Abstract
Acute Myeloid Leukemia is a cancer of leukemic stem cells (LSCs) with a rapid progression. It is characterized by overproduction of immature myeloid cells in bone marrow which crowds out normal hematopoietic stem cells (HSC). TIM-3, an immune regulatory molecule, is an LSC specific surface marker in AML with high expression on these cells compared to HSCs. Studies have indicated that micro RNAs (miRNAs) may play an important role in either cancer progression or suppression. Based on bioinformatics assessments, we have predicted that miR-125a-3p could be a miRNA with high suppressive activity on TIM-3 expression. The purpose of this study was to investigate the inhibitory effect of miR-125a-3p on TIM-3 gene expression in an AML cell line, HL-60, in vitro. HL-60 cells were cultured in RPMI 1640 supplied with 10 % FBS. TIM-3 expression was induced on the cells using phorbol miristate acetate. The cells were transfected with miR-125-3p for 24 h and the gene and protein expression of TIM-3 were measured using q-RT-PCR and flow-cytometery methods, respectively. The results of this study showed that miR-125a-3p has a strong silencing effect on TIM-3 gene and protein expression on HL-60 cell line. Based to our results, miR125a-3p can strongly silence TIM-3 expression in AML cell line. Thus, our results have confirmed the bioinformatics prediction of suppressive effect of miR-125a-3p on TIM-3with Mirwalk and Target Scan softwares.
Keywords: AML, TIM-3, miR-125a-3p
Introduction
Acute Myeloid Leukemia (AML) is a non-lymphoid leukemia with high morbidity and mortality [1, 2]. Until now, specific gene mutations, unregulated expression of genes and non-coding RNAs such as micro RNAs (miRNA) have been presented as possible causes for AML development [3, 4]. Because of many problems that are with conventional therapies of AML, new therapeutic approaches try to target leukemic stem cells (LSCs) [5].
Human TIM-3 (T cell immunoglobulin and mucin-3) is a specific protein including 301 amino acids and coded by HAVCR2 gene [5]. This is a membrane glycoprotein which was firstly discovered as a cell marker for Th1 CD4+ differentiated cells.
The difference of TIM-3 with other immune controller molecules is that, its expression is highly elevated in specific T cells rather than all activated T cells [6, 7]. Hence, considering various roles of TIM3, focusing on this protein may be a way for recognizing and treating immune system related diseases [8].
TIM-3 was firstly distinguished as a receptor for IFNγ which is the final product of CD4+, Th1 and CD8 + T cytotoxic-1 (TC1) [6]. Galectin-9 (Gal-9) is one of the TIM-3 ligands. Gal-9 is a soluble molecule can be extensively produced by immunological cells (especially in T and B cells), intestinal epithelial cells, endothelial must cells and fibroblasts [8]. Gal-9 expression can be increased by IFNγ which causes Gal-9 attachment to oligosaccharides sitting on TIM-3 IgV domain. Such a binding in Th1 cells induces apoptosis [6]. Therefore, TIM-3 is considered as a regulator for removing immunological responses generated by Th1 and Tc1 [7].
Gal-9/TIM-3 pathway is an index for inhibiting Th1 through TIM-3 in response to different stimuli [6]. Therefore, Gal-9/TIM-3 interaction mediates both activating and inhibiting signals, which have important roles in self-immunity and inflammation in cancers [8].
Recent studies have shown that TIM-3 expression on LSCs is higher than on HSCs. Also, TIM-3 expression on CD34+/CD38− in AML LSCs is higher than that of HSCs [7, 9]. Using anti-TIM-3 antibodies researchers have successfully inhibited AML in animal models, which introduces TIM-3 as a candidate for therapeutic approaches against AML [7].
There are extensive researches indicating the role of micro RNAs on altered expression of different proteins in cancers [10].
Micro RNAs (miRNAs) are non-coding RNAs having 18–25 nucleotides in length [11]. They are intra-cell regulatory RNAs, which regulate genes expression and do so through RNA interference pathway. The miRNAs partially bind with target mRNA via 3′-UTR region and degenerate it or inhibit its translation process [12]. MiRNA has an intricate targeting process; as one miRNA can target various mRNAs or one mRNA might be controlled by various miRNAs [13]. Although, there are various bioinformatics tools for predicting target mRNAs based on miRNA seed sequence, predicting exact targets for miRNAs is still difficult [10].
