Abstract
Introduction
The aim of this study was to confirm the efficacy of patient-driven titration of BIAsp 30 in terms of glycemic control, by comparing it to physician-driven titration of BIAsp 30, in patients with type 2 diabetes in North Africa, the Middle East, and Asia.
Methods
A 20-week, open-label, randomized, two-armed, parallel-group, multicenter study in Egypt, Indonesia, Morocco, Saudi Arabia, and Vietnam. Patients (n = 155) with type 2 diabetes inadequately controlled using neutral protamine Hagedorn (NPH) insulin were randomized to either patient-driven or physician-driven BIAsp 30 titration.
Results
The noninferiority of patient-driven compared to physician-driven titration with respect to the reduction in HbA1c was confirmed. The estimated mean change in HbA1c from baseline to week 20 was −1.27% in the patient-driven arm and −1.04% in the physician-driven arm, with an estimated treatment difference of −0.23% (95% confidence interval: −0.54; 0.08). After 20 weeks of treatment, the proportions of patients achieving the target of HbA1c <7.5% were similar between titration arms; the proportions of patients achieving the target of ≤6.5% were also similar. Both titration algorithms were well tolerated, and hypoglycemic episode rates were similar in both arms.
Conclusion
Patient-driven titration of BIAsp 30 can be as effective and safe as physician-driven titration in non-Western populations. Overall, the switch from NPH insulin to BIAsp 30 was well tolerated in both titration arms and led to improved glycemic control. A limitation of the study was the relatively small number of patients recruited in each country.
Clinical trial registration: ClinicalTrials.gov NCT01589653.
Funding
Novo Nordisk A/S, Denmark.
Keywords: Biphasic insulin aspart 30, HbA1c, NPH insulin, Titration
Introduction
The prevalence of type 2 diabetes is increasing worldwide, but the rate of increase is particularly dramatic in non-Western countries that are experiencing rapid socioeconomic growth and urbanization, along with the associated changes in diet and life expectancy. Some 8.8% of adults worldwide are estimated to currently have diabetes, with type 2 diabetes constituting 95% of the burden [1]. The prevalence of diabetes is currently highest in Saudi Arabia (17.6%), Egypt (16.7%), Bahrain (15.6%), UAE (14.6%), and Kuwait (14.3%) [1]. By 2040, the prevalence of diabetes is forecast to increase such that it will affect 10% of the global population, placing growing pressure on healthcare resources and imposing a large economic burden [1].
The aim of type 2 diabetes management is to minimize long-term complications, including micro- and macrovascular diseases, while avoiding any unwanted effects of treatment, such as hypoglycemia and weight gain. Current guidelines, supported by data from the UK Prospective Diabetes Study, recommend intensive blood glucose (BG) control to lower HbA1c below a target of 7.0% in most patients [2–4].
Type 2 diabetes is a progressive disease that requires continuous intensification and optimization of treatment to achieve and maintain HbA1c targets. Intensification strategies depend on an individual’s characteristics, needs, and preferences, but most patients will eventually require insulin therapy due to the loss of beta-cell function [2, 5, 6]. Basal insulin, such as long-acting insulin analogs or intermediate-acting neutral protamine Hagedorn (NPH) insulin, are commonly used as initial insulin therapies. When basal insulin fails to provide adequate glycemic control, the addition of rapid-acting mealtime insulin can be considered (basal–bolus), with progressive escalation in the number of injections [2]. A premixed insulin regimen, providing both rapid-acting and intermediate-acting components in one formulation, can also be used to initiate or intensify insulin treatment [3, 7], and is widely used as the initial insulin therapy in African, Middle Eastern, and South-East Asian regions [1, 8–10].
Biphasic insulin aspart 30 (BIAsp 30: NovoMix 30) is a premix of insulin aspart (IAsp) in which 30% is soluble short-acting IAsp and the remaining 70% is an intermediate-acting protamine cocrystallized IAsp [11]. BIAsp 30 provides prandial insulin coverage while also offering adequate basal coverage, and can improve glycemic control in patients with type 2 diabetes previously inadequately controlled with basal insulins [12].
Despite detailed guidelines on the use of insulin therapies, for many patients, achieving and maintaining HbA1c targets in routine clinical care represents a major challenge [13]. Regional data indicate that only 16.4% of patients with type 2 diabetes in Egypt, 26.8% of patients in Morocco, 27.0% of patients in Saudi Arabia, and 32.2% of patients in Indonesia achieve HbA1c targets <7% [8, 10, 14, 15]. There are many patient and physician barriers to insulin initiation and intensification, including a lack of education and awareness, and concerns about hypoglycemia and weight gain [16]. Additional regional and cultural barriers exist in developing countries. In the Middle East, Africa, and South-East Asia in particular, there is often a gap between guideline-driven and actual diabetes care practices, and access to healthcare can be limited due to limited insurance coverage, low physician:population ratios, rural and inaccessible communities, and cost [17–19].
The titration of insulin doses over time is needed to achieve and maintain glycemic targets. Currently, many patients with type 2 diabetes have their insulin doses adjusted by physicians in a primary care setting [20]. Dose adjustment is a time-consuming process, and additional barriers include the need for regular visits to clinics, the cost of glucose-testing strips, and the lack of education and experience of primary healthcare providers in titration.
Self-monitoring of BG and self-titration of insulin dose based on predefined algorithms (with clinical oversight) is well established in type 1 diabetes, and similar therapeutic self-management of various insulin regimens has been shown to be feasible in patients with type 2 diabetes [6, 21–23]. A therapy protocol that is easily taught and self-titrated may allow more patients to initiate and optimize insulin therapy, thus reducing follow-up clinic visits and telephone calls. Premixed insulin analogs such as BIAsp 30 are simple and convenient to use, require fewer injections than basal–bolus treatment, and have relatively predictable time–action profiles that offer the potential for patients to effectively adjust their daily dose [24, 25].
The aim of this study was to confirm the efficacy of patient-driven titration of BIAsp 30 in terms of glycemic control, by comparing it to physician-driven titration of BIAsp 30, in patients with type 2 diabetes inadequately controlled with NPH insulin in North Africa, Asia, and the Middle East using a noninferiority approach.
