KA has an economic importance in the medical field where it can be used as an anti-inflammatory drug and painkiller.
The synthesis of KA by chemical methods results in free radical production in the living cell (Hazra et al. 2008). To avoid this risk, many attempts were carried out to select an alternative method for KA production. Recent studies have focused on KA production by microorganisms as alternative non-toxic and safe methods. The production of KA by aerobic fermentation of Aspergillus species is considered one of the best techniques used in industries. There are 58 different fungal species used for production of KA such as Penicillium, Mucor, Aspergillus, etc. (Abd El-Aziz 2013). Several Aspergillus species including A. oryzae, A. tamari, A. parasiticus, and A. flavus have the ability to produce considerable amounts of KA in the culture medium (Rosfarizan et al. 2010). Screening of high KA secretor from various strains and improvement of strains through various mutation processes were conducted in the last few decades (Rosfarizan et al. 1998). Although several potential Aspergillus strains for KA production have been isolated, very little studies about the improvement of KA production by these strains through either mutation or genetic recombination techniques have been reported. Abd El-Aziz (2013) reported that the mutagenesis of KA biosynthesizing genes by ultraviolet or gamma radiation caused overproduction of KA secreted by different fungi. Wan et al. (2004) reported that the mutation of A. oryzae ATCC 22788 via chemical treatment and UV irradiation was also found to improve KA production with about 100 times higher than the parent strain. Irradiation by gamma ray may cause some mutations to the genes of cells through the DNA repair mechanisms within cells (Ellaiah et al. 2002).
Solid substrate fermentation (SSF), the cultivation of microorganisms under controlled conditions with the absence of free water, was used in biosynthesizing of various commercial products by Aspergillus strains (Prabhakar et al. 2011). SSF is frequently applied in agro-waste bioprocess industry due to the close resemblance of the method to the natural condition of microbial cultivations. Different types of raw materials, which include various synthetic carbon sources like glucose, sucrose, maltose, xylose, and alcohols were used by the earlier researchers to obtain better yields of KA (Barnard and Challenger 1949). Agro-waste by-products such as industrial wastes, fruit wastes, vegetable wastes, etc. were used as cheapest sources for production of KA (Abd El-Aziz 2013; Nurashikin et al. 2013; Chaudhary et al. 2014; El-Kady et al. 2014). Molasses, the main by-product of sugar production, is the final syrup spun off after repeated crystallization in the extraction of sucrose (Douglas and Glenn 1982). It is the cheapest raw materials which contains high amounts of sucrose. Therefore, it has been used in different industrial fermentation as a carbohydrate source (Gad 2003).
This study aims to reduce the cost of industrial fermentation for KA production through improvement the KA producing strains by mutagenesis, and the increment of KA production through optimization of environmental conditions and using different cheapest agriculture raw materials. In addition, it aims to confirm KA application by studying the antioxidant activity of fungal filtrate and its relation to KA production at all conditions.
Materials and methods
Chemicals and solvents
Potato dextrose agar (PDA), yeast extract, malt extract, peptone, glucose, sucrose, and all other constituents of culture media were obtained from Sigma-Aldrich, Lyon, France. Kojic acid standard, 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), methanol, ferric chloride, and hydrochloric acid were purchased from Sigma- Aldrich Chemical Co. (St. Louis, MO, USA). The water was double distilled with Millipore water purification system (Bedford, MA, USA).
Isolation and identification of fungal strains
Nine seed samples including rice, wheat, corn, lentils, cowpea, bean, maize, peanuts, and barley were used for fungal isolation according to Roy and Kumarin (1991). The seed samples were sterilized with 2.0% NaOCl solution for 10 min and washed with sterilized distilled H2O. The surface sterilized seed samples were planted on PDA medium to which rose Bengal (65 ppm) and dihydrostreptomycin (30 µg/ml) were added as bacteriostatic agent. Three replicate plates were used each containing 10 seeds. The plates were incubated at 28 °C ± 2 for 7–10 days. The growing moulds were isolated and purified. The isolated fungi were identified morphologically according to the methods of Raper and Fennell (1965). Molecular confirmation of the two selected KA producer strains, A. flavus HAk1 and A. oryzae HAk2 and their mutants, A. oryzae HAk2-M26 and A. oryzae HAk2, was conducted using ITS1/ITS4 primers, 5′-TCCGTAGGTGAACCTGCGG-3′ and 5′-TCCTCCGCTTATTGATATGC-3′, respectively (Henry et al. 2000). The retrieved sequences were deposited in GenBank under accession numbers, KR364881, KU052567, KU310584, and KU310585, for A. flavus HAk1, A. flavus HAk1-M2, A. oryzae HAk2, and A. oryzae HAk2-M26, respectively.
Culture media
The experimental strains were isolated and preserved on PDA medium (Sigma-Aldrich Company). Five different culture media were used for estimation of KA through this study. Czapek’s Dox medium (CD) (Oxoid, 1982) contains 3% sucrose, 0.3% NaNO3, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.05% KCl, 0.001% FeSO4·7H2O, and 0.5% yeast extract. Sucrose–yeast extract medium (YS) (Scott 1957) contains 4% sucrose and 2% yeast extract. Glucose peptone medium (LCG150) (Adriana and Takahashi 2014) contains 15% d-glucose, 0.5% bacteriological peptone, 0.1% K2HPO4, 0.05% MgSO4·7H2O, and 0.5% NaCl. Yeast peptone glucose medium (YPD) (Ausubel et al. 1994) contains 2% glucose, 2% peptone, and 1% yeast. Malt extract sucrose medium (MES) (Adriana and Takahashi 2014) contains 15% sucrose, 2% malt extract, and 0.05% MgSO4·7H2O. A set of triplicates of each sterilized medium were inoculated with 1 ml of 107 spore suspensions of each fungal strain.
Estimation of kojic acid in culture filtrates
All fungal isolates were inoculated into sterilized Czapek’s Dox liquid culture and incubated statically for 7 days at 30 °C. After incubation time, the culture broth was filtered and the supernatant was used for determination of KA. Qualitative determination of KA was carried out using silica gel F254 as stationary phase and toluene–ethyl acetate–formic acid (3:6:1) as mobile phase. The plate was sprayed with 1% ferric chloride solution and the color change was detected under UV lamp at 336. The quantitative determination was carried out according to the colorimetric method of Bentley (1957). 1 ml of fungal filtrate was mixed with 4 ml of 1% (W/V) freshly prepared FeCl3·6H2O. The optical density of the red–purple colored complex was determined spectrophotometrically at wavelength 500 nm (Milton Roy, Spectronic 20D). The reagent was added into uninoculated culture medium with the same ratio and used as control. Aqueous stock solutions of KA were freshly prepared by dissolving known weight of authentic solid material in deionized distilled water. Different concentrations were prepared and used as working standard solutions. Calibration curve was constructed and used for determination of KA.
Optimization of kojic acid production
Sterilized triplicate flasks, containing 50 ml of MES broth medium, were inoculated with 107 of freshly prepared spore suspension of 5 days old of either Aspergillus flavus or Aspergillus oryzae and incubated statically for different incubation periods (5, 7, 10, and 14 days) at different incubation temperatures (20, 30, 40, and 50 °C). At the end of each incubation period, the dry biomass and KA amount were estimated. To evaluate the optimum pH values for maximum production of KA, the pH of the MES broth medium was adjusted by Citrate phosphate buffer from pH 2 to pH 8 according to Malic and Singh (1980).
