Abstract
Introduction
Previous research has found that the percentage of US adults with diabetes achieving a glycated hemoglobin (HbA1c) target of <7.0% with currently available treatments has been fairly constant from 2003 to 2010, remaining at just over 50% [1]. The objective of this study was to compare the most recent data (2011–2014) with earlier data to track progress on HbA1c target achievement, for both the general target of <7.0% and inferred individualized targets based on age and the presence of complications.
Methods
Data from 2677 adults with self-reported diabetes from the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2014 were examined to determine the percentage of adults who achieved HbA1c targets of <7% and an individualized target based on age and comorbidities. National estimates are reported by using weights that account for the complex sampling design of the NHANES.
Results
The percentage of people with diabetes and HbA1c <7.0% slightly declined from 52.2% (95% CI 48.7–55.7%) to 50.9% (95% CI 47.2–54.7%) between the two most recent waves of data. Achievement of individualized targets declined from 69.8% (95% CI 66.5–73.0%) to 63.8% (95% CI 60.1–67.5%). The percentage with HbA1c >9.0% increased from 12.6% (95% CI 10.5–14.8%) to 15.5% (95% CI 12.9–18.2%). Achievement of individualized targets varied by age group and presence of comorbidities, but exhibited similar trends as general target achievement.
Conclusions
Despite the development of many new medications to treat diabetes during the past decade, the proportion of patients achieving glycemic control targets has not improved.
Funding
Intarcia Therapeutics.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-017-0280-5) contains supplementary material, which is available to authorized users.
Keywords: Diabetes, Glycemic control, United States, Trends
Introduction
Diabetes continues to be a growing epidemic in the US, with the number of Americans diagnosed with diabetes increasing fourfold from 1980 to 2014 [2]. The majority of this increase is due to an increase in type 2 diabetes (T2D), partially influenced by a concomitant increase in rates of obesity. Therapy is typically guided by HbA1c, as this metric is strongly correlated with microvascular complications among patients with diabetes [3]. Multiple organizations have developed measures of the quality of T2D care based on HbA1c including the National Quality Forum (NQF) [4]; these are used in Medicare star ratings and are also included in the Healthcare Effectiveness Data and Information Set (HEDIS) [5, 6]. Recently, the American Diabetes Association and European Association for the Study of Diabetes (ADA-EASD) position statement and 2015 update recommended individualized HbA1c targets for patients with T2D, based on a range of factors including patient age, presence of comorbidities, patient attitudes, resources and support system, life expectancy, and disease duration [3, 7]. Previously, a target of <7.0% has been recommended [8].
The objective of this study was to use the most recent NHANES to assess the trend in glycemic control (measured by HbA1C levels) among diabetic adults in the US from 1999 to 2014 and to determine whether the previously observed improvements in glycemic control have continued. Previous analysis of the NHANES estimated that between 1999 and 2006, the percentage of US adults with diabetes achieving HbA1c <7.0% increased between 1999 and 2003 and has remained constant at just over 50% between 2003 and 2010 [1]. Our study updates this prior analysis with the most recent wave of the NHANES (2011–2014).
Methods
Publicly available NHANES data were downloaded from the Centers for Disease Control and Prevention (CDC) website in January 2016 [9]. The portion of data for this analysis comprised the continuous NHANES era, beginning in 1999 when the survey was conducted on an annual basis. Using continuous data allows researchers to study disease prevalence, monitor risk factors, and assess trends over time [5]. Data were analyzed in four waves: 1999–2002, 2003–2006, 2007–2010, and 2011–2014. The NHANES uses a complex multistage probability sampling design to select approximately 5000 participants annually from 15 locations within the sampling frame [5]. The participant population provides a representative sample of US non-institutionalized persons residing in all 50 states and the District of Columbia. Participants were included in the analysis population if they had a self-reported diagnosis of diabetes by a physician [type 1 diabetes (T1D) or T2D is not differentiated in the survey], had valid HbA1c test results, and were aged 18 years or older. Data from 2677 participants were extracted from interview questions and laboratory metrics. Approximately 5% of patients diagnosed with diabetes in the US have T1D, and between 90% and 95% have T2D [10]. As this study includes a representative sample of US non-institutionalized persons, the vast majority (>90%) of participants were assumed to have T2D.