Methods
Patients
Patients were enrolled at 18 sites across Egypt, Indonesia, Morocco, Saudi Arabia, and Vietnam between May 2012 and July 2015. Eligible patients were aged ≥18 years, had a diagnosis of type 2 diabetes for at least 12 months before screening, were currently being treated with NPH insulin (for at least 3 months) in combination with a stable total daily dose of at least 1500 mg or a maximum tolerated dose (minimum 1000 mg) of metformin for at least 2 months (with the exception of Indonesia, where the recommended daily dose was 750 mg) with or without additional oral antidiabetic drug (OAD) treatment, had HbA1c ≥7.0% (≥53.0 mmol/mol) and ≤10% (≤85.8 mmol/mol), and had a body mass index (BMI) of ≤40.0 kg/m2.
Key exclusion criteria included treatment with any thiazolidinedione, glucagon-like peptide-1 receptor agonists, and/or pramlintide within the 3 months before screening; treatment with more than 1 IU/kg NPH insulin daily; previous use of premixed or bolus insulins; more than one severe hypoglycemic episode during the previous 12 months; impaired kidney or hepatic function; or proliferative retinopathy or maculopathy requiring treatment.
Study Design
This was an open-label, randomized, two-armed, parallel-group, multicenter trial consisting of a 2-week screening period, a 4-week training period, and a maintenance period of 16 weeks. Patients were randomized 1:1 to receive patient-driven or physician-driven titration of BIAsp 30 twice daily (BID). All patients discontinued their previous NPH insulin and OADs, except for their pre-trial metformin. The dose and regimen of metformin was not changed throughout the trial period. Patients had a starting BIAsp 30 dose equivalent to their previous NPH insulin dose split into two equal doses, administered subcutaneously via FlexPen® (Novo Nordisk, Bagsvaerd, Denmark) immediately before breakfast and immediately before dinner. Titration of BIAsp 30 in both arms was performed according to the titration protocol recommended by the BIAsp 30 summary of product characteristics [26], and was based on self-measured plasma glucose (SMPG) values measured twice daily on three preceding days (Table 1). Patients were provided with BG meters and plasma-calibrated BG test strips to record SMPG. All measurements performed with capillary blood were automatically calibrated to equivalent plasma glucose (PG) values. BIAsp 30 dose adjustments were made once a week in the training period and every second week in the maintenance period. Participants in the patient-driven arm had three clinic visits after randomization (weeks 4, 12, and 20) and telephone contact whenever deemed necessary. Participants in the physician-driven arm had six clinic visits (weeks 2, 4, 8, 12, 16, and 20) and telephone contact 1 week after the previous visit if one of the doses was changed, and at any other time if deemed necessary.
Table 1.
Protocol for physician-driven and patient-driven titration of BIAsp 30
| SMPG value | BIAsp 30 dose adjustment (U)a | |
|---|---|---|
| mmol/L | mg/dL | |
| <4.4 | <80 | −2 |
| 4.4–6.1 | 80–110 | 0 |
| 6.2–7.8 | 111–140 | +2 |
| 7.9–10 | 141–180 | +4 |
| >10 | >180 | +6 |
BIAsp dose adjustment was based on the lowest SMPG measured before breakfast and before dinner in the preceding 3 days. The lowest SMPG value before breakfast was used to adjust the following dinner dose and the lowest SMPG value before dinner was used to adjust the following breakfast dose
SMPG self-measured plasma glucose, U units
aNo increase in dose if hypoglycemia [≤3.9 mmol/L (70 mg/dL)] occurred in the preceding 3 days
The titration algorithms, efficacy and safety assessments, and statistical analysis methods used in this study were the same as those described for another study in a previous publication [27].
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent to be included in the study was obtained from all patients. The study protocol was approved by the Ministry of Health of Egypt, as well as by the Ethics Committee of the Faculty of Medicine at Alexandria University, Alexandria, Egypt. The ClinicalTrials.gov clinical trial identifier number is NCT01589653.
Efficacy and Safety Assessments
The primary endpoint was change in HbA1c from baseline to week 20. Secondary supportive endpoints included: proportion of patients achieving the American Diabetes Association (ADA) target of HbA1c <7.0% and the HbA1c target of ≤6.5% after 20 weeks of treatment with and without severe and minor hypoglycemic episodes during the last 12 weeks of the trial; change in fasting plasma glucose (FPG) levels from baseline; a 7-point SMPG profile (PG values measured before and 90 min after the start of breakfast, lunch, and main evening meal, and prior to bedtime); time to PG target using 2-point SMPG profile [first time PG values before breakfast and before dinner both reached 4.4–6.1 mmol/L (80–110 mg/dL)]; patient satisfaction assessed by Treatment-Related Impact Measures for Diabetes (TRIM-D) scores [28]; and assessment of healthcare resource utilization (clinic visits, telephone calls to the clinic, and BG strips).
Safety and tolerability were assessed in terms of treatment-emergent hypoglycemic episodes and treatment-emergent adverse events (AEs). A hypoglycemic episode was defined as treatment-emergent if the onset occurred after the first administration of BIAsp 30 and no later than the last day of administration within the trial. An AE was defined as treatment-emergent if the onset occurred on or after the first day of BIAsp 30 administration and no later than 7 days after the last day.
Minor hypoglycemic episodes were defined as an episode with symptoms consistent with hypoglycemia [as confirmed by PG <3.1 mmol/L (<56 mg/dL) or full BG value <2.8 mmol/L (<50 mg/dL)] which was handled by the patient himself/herself, or any asymptomatic PG value <3.1 mmol/L (56 mg/dL) or full BG value <2.8 mmol/L (<50 mg/dL). Severe hypoglycemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Documented symptomatic hypoglycemia was defined as an episode during which typical symptoms of hypoglycemia are accompanied by a measured PG concentration of ≤3.9 mmol/L (≤70 mg/dL). Asymptomatic hypoglycemia was defined as an episode not accompanied by typical symptoms of hypoglycemia, but with a measured PG concentration of ≤3.9 mmol/L (≤70 mg/dL). Probable symptomatic hypoglycemia was defined as an episode during which symptoms of hypoglycemia are not accompanied by a PG determination [but the episode was presumably caused by a PG concentration of ≤3.9 mmol/L (≤70 mg/dL)]. Relative hypoglycemia was defined as an episode during which the person with diabetes reports any of the typical symptoms of hypoglycemia and interprets those as indicative of hypoglycemia, but with a measured PG concentration of >3.9 mmol/L (>70 mg/dL) [29]. An episode with an onset time between 00:00 and 05:59 (inclusive of both) was defined as a nocturnal episode.