Mutagenesis of kojic acid producer strains
Spores suspension of both WT fungal strains (A. flavus HAk1 and A. oryzae HAk2) was washed twice with sterile distilled water and then re-suspended in sterile water to concentration of 106 ml−1. Spores suspension was irradiated by different doses (50, 100, 150, 200, and 250 Gy) of 60Co γ rays emitted by an Indian gamma rays through Indian gamma cell which is located at the National Center for Radiation Research and Technology (NCRRT), Nasr City, Cairo, Egypt. Serial dilution of the irradiated suspension was prepared for each fungal strain and inoculated separately into MES solid media containing 1% FeCl2. The inoculated plates were incubated for 5 days at 30 °C, and the mutagenic colonies were selected according to their morphological variation about control and according to the intensity of red–purple color of FeCl2-culture medium. Selected colonies from each fungal strain were inoculated into KA production medium and incubated for 7 days at 30 °C. The potentiality of selected mutants for KA production was determined quantitatively. Stability study for each strain has been tested for fourth generation according to Luthra et al. (2014). The most stable mutant (MT) of each fungal strain has been selected for our study. To determine the survival level, the number of growing colonies was counted. The number of colonies obtained from the non-irradiated conidia was considered to be 100% survival.
KA production using agricultural raw materials (SSF) and industrial by-products
Depending on our local resources, different agricultural raw materials including, wheat stalks, wheat bran, rice stalks, corn cob, rice straw, sugarcane bagasse, banana peel, pineapple peel, potato peel, orange peel, and apple peel, and industrial by-products, including sugar-beet molasses (SBM), sugarcane molasses (SCM), were selected as cheap sources for production of KA by WT and MT strains of A. oryzae and A. flavus. The agricultural raw materials were dried in oven at 60 °C, and then grinded and sieved. 20 g of each agricultural material were suspended in 100 ml distilled water containing 0.5% yeast extract (El-Kady et al. 2014). All flasks containing raw materials were sterilized for 20 min at 121 °C. The sterilized flasks were inoculated with 107 spore suspension of each WT and MT strain and incubated at the optimum conditions. After the incubation period, KA amounts were estimated and the fungal growth was observed.
Determination of antioxidant activity
The antioxidant activity was determined through the ability of KA to scavenge 1, 1-diphenyl-2-picryl-hydrazyl (DPPH). DPPH is a stable free radical with violet color which turns to yellow in the presence of antioxidant and scavenging agents. The percentage of radical scavenging activity was calculated according to Elmastas et al. (2007) using the following formula: radical scavenging (%) = [(A 0 − A 1/A 0) × 100]. A 0 is the absorbance of the control, whereas A 1 is the absorbance of the sample extracts.
Statistical analysis
The results have been expressed as ±standard deviation of mean (SD). The difference in means was compared using one-way analysis of variance (ANOVA) according to Duncan (1955).
Results and discussion
Fungal isolates and kojic acid production
Fifty-six fungal species belonging to three genera, Aspergillus, Penicillium, and Fusarium, were isolated from nine different Egyptian seeds and identified morphologically, as shown in Table 1. The class Ascomycetes comprise 53 fungal species related to two genera namely, Aspergillus and Penicillium, that include 42 and 7 species, respectively. The class of Deuteromycetes was represented by 3 fungal species of one genus, namely, Fusarium. It was observed that both Aspergillus and Penicillium contribute the greatest number of isolates. The potentiality of these fungal isolates to produce KA in their culture filtrate was tested. Qualitative detection of KA production by all fungal isolates was done by TLC analysis, as shown in Fig. 1. Current results showed that seven fungal species including A. flavus, A. oryzae, A. parasiticus, A. brevipes, and A. duricaulis have the ability to produce considerable amounts of KA in the culture medium. The highest amounts of KA (0.1591 and 0.0131 g/100 ml) were produced by A. flavus and A. oryzae, respectively. Several studies reported that various Aspergillus species such as A. oryzae, A. tamari, A. parasiticus, and A. flavus have the ability to produce large amount of KA in their metabolites (Rosfarizan et al. 2010; Abd El-Aziz 2013; El-Kady et al. 2014).
Table 1.
Survey on KA production by fungal isolates
| Fungal species | Screened strains | Number of KA producing isolates | Isolation source | Concentration of KA (g/100 ml) culture media | % KA producing strains |
|---|---|---|---|---|---|
| Aspergillus vesicolor | 2 | 0 | Lentils, cowpea | 0.00 | 0 |
| Aspergillus brevipes | 4 | 1 | Barely, beans, rice, cowpea | 0.005 | 25 |
| Aspergillus japonicus | 5 | 0 | Bean, rice | 0.00 | 0 |
| Aspergillus parasiticus | 2 | 1 | Bean | 0.01 | 50 |
| Aspergillus duricaulis | 3 | 2 | Bean, lentils, barely | 0.006–0.012 | 66.6 |
| Aspergillus candidus | 3 | 0 | Rice, bean | 0.00 | 0 |
| Aspergillus flavus | 6 | 2 | Lentils, rice, cowpea, bean, maize | 0.01–1591 | 33.3 |
| Aspergillus oryzae | 4 | 1 | Lentils, rice, barely | 0.0131 | 25 |
| Aspergillus fumigatus | 3 | 0 | Lentils, bean | 0.00 | 0 |
| Aspergillus aculeatus | 2 | 0 | Peanuts, cowpea | 0.00 | 0 |
| Aspergillus niger | 3 | 0 | Bean | 0.00 | 0 |
| Aspergillus viridi-nutans | 1 | 0 | Barely | 0.00 | 0 |
| Aspergillus terreus | 3 | 0 | Maize, rice | 0.00 | 0 |
| Aspergillus foetidus | 5 | 0 | Rice, barely, bean, maize | 0.00 | 0 |
| Penicillium notatum | 1 | 0 | Rice | 0.00 | 0 |
| Penicillium nalgiovense laxa | 1 | 0 | Lentils | 0.00 | 0 |
| Penicillium baarnense | 1 | 0 | Peanuts | 0.00 | 0 |
| Penicillium digitatum | 1 | 0 | Beans | 0.00 | 0 |
| Penicillium citrinum | 1 | 0 | Rice | 0.00 | 0 |
| Penicillium chrysogenum | 2 | 0 | Rice | 0.00 | 0 |
| Fusarium solani | 1 | 0 | Beans | 0.00 | 0 |
| Fusarium oxysporum | 2 | 0 | Peanuts, maize | 0.00 | 0 |
Fig. 1.