Studies on miRNAs’ expression and function have indicated that many of them have an unnatural expression in cancers. Some of them make oncogenic phenotype through decreasing expression of regulatory genes for differentiation and apoptosis. For example in stomach cancer mir-512-3p, which induces apoptosis in cancer cells via targeting Mc1-1, has low expression [14]. Some other miRNAs attenuate cancer development through targeting proto-oncogenic mRNAs and silencing them [15].
According to our bioinformatics predictions and to the increased TIM-3 expression on AML cancer cells, we planned to investigate the impact of miR-125a-3p on TIM-3 expression in AML cell line HL-60. This study might contribute to development of new micro-RNA-based treatments in future.
Materials and Methods
Bioinformatics Prediction
At the first step, Mirwalk 2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) was used for bioinformatics prediction of miRNA-TIM-3 interaction. In the “predicted targets module”, we selected “Gene-miRNA target” tab and then, we put the TIM3 RefSeq ID (NM_032782.4) in the relevant box. Input parameters was adjusted on finding 3´-UTR and 10 databases were selected. At the second step, Target Scan 7.0 (http://www.targetscan.org/) was applied to confirm bioinformatics prediction of miRNA-TIM-3 interaction predicted by Mirwalk. We put the formal gene symbol of TIM-3 (HAVCR2) in the relevant box and then, submitted the query.
Cell Culture
In current study, HL-60 cell line (Pasture Inst, Iran) was cultured in RPMI-1640 supplied with 10 % Fetal Buvin Serum (FBS) and 1 % Pen/Strep (PSF1) (Farabi, Iran).
Transfection with miRNA-125a-3p Mimic
Approximately 24 h before transfection, 105 HL-60 cells were cultured in each well of 24 well plate, in 450 μl medium. To induce the TIM-3 expression, cells were treated with 50 ng PMA (Sigma Aldrich, Germany). Afterward, the cells were transfected with 50 nM final concentration of has-miRNA-125a-3p mimic (Qiagene, Germany) using Xtremegene transfection reagent (Roche, Germany). LableIT siRNA delivery control labled with FITC (Mirus, USA) was applied as both transfection indicator and scrambled siRNA. Cells treated with only Xtremegene transfection reagent used as mock control, and cells without treatment used as negative control.
After an overnight incubation of cells, the cell culture medium (except for transfected polyplexes) was refreshed.
Cell Viability Test
Methylthiazole terazolium (MTT) assay was used for assessing the metabolic activities of HL-60 cells. MTT assay was in brief as follows: 10 µl of a 5 mg/ml MTT solution in PBS buffer (Sigma-Aldrich, Germany) was added to each well of 96-well plate (Orang, Belgium). Each well contained 90 µl medium. The medium was emptied and wells were covered after 1 h of incubation at 37 °C and 5 % CO2. Then the cells were frozen for 1 h at −80 °C (New Brunswick scientific, USA). At this time, the purple formazan product was dissolved in 100 µl/well dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Germany) for 30 min while shaking. A microplate reader (M680 Bio rad, USA) at 590 nm (reference wavelength 630 nm) was used to quantify the optical density, and viability of the cells was reported as a percentage compared with un-transfected (negative) control cells.
RNA Extraction, cDNA Synthesis and Quantitative RT-PCR
All the RNA was extracted from HL-60 cell line through RNX Plus kit (Cinnagen, Inc. Iran). The cDNA was synthesized using Revert Identify Transcription Kit (Fermentase, Germany) as instructed by its manufacturer. The resulting transcripts quantified by real time quantitative PCR on a Step One Plus™ real time DNA amplification system (Applied Biosystem, USA) along with maxims SYBER Green qPCR master mix kit (Fermentase, Germany) and specific primers for each sample. The expression level of TIM-3 was optimized versus beta-actin (ACTB) expression as house-keeping gene.