Statistical Analysis
The analysis sets were defined in accordance with ICH-E9 guidance [30]. The full analysis set (FAS) included all randomized patients, and the statistical evaluation of the FAS followed the intention-to-treat (ITT) principle and patients contributed to the evaluation “as randomized.” Missing values were imputed using the last observation carried forward (LOCF) method. The per protocol (PP) analysis set included all patients who were exposed to patient-driven titration or physician-driven titration for more than 12 weeks and had a valid assessment for deriving the primary endpoint without any major protocol violations that could affect the primary endpoint. The safety analysis set (SAS) included all patients who received at least one dose of BIAsp 30. Patients in the PP set and the SAS contributed to the evaluation “as treated.”
Sample size calculation was determined using a t statistic under the assumption of a one-sided test of size 2.5% and a zero mean treatment difference in HbA1c between treatment arms (primary endpoint). The minimum sample size required to meet the primary endpoint with at least 80% power using the PP analysis set was 162 with an assumed standard deviation (SD) of 0.9%. Based on an expected exclusion of 10%, a randomization of 180 patients was planned. Despite the recruitment period being extended, the study was prematurely terminated as the required numbers of eligible patients were not available.
The primary endpoint of the study was change in HbA1c from baseline to week 20, analyzed using a normal linear regression model with treatment, stratum (metformin monotherapy versus metformin + additional OAD therapy), and country as factors, and baseline HbA1c as covariate. Patient-driven titration was considered noninferior to physician-driven titration if the upper boundary of the two-sided 95% confidence interval (CI) for the difference in change from baseline in HbA1c after 20 weeks of treatment between titration arms was ≤0.4%.
Sensitivity analyses were performed with all available HbA1c measurements taken post-randomization at scheduled measurement times using a mixed model for repeated measurements (MMRM). The model included treatment, time, treatment-by-time interaction, baseline HbA1c, stratum, and region as fixed factors, and subject as a random effect. Based on this model, treatment differences were estimated after 4, 12, and 20 weeks.
The proportions of patients achieving the target of HbA1c <7.0% and ≤6.5% after 20 weeks of treatment with and without hypoglycemic episodes (severe and documented symptomatic episodes, and severe and minor episodes) were analyzed separately based on a logistic regression model using treatment, strata, and country as factors and baseline HbA1c as covariate.
Change from baseline in FPG after 20 weeks of treatment was analyzed using a normal linear regression model using treatment, stratum, and country as factors, and baseline FPG as covariate.
A mixed-effect model was fitted to the 7-point SMPG profile data at week 20. The model included treatment, time, interaction between treatment and time, stratum, and country as fixed factors, and patient as a random effect.
The prandial PG increment for each meal was derived from the 7-point SMPG profile as the difference between PG values available before a meal and 90 min after a meal. Prandial PG increments for each meal were analyzed using a normal linear regression model, with treatment, stratum, and country as factors, and the corresponding baseline value as covariate.
Time to reach PG target was assessed using the 2-point SMPG profile. The event-free survival endpoint was analyzed using a Cox proportional hazards model including treatment, stratum, and country as factors. Subjects who were lost to follow-up without meeting the target and subjects who never met the target during treatment were censored at the last day of treatment.
The PRO questionnaire included TRIM-D, which was scored as an overall score, as well as five subscale scores. After recording the 20 required items, the scores were transformed to a 0–100 scale, with higher scores indicating a better health state than lower scores. Each of the subscale scores and the overall score were analyzed separately using a normal linear regression model including treatment, stratum, and country as factors, and the corresponding baseline score as covariate.
The number of hypoglycemic episodes was analyzed using a negative binomial regression model with a log-link function, offset by the logarithm of the time period in which a hypoglycemic episode was considered treatment-emergent. The model included treatment, stratum, and country as factors.
Change from baseline in body weight after 20 weeks of treatment was analyzed using a normal linear regression model, with treatment, stratum, and country as factors, and baseline weight as covariate.
Results
Patients and Physicians
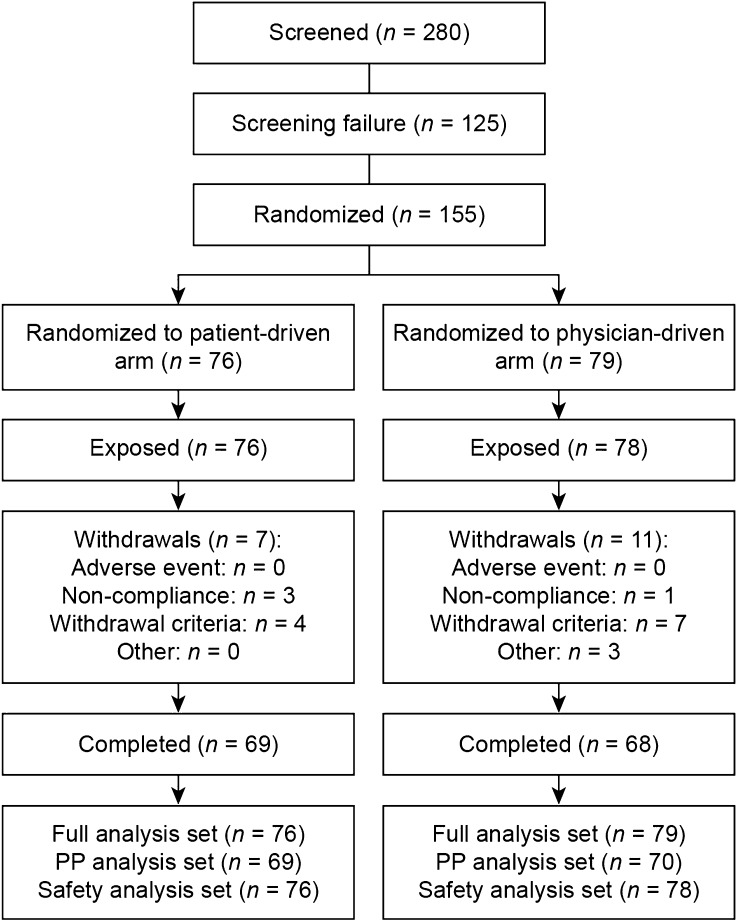
Of the 280 patients screened, 155 were randomized, among whom 88.4% completed the study (Fig. 1). There were 125 screening failures; 84 patients did not meet the HbA1c inclusion criteria (≥7% and ≤10%) and 26 patients were lost after a study site was discontinued. The dropout rates were 9.2% (seven of 76 patients) and 13.9% (11 of 79 patients), respectively, for the patient-driven and physician-driven arms. Baseline characteristics were comparable across titration arms (Table 2). Overall, mean age was 54.7 years, diabetes duration was 9.5 years, HbA1c was 8.6%, and BMI was 29.0 kg/m2, with males comprising 25.2% of the study enrollment. At screening, 16.1% of patients had diabetic neuropathy, 5.2% had macroangiopathy, 3.2% had diabetic retinopathy, and 3.2% had diabetic nephropathy.
