Abstract
Introduction
Combination therapy with insulin and glucagon-like peptide-1 receptor agonists (GLP-1RAs) has already been proven an efficient treatment option for type 2 diabetes. This combination can effectively improve glycated hemoglobin levels, cause weight loss and reduce the dosage of insulin. In addition, it can also reduce the risk of hypoglycemia. Several randomized controlled trials have confirmed that this treatment may be just as effective for type 1 diabetes mellitus (T1DM) patients. The objective of this meta-analysis was to assess the effects and efficacy of the treatment on glycemic changes, weight loss and insulin dosage in type 1 diabetes mellitus patients.
Methods
We searched Embase, PubMed and Cochrane for randomized controlled trials (no time restrictions) that investigated combined insulin and GLP-1 treatment. The main endpoints were measurements of glycated hemoglobin and changes in the weight and the dosage of insulin.
Results
In total, 1093 were studies identified, and 7 studies were included in our meta-analysis. GLP-1 agonist and insulin combination therapy led to greater reductions in HbA1c levels [P = 0.03; mean difference −0.21; 95% confidence intervals (CI) (−0.40, 0.02)] and weight [P < 0.05; −3.53 (−4.86, 2.19)] compared to control treatments. The combination therapy did not significantly influence the daily weight-adjusted total insulin dose [P = 0.05; −0.11 (−0.23, 0)], but it did reduce the daily weight-adjusted bolus insulin dose [P = 0.001; −0.06 (−0.1, 0.02)].
Conclusion
Our meta-analysis supports the use of a combined therapeutic regimen of insulin and GLP-1RAs for treating patients with T1DM. Combination therapy with GLP-1 and insulin could achieve an ideal treatment effect on glycemic control, weight loss and bolus insulin dose in patients with T1DM.
Keywords: Glucagon-like peptide-1 receptor agonists (GLP-1RAs), Insulin, Meta-analysis, Type 1 diabetes (T1DM)
Introduction
Type 1 diabetes mellitus (T1DM) is characterized as an autoimmune disease caused by damage to insulin-producing pancreatic β-cells with or without residual functional tissue [1]. There are many causes of type 1 diabetes, including viral infection, drug effects and autoimmune causes. These factors lead to β-cell apoptosis and an absolute lack of insulin in serum, which causes high blood glucose.
Since Best and Banting identified insulin in 1921, it has been the leading treatment choice for T1DM. However, insulin cannot improve the excessive secretion of glucagon in patients with T1DM [2]. With the wide application of controlled-release insulin-pump technology and genetically engineered long-acting insulin in recent years, glucose levels in patients with T1DM have been effectively controlled. In fact, it is easy to cause hypoglycemia syncope because of large fluctuations in glucose resulting from uncontrolled release of blood glucose or false physiological rhythms. Tight glycemic control can decrease the probability of diabetes complications, such as hypoglycemia and weight gain [3]. Currently, drugs that can prevent further progression of the disease or restore the function or quantity of pancreatic β-cells are not available.
Researchers have begun to explore new methods for the treatment of T1DM as they deepen their understanding of the underlying pathogenic mechanism. Gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are secreted by intestinal mucosal endocrine cells after the intake of food. GIP can reduce postprandial blood glucose, promote the synthesis of lipids and stimulate the secretion of glucagon. GLP-1 could be used as an injectable anti-diabetic drug because it has glucose-independent characteristics that promote insulin secretion.
GLP-1 can regulate blood glucose by promoting insulin secretion from pancreatic β-cells, inhibiting inappropriate glucagon secretion from pancreatic α-cells, delaying gastric emptying and controlling appetite. In addition, GLP-1 does not induce insulin secretion at low levels of blood glucose; therefore, it can effectively reduce both glycated hemoglobin levels and the risk of hypoglycemia [4, 5].
Based on previous studies, combination therapy with insulin and GLP-1RAs can effectively improve glycated hemoglobin levels, cause weight loss and reduce the dosage of insulin in patients with type 2 diabetes mellitus (T2DM) [6]. According to the 2013 American Association of Clinical Endocrinologists (AACE) drug recommendations, early application of GLP-1RAs has been suggested for patients with T2DM. Many clinical trials have begun to explore the potential of GLP-1RA treatment among patients with T1DM. Several preclinical trials have confirmed that GLP-1 can reduce pancreatic β-cell apoptosis, stimulate their proliferation and enhance their survival rate [7].
The aim of this article was to provide a meta-analysis regarding the positive effects of GLP-1RAs combined with insulin for the treatment of patients with T1DM.