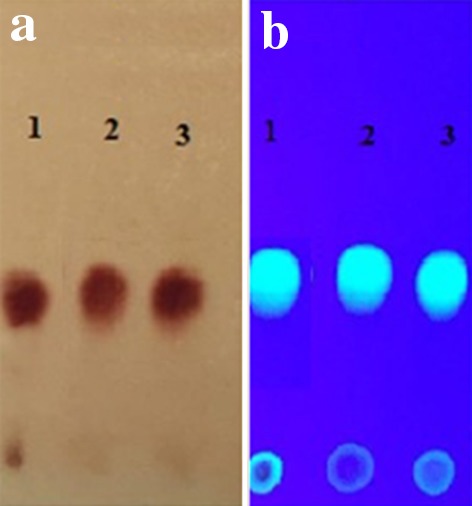
Thin layer chromatogram of KA extracted from fungal filtrate of A. oryzae (2) and A. flavus (3) under UV lamp in comparison with authentic KA(1), after spraying with ferric chloride (a) and before spraying by ferric chloride (b)
The morphological identification of the two highest KA producer strains was confirmed by molecular methods, based on the 18S–28S rRNA sequence (Henry et al. 2000) and nomenclature as A. flavus HAk1 and A. oryzae HAk2. Genomic DNA sequence of ITS1 and ITS2 regions was submitted in the GenBank under the accession numbers, KR364881 and KU052567 for A. flavus HAk1 and A. oryzae HAk2, respectively. Figure 2 shows the phylogenetic relationship of both Aspergillus strains with the nearest fungal strains deposited in the GenBank database. The alignment analysis confirms that our strains are A. flavus and A. oryzae where their similarity with another related species is 99–100%.
Fig. 2.
Phylogenetic relationship between the four KA producing fungal strains, A. flavus HAk1 (KR364881), A. flavus HAk1-M2 (KU052567), A. oryzae HAk2 (KU310584), and A. oryzae HAk2-M26 (KU310585), respectively, and the ITS sequences of closely related fungal strains retrieved from NCBI Gene Bank. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA6
Optimization of kojic acid production
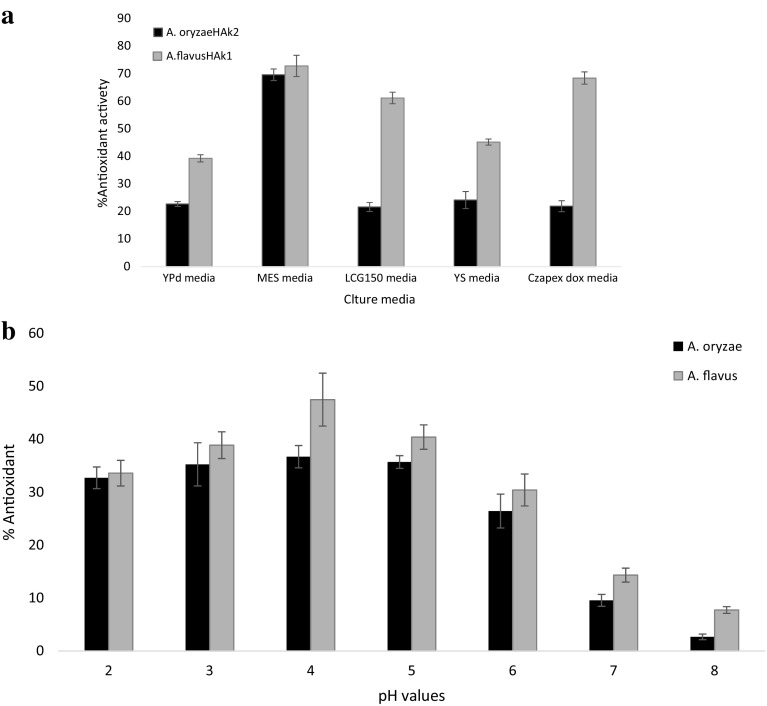
In general, the biotechnological production of industrial metabolites from different fungi is based on the adaptations of these microbes to their environment. Growth media and incubation conditions have a strong influence on KA production. To select the optimum conditions for maximum KA production by our isolates, the environmental factors were studied. Up till now, there has been no unanimity on which media are the optimal for KA production. Therefore, five different culture media, YPD, YS, LCG150, MES, and CD, were screened for KA production by the WT strains, A. oryzae HAk2, and A. flavus HAk1, as shown in Fig. 3. The MES broth proved to be the most suitable medium for maximum production of KA by A. flavus and A. oryzae, 0.6489 ± 0.03 g/100 ml and 0.1504 ± 0.03 g/100 ml, respectively. Our results indicate that KA producing isolates have the ability to convert sucrose of MES medium into glucose through the secretion of invertase enzyme in the culture medium. Glucose molecule is converted directly into KA without any additional cleavage of the carbon chain into smaller fragments. This indicates that the component of culture medium and type of sugars play a significant role in biosynthesis of KA by Aspergillus spp. Our results agree with Wan et al. (2004) and Rosfarizan and Ariff (2006), who reported that Aspergillus spp. has the ability to produce invertase enzyme for the hydrolysis of sucrose to glucose and fructose for subsequent transformation into KA. Rasmey et al. (2016) reported that glucose and sucrose followed by starch are most suitable sources for KA production, while no detected acid was produced in the presence of maltose and cellulose as carbon sources.
Fig. 3.
Kojic acid concentration (g/100 ml culture filtrate) of A. oryzae HAK2 and A. flavus HAk1 grown in different culture media
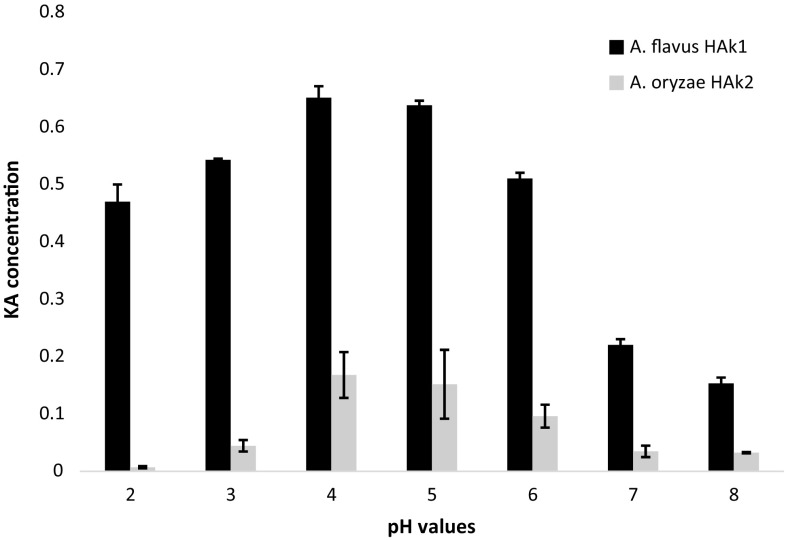
The pH of the medium determines the rate of growth and fermentation processes of microorganisms. KA production was significantly affected by pH value of the culture medium. The results showed a significant variation in KA production by the two experimental strains when the pH value of growing medium varied. Figure 4 shows that the acidic medium ranging from 4 to 5 was the best culture for maximum production of KA by the experimental WT strains. A negligible amount of KA (0.0071 ± 0.002 g/100 ml culture medium) was produced by A. oryzae HAK2 at pH 2 compared with KA amount produced by A. flavus HAK1 (0.542 ± 0.03 g/100 ml culture medium) at the same pH value. This variation between the two strains indicates that A. flavus is more tolerant to acidic medium and could produce a significant amount of KA. In general, most of fungi show an extensive growth and sporulation in weak acidic-to-neutral environment. Usually, the highest amounts of KA are produced in acidic medium with pH values ranged from 4 to 6 (Hassan et al. 2014; Durgadevi et al. 2015).