Fig. 1.
Patient enrollment, randomization, and analysis. PP per protocol
Table 2.
Demographics and baseline characteristics of randomized patients switching from an NPH insulin regimen to BIAsp 30 BID
| Patient-driven titration (n = 76) |
Physician-driven titration (n = 79) |
|
|---|---|---|
| Age, years | 54.4 (10.2) | 54.9 (9.8) |
| Gender M/F, n (%) | 16/60 (21.1/78.9) | 23/56 (29.1/70.9) |
| Country | ||
| Egypt, n (%) | 18 (23.7) | 22 (27.8) |
| Indonesia, n (%) | 14 (18.4) | 18 (22.8) |
| Morocco, n (%) | 21 (27.6) | 22 (27.8) |
| Saudi Arabia, n (%) | 10 (13.2) | 7 (8.9) |
| Vietnam, n (%) | 13 (17.1) | 10 (12.7) |
| BMI, kg/m2 | 29.2 (4.9) | 28.9 (4.9) |
| Duration of diabetes, years | 8.7 (5.1) | 10.3 (6.5) |
| HbA1c, % | 8.5 (0.88) | 8.7 (0.78) |
| FPG | ||
| mmol/L | 8.7 (3.3) | 8.2 (3.0) |
| mg/dL | 156.8 (59.0) | 148.5 (54.2) |
| Stratum, n (%) | ||
| Metformin | 32 (42.1) | 35 (44.3) |
| Metformin + additional OADs | 44 (57.9) | 44 (55.7) |
| Diabetic nephropathy, n (%) | 5 (6.6) | 0 (0.0) |
| Diabetic neuropathy, n (%) | 10 (13.2) | 15 (19.0) |
| Diabetic retinopathy, n (%) | 2 (2.6) | 3 (3.8) |
| Macroangiopathy, n (%) | 4 (5.3) | 4 (5.1) |
Data are mean (SD) unless stated otherwise
BID twice daily, BMI body mass index, FPG fasting plasma glucose, M/F male/female, NPH neutral protamine Hagedorn, OAD oral antidiabetic drug
In the physician-driven arm, dose adjustment was performed by general physicians (n = 2), specialists (n = 21), and internists (n = 15).
Efficacy
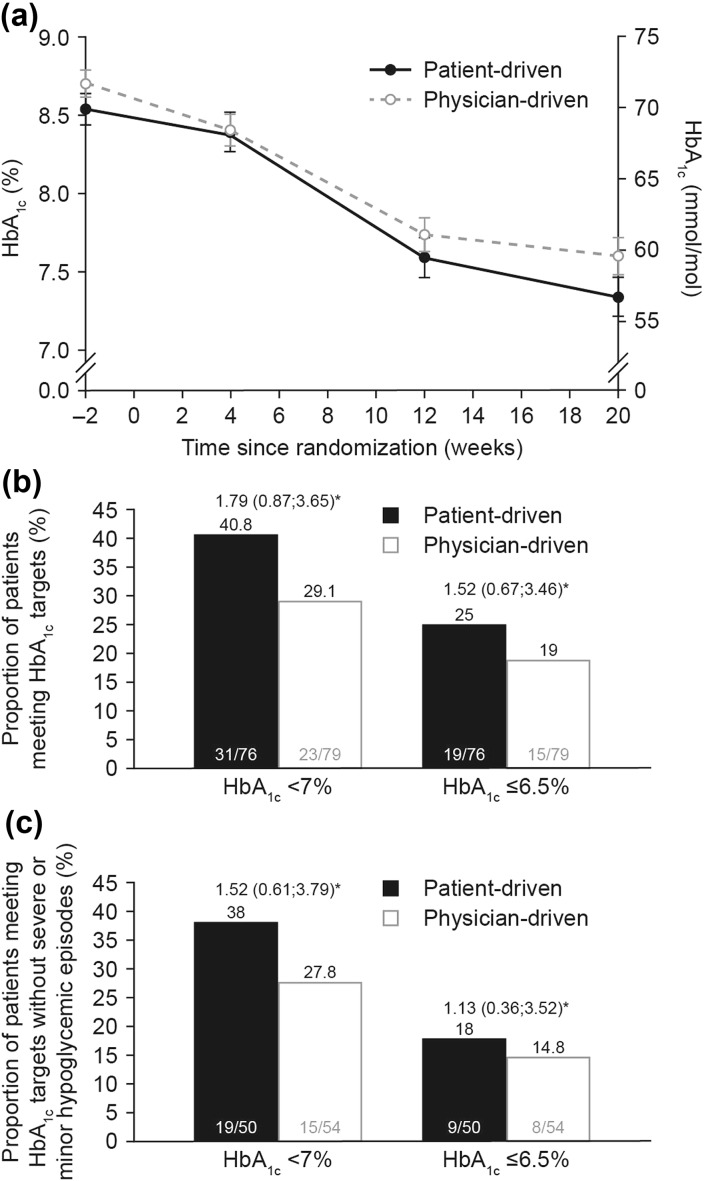
Observed mean HbA1c decreased throughout the study in both titration arms (Fig. 2a). The reduction in HbA1c for the FAS was numerically higher in the first 4 weeks of treatment in the physician-driven than in the patient-driven arm.