Methods
Sources
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8]. We performed a search for relevant literature in the PubMed, Embase and Cochrane Central Register of Controlled Trials databases for randomized controlled trials (RCTs) conducted between January 1990 and October 2016. The search strategy did not include restrictions on language or race, and the search was performed using the following key words in Embase: ‘diabetes mellitus, insulin-dependent’:ab,ti OR ‘diabetes mellitus, insulin dependent’:ab,ti OR ‘insulin-dependent diabetes mellitus’:ab,ti OR ‘diabetes mellitus, insulin-dependent, 1’:ab,ti OR ‘diabetes mellitus, juvenile-onset’:ab,ti OR ‘diabetes mellitus, juvenile onset’ OR ‘juvenile-onset diabetes mellitus’:ab,ti OR ‘type 1 diabetes mellitus’:ab,ti OR ‘diabetes mellitus, sudden-onset’:ab,ti OR ‘diabetes mellitus, sudden onset’:ab,ti OR ‘mellitus, sudden-onset diabetes’:ab,ti OR ‘sudden-onset diabetes mellitus’:ab,ti OR ‘diabetes mellitus, type i’:ab,ti OR ‘iddm’:ab,ti OR ‘insulin-dependent diabetes mellitus 1’:ab,ti OR ‘insulin dependent diabetes’ OR ‘juvenile-onset diabetes mellitus 1’:ab,ti OR ‘diabetes, juvenile-onset’:ab,ti OR ‘juvenile onset diabetes’:ab,ti OR ‘diabetes mellitus, brittle’:ab,ti OR ‘brittle diabetes mellitus’:ab,ti OR ‘diabetes mellitus, ketosis-prone’:ab,ti OR ‘diabetes mellitus, ketosis prone’:ab,ti OR ‘ketosis-prone diabetes mellitus’:ab,ti OR ‘diabetes, autoimmune’:ab,ti AND ‘glucagon like peptide 1’:ab,ti OR ‘glucagon-like peptide-1’:ab,ti OR ‘glp-1’:ab,ti OR ‘glp 1’:ab,ti OR ‘exenatide’:ab,ti OR ‘liraglutide’:ab,ti OR ‘lixisenatide’:ab,ti OR ‘albiglutide’:ab,ti OR ‘semeglutide’:ab,ti OR ‘incretin’:ab,ti OR ‘glucagon like peptide-1’:ab,ti OR ‘byetta’:ab,ti OR ‘bydureon’:ab,ti OR ‘victoza’:ab,ti OR ‘saxenda’:ab,ti OR ‘lyxumia’:ab,ti OR ‘tanzeum’:ab,ti OR ‘dulaglutide’:ab,ti OR ‘trulicity’:ab,ti OR ‘taspoglutide’:ab,ti AND ‘insulin, regular’:ab,ti OR ‘regular insulin’:ab,ti OR ‘soluble insulin’:ab,ti AND ‘insulin, soluble’:ab,ti OR ‘insulin a chain’:ab,ti OR ‘sodium insulin’:ab,ti OR ‘insulin, sodium’:ab,ti OR ‘novolin’:ab,ti OR ‘iletin’:ab,ti OR ‘insulin b chain’:ab,ti OR ‘chain, insulin b’:ab,ti AND ‘randomized controlled trial’ OR ‘placebo’ OR ‘randomized.’ We searched all potentially eligible papers for inclusion in the meta-analysis.
Eligibility Criteria and Data Extraction
Studies were included if they satisfied the following criteria: (1) randomized clinical trial with at least 8 weeks of intervention, (2) compared insulin and GLP-1 agonist combination therapy with other treatments in patients with type 1 diabetes and (3) contained adequate data to calculate 95% confidence intervals (CIs) and mean differences (mds). Retrospective studies were excluded. Trials were selected by two independent investigators (Weihao Wang and Hongyan Liu); a third investigator (Shumin Xiao) was recruited if there were disagreements.
Data were extracted as follows for every included study: first author, year of publication, differential interventions in study groups, duration of interventions, number of patients, mean age, mean baseline glycated hemoglobin (HbA1c) (%), mean baseline weight (kg), mean body mass index (BMI) (kg/m2) and mean duration of diabetes. We extracted the mean ± standard deviation (SD) for HbA1c, weight and the insulin dose at the end of intervention. The primary outcome was HbA1c levels at the end of intervention. The authors were contacted if additional data were needed.
This article was based on results from previously conducted studies, and no new studies of human or animal subjects were performed by any of the authors. In the studies included in this meta-analysis, all procedures were performed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Declaration of Helsinki (1964).
Statistical Analyses
We focused on three indicators of the effect of insulin and GLP-1 combination therapy in patients with type 1 diabetes, including glycogenic control based on HbA1c, change in weight and change in insulin dose. Data were analyzed using Review Manager (RevMan), and the results were expressed with forest plots. We assessed HbA1c, weight and insulin dose as continuous variables. The choices of statistical method, analysis and effect measurement were inverse variance, random effects and mean difference, respectively. I 2 testing was used to evaluate the heterogeneity between each study. I 2 values of more than 50% indicated high heterogeneity, and additional analyses were performed [9, 10].