Glycemic control was assessed in three ways: a general target of <7.0% and an individualized target based on the person’s age and diabetes-related comorbidities and poor control (HbA1c >9.0%), shown in Table 1. The HbA1c target <7.0% aligns with American Diabetes Association (ADA) guidelines before 2012 and one of the HEDIS quality metrics (only applied to members under 65 without certain comorbidities) [5, 8]. The individualized guidelines were adapted based on guidelines introduced in 2012 [3, 7]. HbA1c >9.0% is considered poor control in various quality metrics, including the National Quality Forum (NQF) [4, 8]. Individualized targets were initially developed to offer a patient-centered approach to diabetes management and were introduced in 2012 [3]. The ADA-EASD guidelines provide several factors that can be used to guide the development of individualized HbA1c targets [3]. As individualized HbA1c targets were not collected by the NHANEs (i.e., reported by the patient or a treating physician), this analysis followed the same approach to developing individualized targets as Ali and used two factors (age and the presence of complications) to infer individualized targets [1]. These factors were chosen as they were the only two factors that could be objectively observed in our data set. We further modified the individualized targets used by Ali for two groups of patients. First, for persons aged 18–44 years without complications, Ali used the intensive treatment target of <6.5%, while this analysis used a more conservative target of ≤7.0% [1]. Second, for patients aged 65 years and older without complications, Ali used two target values (≤7.0 or ≤7.5%) because of the lack of consensus regarding the most appropriate target for this risk group, while this analysis used the more conservative target of ≤7.5% [1]. Determination of complications was based on the presence of any of the following conditions: self-reported retinopathy, self-reported cardiovascular disease (heart attack, coronary heart disease, or stroke), or measured albumin/creatinine ratio of 30 mg of albumin per gram of creatinine or higher. HbA1c (%), albumin (per milligram), and creatinine (per gram) were all measured directly from blood samples provided by survey participants. All HbA1c measurements were collected during the time of the interview and therefore offer accurate and timely assessments.
Table 1.
HbA1c targets
| Measure | Target HbA1c, % | Guideline |
|---|---|---|
| General target | <7.0 | ADA [8] |
| Poor control | >9.0 | NQF [4] |
| Individualized targets | ADA-EASD [3] as adapted by Ali et al. [1] | |
| Age 18–44 years |
≤7.0, without complicationsa ≤7.0, with complications |
|
| Age 45–64 years |
≤7.0, without complications ≤8.0, with complications |
|
| Age 65 years and older |
≤7.5, without complicationsb ≤8.0, with complications |
|
ADA American Diabetes Association, EASD European Association for the Study of Diabetes, HbA1c glycated hemoglobin, NQF National Quality Forum
Table adapted from Ali et al. [1]
aAli et al. [1] used the intensive treatment target of <6.5% for persons 18–44 years old. This analysis used a more conservative target of ≤7.0%
bThis analysis used the more conservative target of ≤7.5%
Patient characteristics were summarized for the sample population from interview questions and physical examination. Characteristics were weighted to represent the US population of persons with diagnosed diabetes. Participant age was imputed from date of birth or approximated when the month and/or day was missing. Under circumstances where the date of birth was not provided, the reported age was used instead. Gender (male/female) was recorded under demographic information. Race or ethnic group categories were mutually exclusive (“other” included multiracial individuals). Education level was recorded separately for youth participants (aged 6–19 years) and adults (aged 20 years and older), and our sample included individuals aged 18 years and older. Both variables were used to categorize education level in a manner consistent with Ali 2013 [1]. Annual household income was originally categorized in $5000 to $15,000 increments. The earlier years (1999–2006) had a maximum category of $75,000 and over. Beginning in 2007, the maximum category was changed to $100,000 and over. Both variables were recoded into a binary measurement of less than $20,000 per year or greater than or equal to $20,000. Insurance coverage included private, Medicare, Medicaid/Chip, or other government insurance and was consolidated into a variable to capture any coverage. Uninsured participants were those who had no coverage. Time since diabetes diagnosis was calculated from the participant’s age and the reported age when he or she was first told he or she had diabetes. Body mass index (BMI) was calculated from weight and height measurements collected during the physical examination.