The PCR holding stage was performed one time for 10 min at 95 °C. PCR was performed for 40 cycles while the temperatures for Denaturation, Annealing and Extension were 95 °C for 15 s, 60 °C for 1 min, and 72 °C for 15 s, respectively. Quality control of the results were done by gel electrophoresis of PCR product along with melting curve analysis. Specific primers for TIM-3 and ACTB were designed with Allele ID 7.0 and were synthesized by Tag Copenhagen, Inc., Denmark. The sequences of these primers were shown in Table 1.
Table 1.
The sequences of β-Actin and TIM-3 specific primers
| β-Actin | Primer sequence |
|---|---|
| Forward | 5-TTC GAG CAA GAG ATG GCC A-3 |
| Reverse | 5-CAC AGG ACT CCA TGC CCA G-3 |
| TIM-3 | Primer sequence |
|---|---|
| Forward | 5′-CCA TCA GAA TAG GCA TCT ACA TC-3′ |
| Reverse | 5′-CCA TCA GAA TAG GCA TCT ACA TC-3′ |
Flow Cytometry Analysis
Flow cytometric analysis was done by FACS Callibuor instrument (BD Bioscience, San Jose, USA). Anti-human TIM-3-PE (eBiosciences, USA) was used to measure the expression of TIM-3 protein on HL-60 cells. The results were analyzed using Cell Quest Pro software (BD Bioscience, San Jose, USA).