Fig. 4.
Kojic acid amounts produced by A. flavus HAk1 and A. oryzae HAk2 grown on SEM medium with different pH values
The yield of KA can be substantially increased by the optimization of environmental conditions. To select the best incubation conditions for maximum production of KA, the experimental organisms were inoculated into the KA producer medium, MES medium, and incubated for different incubation time at different temperature. The results presented in Table 2 showed that A. flavus HAk1 and A. oryzae HAk2 gave maximum amounts of KA (0.4557 ± 0.06 and 0.2275 ± 0.03 g/100 ml, respectively) after 7 and 10 days of incubation, respectively, at 30 °C. The result refers that optimum time for maximum KA production is ranged from 7 to 10 days depending on the producer strains. The differences in optimum KA production by different microorganisms may be ascribed to either condition of fermentation process or species differences (Mohamad et al. 2010). Significant amounts of KA were produced by A. flavus and A. oryzae after 8–12 days of incubation at 30 °C (Abd El-Aziz 2013, Hassan et al. 2014). Durgadevi et al. (2015) reported that the maximum production of KA was obtained at 28 °C using starch as a carbon source. Moreover, Rosfarizan et al. (2000) suggested that the cell bound enzyme system for KA synthesis was stable for prolonged incubation.
Table 2.
Optimization of KA production through selection of incubation temperature after different incubation time
| Incubation period (days) | Temperature (°C) | A. flavus HAk1 | A. oryzae HAk2 | ||||
|---|---|---|---|---|---|---|---|
| Dry weight (g/100 ml) culture medium | KA (g/100 ml) culture medium | % Antioxidant | Dry weight (g/100 ml) culture medium | KA (g/100 ml) culture medium | % Antioxidant | ||
| 5 | 20 | 0.9067 ± 0.03ab | 0.0466 ± 0.006ab | 39.36 ± 2.01 | 1.253 ± 0.06ab | 0.0397 ± 0.01ab | 34.12 ± 1.02 |
| 30 | 1.2933 ± 0.19ac | 0.1489 ± 0.04ac | 49.2 ± 1.89 | 1.36 ± 0.04ab | 0.1071 ± 0.009ad | 53.6 ± 1.91 | |
| 40 | 1.1133 ± 0.04ac | 0.1132 ± 0.04ab | 42 ± 2.68 | 0.92 ± 0.01ab | 0.0682 ± 0.007ac | 51.68 ± 3.02 | |
| 50 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0 | |
| 7 | 20 | 1.0876 ± 0.1b | 0.2005 ± 0.01bc | 52.4 ± 3.42 | 0.933 ± 0.18ab | 0.0359 ± 0.01ab | 48 ± 2.00 |
| 30 | 1.554 ± 0.05bc | 0.4557 ± 0.06c | 63.08 ± 3.21 | 1.366 ± 0.15ac | 0.1126 ± 0.03ad | 56.28 ± 2.22 | |
| 40 | 1.100 ± 0.17bc | 0.2722 ± 0.03bc | 53.96 ± 1.69 | 1.07 ± 0.03ab | 0.1019 ± 0.04ac | 39.16 ± 2.43 | |
| 50 | 0.00 ± 0.00ab | 0.00 ± 0.00a | 0.00 ± 0 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0 | |
| 10 | 20 | 1.1467 ± 0.09b | 0.1563 ± 0.06b | 61.92 ± 2.74 | 1.046 ± 0.11cb | 0.0566 ± 0.01b | 51.48 ± 1.89 |
| 30 | 1.160 ± 0.06abc | 0.2146 ± 0.07abc | 62.88 ± 2.35 | 1.54 ± 0.21c | 0.2275 ± 0.03bd | 62.48 ± 2.12 | |
| 40 | 1.30 ± 0.08abc | 0.1034 ± 0.01b | 48.88 ± 1.77 | 1.42 ± 0.04cb | 0.1513 ± 0.05bc | 51.4 ± 2.76 | |
| 50 | 0.00 ± 0.00ab | 0.00 ± 0.00a | 0.00 ± 0 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0 | |
| 14 | 20 | 1.16 ± 0.17b | 0.0694 ± 0.03ab | 48.6 ± 2.09 | 1.186 ± 0.25abc | 0.0543 ± 0.01ab | 46.8 ± 1.42 |
| 30 | 1.12 ± 0.02bc | 0.1403 ± 0.04c | 48.08 ± 2.99 | 1.44 ± 0.06abc | 0.1035 ± 0.05ad | 55.28 ± 3.2 | |
| 40 | 1.4467 ± 0.12bc | 0.0643 ± 0.01ab | 44.8 ± 0.89 | 1.246 ± 0.14abc | 0.0318 ± 0.04ac | 50.08 ± 2.06 | |
| 50 | 0.00 ± 0.00ab | 0.00 ± 0.00a | 0.00 ± 0 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0 | |
Calculated mean is for triplicate measurements from two independent experiments ±SD; the values followed by different letters vary significantly at p ≤ 0.05 (Duncan test, p ≤ 0.05)
Improvement of kojic acid production by mutagenesis
The use of mutation to improve many organisms for overproduction of industrial compounds has been strongly established for over 50 years and is still recognized as a valuable tool for strain improvement. To improve the production of KA in the culture medium, the two wild-type (WT) fungal isolates, A. oryzae HAk2 and A. flavus HAk1, were subjected to different doses of gamma radiation (50, 100, 150, 200, and 250 Gy). According to the intensity of color change of FeCl2 containing medium, 16 colonies of A. flavus HAk1 and A. oryzae HAk2, from the last dose, were selected for KA screening (Table 3). The selected mutants of A. oryzae HAK2 and A. flavus HAK1 were cultivated on MES medium and incubated at optimum conditions; then, KA was quantitatively estimated in the culture filtrate. The data in Table 3 revealed that the mutant isolates of A. oryzae HAk2, 26, 32, 24, 29, and 31 M, produced the maximum amounts of KA (0.3056 ± 0.005, 0.2764 ± 0.002, 0.2443 ± 0.004, 0.2063 ± 0.003, and 0.1968 ± 0.003 g/100 ml, respectively), while the MT isolates of A. flavus 2, 14, 3, 17, and 4 M produced the maximum concentrations of KA (0.8329 ± 0.002, 0.7847 ± 0.0005, 0.7605 ± 0.0005, 0.7047 ± 0.0005, and 0.6428 ± 0.0002 g/100 ml, respectively). These results indicate that gamma irradiation is an effective agent for strain mutagenesis and has a profound effect on improvement of A. flavus and A. oryzae strains for enhancement of KA production. Gamma ray may cause different mutations in the gene clusters which are responsible for KA biosynthesis leading to decrease or increase in KA production (Ellaiah et al. 2002). Abd El-Aziz (2013) investigated that gamma-mutated parent strain AFNS9 of A. flavus gave high amount of KA as compared with its parent strain.
Table 3.