We conducted the statistical analysis in SAS version 9.4 (Cary, NC). Sample weights provided in the NHANES documentation were applied to individual participants to account for selection probability for each demographic domain, survey nonresponse, and differences between the sample and total population [5]. Combining data into 4-year waves increases the precision and reduces sampling error. Multiple survey weights are available based on the data for the sample population. Four-year weights were calculated according to the NHANES documentation by dividing the 2-year sample weight by two for years 2003–2014. For the 1999–2002 waves, 4-year sample weights were provided by NHANES and no additional calculations were necessary. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
The number of survey respondents with diabetes and included in the study was 1326, representing 21.3 million adults with diabetes in the US. In prior waves, the number of people with diabetes participating in the survey ranged from 857 to 1351, representing 12.6–17.6 million US adults with diabetes. Characteristics of US adults with diagnosed diabetes are reported in Table 2. In the most recent wave of data, demographics including age, sex, and race/ethnicity have remained constant relative to prior waves. There was an overall trend toward higher levels of education attainment across waves; under a quarter (23.8%) of patients reported less than a high school level of education (a decrease from previous years) and just over half (50.6%) reported at least some college education (an increase from previous years). A decreasing proportion of patients were recorded as having a BMI <25.0, while an increasing proportion of patients were recorded as having a BMI ≥30.0 across the four waves of data.
Table 2.
Characteristics of US adults with diagnosed diabetes
| Survey population | 1999–2002 | 2003–2006 | 2007–2010 | 2011–2014 |
|---|---|---|---|---|
| N = 857 | N = 943 | N = 1351 | N = 1326 | |
| Weighted population, millions | 12.6 | 15.4 | 17.6 | 21.3 |
| Age (years), % (SE) | ||||
| 18–44 | 17.9 (2) | 15.9 (1.7) | 13.1 (1.3) | 13.7 (1.3) |
| 45–64 | 44.4 (2.3) | 44.9 (2.1) | 46.4 (1.8) | 47.8 (1.9) |
| ≥65 | 37.7 (2.1) | 39.2 (2.0) | 40.5 (1.7) | 38.5 (1.8) |
| Mean, years (SE) | 58.7 (0.7) | 59.1 (0.6) | 58.0 (0.6) | 58.7 (0.5) |
| Female sex, % (SE) | 49.8 (2.3) | 53.6 (2.1) | 50.3 (1.8) | 50.5 (1.9) |
| Race or ethnic group, % (SE) | ||||
| Non-Hispanic white | 62.0 (2.1) | 64.8 (1.9) | 62.5 (1.6) | 61.4 (1.7) |
| Non-Hispanic black | 15.0 (1.2) | 15.8 (1.1) | 16.5 (1) | 15.1 (0.9) |
| Mexican-American | 7.0 (0.6) | 8.0 (0.7) | 8.6 (0.6) | 9.2 (0.8) |
| Other | 15.9 (1.8) | 11.4 (1.5) | 12.4 (1.2) | 14.3 (1.1) |