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc, Chicago, USA). In order to compare the groups, one-way ANOVA test was performed. All experiments were carried out in triplicate. Results are expressed as mean percentage ± relative standard deviation, and p values <0.05 are considered as significant.
Results
The miR-125a-3p was Predicted as a TIM-3 Silencer miRNA
In the Mirwalk output, miR-125a-3p was predicted to suppress TIM-3 expression by 5/10 of chosen algorithms (DIANAmT, miRanda, miRWalk, PICTAR5 and Targetscan). “Validated targets module” showed nothing for miR-125a-3p-TIM-3 interaction which represented that no experimental study had been done to validate this bioinformatics prediction. In the Targer Scan output, context score percentile of miR-125a-3p for silencing TIM-3 expression was predicted as 97 % with an eight mer seed region.
Induction of TIM3 Expression on HL-60 Cell Using PMA
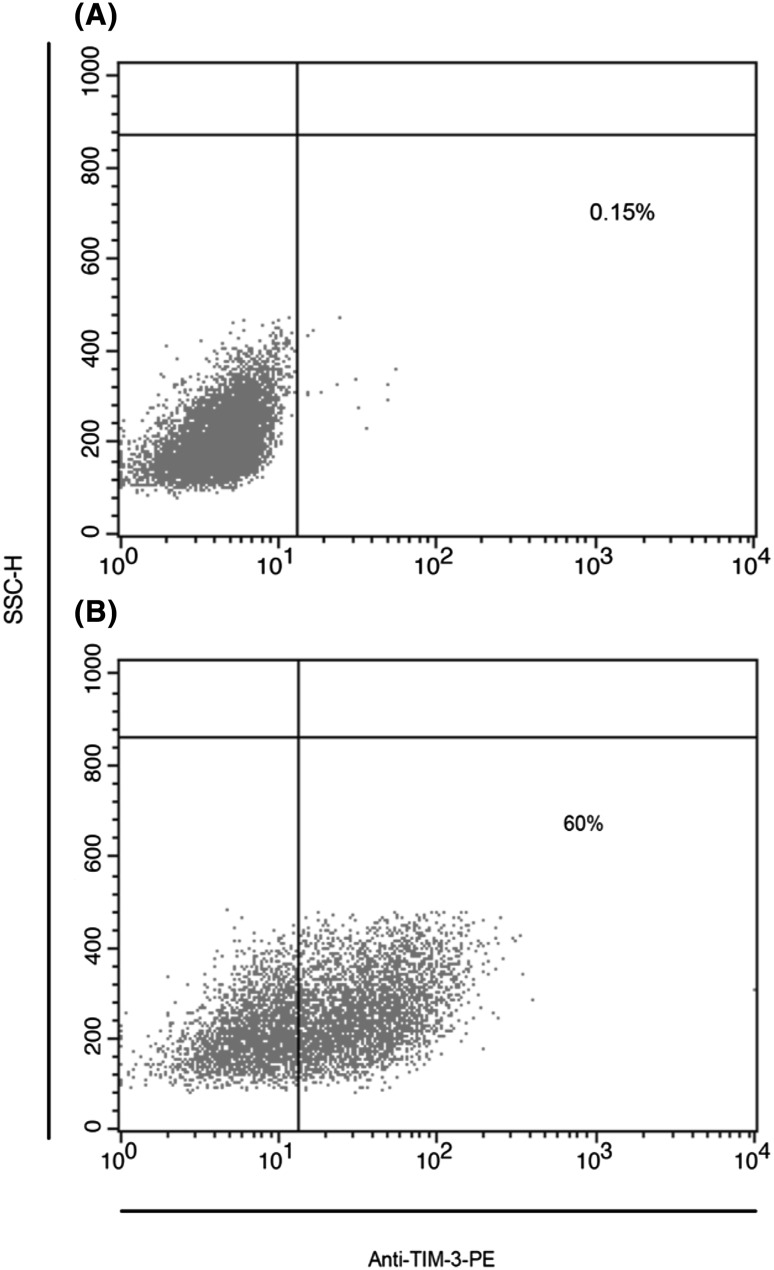
At the beginning of culturing, 50 ng PMA was added to the cultured HL-60 cells. The expression of TIM-3 protein on Hl-60 cell line was 60 % after 72 h of PMA treatment, but it was 0.08 % without PMA (Fig. 1).
Fig. 1.