Assessment of Bias
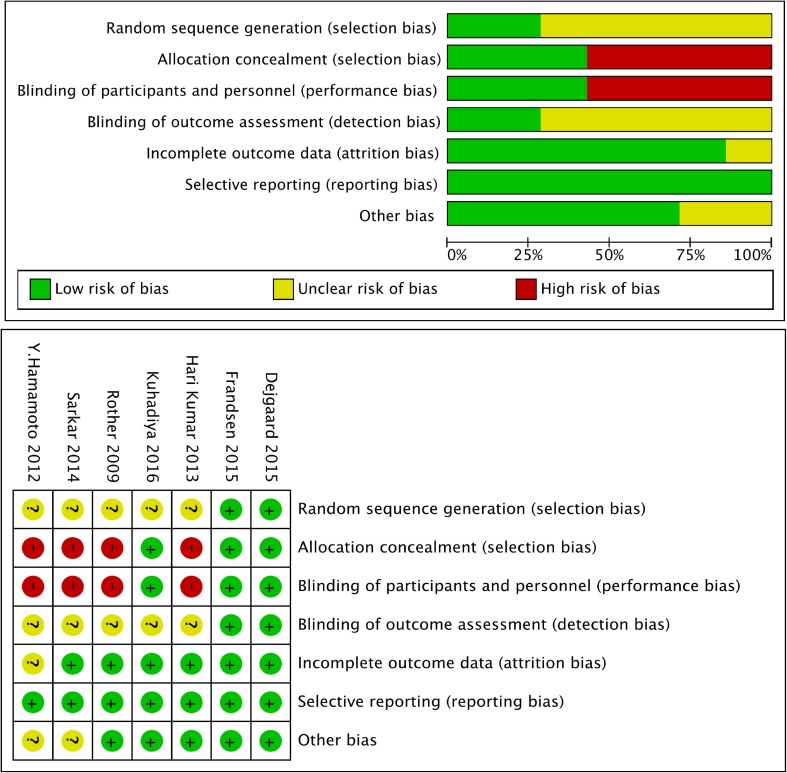
Bias was analyzed using the Cochrane Collaboration’s tool. Every study was scored as “high,” “unclear” or “low” risk. The bias evaluation was based on random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other biases. Two independent investigators (Weihao Wang and Hongyan Liu) evaluated the quality of the included articles.
Results
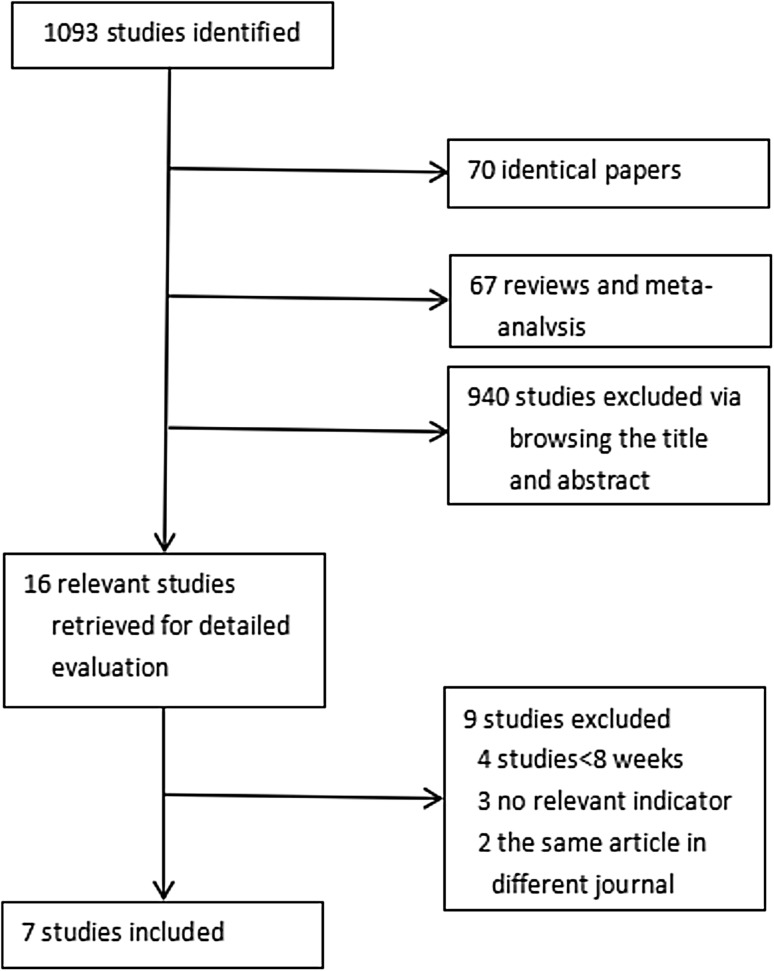
We identified 1093 studies, and 7 were ultimately included in our analysis. Studies were excluded because they were duplicate studies, not relevant, etc. (Fig. 1). The baseline data for all included studies, including publication year, intervention method and duration, number, mean age, mean baseline HbA1c, mean weight, mean BMI and mean duration of diabetes, are shown in Table 1. There were differential interventions in the study groups, which included four liraglutide groups and three exenatide groups. All studies were published between 2009 and 2016 [11–17]. There were a total of 206 patients, including 101 patients treated with GLP-1 agonist and 105 patients treated with a control. All included RCTs analyzed the curative effect as decreased glycated hemoglobin levels compared between the experimental group and the control group of patients with T1DM.