| Education level, % (SE) | ||||
| <High school | 36.4 (2.1) | 28.5 (1.8) | 31.0 (1.5) | 23.8 (1.4) |
| High school graduate | 25.1 (2) | 26.0 (1.9) | 23.0 (1.5) | 25.6 (1.7) |
| At least some college | 38.5 (2.3) | 45.6 (2.1) | 46.0 (1.8) | 50.6 (1.9) |
| Annual household income <$20,000, % (SE) | 37.1 (2.3) | 28.6 (1.8) | 22.5 (1.3) | 24.2 (1.5) |
| Uninsured, % (SE) | 10.2 (1.4) | 10.6 (1.3) | 11.4 (1) | 11.8 (1.1) |
| Time since diabetes diagnosis, % (SE) | ||||
| 0–<5 years | 37.2 (2.3) | 34.3 (2.1) | 34.2 (1.8) | 27.6 (2.3) |
| 5–15 years | 34.7 (2.2) | 42.3 (2.1) | 39.3 (1.7) | 43.7 (2.6) |
| ≥15 years | 28.1 (2.1) | 23.4 (1.7) | 26.5 (1.6) | 28.7 (2.3) |
| BMI, % (SE)a | ||||
| <25.0 | 17.0 (1.8) | 14.9 (1.5) | 13.3 (1.2) | 12.5 (1.2) |
| 25.0–29.9 | 30.7 (2.2) | 28.0 (1.9) | 23.3 (1.5) | 26.2 (1.6) |
| ≥30.0 | 52.4 (2.4) | 57.1 (2.1) | 63.5 (1.7) | 61.3 (1.8) |
| Age and comorbidity status | Number of people with diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Survey, N | US pop., mil N | Survey, N | US pop., mil N | Survey, N | US pop., mil N | Survey, N | US pop., mil N | |
| 18–44 year without complications | 55 | 1.2 | 62.0 | 1.4 | 68 | 1.2 | 97 | 1.6 |
| 18–44 year with complications | 54 | 1.1 | 48 | 1.0 | 65 | 1.1 | 69 | 1.3 |
| 45–64 year without complications | 160 | 2.8 | 170 | 3.4 | 293 | 4.7 | 306 | 5.9 |
| 45–64 year with complications | 183 | 2.8 | 202 | 3.5 | 296 | 3.5 | 285 | 4.3 |
| ≥65 year without complications | 121 | 1.4 | 165 | 2.2 | 253 | 2.8 | 205 | 3.3 |
| ≥65 year with complications | 284 | 3.3 | 296 | 3.8 | 396 | 4.3 | 364 | 4.9 |
Percentages reported unless otherwise specified
Data values are weighted percentages (standard error) unless otherwise noted
BMI body mass index, SE standard error
aThe body mass index is the weight in kilograms divided by the square of the height in meters
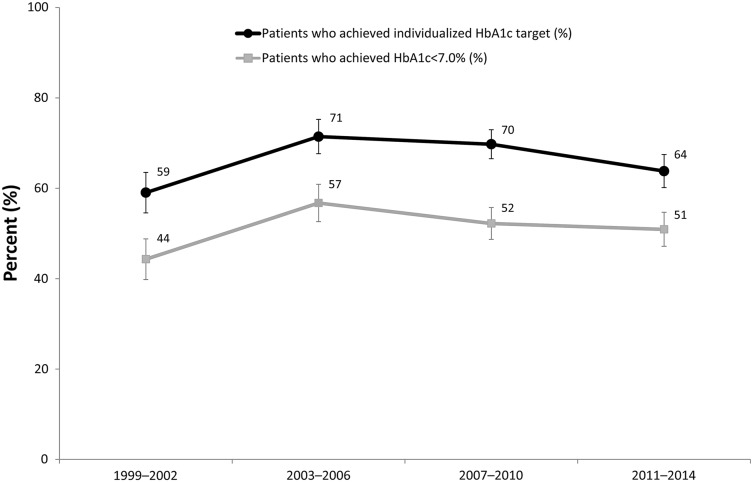
Results for achievement of individualized and general HbA1c targets are reported in Fig. 1. In the most recent wave (2011–2014), about half (50.9%, 95% CI 47.2–54.7%) of people with diabetes had HbA1c <7.0% and under two-thirds (63.8%, 95% CI 60.1–67.5%) achieved the individualized targets. For the target of HbA1c <7.0%, this was unchanged from 52.2% (CI 48.7–55.7%) observed in the previous wave (2007–2010), but achievement of individualized targets declined from 69.8% (95% CI 66.5–73.0%) in the most recent two waves of data, although the decline was not statistically significant at 95% confidence levels. Between the first two waves of data (1999–2002 and 2003–2006), target achievement increased significantly and was flat between the next two waves (2003–2006 and 2007–2010) for both individualized targets and the target of HbA1c <7.0%.