Kojic acid concentration (g/100 ml) of mutants after exposer to 250 Gy of A. flavus HAK1 and A. oryzae HAk2
| A. flavus HAK1 | A. oryzae HAk2 | ||
|---|---|---|---|
| Mutant isolates | KA amounts (g/100 ml) culture media | Mutant isolates | KA amounts (g/100 ml) culture media |
| Control | 0.448 ± 0.03 | Control | 0.1504 ± 0.03 |
| 1 M | 0.0359 ± 0.0001 | 20 M | 0.069 ± 0.001 |
| 2 M | 0.8329 ± 0.002 | 21 M | 0.097 ± 0.002 |
| 3 M | 0.7605 ± 0.0005 | 22 M | 0.034 ± 0.004 |
| 4 M | 0.6428 ± 0.0002 | 23 M | 0.018 ± 0.001 |
| 5 M | 0.0434 ± 0.0004 | 24 M | 0.2443 ± 0.004 |
| 6 M | 0.0486 ± 0.0003 | 25 M | 0.0233 ± 0.001 |
| 7 M | 0.01 ± 0.001 | 26 M | 0.3056 ± 0.005 |
| 8 M | 0.0286 ± 0.0006 | 27 M | 0.025 ± 0.005 |
| 9 M | 0.0138 ± 0.005 | 28 M | 0.042 ± 0.002 |
| 10 M | 0.005 ± 0.0003 | 29 M | 0.2063 ± 0.003 |
| 11 M | 0.09 ± 0.03 | 30 M | 0.003 ± 0.001 |
| 12 M | 0.007 ± 0.002 | 31 M | 0.1968 ± 0.003 |
| 13 M | 0.14 ± 0.04 | 32 M | 0.2764 ± 0.002 |
| 14 M | 0.7847 ± 0.0005 | 33 M | 0.042 ± 0.002 |
| 15 M | 0.138 ± 0.0003 | 34 M | 0.031 ± 0.001 |
| 16 M | 0.131 ± 0.001 | 35 M | 0.033 ± 0.003 |
±SD means standard deviation of triplicate measurements from two independent experiments
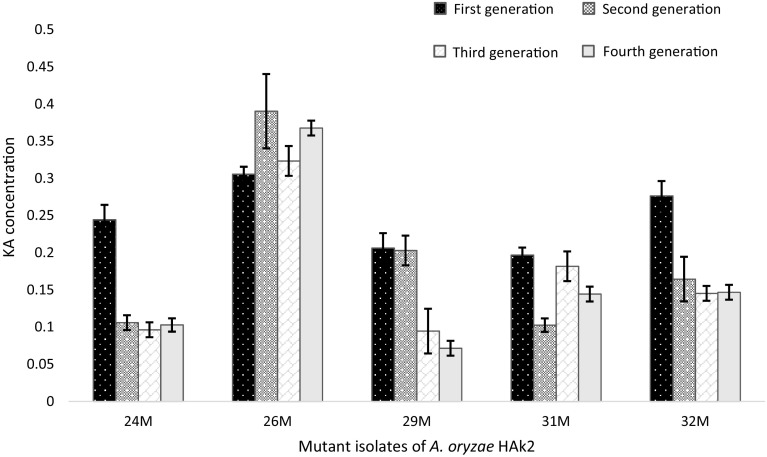
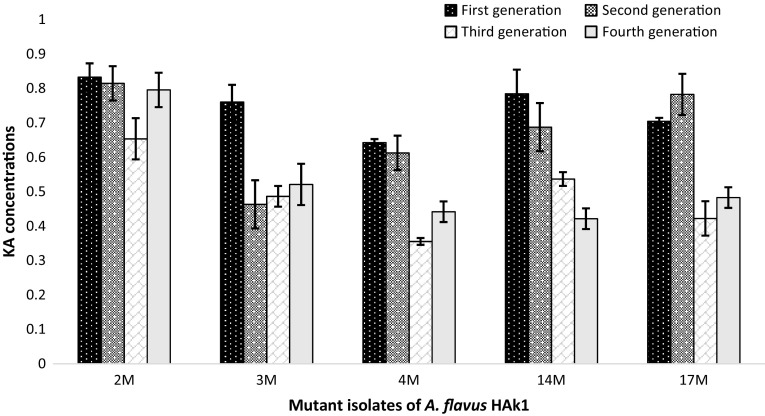
The isolated mutants were tested for their KA stability through four generations, as shown in Figs. 5 and 6. It was observed that the mutant strains M26 of A. oryzae HAk2 and M2 of A. flavus HAk1 gave constant amounts of KA through the four generations, whereas there was non-significant variation in KA amounts (p < 0.05) through these generations. MT strains A. oryzae HAk2-M26 and A. flavus HAk1-M2 produced KA with 2.03- and 1.9-fold increase compared to their parent strains. Therefore, they were selected as high producer strains for KA. The mutant strains were identified through ITS1, ITS4 sequencing analysis, and deposited in the GenBank under the accession numbers, KU052567 for A. flavus HAk1-M2 and KU310585 for A. oryzae HAk2-M26. From the alignment profile results, it was clear that the A. flavu HAk1-M2 and A. oryzae HAk2-M26 exhibited a 99–100% identity with WT A. flavus HAk1 and A. oryzae HAk2, respectively, and with the other related strains submitted in the GenBank. Phylogeny of both MT and WT strains showed in Fig. 2 indicates the closely relation between all KA producing strains.
Fig. 5.
Stability study of the MT isolates of A. oryzae HAk2 grown on SEM medium through four generations showing KA amounts (g/100 ml culture medium)
Fig. 6.