Fig. 1.
Selection process for the included studies
Table 1.
Baseline data from the included studies
| Year | Differential interventions in study groups | Durations of interventions | Number | Mean age | Mean baseline HbA1c | Mean baseline weight (kg) | Mean body-mass index (kg/m2) | Mean duration of diabetes | |
|---|---|---|---|---|---|---|---|---|---|
| Kristina I. Rother | 2009 | Exenatide versus no exenatide | 15 months | 20 | Not available | 7.6% | Not available | 25.9 | 21.3 years |
| Y. Hamamoto | 2012 | Liraglutide versus no liraglutide | 12 months | 10 | 48.5 | Not available | Not available | 22.8 | Not available |
| K. V. S. Hari Kumar | 2013 | Exenatide versus no exenatide | 12 months | 18 | 27.7 | 9.7% | 57.3 | 21.5 | 1.1 months |
| Gayathri Sarkar | 2014 | Exenatide versus no exenatide | 12 months | 14 | 37.3 | 7.0% | Not available | 26.1 | 20.5 years |
| Thomas Fremming Dejgaard | 2015 | Liraglutide versus no liraglutide | 24 weeks | 50 | 47 | 8.7% | 93.4 | 30.3 | 20 years |
| 50 | 49 | 8.7% | 94 | 29.8 | 25 years | ||||
| Nitesh D. Kuhadiya | 2016 | Liraglutide versus no liraglutide | 12 weeks | 16 | 42 | 7.84% | 96 | 33 | 21 years |
| 17 | 50 | 7.69% | 80 | 28 | 30 years | ||||
| Christian S. Frandsen | 2016 | Liraglutide versus no liraglutide | 12 weeks | 18 | 39.5 | 8.8% | 75.83 | 24.17 | 18.33 years |
| 18 | 36.1 | 8.7% | 74.89 | 22.75 | 19.56 years |
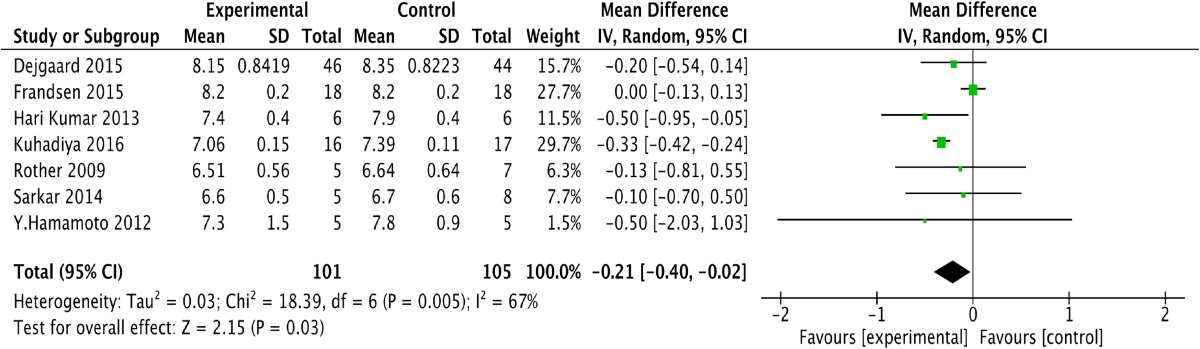
We obtained one forest plot on the effect of the GLP-1 and insulin combination treatment in patients with T1DM. One study reported that the level of HbA1c at the end of intervention in the placebo group was calculated using the following formula: S change2 = S baseline2 + S endpoint2-2*R*S baseline*S end point [17]. Table 2 shows the changes in HbA1c levels in both groups and illustrates that GLP-1 and insulin combination therapy led to a greater reduction in HbA1c [P = 0.03; −0.21; (−0.40, 0.02)] than the control treatment, with higher heterogeneity (I 2 = 67%). Sensitivity analysis indicated that this heterogeneity may have resulted from a study by the Frandsen group. We determined that the possible reasons for this outcome may have been a shorter intervention interval (12 weeks) [14]. Therefore, we think that the GLP-1 and insulin combination treatment can lead to an obvious reduction in HbA1c compared to control treatments.
Table 2.