Flow cytometry analysis of TIM-3 expression on HL-60 cell line. Dot plot analysis of TIM-3 expression on PMA treated HL-60 cells comparing with un-treated cells after 72 h
TIM3 Expression Suppressed on HL-60 Cell Line After Transfection with miR-125a-3p Mimic
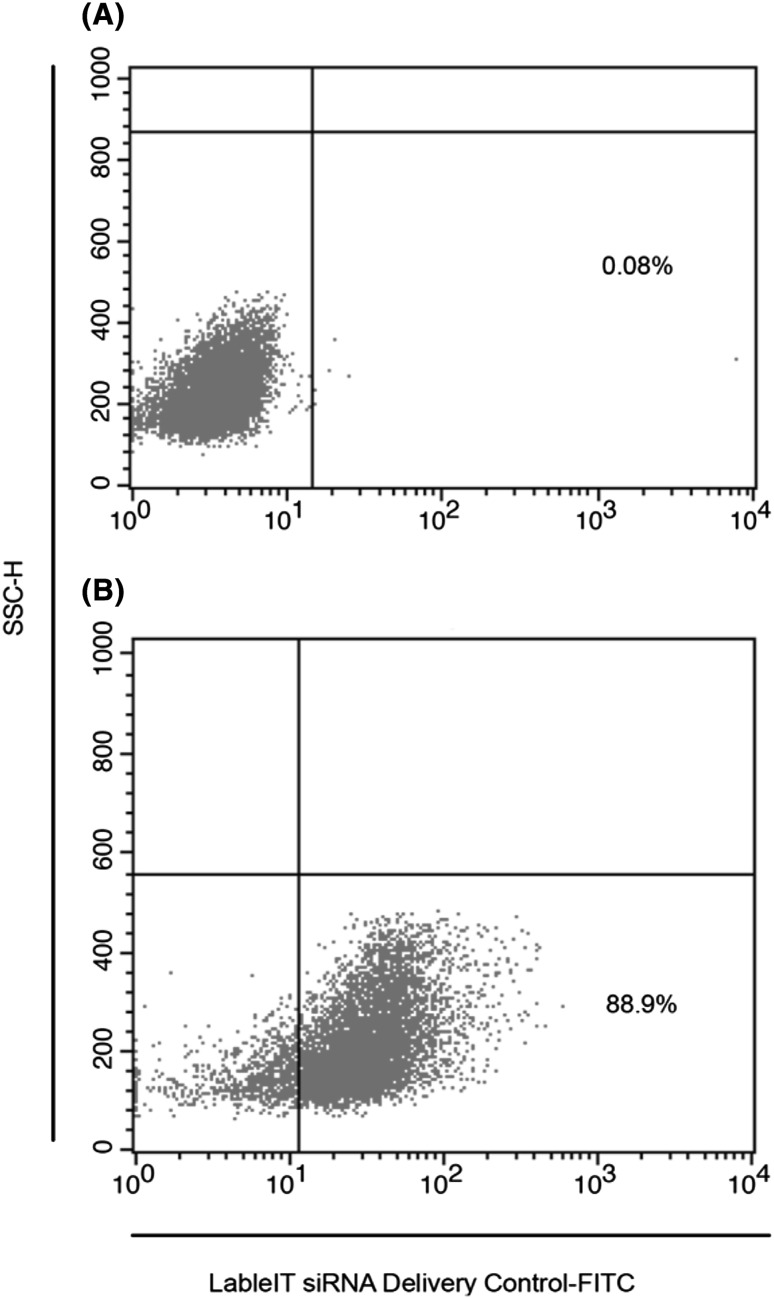
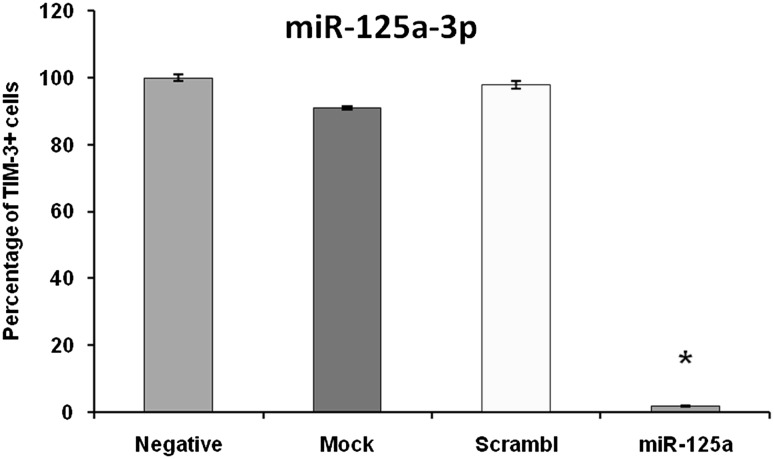
After 24 h of treatment of HL-60 cells with PMA, they were transfected with has-miR-125a-3p mimic. Using 50 nM final concentration of LableIT siRNA Delivery Control-FITC for 24 h, successful transfection of more than 90 % of the cells was confirmed with flow cytometry method (Fig. 2). After 72 h, using anti TIM-3-PE antibody, the cells were analyzed for TIM-3 expression. Comparing with un-transfected control group, flow cytometry analysis revealed that only 1.75 ± 0.04 of transfected cells expressed TIM-3 protein, which shows more than 98 % gene silencing (Fig. 3). As it is shown in Fig. 3, there was no significant difference between negative, mock and scrambled controls for TIM-3 expression. This finding confirms that the silencing was specific.
Fig. 2.
Flow cytometry analysis of HL-60 cells after transfection with FITC labled siRNA. The Dot plot shows that 90 % of cells are successfully transfected
Fig. 3.