Fig. 2.
a Mean (±SE) HbA1c levels (LOCF) over time; b observed proportions of patients who had HbA1c levels (LOCF) of <7% or ≤6.5% at week 20; c observed proportions of patients reaching HbA1c targets (LOCF) without severe and minor hypoglycemic episodes at week 20. Data from FAS. *Estimated odds ratio (95% CI) for patient-driven versus physician-driven titration. Logistic regression with treatment, stratum, region, and baseline HbA1c as explanatory variables. Minor hypoglycemic episodes were defined as episodes with symptoms consistent with hypoglycemia as confirmed by PG <3.1 mmol/L (<56 mg/dL) or full BG value <2.8 mmol/L (<50 mg/dL) and handled by the patient himself/herself, or any asymptomatic PG value <3.1 mmol/L (56 mg/dL) or full BG value <2.8 mmol/L (<50 mg/dL). Severe hypoglycemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. BG blood glucose, CI confidence interval, FAS full analysis set, LOCF last observation carried forward, PG plasma glucose, SE standard error
The estimated mean [standard error (SE)] reduction in HbA1c from baseline to week 20 was −1.27 (0.11)% and −1.04 (0.11)% in the patient-driven and physician-driven arms, respectively, for the FAS, with an estimated treatment difference (ETD; patient-driven minus physician-driven) of −0.23% (95% CI −0.54; 0.08). Noninferiority of patient-driven compared to physician-driven titration with respect to reduction in HbA1c was confirmed for the FAS, as the upper bound of the two-sided 95% CI was ≤0.4%. Repeating the primary analysis on the PP analysis set gave similar results. The MMRM sensitivity analysis supported the findings from the primary analysis, with an ETD of −0.15% (95% CI −0.49; 0.19) after 20 weeks of treatment.
More patients reached the HbA1c target levels of <7.0% (<53.0 mmol/mol) and ≤6.5% (≤47.5 mmol/mol) in the patient-driven compared to the physician-driven titration arm at week 20 (Fig. 2b). Furthermore, there were more patients who reached target HbA1c levels of <7.0% (<53.0 mmol/mol) or ≤6.5% (≤7.5 mmol/mol) without severe or minor hypoglycemic episodes in the patient-driven than in the physician-driven arm at week 20 (Fig. 2c).
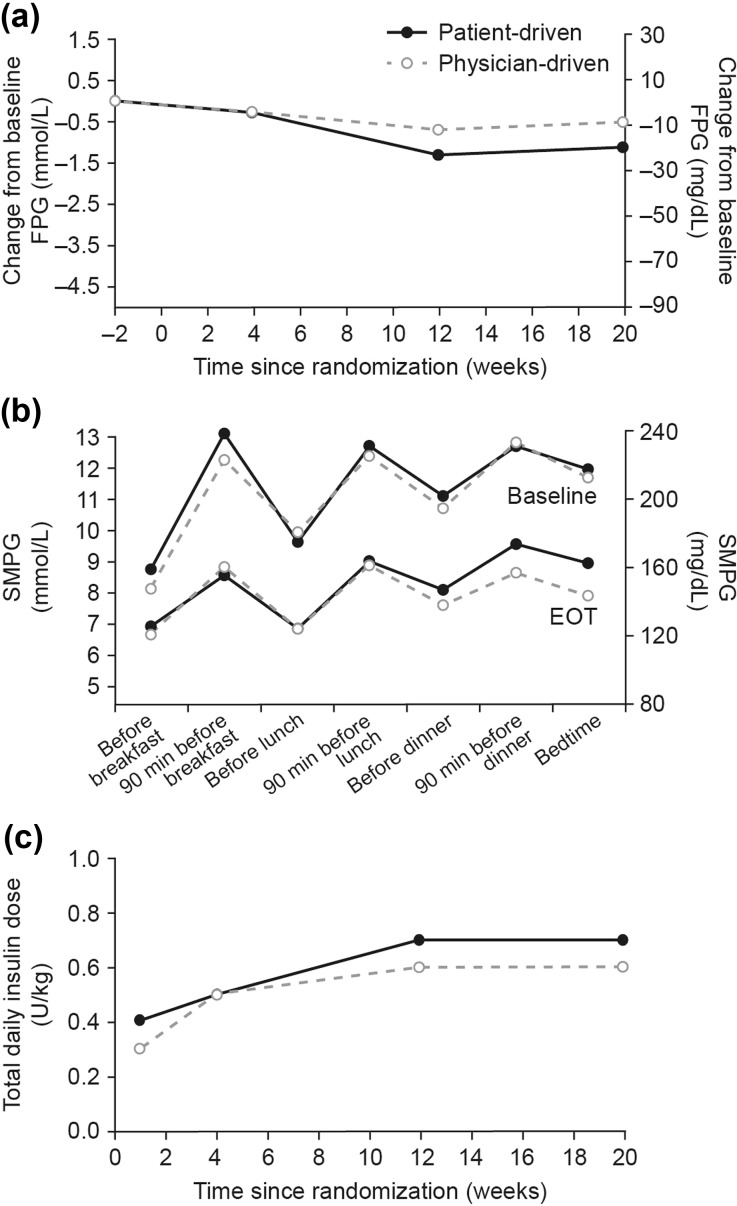
FPG decreased in both titration arms throughout the study (Fig. 3a). The estimated mean (SE) FPG change from baseline to week 20 was −0.95 (0.28); −17.04 (5.10) mg/dL in the patient-driven and −0.67 (0.28); −12.05 (5.10) mg/dL in the physician-driven arm, with an ETD between titration arms of −0.28 mmol/L (95% CI −1.07; 0.52); −4.99 mg/dL (95% CI −19.32; 9.34).
Fig. 3.
a Mean change from baseline FPG (LOCF); b 7-point SMPG profiles from baseline and after 20 weeks of treatment (EOT); c total daily insulin dose. Data from FAS. EOT end of trial, FPG fasting plasma glucose, LOCF last observation carried forward, SMPG self-measured plasma glucose
Treatment with BIAsp 30 decreased the 7-point SMPG profile measurements from baseline in both the patient- and the physician-driven arms [ETD in overall profile: 0.2 mmol/L (95% Cl −0.3; 0.7); 4.3 mg/dL (95% CI −4.72;13.37)] (Fig. 3b). After 20 weeks of treatment, the observed mean prandial PG increments were similar for the two arms at breakfast, lunch, and dinner. The observed mean increment across all meals was 1.8 mmol/L (33.3 mg/dL) in the patient-driven arm and 2.0 mmol/L (35.1 mg/dL) in the physician-driven arm.
By the end of the study, 57.9% of the patients in the patient-driven arm and 70.9% of the patients in the physician-driven arm had reached the 2-point SMPG target. Time to first reach the 2-point SMPG target was also similar in both arms [14.4 weeks (95% CI 12.3; 19.9) for the patient-driven arm and 13.9 weeks (95% CI 10.1; 14.1) for the physician-driven arm].
Patient-Reported Outcomes and Healthcare Utilization
Total and individual domain TRIM-D questionnaire scores in the titration arms were similar. The total mean TRIM-D score increased from 66.9 to 75.1 out of 100 in the patient-driven arm and from 65.9 to 72.5 out of 100 in the physician-driven arm.