Fig. 1.
Percentage of all adults with diabetes: HbA1c targets (individualized and general target HbA1c <7.0%); 95% confidence intervals are shown. Individualized targets are defined in Table 1
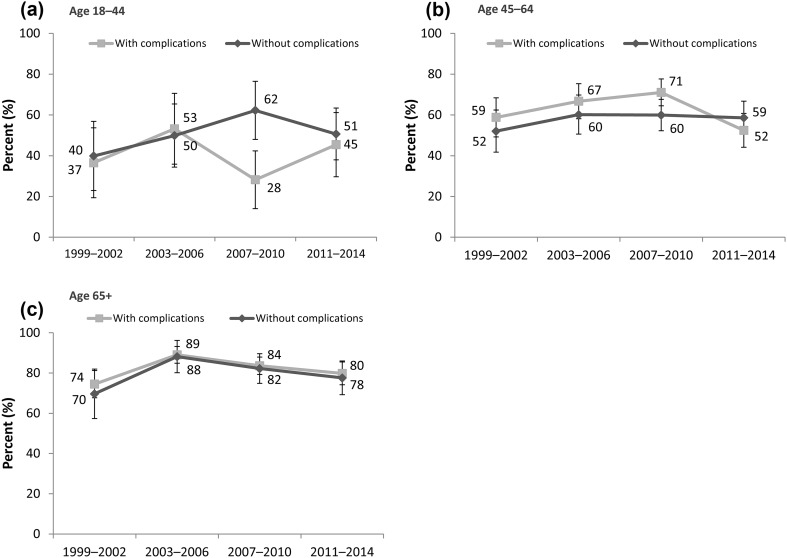
Results for achievement of individualized targets by age group and the presence of complications are reported in Fig. 2 and are similar to the aggregate estimate reported in Fig. 1. For patients aged >65 years, trends were similar, with an improvement between 1999 and 2006 and a small decline between 2007 and 2014. Target achievement for adults 45–64 years without complications (individualized target HbA1c ≤7.0%) was similar in the most recent waves, but adults aged 45–64 years with complications had a significant decline in target achievement (HbA1c ≤8.0%) from 71.1% (CI 64.5–77.6%) to 52.5% (CI 44.1–60.8%). Estimated confidence intervals were large among adults in the youngest age group, 18–44 years.
Fig. 2.