Flow cytometry analysis for TIM3 expression on HL-60 cells after transfection with miR-125a-3p mimic. Expression of TIM-3 on transfected cells was strongly inhibited in compare with negative control (P < 0.05). There was no meaningful difference between negative, mock and scrambled controls for TIM-3 expression. Data are shown as mean percentage ± relative SD of three identical repeats of each experiment. Asterisk (*) indicates statistical significance
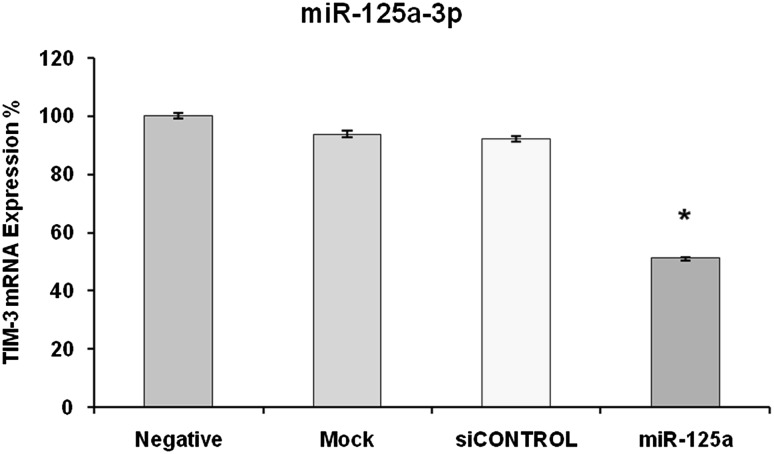
Transcript level of TIM-3 was also measured in transfected and un-transfected HL-60 cells with qRT-PCR. As it is shown in Fig. 4, TIM3 mRNA level was only 51 ± 0.56 % in compare with negative control (P < 0.05), which indicates 49 % silencing of TIM-3 gene expression. There was no significant difference for TIM-3 transcript level between negative, mock and scrambled controls (Fig. 4).
Fig. 4.
Measurement of TIM-3 mRNA level in HL-60 cells using qRT-PCR after transfection with miR125-3p mimic. In compare with negative control, TIM-3 mRNA was significantly reduced in transfected cells (P < 0.05). There was no meaningful difference between negative, mock and scrambled controls for TIM-3 mRNA level. Data are shown as mean percentage ± relative SD of three identical repeats of each experiment. Asterisk (*) indicates statistical significance
Cell line Viability Test for HL-60 After Transfection with miR-125-3p Mimic
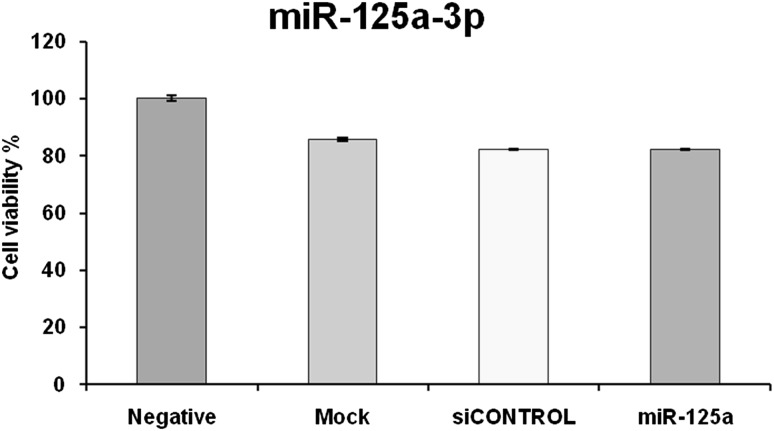
In Fig. 5, it is shown that the viability of transfected cells is 82.14 ± 0.20 % and MTT assay has not revealed any significant difference between the viability of transfected cells with negative, mock and scrambled controls (P > 0.05). This finding proved that no toxic effect of transfection reagent and/or RNA structure has affected TIM-3 gene silencing.
Fig. 5.
Cell viability assessment with MTT assay. As it can be seen in the graph, the cell viability in transfected HL-60 cells was 82.14 % which has no significant difference with negative control. There was also no meaningful difference between negative, mock and scrambled controls for cell viability. Data are shown as mean percentage ± relative SD of three identical repeats of each experiment. Asterisk (*) indicates statistical significance
Discussion
AML is a heterogenic disease characterized by defective myeloid cell lineage differentiation and proliferation which lead to their accumulation in bone marrow. Recent studies have shown that TIM-3 protein has high expression on LSCs. It is shown that TIM-3 is mainly expressed on Th1 cells and suppresses their function against cancers [16]. High expression of TIM-3 in cancers like AML suggests that its expression might be responsible for LSCs survival. In one study, Kikushige et al. [17] added Gal-9 and HMGBI to AML TIM-3 + cells and find low or zero expression of pro-apoptotic gene, BAX. Considering this finding and miRNA therapeutic function, we planned to study the effect of mir-125a-3p on TIM-3 expression on an AML cell line, HL-60.