In accordance with the trial design, clinic visits to healthcare professionals were less frequent in the patient-driven arm [mean (SD) 4.8 (0.65) visits/patient] than in the physician-driven arm [7.5 (1.42) visits/patient], and the mean (SD) total number of BG strips used per patient during the 20-week study was moderately higher in the patient-driven arm [37.5 (15.22) per patient] compared with the physician-driven arm [31.3 (20.33) per patient].
BIAsp Dose
The majority of subjects included in the SAS in the patient-driven arm (84.2%) and physician-driven arm (75.9%) were exposed to BIAsp 30 for >20 weeks, with no apparent differences in exposure time between arms (27.45 and 28.13 subject-years in the patient-driven and physician-driven arms, respectively). The mean total daily dose of BIAsp 30 was numerically higher in the patient- versus the physician-driven arm throughout the study. After 20 weeks of treatment, the mean (SD) total daily insulin dose in the FAS was 0.7 (0.31) U/kg in the patient-driven arm and 0.6 (0.23) U/kg in the physician-driven arm (Fig. 3c).
Safety
Of the 154 participants included in the SAS, 77 experienced one or more treatment-emergent hypoglycemic episodes during the 20 weeks of treatment (Table 3). The rate of hypoglycemic episodes was 608.4 versus 789.2 per 100 patient-years of exposure in the patient- and physician-driven arms, respectively (Table 3). There were only three episodes of severe hypoglycemia: two episodes reported by one participant in the patient-driven arm and one episode in the physician-driven arm. The estimated rate ratio (RR) between patient-driven and physician-driven titration was 0.74 (95% CI 0.44; 1.23) for overall ADA-classified treatment-emergent hypoglycemic episodes, 0.66 (95% CI 0.32; 1.34) for severe or minor episodes, and 1.07 (95% CI 0.46; 2.49) and 0.82 (95% CI 0.27; 2.53) for nocturnal and severe or minor nocturnal episodes, respectively.
Table 3.
Summary of treatment-emergent hypoglycemic episodes by ADA classification
| ADA classification | Patient-driven titration (n = 76) |
Physician-driven titration (n = 79) |
|---|---|---|
| All | 36 (47.4) [167] {608.4} | 41 (52.6) [222] {789.2} |
| Severe | 1 (1.3) [2] {7.3} | 1 (1.3) [1] {3.6} |
| Documented symptomatic | 23 (30.3) [90] {327.9} | 31 (39.7) [120] {426.6} |
| Relative | 7 (9.2) [9] {32.8} | 14 (17.9) [18] {64} |
| Probable symptomatic | 7 (9.2) [18] {65.6} | 5 (6.4) [9] {32.0} |
| Asymptomatic | 12 (15.8) [23] {83.8} | 13 (16.7) [26] {92.4} |
| Unclassifiable | 9 (11.8) [25] {91.1} | 16 (20.5) [48] {170.6} |
Data are shown as n (%) [E] {rate}
% percentage of subjects, ADA American Diabetes Association, E number of episodes, n number of subjects, rate rate per 100 patient-years of exposure
Patients gained weight in both titration arms. The mean absolute change in body weight (SD) was 1.4 (3.5) kg in the patient-driven arm and 1.9 (4.1) kg in the physician-driven arm [ETD −0.26 kg (95% CI −1.45; 0.93)].
The overall rate of treatment-emergent AEs was numerically higher in the patient-driven than in the physician-driven arm (324.2 vs. 302.2 events per 100 patient-years of exposure) (Table 4). Five serious AEs were reported in the physician-driven arm and all were judged as “unlikely to be related to trial product” by an investigator.
Table 4.
Summary of treatment-emergent AEs
| Patient-driven titration (n = 76) |
Physician-driven titration (n = 79) |
|
|---|---|---|
| All | 28 (36.8) [89] {324.2} | 37 (47.4) [85] {302.2} |
| Serious | 0 (0.0) [0] {0} | 5 (6.4) [5] {17.8} |
| Severe | 2 (2.6) [2] {7.3} | 4 (5.1) [4] {14.2} |
| Moderate | 14 (18.4) [20] {72.9} | 12 (15.4) [15] {53.3} |
| Mild | 22 (28.9) [67] {244.1} | 31 (39.7) [66] {234.6} |
| Possibly related to trial product | 0 (0.0) [0] {0} | 0 (0.0) [0] {0} |
| Probably related to trial product | 2 (2.6) [2] {7.3} | 1 (1.3) [2] {7.1} |
| Unlikely to be related to trial product | 28 (36.8) [87] {316.9} | 37 (47.4) [83] {295.1} |
Data are shown as n (%) [E] {rate}
% percentage of subjects, ADA American Diabetes Association, AE adverse event, E number of episodes, n number of subjects, rate rates per 100 patient-years of exposure
No safety issues were observed in the assessment of vital signs, physical examinations, or laboratory tests.
Discussion
This multicenter, randomized controlled trial (RCT) showed that, after switching from NPH insulin to BIAsp 30, patient-driven titration was noninferior to physician-driven titration with respect to change in HbA1c from baseline after 20 weeks of treatment. It is important to note that a key limitation of this study was the low number of randomized patients. Recruitment of patients was prematurely terminated due to the small number of eligible patients receiving treatment with NPH insulin at the study sites. However, the results of this study are in line with previous RCTs that have reported self-titration of insulin to be effective in improving glycemic control in patients with type 2 diabetes [6, 21–23]. For example, the ATLAS study reported that patient-driven insulin glargine titration was noninferior to physician-driven titration with near-target glycemic control in Asian patients with type 2 diabetes uncontrolled with oral glucose-lowering drugs [31, 32].
In this study, the reduction in HbA1c was numerically greater in the first 4 weeks of treatment in the physician-driven than in the patient-driven arm. However, after 12 weeks, the reduction in HbA1c was comparable between the arms. In addition, the uptitration of insulin dose was more aggressive in the physician-driven arm in the first 4 weeks of the study. This observation indicates that, initially, patients were learning how to titrate using the algorithm and, when comfortable, were able to achieve expected glycemic control. Other outcomes of efficacy (FPG, PG increments, and SMPG profiles) appear to be comparable between titration arms, further suggesting that patient-driven titration can be as successful as physician-driven titration. Patient satisfaction, as measured by TRIM-D scores, was also similar in both titration arms.