Forest plot showing the effect of combined glucagon-like peptide-1 receptor agonist (GLP-1RA) and insulin therapy versus control treatments on changes in HbA1c
CI confidence interval, SD standard deviation, df degrees of freedom, IV inverse variance, HbA1c glycated hemoglobin
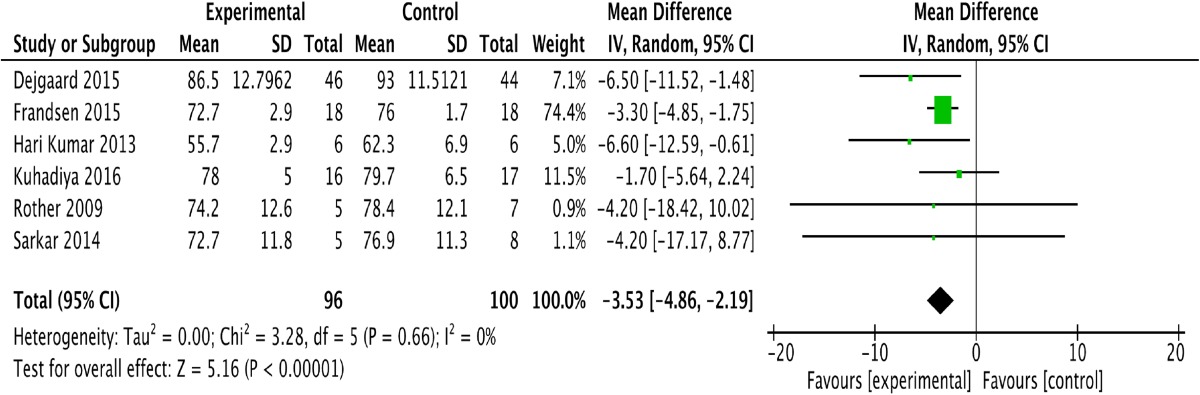
Six of the included studies assessed weight change before and after treatment [12–17]. One study conducted in Japan did not assess the effect of the GLP-1 and insulin combination treatment on T1DM [11]. We evaluated the remaining six studies based on weight change. As shown in Table 3, the GLP-1 and insulin combination therapy resulted in an obvious reduction in weight [P < 0.05; −3.53; (−4.86, 2.19)] in T1DM patients, without heterogeneity (I 2 = 0).
Table 3.
Forest plot showing the effect of combined glucagon-like peptide-1 receptor agonist (GLP-1RA) and insulin therapy versus control treatments on changes in weight
CI confidence interval, SD standard deviation, df degrees of freedom, IV inverse variance
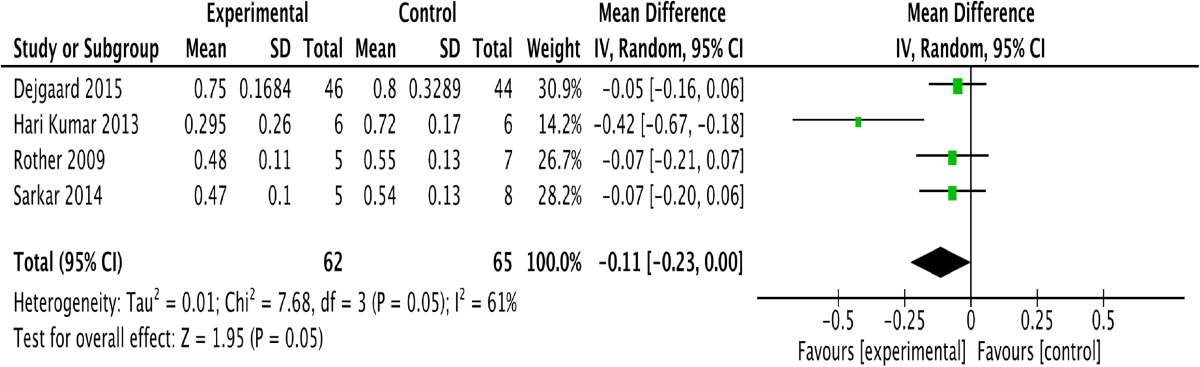
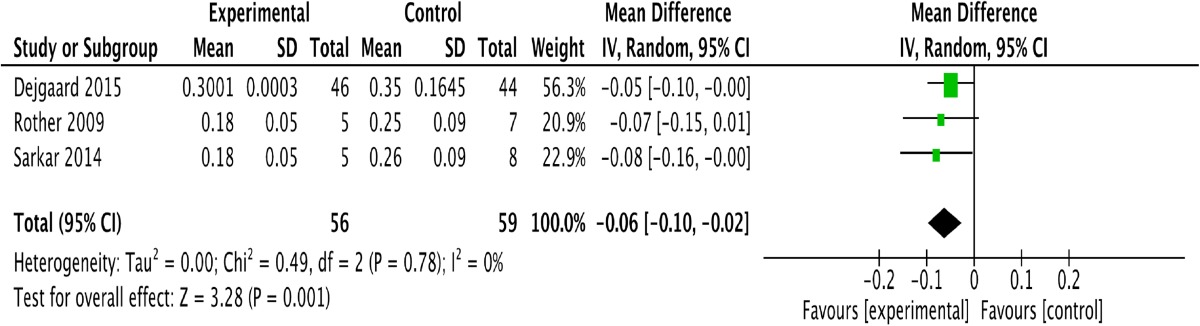
Five studies assessed changes in the daily weight-adjusted total insulin dose [12, 13, 15, 16]; one study among them did not provide these data at the end of intervention [11]. Table 4 shows that the GLP-1 and insulin combination therapy did not influence the daily weight-adjusted total insulin dose [P = 0.05; −0.11; (−0.23, 0.00)]. Four studies provided data on changes in the daily weight-adjusted bolus insulin dose after intervention [12, 14–16]. We excluded one study [14] based on the results of sensitivity analysis because the difference in baseline data between the experimental group and the control group interfered with the conclusion; this excluded paper’s conclusion regarding the bolus insulin dose was the same as ours. Table 5 shows that the combined treatment of GLP-1 and insulin led to a decrease in the daily weight-adjusted bolus insulin dose [P = 0.001; −0.06; (−0.10, 0.02)], without heterogeneity (I 2 = 0).