Bioinformatically, we predicted that hsa-miR-125a-3P with a probability of 98 % may prevent the expression of TIM3 gene through an 8 mer seed region. According to our survey in the available data in different databases, inhibitory effect of this miRNA on TIM3 gene has not been yet tested or proved in vitro.
Based on our flow cytometric analyses, miR-125a-3P showed a strong suppressive effect on TIM3 protein expression on HL-60 cell line. Also, the results of our Real-time PCR demonstrated about 50 % decrease in TIM3 mRNA level, which was in line with our flow cytometry findings. Therefore, miR-125a-3P presumably inhibits TIM3 mRNA translation by steric hindrance rather than its degeneration. Despite of the extensive surveys we performed in various databases, no similar report was found for comparing with ours.
Jiang et al. [18] reported that expression of hsa-miR-125a-3p/5p was lower in NSCLC tissues than in adjacent normal lung tissues (LAC). They have shown a negative relationship between hsa-miR-125a-3p expression and pathological stage or lymph node metastasis and a contrary relationship between hsa-miR-125a-5p expression and pathological stage or lymph node metastasis. Hsa-miR-125a-3p and hsa-miR-125a-5p play distinct roles in regulation of invasive and metastatic capabilities of lung cancer cells, consistent with the opposing correlations between the expression of these miRNAs and lymph node metastasis in NSCLC. These results suggest that more studies on miR-125a family members should be performed [18].
Hashiguchi et al. [19] studied the function and clinical importance of miR-125a-3p in human gastric cancer, as well as its effect on the spreading of gastric cancer cells. They indicated that miR-125a-3p suppresses the spread of gastric cancer cells. Reduced level of miR-125a-3p expression has been reported to be associated with indicators of malignancy such as tumor size, tumor invasion, lymph node metastasis, liver metastasis, peritoneal dissemination, and advanced clinical stage. Clinical importance and tumor-suppressing effect of miR-125a-3p suggest that the role of miR-125a-3p as a strong diagnostic factor for gastric cancer cannot be ignored [19].
Lihi et al. (2014) have reported that miR-125a-3p may play a role in cellular pathways created for movement and migration of prostate cancer cells, probably through affecting the expression of Fyn and downstream proteins of Fyn. Overexpression of miR-125a-3p in PC3 prostate cancer cells reduced the migration of PC3 cells and increased the apoptosis. Confocal imaging of living cells showed that overexpression of miR-125a-3p causes a reduction in the length of path and speed of cancer cells and also a phenotypic disorder in. Studies have also shown that increased expression of miR-125a-3p reduces the activities of some proteins such as Fyn, FAK, and paxillin. According to these results, it can be stated that miR-125a-3p is able to regulate and adjust the path of movement and migration of prostate cancer cells [20].
Hao Tang et al. (2014) have shown that miR-125a can suppress the invasive ability of hepatocellular carcinoma cells by regulating the path of PI3 K/AKT/mTOR [21].
Ufkin et al. [12] studied the role of miR-125a in normal hematopoiesis. Profiling showed that the ErbB signaling pathway had significantly decreased with ectopic miR-125a expression which resulted in inhibition of cell cycle proliferation and progression with enhanced apoptosis. This revealed that ErbB inhibitors could be potential therapeutic agents for treating miR-125a-low AML [22].
All of the above studies suggest a tumor suppressor function for miR-125a-3p and its probable negative effect on AML progression which are in line with our finding showing suppressive effect of miR-125a-3p on TIM3 expression on an AML cell line.
Conclusion
In the current study, we investigated the effect of miR-125a-3p on TIM-3 expression in an AML cell line. Based on the obtained results, we suggest that miR-125-3p has a high suppressive effect on TIM-3 expression. Since TIM-3 expression is high in almost all types of AML LSCs, and because it suppresses T cell anti-tumor responses; miR-125-3p can be considered as a critical target for further studies on other aspects of AML, like leukemic cells apoptosis, proliferation and differentiation. Such studies can pave the way for future therapeutic attempts for AML and even other cancers.
Acknowledgments
This research has been done as a self-financed project in both Isfahan University of Medical Sciences and Azad University of Shahr-e kord, Iran. We would like to thank to Vida Homayouni, Zahra Hejazi and Mohammad Sadegh Hesamian for their generous technical helps.