The numerically greater reduction in HbA1c from baseline to week 20 in the patient-driven arm may be due to the more aggressive titration of the insulin dose by patients compared to that by physicians in the maintenance period of the study. However, the higher end of the treatment insulin dose and the reduction in HbA1c were not achieved at the expense of increased hypoglycemic episodes, indicating that, with adequate training, patient-driven titration of BIAsp 30 does not raise additional safety concerns.
This is the first RCT to demonstrate that patient-driven titration was as effective as physician-driven titration in achieving recommended HbA1c targets in patients switching from NPH insulin to BIAsp 30 in non-Western countries. A small real-world study in a Dutch clinical practice has shown that patients with type 2 diabetes who switch from previous insulin regimens, including NPH insulin, to BIAsp 30 and are willing to self-titrate can improve glycemic control [33].
Previous RCTs have demonstrated noninferiority of patient-driven versus physician-drive titration of BIAsp 30 but in patient populations different from the current study. The INITIATEplus trial enrolled insulin-naïve patients with type 2 diabetes poorly controlled on OADs [20], and another study enrolled Chinese patients switching from premixed/self‐mixed human insulin to BIAsp 30 [34]. In contrast, the SimpleMix trial, conducted in Argentina, China, India, Poland, and the UK in patients with type 2 diabetes inadequately controlled on basal insulin analogs, could not confirm the noninferiority of patient-driven versus investigator-driven titration of BIAsp [27].
In the current study, glycemic control was improved after switching from NPH insulin to BIAsp 30 in both arms, highlighting the value of insulin intensification in patients with type 2 diabetes. Modern insulin analogs have the advantage of a lower risk of hypoglycemia and weight gain, as well as more predictable insulin action, compared with traditional human insulin formulations [35]. Additionally, premixed insulin analogs offer the benefit of being simple to use, with fewer injections, and being similarly efficacious and safe to a basal–bolus regimen [36, 37].
With increasing numbers of patients with diabetes and limited healthcare resources, especially in non-Western countries, there will be a greater need for patients to self-manage their disease. In this study, fewer clinic visits were made per person in the patient-driven than in the physician-driven arm, suggesting that self-titration reduces healthcare utilization. In a similar trial in a Chinese population, patient-driven titration of BIAsp 30 was associated with less healthcare utilization and lower direct and nondirect costs compared to physician-driven titration, without compromising glycemic control [38].
Key issues when deciding whether the patient or a physician should titrate insulin and in the success of patient-driven titration are the patients’ ability and motivation to manage their disease. This is influenced by factors such as age, socioeconomic status, health literacy and numeracy, and cultural aspects of healthcare in different countries. Poor health literacy and numeracy are associated with a low level of disease knowledge, fewer self-management behaviors, difficulty understanding titration instructions, and poorer diabetes outcomes [6]. Disease empowerment and education play significant roles in improving self-care behavior, quality of life, and outcomes in diabetes patients [39, 40]. This study demonstrates that, after a 4-week training period, patients in non-Western countries are able to effectively and safely self-titrate BIAsp 30 using a predefined algorithm.
Conclusions
This was the first randomized study to demonstrate that patient-driven titration of BIAsp 30 BID can be at least as effective and safe as physician-driven titration of BIAsp 30 BID in non-Western patients with type 2 diabetes that is no longer controlled with NPH insulin.
Acknowledgements
The study was funded by Novo Nordisk A/S, Denmark. Article processing charges were funded by Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc.
Medical writing and submission support were provided by Helen Parker and Helen Marshall of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk A/S.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
We thank all the patients, investigators, and trial site staff members who were involved in the conduct of this trial.
Disclosures
Anil Shinde is an employee of Novo Nordisk. Balasubramanian Lakshmivenkataraman is an employee of Novo Nordisk. Abdelmjid Chraibi received honoraria from Novo Nordisk to perform this study and reports speaker fees and conference grants from Novo Nordisk outside of the submitted work. Shoorook Al-Herz received honoraria to perform this study. Djoko W. Soeatmadji was an employee of Bwarijaya University, received honoraria from Novo Nordisk to perform this study, and reports speaker fees and conference grants from Novo Nordisk outside of the submitted work. Samir H. Assaad-Khalil and Bich Dao Nguyen have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent to be included in the study was obtained from all patients. The study protocol was approved by the Ministry of Health of Egypt, as well as by the Ethics Committee of the Faculty of Medicine at Alexandria University, Alexandria, Egypt.
Data Availability
The datasets obtained during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D848F060741D0377.
An erratum to this article is available at https://doi.org/10.1007/s13300-017-0275-2.
Change history
6/20/2017
An erratum to this article has been published.
References
- 1.International Diabetes Federation . IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S1–109.
- 3.IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation; 2012. www.idf.org. Accessed Aug 2016.
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed]
- 5.Colagiuri S, Dickinson S, Girgis S, Colagiuri R. National evidence based guideline for blood glucose control in type 2 diabetes. Canberra: Diabetes Australia and the NHMRC; 2009. www.diabetesaustralia.com.au/For-Health-Professionals/Diabetes-National-Guidelines/. Accessed Feb 23, 2017.