Stability study of the MT isolates of A. flavus HAk1 grown on SEM medium through four generations showing KA amounts (g/100 ml culture medium)
Kojic acid production by WT and MT strains of A. oryzae and A. flavus using agriculture raw materials through solid-state fermentation (SSF)
Fermentation of the agriculture raw materials by fungal strains, which cause pollution for environment, is considered an essential part for reduction of the industrial costs of KA synthesis. The ability of experimental mutagenic strains A. flavus HAk1-2M and A. oryzae HAk2-M26 for production of KA from such materials, in comparison with parent strains, was studied. A total of 11 agricultural by-products (wastes) were tested as cheapest solid substrates for KA production by experimental strains, including banana peel, pine apple peel, potato peel, orange peel, wheat bran, rice straw, rice stalk, wheat straw, bagasse, corn cob, and apple peel. The results presented in Tables 4 and 5 show that the raw materials, apple peel, and orange peel were the best raw materials for production of KA by WT strains A. flavus HAk1 and A. oryzae HAk2 (0.8282 ± 0.001 and 1.1786 ± 0.003 g/g dry matter, respectively), while orange peel and rice straw were the most suitable materials for maximum production of KA by MT strains A. flavus HAk1-M2 and A. oryzae HAk2-M26 (0.1892 ± 0.001 and 0.1601 ± 0.02 g/g dry, respectively). The minimum yield of KA by A. oryzae HAK2, A. oryzae HAk2-M26 and A. flavus HAk1-M2 was recorded by potato peel (0.0110 ± 0.001, 0.0103 ± 0.002, and 0.0109 ± 0.0002 g/g dry matter, respectively), while the minimum yield of KA by A. flavus HAK1 was recorded by rice straw (0.0064 ± 0.0002 g/100 g dry matter). All WT and MT strains failed to produce KA in the presence of banana peel. It has been observed that the KA production varied according to fungal strains and raw materials. There is no correlation between fungal growth and KA production as shown in Tables 4 and 5. These results prove that KA biosynthesis depends on the capability of fungal strain to convert lignocellulosic wastes into fermentable sugars and on the type of sugars produced from this process, not on the intensity of fungal growth. The degradation of lignocellulosic wastes is accomplished by different types of hydrolytic enzymes which known as cellulases. The initial conversion of these compounds into fermentable sugars is the key factor controlling KA production. The previous results about the variation of KA biosynthesizing from different agriculture raw materials have been recorded by some authors. Chaudhary et al. (2014) reported that the use of mutated strains of A. flavus showed good yield of KA using potato, sago, and corn starch. On the other hand, El-Kady et al. (2014) used different agro-industrial raw materials including molasses, rice fragments, kidney bean, and carrot for KA production by A. flavus. An observed change in the productivity pattern of the mutant strains, A. flavus HA1 and A. oryzae HA2, compared with their parent strains was detected through this experiment. The mutant strains produced lower amount of KA in the presence of some agricultural raw materials such as pine peel, potato peel, and apple peel than WT strains. The variation in their productivity may result in the effect of γ-radiation on one or more genes encoding cellulase enzymes. The mutation of these genes may lead to inactivate some of hydrolytic enzymes, which consequently affects the type of sugars resulting from fermentation process. The releasing sugars significantly affect the KA production by the experimental mutant strains. The previous studies recorded that the amounts of KA excreted in the culture medium depend significantly on the type of fermentable sugars consumed by fungal strains (Rasmey et al. 2016). The differences between both mutant strains in their productivity of KA from raw materials, either with increase or decrease about WT strain, may result in difference in their ability to recover the mutated genes. Wang et al. (2006) and El-Tablawy (2014) reported that γ-radiation inactivates some enzymes required for fermentation of lignocellulosic materials such as lipoxygenase, polyphenol oxidase, peroxidase, and cellulose.
Table 4.
KA concentration (g/g dry matter) and antioxidant percentage of A. oryzae HAk2 and A. oryzae HAk2-M26 grown on different raw materials
| Raw materials | A. oryzae HAk2 | A. oryzae HAk2-M26 | ||||
|---|---|---|---|---|---|---|
| Growth | KA (g/g dry matter) | % Antioxidant | Growth | KA (g/g dry matter) | % Antioxidant | |
| Banana peel | + | 0.00 ± 0.00a | 0.00 ± 0.00 | + | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Pine apple peel | ++ | 0.4011 ± 0.09e | 70.12 ± 3.87 | + | 0.0966 ± 0.001d | 55.79 ± 1.01 |
| Potato peel | +++ | 0.0110 ± 0.001a | 41.16 ± 1.82 | ++ | 0.0103 ± 0.002ab | 35.16 ± 1.89 |
| Orange peel | +++ | 0.2986 ± 0.02d | 74.74 ± 3.14 | + | 0.1055 ± 0.009d | 69.05 ± 2.25 |
| Wheat bran | +++ | 0.2218 ± 0.02c | 69.47 ± 2.79 | +++ | 0.0203 ± 0.002ab | 50.63 ± 1.86 |
| Rice straw | +++ | 0.00 ± 0.00a | 0.00 ± 0.00 | +++ | 0.0268 ± 0.001b | 53.68 ± 1.59 |
| Rice stalk | + | 0.00 ± 0.00a | 0.00 ± 0.00 | + | 0.1601 ± 0.02e | 75.14 ± 3.29 |
| Wheat straw | ++ | 0.00 ± 0.00a | 0.00 ± 0.00 | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Bagasse | ++ | 0.1396 ± 0.01b | 49.68 ± 2.68 | ++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Corn cob | + | 0.0179 ± 0.0003a | 34.42 ± 1.39 | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Apple peel | +++ | 0.8282 ± 0.001f | 92.74 ± 2.79 | +++ | 0.0575 ± 0.03c | 52.63 ± 3.98 |
Calculated mean is for triplicate measurements from two independent experiments ±SD; the values followed by different letters vary significantly at p ≤ 0.05 (Duncan test, p ≤ 0.05). Plus sign indicates fungal growth; progressive increase in plus sign indicates more fungal growth
Table 5.
Kojic acid concentration (g/g dry matter) and antioxidant percent of A. flavus HAk1 and A. flavus HAk1-M2 strains grown on different raw materials
| Raw materials | A. flavus HAK1 | A. flavus HAk1-M2 | ||||
|---|---|---|---|---|---|---|
| Growth | KA (g/g dry matter) | % Antioxidant | Growth | KA (g/g dry matter) | % Antioxidant | |
| Banana peel | + | 0.000 ± 0.00a | 0.00 | + | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Pine apple peel | + | 0.0196 ± 0.001b | 72.74 ± 1.21 | + | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Potato peel | +++ | 0.0127 ± 0.001ab | 54.32 ± 0.98 | ++ | 0.0109 ± 0.0002b | 56.32 ± 2.22 |
| Orange peel | +++ | 0.0418 ± 0.0007c | 75.36 ± 1.51 | ++ | 0.1892 ± 0.001e | 86.84 ± 2.91 |
| Wheat bran | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 | + | 0.0197 ± 0.01c | 63.68 ± 3.01 |
| Rice straw | +++ | 0.0064 ± 0.0002ab | 12.16 ± 0.09 | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Rice stalk | + | 0.000 ± 0.00a | 0.00 ± 0.00 | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Wheat straw | ++ | 0.000 ± 0.00a | 0.00 ± 0.00 | +++ | 0.0126 ± 0.001c | 58.42 ± 2.54 |
| Corn cob | +++ | 0.4109 ± 0.02d | 85.26 ± 1.90 | +++ | 0.000 ± 0.00a | 0.00 ± 0.00 |
| Baggase | ++ | 0.000 ± 0.00a | 0.00 ± 0.00 | ++ | 0.0110 ± 0.002b | 58.95 ± 3.10 |
| Apple peel | +++ | 1.1786 ± 0.003e | 95.03 ± 2.49 | +++ | 0.1021 ± 0.001d | 69.32 ± 1.73 |
Calculated mean is for triplicate measurements from two independent experiments ±SD; the values followed by different letters vary significantly at p ≤ 0.05 (Duncan test, p ≤ 0.05). Plus sign indicates fungal growth; progressive increase in plus sign indicates more fungal growth
Kojic acid production from sugarcane molasses (SCM) and sugar-beet molasses (SBM) by WT and MT strains of Aspergillus spp.