Achievement of individualized targets by age group and the presence of complications. Based on N = 1326 (2011–2014), 1351 (2007–2010), 943 (2003–2006), and 857 (1999–2002) survey respondents; weighted to represent US adults with diabetes; 95% confidence intervals are shown. Individualized targets are defined in Table 1
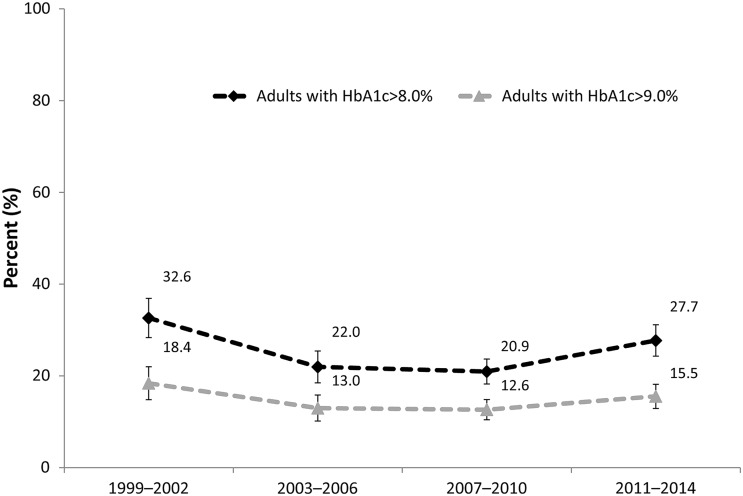
Statistically significant declines in the percentage of adults with diabetes with HbA1c >8.0 or 9.0% were observed between the first two waves of data (1999–2006). There was no statistically significant difference between the middle two waves (2003–2010). Between the two most recent waves of data (2007–2010 and 2011–2014), the percentage of adults with Hb1c >8.0% increased at statistically significant levels from 20.9% (95% CI 18.2–23.7%) to 27.7% (95% CI 24.3–31.3%), respectively. The increase in the percentage of adults with HbA1c >9.0% was not, however, statistically significant at 12.6% (95% CI 10.5–14.8%) and 15.5% (95% CI 12.9–18.2%) between 2007–2010 and 2011–2014, respectively (Fig. 3).
Fig. 3.
US adults with HbA1c exceeding 8.0% and 9.0% (poor control). Based on N = 1326 (2011–2014), 1351 (2007–2010), 943 (2003–2006), and 857 (1999–2002) survey respondents; weighted to represent US adults with diabetes; 95% confidence intervals are shown
Discussion
This analysis has demonstrated that after improvements in glycemic control between 1999 and 2006, the HbA1c level has plateaued through 2014, whether measured using a target of HbA1c <7.0% or individualized targets based on patient age and diabetes-related comorbidities. This study confirms and extends the analysis of glycemic control reported by Ali et al. [1].
One of the strengths of this study is that it is based on a nationally representative survey that collected blood samples, ensuring an accurate estimate of HbA1c, but it does have several limitations. First, diagnosis of diabetes and comorbidities are self-reported and thus subject to biases in these data, such as recall bias. We do not observe the actual glycemic target (i.e., from patient or treated physician), but infer it based on two of several factors that can be considered in setting an appropriate target: age and the presence of diabetes-related comorbidities. Finally, the number of survey respondents with diabetes under age 45 years was relatively small; thus, trends in this group should be interpreted with appropriate caution.
Study results are consistent with findings in non-representative subpopulations with health insurance, including commercial, Medicare, and Medicaid enrollees. An analysis of privately insured and Medicare Advantage enrollees with T2D found that between 2006 and 2013, the proportion of patients with HbA1c <7.0% declined and the proportion with an HbA1c >9.0% increased (p < 0.001) [11]. Healthcare Effectiveness Data and Information Set (HEDIS) measures in commercial, Medicare, and Medicaid plans are flat, with the exception of commercial and Medicare PPO plans 2006–2009, shown in the Appendix [5]. We suspect that the earlier very low target achievement in PPO may have been due to limited collection and reporting of these data, as this low level of target achievements in 2007–2009 is not observed in other populations or data sets.
This plateau in glycemic control has occurred despite continued innovations in glucose-lowering pharmaceuticals. Greater availability and a wider range of safe and effective medicines that improve disease management and reduce the risk of hypoglycemia might increase attainment of glycemic control. One factor that could limit the benefit of drug innovation is the trend toward increased patient cost-sharing overall in US healthcare plans, although there are exceptions where plans have lowered cost sharing for diabetes drugs to encourage treatment as part of value-based insurance design [12, 13]. Even without changes in drug coverage, patients may not be optimally treated with these newer medications because of the cost (e.g., when placed in a higher tier of a drug plan).