- 6.Khunti K, Davies MJ, Kalra S. Self-titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary care. Diabetes Obes Metab. 2013;15(8):690–700. doi: 10.1111/dom.12053. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Assaad-Khalil SH, Zaki A, Guindy MAS, Megallaa MH. Assessment of patient characteristics, metabolic control and therapeutic management of diabetic patients in Egypt: results from the cross-sectional wave-2006 of the International Diabetes Management Practices Study (IDMPS) J Egypt Soc Endocrinol Metab Diabetes. 2011;43(2):3–14. [Google Scholar]
- 9.Al-shaqha WM, Al-Janabi FA, Chaudhary AA, Alkharfy KM. Insulin prescribing practices in Saudi Arabia. World J Pharm Sci. 2015;4(2):29–40. [Google Scholar]
- 10.Chadli A, El Aziz S, El Ansari N, Ajdi F, Seqat M, Latrech H, Belmejdoub G. Management of diabetes in Morocco: results of the International Diabetes Management Practices Study (IDMPS)—wave 5. Ther Adv Endocrinol Metab. 2016;7(3):101–9. [DOI] [PMC free article] [PubMed]
- 11.Weyer C, Heise T, Heinemann L. Insulin aspart in a 30/70 premixed formulation. Pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care. 1997;20(10):1612–4. [DOI] [PubMed]
- 12.Gumprecht J, Benroubi M, Borzi V, et al. Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix® 30) can improve glycemic control in patients treated with basal insulins: a subgroup analysis of the IMPROVE™ observational study. Int J Clin Pract. 2009;63(6):966–972. doi: 10.1111/j.1742-1241.2009.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401–409. doi: 10.1111/dom.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Elq AH. Current practice in the management of patients with type 2 diabetes mellitus in Saudi Arabia. Saudi Med J. 2009;30(12):1551–1556. [PubMed] [Google Scholar]
- 15.Soewondo P, Soegondo S, Suastika K, Pranoto A, Soeatmadji D, Tjokroprawiro A. The DiabCare Asia 2008 study—outcomes on control and complications of type 2 diabetic patients in Indonesia. Med J Indones. 2010;9(4):235–244. doi: 10.13181/mji.v19i4.412. [DOI] [Google Scholar]
- 16.Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med. 2015;32(3):407–13. [DOI] [PubMed]
- 17.Almaatouq MA, Al-Arouj M, Amod A, Assaad-Khalil SH, Assaad SN, Azar ST, Esmat K, Hassoun AA, Jarrah N, Zatari S. Barriers to the delivery of optimal antidiabetic therapy in the Middle East and Africa. Int J Clin Pract. 2014;68(4):503–511. doi: 10.1111/ijcp.12342. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Density of physicians (total number per 1000 population, latest available year). 2016. http://www.who.int/gho/health_workforce/physicians_density/en/. Last accessed Oct 2016.
- 19.Soewondo P, Ferrario A, Tahapary DL. Challenges in diabetes management in Indonesia: a literature review. Glob Health. 2013;9:63. doi: 10.1186/1744-8603-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolla A. Pharmacokinetic and pharmacodynamic advantages of insulin analogues and premixed insulin analogues over human insulins: impact on efficacy and safety. Am J Med. 2008;121(6 Suppl):S9–S19. doi: 10.1016/j.amjmed.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Oyer DS, Shepherd MD, Coulter FC, Bhargava A, Brett J, Chu PL, Trippe BS, INITIATEplus Study Group A(1c) control in a primary care setting: self-titrating an insulin analog pre-mix (INITIATEplus trial) Am J Med. 2009;122(11):1043–1049. doi: 10.1016/j.amjmed.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. Improvement of glycemic control in patients with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 23.Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9:902–913. doi: 10.1111/j.1463-1326.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 24.Bell DS, Clements RS, Jr, Perentesis G, Roddam R, Wagenknecht L. Dosage accuracy of self-mixed vs premixed insulin. Arch Intern Med. 1991;151(11):2265–2269. doi: 10.1001/archinte.1991.00400110111022. [DOI] [PubMed] [Google Scholar]
- 25.Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther. 2005;27(8):1113–1125. doi: 10.1016/j.clinthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Novo Nordisk A/S. EU SmPC for Insulin NovoMix® 30. 2010. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000308/WC500029441.pdf. Accessed Nov 2016.
- 27.Gao Y, Luquez C, Lynggaard H, Andersen H, Saboo B. The SimpleMix study with biphasic insulin aspart 30: a randomized controlled trial investigating patient-driven titration versus investigator-driven titration. Curr Med Res Opin. 2014;30(12):2483–2492. doi: 10.1185/03007995.2014.960512. [DOI] [PubMed] [Google Scholar]
- 28.Brod M, Hammer M, Christensen T, et al. Understanding and assessing the impact of treatment in diabetes: the Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device) Health Qual Life Endpoints. 2009;9(7):83. doi: 10.1186/1477-7525-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workgroup on Hypoglycemia. American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 30.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: statistical principles for clinical trials. Geneva: ICH; 1998. [PubMed]
- 31.Garg SK, Admane K, Freemantle N, et al. Patient-led versus physician-led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS study. Endocr Pract. 2015;21(2):143–157. doi: 10.4158/EP14079.OR. [DOI] [PubMed] [Google Scholar]
- 32.ATLAS Study Group Titration of insulin glargine in patients with type 2 diabetes mellitus in Asia: physician-versus patient-led? Rationale of the Asian Treat to Target Lantus Study (ATLAS) Diabetes Technol Ther. 2011;13(1):67–72. doi: 10.1089/dia.2010.0170. [DOI] [PubMed] [Google Scholar]
- 33.Ligthelm RJ. Self-titration of biphasic insulin aspart 30/70 improves glycemic control and allows easy intensification in a Dutch clinical practice. Prim Care Diabetes. 2009;3:97–102. doi: 10.1016/j.pcd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Yang W, Zhu L, Meng B, et al. Subject-driven titration of biphasic insulin aspart 30 twice daily is non-inferior to investigator-driven titration in Chinese patients with type 2 diabetes inadequately controlled with premixed human insulin: a randomized, open-label, parallel-group, multicenter trial. J Diabetes Investig. 2016;7(1):85–93. doi: 10.1111/jdi.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res. 2008;6(2):54–67. doi: 10.3121/cmr.2008.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giugliano D, Chiodini P, Maiorino MI, Bellastella G, Esposito K. Intensification of insulin therapy with basal-bolus or premixed insulin regimens in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2016;51:417–428. doi: 10.1007/s12020-015-0718-3. [DOI] [PubMed] [Google Scholar]
- 37.Malek R, Ajili F, Assaad-Khalil SH, Shinde A, Chen JW, Van den Berg E. Similar glucose control with basal-bolus regimen of insulin detemir plus insulin aspart and thrice-daily biphasic insulin aspart 30 in insulin-naïve patients with type 2 diabetes: results of a 50-week randomized clinical trial of stepwise insulin intensification. Diabetes Metab. 2015;41(3):223–230. doi: 10.1016/j.diabet.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Liu K, Kiu L, et al. Health care utilizations and costs of insulin patient-driven titration versus physician-driven titration: evidence based on a clinical trial of biphasic insulin aspart 30 twice daily in people with type 2 diabetes in China. Value Health. 2014;17:A719–A813. doi: 10.1016/j.jval.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Tejada MA, Campbell JA, Walker RJ, Smalls BL, Davis KS, Egede LE. Diabetes empowerment, medication adherence and self-care behaviors in adults with type 2 diabetes. Diabetes Technol Ther. 2012;14(7):630–634. doi: 10.1089/dia.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22(3):123–127. doi: 10.2337/diaclin.22.3.123. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained during and/or analyzed during the current study are available from the corresponding author on reasonable request.