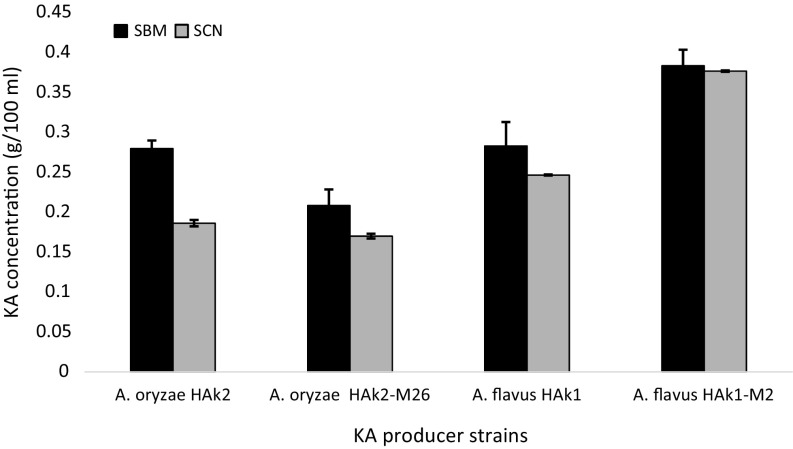
The screenings of KA production from SCM and SBM by WT and MT of experimental strains were shown in Fig. 7. The results show that A. flavus HAk1-M2 was the highest producer strain for KA from SBM and SCN (0.383 and 0.376 g/100 ml culture medium, respectively). SBM was more suitable substrate than SCM for KA production by all tested strains. The high production of KA from molasses can be due to the composition of molasses. Approximately, 52% of Egyptian molasses are glucose, sucrose, and fructose, while 0.46% are total nitrogen in addition to some vitamins such as riboflavin and thiamin (Khalifa 2003). Bioconversion of molasses into industrial products by different Aspergillus species was recorded by various authors (Shetty 2015; El-Kady et al. 2014). Gad (2003) agrees with our results, who found that beet molasses was suitable source for KA production by A. parasiticus. Abd El-Aziz (2013) revealed that the medium which contains potato starch and molasses produces a higher concentration of KA than that of sugarcane bagasse and barely.
Fig. 7.
Kojic acid production (g/100 ml culture filtrate) by WT and MT strains of A. flavus HAk1 and A. oryzae growing on SCM and SBM. Data are shown as the mean ± SD of triplicate measurements
Antioxidant activity of kojic acid produced by A. flavus and A. oryzae grown under different environmental and nutritional conditions
Although KA has many potential industrial applications, no previous study on its antioxidant application has been investigated. In this study, the antioxidant activity of KA produced by our strains was tested by their scavenging effect on DPPH radicals under all cultural conditions. Our results revealed that the antioxidant activity of KA extract produced by A. flavus HAk1 and A. oryzae HAk2 was increased with increasing of KA in the fungal filtrate. The maximum antioxidant activity of KA extract produced by A. oryzae HAk2 and A. flavus HAk1 was 69.63 and 72.85%, respectively, when they grew on MES medium, and were 36.703% and 47.48, respectively, when they grew on culture medium adjusted to pH value 4 (Fig. 8 a and b, respectively). The highest antioxidant activity (63.08 and 62.48%) was recorded when A. flavus HAk1 and A. oryzae HAk2 were incubated at 30 °C for 7 and 10 days, respectively (Table 2). The antioxidant activity of fungal filtrate of both MT and WT strains was recorded when they grew on different agriculture raw materials (Tables 4, 5). Maximum percentage of antioxidant by fungal extract of A. oryzae HAk2 and A. flavus HAk1 was 92.74 and 95.03%, respectively, when they grew on Apple peel, while the minimum antioxidant percentage were 41.16 and 12.16% when they grew on potato peel and rice stalk, respectively. On the other hand, the maximum antioxidant percentage by fungal extract of A. flavus HAk1-M2 and A. oryzae HAk2-M26 were 86.84 and 75.14% when grew on orange peel and potato peel, respectively. The minimum antioxidant percentage by fungal extract of MT strains A. flavus HAk1-M2 and A. oryzae HAk2-M26 was 56.32% and 35.16, respectively, when they grew on potato peel material. There is no antioxidant activity in the culture filtrate free from KA at all tested conditions. These results indicate that the antioxidant percentage is correlated with amounts of KA in the culture medium. KA is a good chelator of transition metal ions and a good scavenger of free radical DPPH. Moreover, KA has potential activity in depigmentation processes through chelating the copper ion presenting in the active site of tyrosinase, which mediates the formation of melanin from the amino acid tyrosine (Gonçalez et al. 2013). The potential antioxidant activity of KA confirms its application in food industry, where it has been used in post-harvest process as an anti-speck and an anti-browning agent for agricultural product (Chaudhary et al. 2014). The antioxidant activity of kojic dipalmitate was previously studied by Gonçalez et al. (2015).
Fig. 8.
a Antioxidant activity of KA produced by A. flavus HAk1 and A. oryzae HAk2 grown in different culture media. b Antioxidant activity of KA produced by A. flavus HAk1 and A. oryzae HAk2 grown in MES culture medium with different pH values
From this study, we conclude that the highest amounts of KA can be obtained when fungal strains were incubated at 30 °C for 7–10 days and grown on MES culture medium adjusted to pH 4. KA can be significantly increased by mutagenesis of A. flavus and A. oryzae. Various raw materials such as apple peel, potato peel, and rice straw can be used as cheapest substrate for highly significant production of KA instead of using chemical synthetic medium. The mutation of KA producer strains is a crucial step in improvement of KA production. WT and MT strains of A. flavus were the highest strains for KA production compared with WT and MT strains of A. oryzae at all tested conditions. The biosynthesizing KA has the ability to scavenge the free radicals and show intensive antioxidant activity which increases according to the optimum conditions for KA production. This is the first study that focused on the overproduction of KA by mutant strains of A. flavus and A. oryzae using different cheapest raw materials and on the evaluation of antioxidant activity of KA under different environmental conditions.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abd El-Aziz BA. Improvement of kojic acid production by a mutant strain of Aspergillus flavus. J Nat Sci Res. 2013;3(4):31–41. [Google Scholar]
- Adriana APB, Takahashi JA. Modulation of antimicrobial metabolites production by the fungus Aspergillus parasiticus. Braz J Microbiol. 2014;45(1):313–321. doi: 10.1590/S1517-83822014000100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current protocols in molecular biology. Current Protocols, Brooklyn
- Barnard D, Challenger F (1949) The formation of kojic acid from ethyl alcohol by A. oryzae and the action of this mould on some carbohydrate derivatives. J Chem Soc 110–117. doi:10.1039/JR9490000110
- Bentley R. Preparation and analysis of kojic acid. In: Colowick SP, Kaplan NO, editors. Methods enzymology. New York: Academic press; 1957. pp. 238–241. [Google Scholar]
- Chaudhary J, Pathak AN, Lakhawat S. Production technology and applications of Kojic acid. Annu Res Rev Biol. 2014;4(21):3165–3196. doi: 10.9734/ARRB/2014/10643. [DOI] [Google Scholar]
- Chen JS, Wei CI, Rolle RS, Balaban MO, Otwell SW, Marshall MR. Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases. J Agric Food Chem. 1991;39:1396–1401. doi: 10.1021/jf00008a008. [DOI] [Google Scholar]