Compliance with ethical standards
Conflict of interest
There is no conflict of interests among the authors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Becton D, Dahl GV, Ravindranath Y, Chang MN, Behm FG, Raimondi SC, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: pediatric Oncology Group Study 9421. Blood. 2006;107(4):1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperr W, Wimazal F, Kundi M, Fonatsch C, Thalhammer-Scherrer R, Schernthaner G, et al. Survival analysis and AML development in patients with de novo myelodysplastic syndromes: comparison of six different prognostic scoring systems. Ann Hematol. 2001;80(5):272–277. doi: 10.1007/s002770000280. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U, Ritter J, Schellong G. Identification of two risk groups in childhood acute myelogenous. Blood. 1990;75(10):1932–1940. [PubMed] [Google Scholar]
- 4.Sayehmiri K, Eshraghian MR, Mohammad K, Alimoghaddam K, Foroushani AR, Zeraati H, et al. Prognostic factors of survival time after hematopoietic stem cell transplant in acute lymphoblastic leukemia patients: cox proportional hazard versus accelerated failure time models. J Exp Clin Cancer Res. 2008;27(1):1–9. doi: 10.1186/1756-9966-27-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaout M, Radomski K, Srivastava D, Tong X, Belt J, Raimondi S, et al. Treatment of childhood acute myelogenous leukemia with an intensive regimen(AML-87) that individualizes etoposide and cytarabine dosages: short- and long-term effects. Leukemia. 2000;14(10):1736–1742. doi: 10.1038/sj.leu.2401906. [DOI] [PubMed] [Google Scholar]
- 6.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan M, Chao MP, Cha AC, Alizadeh AA, Gentles AJ, Weissman IL, et al. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci. 2011;108(12):5009–5014. doi: 10.1073/pnas.1100551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 9.Kikushige Y, Shima T, S-i Takayanagi, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7(6):708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhong X, Coukos G, Zhang L. miRNAs in human cancer. Next-generation MicroRNA expression profiling technology. Berlin: Springer; 2012. pp. 295–306. [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43(10):1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Giovannetti E, Erozenci A, Smit J, Danesi R, Peters GJ. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit Rev Oncol Hematol. 2012;81(2):103–122. doi: 10.1016/j.critrevonc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42(8):1273–1281. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 16.Pinkel D. Treatment of children with acute myeloid leukemia. Blood. 2001;97(11):3673–3675. doi: 10.1182/blood.V97.11.3673. [DOI] [PubMed] [Google Scholar]
- 17.Kikushige Y, Shima T, Yuda J, Miyamoto T, Akashi K, editors. Leukemogenic function of TIM-3, a leukemia stem cell marker, in acute myelogenous leukemia and myelodysplastic syndromes. ASH Annual Meeting Abstracts; 2012
- 18.Jiang L, Huang Q, Zhang S, Zhang Q, Chang J, Qiu X, et al. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010;10(1):318. doi: 10.1186/1471-2407-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashiguchi Y, Nishida N, Mimori K, Sudo T, Tanaka F, Shibata K, et al. Down-regulation of miR-125a-3p in human gastric cancer and its clinicopathological significance. Int J Oncol. 2012;40(5):1477–1482. doi: 10.3892/ijo.2012.1363. [DOI] [PubMed] [Google Scholar]
- 20.Ninio-Many L, Grossman H, Levi M, Zilber S, Tsarfaty I, Shomron N, et al. MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells. Oncoscience. 2014;1(4):250. doi: 10.18632/oncoscience.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang H, Li RP, Liang P, Zhou YL, Wang GW miR-125a inhibits the migration and invasion of liver cancer cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol Lett [DOI] [PMC free article] [PubMed]
- 22.Ufkin ML, Peterson S, Yang X, Driscoll H, Duarte C, Sathyanarayana P. miR-125a regulates cell cycle, proliferation, and apoptosis by targeting the ErbB pathway in acute myeloid leukemia. Leuk Res. 2014;38(3):402–410. doi: 10.1016/j.leukres.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]