Other possible reasons for the plateau in glycemic control between 2006 and 2010 include changes in the population with T2D in the US. Poor control has been associated with younger age (particularly at time of T2D diagnosis) [14], race/ethnicity, current insulin treatment, and lack of insurance [15], but these factors have not changed much since 2006 with the exception of the implementation of Medicare Part D (which increased insurance coverage for prescription drugs in the over 65 population and was implemented in 2006). Other analyses of the NHANES have found that growth in diagnosed diabetes was faster than growth in undiagnosed diabetes 1999–2012, suggesting a decline in the proportion of undiagnosed diabetes. This could reduce estimates of glycemic control if the trend has continued into 2014 and if these formerly undiagnosed patients are disproportionately from groups predisposed to worse control (e.g., due to age or race/ethnicity) [16].
Whether influenced by increased cost sharing, T2D population changes, or other factors, the benefits realized from new treatments may also be limited by poor medication adherence. Since evidence on long-term trends in medication adherence is not available in NHANES data, it is unclear whether adherence is a key explanatory factor for the plateau in glycemic control. Past studies consistently find poor medication adherence is associated with worse outcomes. Recent studies have found adherence rates among patients with T2D to vary from 20 to 50% [17, 18] when measuring adherence to a specific drug class. Higher rates (65–80%) of adherence [19, 20] were found when the measure is defined as having any diabetes drug on hand, which may count patients taking more than one diabetes drug as adherent even if they may not be adherent to each medication individually. Poor adherence to diabetes medications has been associated with increased healthcare utilization and medical (non-pharmacy) costs [18, 21, 22] as well as worse glucose control [19]. Ta et al. [19] found that each percentage point increase in the share of patients who were adherent (e.g., PDC ≥80%) was associated with 4.69 higher odds of performing in the top quartile of glycemic control (defined as percentage of patients with HbAc1 <8.0%).
Currently available T2D medications are administered either orally or as an injection [23]. While these routes of administration are appropriate for treating patients with acute illness or in a hospital-based setting, they are not ideal for long-term treatment of complicated chronic diseases such as T2D. As the understanding of T2D as a disease has increased in recent decades, so too has the complexity of the therapies used for its treatment [23]. Ultimately, high rates of medication adherence and treatment success rely on consistent patient action to adhere to complex dosing or injection regimens, possibly explaining the continued low rates of medication adherence despite pharmaceutical innovation. A potential solution to overcoming the challenges of medication adherence with treatment would be to design a more appropriate and innovative method of delivery of T2D medication that requires less consistent action on the part of the patient to maintain high adherence rates.
Conclusions
In conclusion, this study of the NHANES has demonstrated that the improvements in glycemic control observed between 1999 and 2006 have remained flat or unchanged over the past decade. Adherence to medications is likely an important reason limiting the benefits of new therapies, given the low levels of adherence observed in patients with T2D and the poor outcomes associated with adherence that have been observed in patient-level studies. A trend toward increased patient cost sharing for diabetes drugs in US healthcare plans and the cost of new medications may also be contributing factors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding for this research was provided by Intarcia Therapeutics (Boston, Mass.). The study sponsor, Intarcia Therapeutics (Boston, Mass.), was involved in all stages of the study research and manuscript preparation, and all authors participated in the design of the study, had access to the data, and contributed to the manuscript development. Analysis Group Inc. funded all article processing charges and provided medical writing assistance. Data were obtained by Analysis Group Inc. and analyzed and interpreted in collaboration with all other authors. All the authors vouch for the accuracy and completeness of the data reported and the adherence of the study to the protocol, and all the authors made the decision to submit the manuscript for publication. Ginger Carls is the guarantor for the contents of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Manuscript drafts were prepared by the authors with editorial assistance from Cody Patton. Kelly Adamski also assisted with data analysis and development of study exhibits. Both Mr. Patton and Ms. Adamski completed their work as part of their employment at Analysis Group Inc.