- Douglas MC, Glenn DC (1982) Foods and food production encyclopedia. Van nostrand Reinhold, New York, p 1941
- Duncan DB. Multiple ranges and multiple F test. Biometrics. 1955;11:1–41. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Durgadevi KB, Vijayalakshmi P, Shilpa V, Prasad VSSLT, Kumar BV. Response surface methodology for the optimization of kojic acid production by Aspergillus flavus using Muntingia calabura fruits as a carbon source. Indian J Sci Technol. 2015;8(6):556–561. doi: 10.17485/ijst/2015/v8i6/67049. [DOI] [Google Scholar]
- El-Kady IA, Zohri AN, Hamed SR. Kojic acid production from agro-industrial by-products using fungi. Biotechnol Res Int. 2014 doi: 10.1155/2014/642385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaiah P, Prabhakar T, Ramakrishna B, Taleb AT, Adinarayana KM. Strain improvement of Aspergillus niger for the production of lipase. Indian J Microbiol. 2002;42:151–153. [Google Scholar]
- Elmastas M, Isildak O, Turkekul I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Comp Anal. 2007;20(3–4):337–345. doi: 10.1016/j.jfca.2006.07.003. [DOI] [Google Scholar]
- El-Tablawy SY. Reduction of some enzymes produced by irradiated fungal strains isolated from certain medicinal plants. J Natl Sci Res. 2014;4(2):30–37. [Google Scholar]
- Gad AS. Modification of molasses for kojic acid production by Aspergillus parasiticus. Egypt J Microbiol. 2003;5:14–26. [Google Scholar]
- Gonçalez ML, Marcussi DJ, Calixto GM, Corrêa MA, Chorilli M. (2013) Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed Res Int 1–9. doi:10.1155/2013/271276 [DOI] [PMC free article] [PubMed]
- Gonçalez ML, Marcussi DG, Calixto G, Correa AC, Chorilli M (2015) Structural characterization and in vitro antioxidant activity of kojic dipalmitate loaded W/O/W multiple emulsions intended to skin disorders. Biomed Res Int 1–8. doi:10.1155/2015/304591 [DOI] [PMC free article] [PubMed]
- Hassan HM, Saad AM, Hazzam MM, Ibrahim EI. Optimization study for the production of kojic acid crystals by Aspergillus oryzae var. effusus NRC 14 isolate. Int J Curr Microbiol App Sci. 2014;3(10):133–142. [Google Scholar]
- Hazra B, Biwas S, Mandal Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Peter CI, Steven HH. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J Clin Microbiol. 2000;38(4):1510–1515. doi: 10.1128/jcm.38.4.1510-1515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn V, Linder P, Zakin V. Effect of kojic acid on the oxidation of o-dihydroxyphenols by mushroom tyrosinase. J Agric Food Chem. 1995;18:253–271. [Google Scholar]
- Khalifa MS (2003) Studies on production of single cell protein from vinasse. M.S. thesis, Sugar Technology Research Institute, Assiut University, Assiut, Egypt
- Luthra U, Khadpeka S, Trivedi, Kumar, Singh N, Tripathi Induced mutation by gamma radiation of Penicillium brevicompactum to enhance production of mycophenolic acid. IJAST. 2014;4:952–957. [Google Scholar]
- Malic CP, Singh MB (1980) Extraction and estimation of amino acids and keto acids. In: Hana L (ed) Plant enzymology and histoenzymology. Kalyani Publishers, New Delhi, p 257
- Mohamad R, Mohamed MS, Suhaili N, Salleh MM, Ariff AB. Kojic acid: applications and development of fermentation process for production. Biotechnol Mol Biol Rev. 2010;5(2):24–37. [Google Scholar]
- Nandan R, Polasa H. Inhibition of growth of kojic acid biosynthesis in Aspergillus by some chlorinated hydrocarbons. Indian J Microbiol. 1985;25:21–25. [Google Scholar]
- Noh JM, Kwak SY, Seo HS, Seo JH, Kim BG, Lee YS. Kojic acid-amino acid conjugate as tyrosinase inhibitors. Bioorg Med Chem Lett. 2009;19:5586–5589. doi: 10.1016/j.bmcl.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Nurashikin S, Rusley EZ, Husaini A. Solid-state bio conversion of pineapple residues into kojic acid by Aspergillus flavus: a prospective study. World Acad Sci Eng Technol. 2013;7(8):825–827. [Google Scholar]
- Ohyama Y, Mishima Y. Melanosis-inhibitory effect of kojic acid and its action mechanism. Fragr J. 1990;6:53–58. [Google Scholar]
- Oxoid L. The oxoid manual of culture media ingredients and other laboratory services. 5. England: Turnergraphic. Ltd; 1982. [Google Scholar]
- Prabhakar M, Lingappa K, Vivek B, Amena S, Vishalakshi N, Mahesh D. Characterization of physical factors for optimum lovastatin production by Aspergillus terreus klvb28mu21 under solid state fermentation. J Adv Appl Sci. 2011;27:1–5. [Google Scholar]
- Raper KB, Fennell DI. The genus Aspergillus. Baltimore: Williams & Wilkins; 1965. [Google Scholar]
- Rasmey AM, Aya H, Basha AH. Isolation and screening of kojic acid producing isolate of Aspergillus oryzae potentially applicable for production from sugarcane molasses. Int J Biol Res. 2016;4(2):119–128. doi: 10.14419/ijbr.v4i2.6434. [DOI] [Google Scholar]
- Rosfarizan M, Ariff AB. Kinetics of kojic acid fermentation by Aspergillus flavus link S44-1 using sucrose as a carbon source under different pH conditions. Biotechnol Bioprocess Eng. 2006;11:72–79. doi: 10.1007/BF02931872. [DOI] [Google Scholar]
- Rosfarizan M, Madihah S, Ariff AB. Isolation of kojic acid-producing fungus capable of using starch as a carbon source. Lett Appl Microbiol. 1998;26:27–30. doi: 10.1046/j.1472-765X.1998.00263.x. [DOI] [Google Scholar]
- Rosfarizan M, Ariff AB, Hassan MA, Karim MIA. Influence of pH on kojic acid fermentation by Aspergillus flavus. Pak J Biol Sci. 2000;3(6):977–982. doi: 10.3923/pjbs.2000.977.982. [DOI] [Google Scholar]
- Rosfarizan M, Mohamed MS, Nurashikin S, Saleh MM, Ariff AB. Kojic acid: applications and development of fermentation for production. Biotechnol Mol Biol. 2010;5(2):24–37. [Google Scholar]
- Roy AK, Kumarin V. Aflatoxin and citrinin in seeds of some medicinal plants under storage. Int J Pharmacogn. 1991;29:62–65. doi: 10.3109/13880209109082851. [DOI] [Google Scholar]
- Scott WJ. Water relation of food spoilage microorganisms. Adv Food Resour. 1957;7:83–127. doi: 10.1016/S0065-2628(08)60247-5. [DOI] [Google Scholar]
- Shetty VG. Production and optimization of citric acid by Aspergillus niger using molasses and corncob. Int J Pharm Pharm Sci. 2015;7(5):152–157. [Google Scholar]
- Wan HM, Chen CC, Chang TS, Giridhar RN, Wu WT. Combining induced mutation and protoplasting for strain improvement of Aspergillus oryzae for KA production. Biotechnol Lett. 2004;26:1163–1166. doi: 10.1023/B:BILE.0000035490.49252.38. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma Y, Zhao G, Liao X, Chen F, Wu J, Chen J, Hu X. Influence of gamma irradiation on enzyme, microorganism and flavor of cantaloupe (Cucumis mello L.) juice. J Food Sci. 2006;71(6):215–220. doi: 10.1111/j.1750-3841.2006.00097.x. [DOI] [Google Scholar]