Table 4.
Forest plot showing the effect of combined glucagon-like peptide-1 receptor agonists (GLP-1RAs) and insulin therapy versus control treatments on changes in the daily total insulin dosage
CI confidence interval, SD standard deviation, df degrees of freedom, IV inverse variance
Table 5.
Forest plot showing the effect of combined glucagon-like peptide-1 receptor agonist (GLP-1RA) and insulin therapy versus control treatment on changes in the daily bolus insulin dosage
CI confidence interval, SD standard deviation, df degrees of freedom, IV inverse variance
Five studies described hypoglycemic events [12–14, 16, 17]. Three studies showed no significant differences between treatment and control groups [13, 14, 17], and two studies showed that combination therapy led to a reduction in hypoglycemic event rates [12, 16]. Based on these findings, we concluded that the combination treatment does not increase the risk of hypoglycemia.
Four studies showed that the administration of GLP-1 agonists was associated with more frequent adverse gastrointestinal events, such as nausea, dyspepsia, diarrhea and vomiting [12–14, 17]. However, most patients could tolerate and gradually adapt to these side effects, which did not obviously impact the curative effect.
The risks of bias are shown in Fig. 2. There was a risk of performance bias and selection bias in our meta-analysis. The reason for the bias was that several included studies involved open-label treatments without a blinded strategy. However, we do not think this had a significant influence on the final result.
Fig. 2.
Assessment of bias among the included studies
Discussion
In the present study, we evaluated the curative effect of GLP-1RA and insulin combination treatment in patients with T1DM. Changes in HbA1c were the primary outcome, and changes in weight and insulin dose were the secondary outcomes. Our results show that the GLP-1RA and insulin combination therapy improved the control of HbA1c better than insulin monotherapy, without an increased risk of hypoglycemia. The combination therapy also led to a significant reduction in weight, which is beneficial for addressing obesity. Additionally, this combination therapy could reduce the daily dosage of bolus insulin without influencing the daily total insulin dose. The only drawback of GLP-1RAs compared with insulin was the occurrence of more frequent adverse gastrointestinal events, which most patients could tolerate. These data support the combined therapeutic regimen of GLP-1RAs and insulin for treating patients with T1DM.
Our meta-analysis confirms that GLP-1RAs plus insulin can improve HbA1c levels in patients with T1DM. Our conclusion is convincing and has acceptable heterogeneity; however, this outcome may be influenced by intervention time, medication dosage, diet, exercise habits and remaining β-cell function. Possible mechanisms include (1) reduced intake of carbohydrates due to delayed gastric emptying caused by the GLP-1 agonist [17], (2) improved insulin sensitivity, (3) stabilization of the daily fluctuation of glucose levels, (4) promotion of the synthesis and secretion of insulin, (5) suppressed glucagon secretion and (6) an unclear mechanism through which GLP-1 improves systematic insulin-mediated blood glucose utilization (may be associated with the phosphatidylinositol 3-kinase pathway) and insulin sensitivity [18–20]. T1DM is an autoimmune disease characterized by the destruction of pancreatic β-cells. Numerous factors, including genetic and environmental factors, may be involved in triggering T1DM. The ideal therapy for T1DM should prevent the immune system from attacking β-cells. Previous studies have demonstrated that GLP-1RAs promote the synthesis and secretion of insulin, stimulate the neogenesis of β-cells, increase the replication of β-cells and reduce β-cell apoptosis [21, 22]. Therefore, GLP-1RAs are a potential treatment option for T1DM. The effect of GLP-1 on fasting glucose in T1DM patients depends on blood glucose levels, glucagon levels and remaining β-cell function [23]. Postprandial blood glucose is regulated by multiple factors, such as the rate and extent of intestinal glucose absorption, the secretion of various hormones (including insulin, glucagon and incretin) and these hormones’ influence on the metabolism of the liver and peripheral tissue. GLP-1RAs may stabilize the daily fluctuation of glucose levels in patients with type 1 diabetes. The mechanism of action may be different depending on the remaining number of β-cells in the patient. Postprandial glucose fluctuations can be controlled by transfusing GLP-1 without a bolus of insulin into T1DM patients with positive C-peptide, and the effects may be associated with delayed gastric emptying and lower glucagon levels; endogenous insulin can also act as a hypoglycemic agent. Gutniak et al. [24] performed the glucose clamp test on T1DM patients with negative C-peptide and reported that infusion of GLP-1 could reduce elevated postprandial blood glucose levels, reduce glucagon levels and increase glucose utilization [25]. Based on these results, GLP-1 may improve insulin sensitivity, although delayed gastric emptying can reduce the amount of insulin needed after a meal. GLP-1 is now thought to improve insulin sensitivity by indirectly causing weight loss, lowering blood glucose levels and lowering glucagon levels. Our data showed an obvious improvement in HbA1c levels, which is consistent with several studies [13, 17, 26, 27]; nevertheless, other studies have not detected a statistically significant difference in the level of HbA1c [11, 12, 14–16].