Disclosures
John Yee is an employee of Intarcia Therapeutics and owns stock options. Ginger Carls is an employee of Analysis Group Inc., which has received consultancy fees from Intarcia Therapeutics. Edward Tuttle is an employee of Analysis Group Inc., which has received consultancy fees from Intarcia Therapeutics. Ruo-Ding Tan is an employee of Analysis Group Inc., which has received consultancy fees from Intarcia Therapeutics. Johnny Huynh is an employee of Analysis Group Inc., which has received consultancy fees from Intarcia Therapeutics. Steven Edelman is on the Intarcia Advisory board and has not received any compensation for any work relating to this manuscript. William Polonsky is on the Intarcia Advisory board and has not received any compensation for any work relating to this manuscript.
Compliance with Ethics Guidelines
The NHANES is a publicly available de-identified data source, and thus this study was exempt from institutional review board (IRB) review. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
Data are available from the US Center for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). Documentation and data are available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/FA98F060431CD742.
References
- 1.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Number (in Millions) of Civilian, Non-Institutionalized Persons with Diagnosed Diabetes, US, 1980–2014; 2015. http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Accessed Dec 1.
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Quality Forum. Comprehensive diabetes care: Hemoglobin A1c (HbA1c) poor control (>9.0%) Washington, DC; 2014. http://www.qualityforum.org. Accessed Sept 1.
- 5.National Committee for Quality Assurance. The State of Health Care Quality 2015; 2015.
- 6.Centers for Medicare and Medicaid Services (CMS). Medicare 2015 Part C and D star rating technical notes; 2015.
- 7.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes A. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2016. http://www.cdc.gov/nchs/nhanes/index.htm. Accessed 5 May 2017.
- 10.Centers for Disease Control and Prevention . National diabetes statistics report: estimates of diabetes and its burden in the US, 2014. Atlanta: US Department of Health and Human Services; 2014. [Google Scholar]
- 11.Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–475. doi: 10.2337/dc16-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claxton G, Raw M, Long M, Panchal N, Damico A. Employer health benefits 2015 annual survey. The Kaiser Family Foundation and Health Research and Educational Trust; 2015.
- 13.Tang KL, Barnieh L, Mann B, et al. A systematic review of value-based insurance design in chronic diseases. Am J Manag Care. 2014;20(6):e229–e241. [PubMed] [Google Scholar]
- 14.Berkowitz SA, Meigs JB, Wexler DJ. Age at type 2 diabetes onset and glycaemic control: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2010. Diabetologia. 2013;56(12):2593–2600. doi: 10.1007/s00125-013-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali MK, Bullard K, Imperatore G, Barker L, Gregg EW. Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes—National Health and Nutrition Examination Survey, US, 2007–2010. Morb Mortal Wkly Rep. 2012;61(2):32–37. [PubMed] [Google Scholar]
- 16.Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by county in the US, 1999–2012. Diabetes Care. 2016;39:1556–1562. doi: 10.2337/dc16-0678. [DOI] [PubMed] [Google Scholar]
- 17.Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287–1305. doi: 10.1007/s12325-014-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–355. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ta JT, Erickson SC, Qiu WA, Patel BV. Is there a relationship between Part D medication adherence and part c intermediate outcomes star ratings measures? J Manag Care Spec Pharm. 2016;22(7):787–795. doi: 10.18553/jmcp.2016.22.7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson SC, Scott LR, Qui W, Patel BV. Adherence trends for 3 chronic disease medication classes among differently insured populations. Am J Pharm Benefits. 2014;6(1):32–37. [Google Scholar]
- 21.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109. doi: 10.1016/j.clinthera.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Project Hope) 2011;30(1):91–99. doi: 10.1377/hlthaff.2009.1087. [DOI] [PubMed] [Google Scholar]
- 23.García-Pérez L-E, Álvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the US Center for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). Documentation and data are available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.