The most common side effects following intensive insulin treatment are weight gain and hypoglycemia [3]. Combination therapy with insulin and GLP-1RAs can effectively fix this problem. We extracted weight data from a group receiving 1.8 mg of the therapy [17] because there was heterogeneity caused by a discrepancy in baseline data in the 1.2-mg group. Our statistical analysis showed that combined treatment with GLP-1RAs plus insulin significantly reduced body weight. All included studies reported the same conclusion, and this change in weight may help improve the quality of life in patients with T1DM [28]. One study [17] provided three outcomes related to weight loss caused by three different dosages of liraglutide. Both the 1.2 and 1.8-mg liraglutide treatment groups exhibited the same reduction in weight (5 ± 1 kg), which was more than that in the 0.6-mg group (3 kg). These results indicate that the degree of weight loss may be related to the dosage of the GLP-1RAs.
According to the available data, there were no statistically significant changes in the daily weight-adjusted total insulin dose. Four studies presented a definite conclusion that GLP-1RAs can reduce the daily weight-adjusted total insulin dose [13, 15–17], and this outcome may be due to the honeymoon phase in T1DM [13, 29], the suppression of glucagon or delayed gastric emptying [30]. However, we concluded that combination therapy obviously reduces the daily weight-adjusted bolus insulin dose, as shown in several studies [12–17], which may influence the daily weight-adjusted total insulin requirement. The limitation of this conclusion is the lack of data from all included studies. We could not obtain a forest plot regarding changes in the daily basal insulin dose because of the lack of data. Data from existing research on the daily insulin requirement did not provide a consistent outcome [14, 15, 17]. Most studies [15, 17, 27, 31], except for two [12, 16], concluded that this type of therapy could not effectively reduce the dosage of basal insulin.
Frandsen and Kuhadiya’s studies also discussed the role of GLP-1RA and insulin combination therapy on systolic blood pressure (SBP) [12, 17], showing an identical mean reduction of 3 mm of mercury (mmHg), compared to control treatments. Furthermore, Kuhadiya’s study reported a reduction in the dosage of β-blockers and angiotensin-converting-enzyme (ACE) inhibitors. Another homologous retrospective study reached the same conclusion regarding SBP [32], and a similar result was found in patients with T2DM [33–35]. The combination therapy may also influence heart rate [12], and cardiovascular effects should be explored in future RCTs.
Different dosages of GLP-1RAs may have different effects on HbA1c levels, bodyweight and postprandial glucagon levels in patients with T1DM [17]. The most concerning side effects of GLP-1RAs are adverse gastrointestinal events, which were reported in most studies; however, most patients were able to tolerate these side effects.
Conclusions
Our meta-analysis provides support for an optional therapeutic regimen for patients with T1DM. Combination treatment with GLP-1RAs and insulin could achieve an ideal treatment effect on HbA1c levels, weight loss and bolus insulin dose in patients with T1DM. Furthermore, the therapy does not increase the risk of hypoglycemia. The side effects of GLP-1RAs are tolerated by most patients. Further studies are needed to investigate the treatment of T1DM patients with both GLP-RAs and insulin.
Acknowledgements
Sponsorship for this study and the article processing charges were funded by the Tianjin Health Industry Key Research Projects 15KG101 and the Tianjin Science and Technology Support Project 13ZCDSY01300. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for this version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
Pei Yu is a professor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University. Weihao Wang is a doctor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University. Hongyan Liu is a doctor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University. Shumin Xiao is a doctor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University. Shuaihui Liu is a doctor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University. Xin Li is a doctor at the Key Laboratory of Hormones and Development (Ministry of Health) in the Metabolic Diseases Hospital & Tianjin Institute of Endocrinology at Tianjin Medical University.
Compliance with Ethics Guidelines
This article was based on results from previously conducted studies, and no new studies of human or animal subjects were performed by any of the authors. In the studies included in this meta-analysis, all procedures were conducted in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Declaration of Helsinki (1964).
Data Availability
The data sets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Weihao Wang and Hongyan Liu are co-first authors of this work.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/0B98F060603CCD39.
References
- 1.Devendra D, Liu E, Eisenbarth GS, et al. Type 1 diabetes: recent developments[J] BMJ. 2004;328(7442):750–754. doi: 10.1136/bmj.328.7442.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinneen S, Alzaid A, Turk D, et al. Failure of glucagon suppression contributes to postprandial hyperglycemia in IDDM[J] Diabetologia. 1995;38(3):337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes. 2011;60:1599–1607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjems LL, Holst JJ, Madsbad S, et al. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and non diabetic subjects. Diabetes. 2003;52:380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 6.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 7.Peffetti R, Zhou J, Doyle ME, et al. Glugacon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose intolerant rats[J] Endocrinology. 2000;141(12):4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Hamamoto Y, Mori K, Honjo S, et al. One-year effects of liraglutide on pancreatic beta cell function and glycemic control in Japanese type 1 diabetes with residual insulin secretion. Diabetologia. 2012;55:S300. [Google Scholar]
- 12.Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycemic control(Lira-1):a randomized, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:221–232. doi: 10.1016/S2213-8587(15)00436-2. [DOI] [PubMed] [Google Scholar]
- 13.Hari Kumar KV, Shaikh A, Prusty P. Addition of exenatide or sitagliptin to insulin in new onset type 1 diabetes: a randomized, open label study. Diabetes Res Clin Pract. 2013;100:e55–e58. doi: 10.1016/j.diabres.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Frandsen CS, Dejgaard TF, Holst JJ, et al. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. 2015;38:2250–2257. doi: 10.2337/dc15-1037. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar G, Alattar M, Brown RJ, et al. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014;37:666–670. doi: 10.2337/dc13-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on β-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhadiya ND, Dhindsa S, Ghanim H, et al. Addition of liraglutide to insulin in patients with type 1 diabetes: a randomized placebo-controlled clinical trial of 12 weeks. Diabetes Care. 2016;39:1027–1035. doi: 10.2337/dc15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergman BC, Howard D, Schauer IE, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97:1663–1672. doi: 10.1210/jc.2011-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idris I, Patiag D, Gray S, Donnelly R. Exendin4 increases insulin sensitivity via PI-3-kinases-dependent mechanism: contrasting effects of GLP-1. Biochem Pharmacol. 2002;63:993–996. doi: 10.1016/S0006-2952(01)00924-8. [DOI] [PubMed] [Google Scholar]
- 20.Gedulin BR, Nikoulina SE, Smith PA, et al. Exenatide(exendin-4) improves insulin sensitivity and beta-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 21.Bregenholt S, Moldrup A, Blume N, et al. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits β-cell apoptosis in vitro. Biochem Biophy Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 23.Creutzfeldt WO, Kleine N, Willms B, et al. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptidel(7-36)amide in type 1 diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 24.Gutniak M, Orskov C, Holst JJ, et al. Antidiabetogenic effect of glucagon-like peptide-1(7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 25.Behme MT, Dupre J, McDonald TJ, et al. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;10:1–9. doi: 10.1186/1472-6823-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011;165:77–84. doi: 10.1530/EJE-11-0330. [DOI] [PubMed] [Google Scholar]
- 27.Harrison LB, Mora PF, Clark GO, Lingvay I. Type 1 diabetes treatment beyond insulin: role of GLP-1 analogs. J Investig Med. 2013;61:40–44. doi: 10.2310/JIM.0b013e318279b7d6. [DOI] [PubMed] [Google Scholar]
- 28.Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes Relat Metab Discord. 2003;27:1227–1232. doi: 10.1038/sj.ijo.0802396. [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Pawlowski B, Greulich B, et al. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care. 1992;15:66–74. doi: 10.2337/diacare.15.1.66. [DOI] [PubMed] [Google Scholar]
- 30.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care. 2011;34:1463–1468. doi: 10.2337/dc11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhadiya ND, Malik R, Bellini NJ, et al. Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. Endocr Pract. 2013;19:963–967. doi: 10.4158/EP13065.OR. [DOI] [PubMed] [Google Scholar]
- 33.Viswanathan P, Chaudhuri A, Bhatia R, et al. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13:444–450. doi: 10.4158/EP.13.5.444. [DOI] [PubMed] [Google Scholar]
- 34.Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 35.Davies MJ, Kela R, Khunti K. Liraglutide-overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207–220. doi: 10.1111/j.1463-1326.2010.01330.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.